Abstract
Objectives
High medication adherence is important for HIV suppression (antiretroviral therapy) and pre-exposure prophylaxis efficacy. We are developing sensor-based technologies to detect pill-taking gestures, trigger reminders, and generate adherence reports.
Materials and Methods
We collected interview, observation, and questionnaire data from individuals with and at-risk for HIV (N = 17). We assessed their medication-taking practices and physical actions, and feedback on our initial design.
Results
While participants displayed diverse medication taking practices and physical actions, most (67%) wanted to use the system to receive real-time and summative feedback, and most (69%) wanted to share data with their physicians. Participants preferred reminders via the wrist-worn device or mobile app, and summative feedback via mobile app or email.
Discussion
Adoption of these systems is promising if designs accommodate diverse behaviors and preferences.
Conclusion
Our findings may help improve the accuracy and adoption of the system by accounting for user behaviors, physical actions, and preferences.
Keywords: medication adherence, HIV infections, gestures, smartphone, human engineering
INTRODUCTION
“USE-MI” (Unobtrusive Sensing of Medication Intake) is a proof-of-concept, low-cost, and innovative system designed to improve medication-taking measurement and adherence. USE-MI employs a wrist-worn device and a tagged medication container to unobtrusively sense individuals’ gestures related to opening a pill bottle followed by hand-to-mouth arm movement. It uses these data to detect pill-taking behaviors, trigger pill-taking reminders to the wrist-worn device, and a smartphone app if pill-taking is not detected, and generate summative data on individuals’ adherence levels. To guide the design of this system, we conducted interviews with and observations of, and administered questionnaires to, individuals with and at-risk for HIV. Our goal was to better understand individuals’ current medication-taking practices and technology preferences. Our approach and findings may provide guidance for the development of similar systems.
BACKGROUND AND SIGNIFICANCE
Studies of HIV treatment have demonstrated that durable viral suppression (the optimal outcome) requires consistently high adherence (>90% of pills taken in previous 4 weeks) to antiretroviral therapy (ART).1–3 Similarly, when antiretroviral medications are used as pre-exposure prophylaxis (PrEP) in HIV-negative individuals, efficacy is highly dependent on adherence.4 For many patients, maintaining high levels of adherence is a challenge and, among both HIV-positive and HIV-negative individuals, self-reported adherence often overestimates adherence compared with more objective indicators.5,6 For providers, lack of reliable, real-time information about adherence makes it difficult to identify patients struggling with adherence until adverse outcomes such as treatment resistance or infection have occurred.
Issues around medication adherence extend well beyond ART and PrEP, especially with respect to the management of other complex chronic diseases. Despite significant work developing and evaluating adherence-promoting interventions, Costa.7 noted in 2015 that the degree of non-adherence had not changed in the previous decade. These adherence-promoting interventions include behavior change, educational, integrated care, self-management, risk communication, and innovative packaging and reminders.7 The theories and models on which these interventions are based are vast, including—among others—behavioral learning theory, the health belief model, social cognitive theory, theory of reasoned action, theory of planned behavior, transtheoretical model, and motivational interviewing.8,9 Clearly, this is an area where significant work remains.
The vast capabilities and low costs of wearable sensors make them an increasingly viable technology for use in medication adherence-promotion devices for HIV and other patient populations. But, these technologies alone are not sufficient. Any adherence-promoting interventions using these sensors must be based on known theoretical frameworks addressing adherence-promotion more generally. Additionally, research in the area of patient work shows that technology-based intervention outcomes are affected by a complex and evolving sociotechnical system composed of work structures and processes.10
The proposed sensor-based technologies may support more tailored reminders based on real-time, objective medication-intake data. One research team member showed that a similar approach could detect smoking gestures, and send real-time, tailored messages to the individual.11 Their algorithm detected smoking gestures with high accuracy (96%), precision (91%) and recall (81%).
MATERIALS AND METHODS
Participants (N = 17) were recruited from two clinics providing medical care and social services for persons living with and at-risk for HIV in Seattle, WA, USA. In semi-structured face-to-face interviews, we asked participants questions about how they stored, took, and remembered to take their medications and audio-recorded their answers. We also elicited their feedback about how two candidate devices (a Microsoft Band and a Sony Android Wear Watch) might support this routine. We then video-recorded participants wearing a wrist device, while taking placebo pills from a bottle. Participants also independently completed an electronic questionnaire assessing their perceived adherence levels, medication-taking practices, preferences for gaining feedback about their medication-taking patterns, and general demographic measures. Interview responses and observations were coded and reviewed by two team members. Questionnaire responses were analyzed using descriptive statistics. This study was approved by the Swedish Medical Center IRB, and all subjects gave consent for participation. Subjects were compensated with a $50 grocery store gift card for their time. Table 1 shows how these insights will guide the design of our system.
Table 1.
Topics assessed and implications for system design
| High-level topic | Sub-topic | How insights will guide design |
|---|---|---|
| Current medication-taking process | Perceived medication adherence | Which individuals should be targeted; USE-MI adoption may be higher among poor adherers |
| Current medication storage method | What storage methods USE-MI may need to accommodate | |
| Schedule of medication-taking | Timing and frequency of gesture-recognition and reminders (affects algorithm design and battery life) | |
| Types of medication dose reminders | Whether USE-MI will support or interfere with current reminder approaches | |
| Whether medications are taken with liquid or food | Whether the gesture detection algorithms should include liquid or food intake | |
| Physical act of taking medications | Hand(s) used to complete medication-taking actions | Types of gestures needing to be recognized, preferred wrist for wearing device |
| Feedback regarding the USE-MI system | Preferences for two different wrist-worn devices | Choice of wrist-worn device (Microsoft Band, Sony Android Wear Watch) |
| Preferences for reminder method | Choice of reminder method(s) (phone call, email, text, app, on wrist-worn device) | |
| Preferences for summative medication-taking feedback method | Choice of summative feedback method (email, portal, app) and recipient (self, physician) | |
| Willingness to adopt the USE-MI system | Tailor USE-MI to persons struggling with adherence to HIV-related medications |
RESULTS
Shown in Table 2, almost all 17 participants were male, HIV-positive, and taking ART. Most were between 40 and 59 years old, and they were relatively diverse in race/ethnicity. Fifteen of the seventeen participants responded via a visual analog scale what percentage of their anti-HIV medications they think they took over the past 4 weeks.12,13 Participants reported taking a median of 95% (average = 88%) of their medications, although 40% of participants reported taking <90% of pills taken in the previous 4 weeks (>90% is considered good). Participants reported taking HIV-related medications once (82%) or twice (18%) per day. Almost all participants took their medications in the morning or at night; only one participant took medications at lunch. Participants reported taking their medications from pill bottles (29%), blister packs (6%), and “pill rolls” with pills heat-sealed into bags labeled with the date and time of prescribed administration (65%). Three participants (18%) reported deviating from their normal method (eg, transferring containers because of travel) five or more days a month. All participants reported taking their medications with liquid; 35% with both liquid and food.
Table 2.
Participant characteristics
| Gender | |
| Male | 16 |
| Female | 1 |
| Age range (years) | |
| 20–29 | 1 |
| 30–39 | 0 |
| 40–49 | 4 |
| 50–59 | 9 |
| 60–69 | 1 |
| Unreported | 2 |
| Status | |
| HIV+ taking ART | 16 |
| HIV− taking PrEP | 1 |
| Race | |
| White | 7 |
| Black | 6 |
| Multiracial | 3 |
| Prefer not to answer | 1 |
| Hispanic or Latino | |
| Yes | 2 |
| No | 13 |
| Prefer not to answer | 2 |
Participants used a range of medication reminder approaches, including placing the medications in visible locations (eg, by the coffee pot) (29%), aspects of the storage method itself (eg, time and date that were stamped on the heat-sealed bags in a “pill roll,”—although some using “pill rolls” were unaware of the date/time stamps on the bags) (12%), annotating calendars or other time-related methods (29%), self-motivation or motivation from others (24%), and eliciting reminders from others (6%).
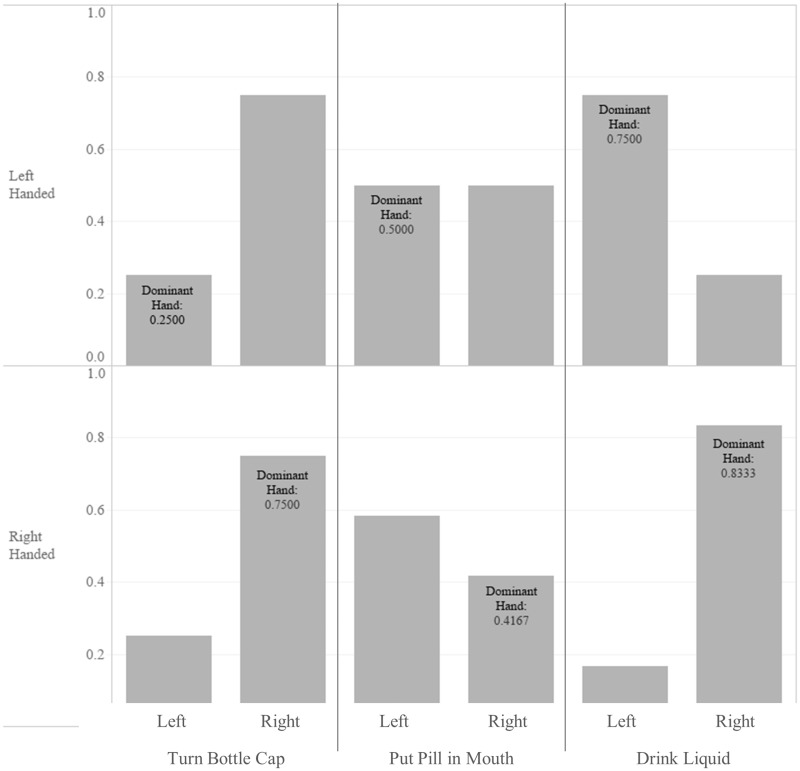
Participants exhibited varied handedness, with 71% right-handed (RH), 23% left-handed (LH), and 6% ambidextrous. When asked to wear the wrist device and take a placebo pill from a bottle (even if they typically took pills a different way), there was high variability in which hands participants used to perform medication-taking tasks. Figure 1 shows what hands participants used to complete each task, separated by the participants’ handedness. Data for the ambidextrous individual is not shown. A majority (75% of RH, 75% of LH) used their right hand to turn the pill bottle cap. Participants were quite evenly split (58.33/41.67% for RH, 50/50% for LH) in terms of which hand they used to put the pill in their mouth. Most participants (83% of RH, 75% of LH) used their dominant hand to drink the liquid.
Figure 1.
Hands used to complete medication-taking tasks.
After testing the devices, two participants (12%) stated they would only wear the Microsoft Band and two (12%) stated they would only wear the Android Wear Watch. Most participants (76%) said they would use either device, although 70% of this group preferred the Microsoft Band over the Android Wear Watch. These preferences related to a variety of criteria, including size, comfort, durability, waterproofness to withstand sweating and showering at bathhouses, style, usability of the interface, other functionality available, battery life, and the charging mechanism. One participant did not want a valuable device that might be sold or stolen (eg, to fund stimulant use).
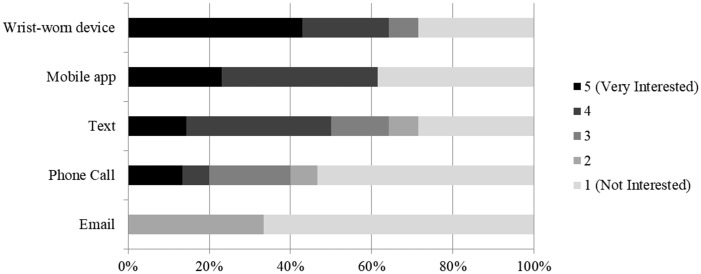
Shown in Figure 2, participants were most interested in receiving medication reminders via the wrist-worn device or a mobile app, somewhat interested in receiving reminders via text messaging. Reminders phone calls and e-mails were not of interest. Most participants (59%) wanted to receive feedback on their pill-taking over time; most (69%) also wanted to share that information with their doctor. Mobile apps (58%) and e-mail (33%) were the preferred mechanisms for receiving this summative feedback. Overall, most participants (67%) stated that they would like to use the system. Some participants felt the feedback would be helpful, but either did not want to wear the device or were concerned about the financial cost of the device.
Figure 2.
Reminder preferences.
DISCUSSION
In this study of persons taking HIV-related medications, many reported taking fewer than 90% of their pills in the previous 4 weeks (>90% is considered good) and most expressed interest in using the system. This combination of clinical need and end-user interest provide support for potential adoption of the proposed system. That said, participants displayed diverse behaviors and had varied preferences for the proposed technologies. We discuss the implications of our findings as they related to the technology form factors, gesture-detection functions, and end-user feedback approaches.
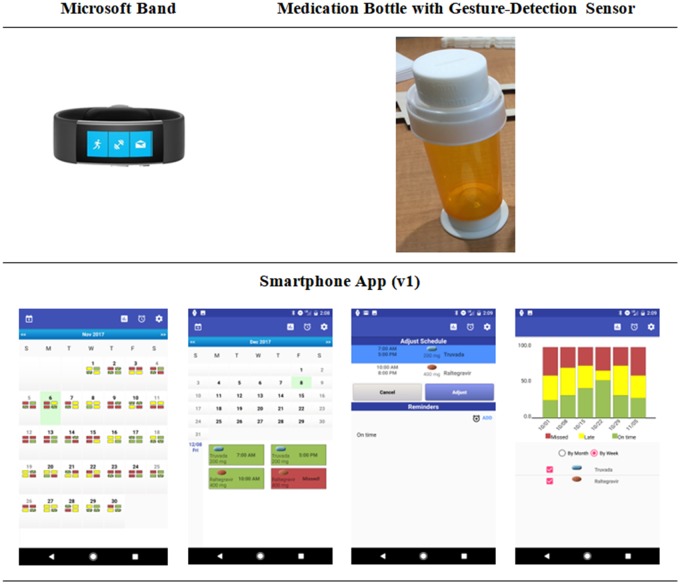
Our proof-of-concept design (Figure 3) employs three physical form factors: a wrist-worn device, a smartphone app, and a small motion-detection sensor attached to a pill bottle. Our initial proof-of-concept design requires users to store their medications in pill bottles, as the gestures related to opening a pill bottle are more distinct than opening other medication storage containers. However, in the future we will need to determine how the system could accommodate medication boxes, blister packs, and heat-sealed bags. Our initial system design uses a Microsoft Band—as it better supports implementation of the gesture detection algorithms. Our observations suggest that being able to offer choice among form factors may increase acceptability and adoption.
Figure 3.
USE-MI system components.
The observations, interviews and questionnaires gave us insight into how the gesture-detection algorithms will need to function. The timing of medications taken (one or twice/day, morning/night) was quite consistent across participants, meaning that we can use this information to anticipate the timing of the gesture-detection and reminders. The limited number of detection timeframes also has positive implications for the battery life of the gesture-detection sensors. The finding that all individuals took their medications with liquid allows us the possibility of adding this gesture to the detection algorithm. The significant variability in what hands individuals use to perform medication-taking gestures presents a significant design challenge. Regardless of handedness, most individuals turned the bottle cap with their right hand, were evenly split on what hand they used to put the pill in their mouth, and tended to drink water with their dominant hand. This finding suggests we cannot reliably detect medication taking using a single gesture. The most complex scenario is a right-handed individual wearing the device on their left wrist, as they are unlikely to turn the bottle cap or drink water with their left hand. For this group, a motion-detection sensor directly on the bottle is especially important.
Finally, there was some diversity in participants’ preferences for system feedback mechanisms, namely how they receive medication-taking reminders and summative reports. The current system design sends medication-taking reminders directly to the wrist-worn device and to the smartphone app (the two most preferred methods). Summative reports can be viewed from within the smartphone app (the most preferred method). While these align with participants’ stated preferences, future iterations of the system will ideally allow some flexibility in these methods. Participants also noted a desire to share the summative reports with their physicians; the system will therefore have the capacity to generate and send reports to the participants’ physicians.
While a large proportion of our sample of participants used pills stored in heat-sealed bags (ie, “pill rolls”), we believe this practice is uncommon based on a larger online survey (n = 96) we conducted. In that survey, 2% of respondents used pill rolls. This large proportion of our participants was likely due to two nearby community pharmacies preferring to use pill rolls. We expect that in other populations, particularly for persons on PrEP, a higher portion of individuals would use pill bottles. Fifty-percent of PrEP survey respondents used pill bottles. This is an important variable to assess in a potential user population.
Our future work will include a pilot validation study, where participants will use the system for six months. This study will allow us to better understand and improve the accuracy and efficiency of the sensors and detection algorithms. We will also be able to understand patterns of adoption and use of the system, including how we can redesign the system to make it more useful and usable.
CONCLUSION
To ensure adoption, interventions using sensor-based technologies must account for user behaviors, physical actions, and preferences. While we focused on designing an intervention for individuals with and at risk for HIV, our findings may align with the preferences and behaviors of patient populations with many other medical conditions for which medication adherence is critical.
FUNDING
This work was supported by the National Institutes of Health grant number 1R01MH109319.
Conflict of interest statement. None declared.
CONTRIBUTORS
All authors conceived of and designed the study. BS conducted participant interviews. JM, SK, and VM analyzed the interview data. JM drafted the manuscript. All authors made critical manuscript revisions, and approved the final version for submission.
REFERENCES
- 1. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 1331: 21–30. [DOI] [PubMed] [Google Scholar]
- 2. Nachega JB, Marconi VC, van Zyl GU, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets 2011; 112: 167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boussari O, Subtil F, Genolini C, et al. Impact of variability in adherence to HIV antiretroviral therapy on the immunovirological response and mortality. BMC Med Res Methodol 2015; 151 doi: 10.1186/1471-2288-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States—2014: A Clinical Practice Guideline Atlanta: Centers for Disease Control and Prevention 2014: 1–67.
- 5. Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis 2001; 338: 1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thirumurthy H, Siripong N, Vreeman RC, et al. Differences between self-reported and electronically monitored adherence among patients receiving antiretroviral therapy in a resource-limited setting. AIDS (London, England) 2012; 2618: 2399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Costa E, Giardini A, Savin M, . Interventional tools to improve medication adherence: review of literature. Patient Prefer Adherence 2015; 9: 1303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munro S, Lewin S, Swart T, Volmink J.. A review of health behaviour theories: how useful are these for developing interventions to promote long-term medication adherence for TB and HIV/AIDS? BMC Public Health 2007; 71: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conn VS, Enriquez M, Ruppar TM, Chan KC.. Meta-analyses of theory use in medication adherence intervention research. Am J Health Behav 2016; 402: 155–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holden RJ, Carayon P, Gurses AP.. SEIPS 2.0: a human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics 2013; 5611: 1669–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parate A, Chiu MC, Chadowitz C, Ganesan D, Kalogerakis E. Risq: recognizing smoking gestures with inertial sensors on a wristband. In: proceedings of the 12th annual international conference on Mobile systems, applications, and services. June 2014, 149–61. [DOI] [PMC free article] [PubMed]
- 12. Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR.. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials 2004; 52: 74–9. [DOI] [PubMed] [Google Scholar]
- 13. Kalichman SC, Cain D, Fuhrel A, Eaton L, Di Fonzo K, Ertl T.. Assessing medication adherence self-efficacy among low-literacy patients: development of a pictographic visual analogue scale. Health Educ Res 2005; 201: 24–35. [DOI] [PubMed] [Google Scholar]