Abstract
Solid organ transplantation tolerance can be achieved following select transient immunosuppressive regimens that result in long-lasting restraint of alloimmunity without affecting responses to other antigens. Transplantation tolerance has been observed in animal models following costimulation or coreceptor blockade therapies, and in a subset of patients through induction protocols that include donor bone marrow transplantation, or following withdrawal of immunosuppression. Previous data from our lab and others have shown that proinflammatory interventions that successfully prevent the induction of transplantation tolerance in mice often fail to break tolerance once it has been stably established. This suggests that established tolerance acquires resilience to proinflammatory insults, and prompted us to investigate the mechanisms that maintain a stable state of robust tolerance. Our results demonstrate that only a triple intervention of depleting CD25+ Tregs, blocking PD-L1 signals, and transferring low numbers of alloreactive T cells was sufficient to break established tolerance. We infer from these observations that Tregs and PD-1/PD-L1 signals cooperate to preserve a low alloreactive T cell frequency to maintain tolerance. Thus, therapeutic protocols designed to induce multiple parallel mechanisms of peripheral tolerance may be necessary to achieve robust transplantation tolerance capable of maintaining one allograft for life in the clinic.
Introduction
Transplantation tolerance is defined as a state of allograft acceptance in the absence of immunosuppression. In the mouse, a short course of costimulation blockade combined with the infusion of donor splenocytes results in long-term cardiac allograft survival and donor-specific tolerance, as a second heart graft of donor origin can be accepted with no further immunosuppression, while maintaining immunocompetence to reject third party allografts. Other tolerizing protocols include treatment with non-depleting anti-CD4 and anti-CD8 antibodies (refs). Our laboratories as well as others have investigated barriers that can challenge these tolerance induction protocols. Viral infections such as LCMV and Pichinde virus, bacterial infections such as Staphylococcus aureus and Listeria monocytogenes, parasitic infections such as Leishmania major, as well as toll like receptor (TLR) agonists can all prevent the induction of tolerance with anti-CD154 (1–5). In addition to infections, manipulations that increase the naïve or memory T cell precursor frequency, or those that directly target mechanisms of peripheral T cell tolerance i.e., preventing apoptosis, depleting/inhibiting regulatory cells or blocking signals through the negative regulatory PD-1/PD-L1 pathway all result in the inability to induce tolerance (6–15).
Interestingly, many of the manipulations that effectively prevent the induction of tolerance are unable to break tolerance once it has been stably established. Viral infections, including infections of graft tissue itself, did not break tolerance (1). S. aureus infections and TLR agonists given during the maintenance phase did not precipitate allograft rejection [T. Wang and L. Chen, unpublished observations and (16)]. Regulatory T cell (Treg) depletion in tolerant cardiac allograft recipients greater than 30 days post-transplantation also failed to break tolerance (11,17). In our model, only infection with Listeria monocytogenes at ≥60 days post transplantation was capable of increasing alloreactivity and precipitating acute cardiac allograft rejection, in a manner dependent on expression of MyD88, type I interferon and interleukin-6 (18). And even in this unique case of breaking of tolerance, donor-specific tolerance was reestablished once the infection was cleared (17).
These data suggest that costimulation blockade-induced transplantation tolerance can be quite robust once established and that the requirements for tolerance maintenance are likely different than those for tolerance induction. This prompted us to investigate the mechanisms controlling its maintenance. Our results from using Listeria monocytogenes to break tolerance indicated that the loss of tolerance was associated with an increase in graft-infiltrating T cells concurrent with an inability of Tregs to adequately suppress them (17,18). Thus, we hypothesized that one facet of tolerance maintenance was to keep alloreactive T cell numbers low. Furthermore, as Treg depletion alone was insufficient to break tolerance even in the presence of a new second donor-matched allograft (11,17), we hypothesized that multiple mechanisms must cooperate to keep residual alloreactive T cells in check. We used CD4+ TCR75 cells that recognize a donor Kd peptide presented on host I-Ab as a tracer population seeded during the induction or maintenance phase of tolerance to fully MHC-mismatched allogeneic cardiac allografts. Our results demonstrate that abortive proliferation of alloreactive T cells occurs during the induction phase of tolerance, and a cooperation between low numbers of alloreactive T cells, PD-L1 signals and presence of CD25+ Tregs exists at the maintenance phase of tolerance. This supports the conclusion that multiple mechanisms of peripheral tolerance cooperate to maintain long-term cardiac allograft acceptance when tolerance is robust.
Materials and Methods
Mice
C57BL/6 and BALB/c mice were purchased from Envigo RMS, Inc. (Indianapolis, IN). CD45.1 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). TCR75 TCR-Tg mice were obtained from R. Pat Bucy (University of Alabama-Birmingham) and crossed to CD45.1 mice. Mice were housed under specific pathogen-free conditions and used in agreement with the University of Chicago’s Institutional Animal Care and Use Committee, according to the National Institutes of Health guidelines for animal use.
Heart transplantation
Cardiac allograft transplantation was performed using a technique adapted from Corry et al. (19). Transplantation of cardiac allografts in the abdominal cavity was performed by anastomosing the aorta and pulmonary artery of the graft end-to-side to the recipient’s aorta and vena cava, respectively. The day of allograft rejection was defined as the first day of cessation of heartbeat as measured by palpation. In certain experiments, mice were treated with a single 400 μg dose of anti-CD25 (PC61) and/or 5 doses of anti-PD-L1 (0.5 mg, 0.25 mg, 0.25 mg, 0.25 mg, 0.25 mg) administered every other day.
Adoptive transfer of cells
Spleen and peripheral lymph node cells (from inguinal, brachial, axillary, cervical and mesenteric lymph nodes) were isolated from donor TCR75 mice. Cell counts were obtained with an Accuri C6 flow cytometer (BD Biosciences) and a subset of cells were stained for CD4 or CD8, Vβ or Vα, the congenic marker CD45.1 and CD44. The percentage of CD44lo, congenically marked TCR-Tg T cells was used to calculate the total number of cells for the adoptive transfer. TCR75 cells were predominantly CD44lo (>97%) at the time of adoptive transfer. Cells were injected retro-orbitally in 200 μl of phosphate-buffered saline (PBS).
Isolation of graft-infiltrating cells
Cardiac allografts were rinsed in situ with Hanks balanced salt solution (HBSS) containing 1% heparin. Explanted hearts were dissected into small pieces and digested for 40 min at 37°C with 400 U/ml collagenase IV (Sigma), 10 mM N-2-hydroxyethylpiperazine-N′−2-ethanesulfonic acid (HEPES, Cellgro) and 0.01% DNase I (MP Biomedicals) in HBSS (Cellgro). Digested cell suspensions were washed with an equal volume of complete Dulbecco’s Modified Eagle medium (DMEM) containing 5% fetal bovine serum, passed through a nylon mesh and centrifuged. Cells were either used directly for flow cytometry or first incubated for 4 h with phorbol myristate acetate (PMA, 50 ng/ml) and ionomycin (0.5 μg/ml) in the presence of brefeldin A (5 μg/ml).
Magnetic enrichment
CD45.1+ TCR75 cells were magnetically enriched from spleen and peripheral lymph nodes of recipient mice following staining with anti-CD45.1-bio and incubation with streptavidin magnetic beads (Miltenyi). The cells were enriched using an AutoMACs machine (Miltenyi).
Intracellular staining and flow cytometry
Single cell suspensions of lymphocytes were prepared from isolated spleens and heart grafts. Cells were stained first with a fixable live/dead stain (Aqua, Invitrogen) and then with anti-CD4 (L3T4), anti-CD8 (Ly2), anti-CD45.1 (A20), anti-Vβ8–3 (1B3.3), anti-PD-1 (J43), anti-CD44 (IM7). For interferon gamma (IFNγ) and tumor necrosis factor (TNFα) intracellular staining, cells were stimulated with PMA (Sigma), ionomycin (Sigma) and brefeldin A (BD Pharmingen) for 4 h, prior to staining for viability and surface markers. Surface-stained cells were then fixed with the Foxp3 fixation permeablization buffer kit (eBioscience) for 15 min at room temperature, washed with 1 x permeabilization buffer, stained using anti-IFNγ (XMG1.2) and anti-TNFα (MP6-XT22) for 30 min at room temperature, washed again, and analyzed by flow cytometry. In certain experiments, unstimulated cells were fixed and stained with anti-Foxp3 (FJK-16s) using the Foxp3 fixation permeablization buffer kit (eBioscience). All mAbs were from BD Biosciences or eBioscience.
Data analysis
Flow cytometry data were analyzed using FlowJo (TreeStar). Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc).
Results
Alloreactive T cells persist in an activated state in tolerant mice
Our previous results using tolerant mice infected with Listeria revealed a clear population of T cells responding to alloantigen following the infection (17,18), suggesting that if clonal deletion occurs during tolerance induction or maintenance, it must be incomplete. An alternative explanation is that clonal deletion was complete following costimulation blockade therapy, but that new thymic emigrants later restored a pool of alloreactive cells. To test the degree of deletion of alloreactive T cells without the confounding factor of new thymic emigrants, a tracer population of CD4+ Kd-reactive TCR75 cells was seeded into naïve C57BL/6 mice, either unimmunized, or prior to immunization with donor BALB/c splenocytes alone (DST), or prior to tolerization with anti-CD154, donor splenocytes and a BALB/c heart transplant (Figure 1A). At doses of 103, 104, and 105 transferred TCR75 cells, tolerance was still successfully induced in the third group, and mice retained their allografts for the duration of the experiment (MST > 60 days). Following isolation of the TCR75 cells from the spleen and peripheral lymph nodes at day 60, a similar percentage and number of TCR75 cells was recovered from tolerant mice as from naïve mice, while ~10-fold more cells were recovered from mice immunized with donor splenocytes alone (Figure 1B and Supplementary Figure 1). Thus, the quantity of alloreactive T cells in tolerant mice was not significantly reduced from that in naïve mice, where cell numbers may be decaying over time due to a lack of antigenic stimulation. The TCR75 cells isolated from tolerant mice were antigen-experienced, with similar percentages of CD44hi cells in the DST-immunized and tolerant groups (Figure 1C). These results indicate that while costimulation blockade-induced tolerance prevents accumulation of alloreactive T cells, it does not cause their complete deletion, nor does it depend on antigen ignorance, as the alloreactive T cells expressed high levels of CD44.
Figure 1: Alloreactive T cells seeded in tolerant mice are not completely deleted and are not ignorant of the graft, but are maintained at low numbers.
A. Experimental design. B. The percentages and total numbers of CD4+ TCR75 cells recovered from the spleen and peripheral lymph nodes 60 days post adoptive transfer into naïve untransplanted C57BL/6 mice, mice receiving BALB/c donor splenocytes (DST) and mice receiving BALB/c donor splenocytes, anti-CD154, and a heart transplant (Tol). n=3 mice per cell dose (103, 104, 105) per group. Different cell doses are represented by different symbols. C. Representative flow plots of percentages of CD44hi cells amongst TCR75 cells and their quantification. Different cell doses were pooled. Mean values were compared using one-way ANOVA with Bonferroni correction for pairwise comparisons ns=not significant, ***p<0.001.
Adoptive transfer of high numbers of alloreactive T cells is sufficient to break tolerance
The persistence of alloreactive T cells at low levels, led us to hypothesize that one requirement for maintaining tolerance is keeping the numbers of alloreactive T cells low. To test this hypothesis, we adoptively transferred a low number (105 cells) or a high number (2–4 × 106 cells) of TCR75 cells into tolerant mice at ≥ 60 days post-transplantation (Figure 2A). The transfer of a low dose of TCR75 cells had no effect on graft acceptance, whereas the transfer of a high dose of TCR75 cells was sufficient to precipitate graft rejection (Figure 2B, low-dose MST > day 60 vs high-dose MST=12, p=0.0096). Thus, the maintenance of tolerance is dependent on keeping the numbers of alloreactive T cells low.
Figure 2: Transfer of a high dose (HD) but not low dose (LD) of alloreactive T cells is sufficient to break established tolerance.
A. Experimental design. B. Graft survival in tolerant mice that were adoptively transferred at least 60 days post-transplantation with 105 TCR75 cells (low dose, LD, n=9) or 2–4 × 106 cells (high dose, HD, n=13), p<0.05 by log-rank test.
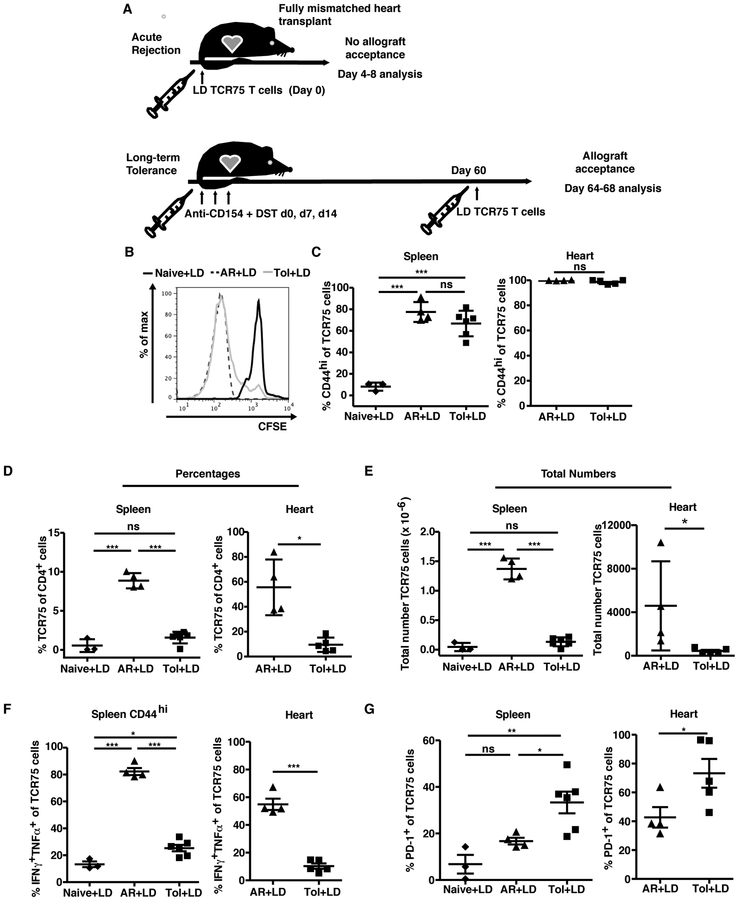
Low numbers of transferred TCR75 cells are controlled in a tolerant environment
The lack of graft rejection in tolerant mice receiving a low dose of alloreactive T cells on day 60 post-transplantation is reminiscent of previous descriptions of ‘infectious tolerance’ (20), whereby adoptively transferred naïve T cells are not only kept in check but can also acquire properties of tolerance. To understand how a low number of new alloreactive T cells could be prevented from destabilizing tolerance, we traced the fate of CFSE-labeled TCR75 cells transferred at low doses (105-2.5×105 cells) into tolerant recipients ≥ 60 days post-transplantation and compared them to those transferred into naïve untransplanted mice, or into naïve mice prior to BALB/c cardiac allograft transplantation and acute rejection (Figure 3A). TCR75 cells in tolerant recipients diluted CFSE (Figure 3B) and upregulated CD44 (Figure 3C) comparably to acutely rejecting mice, indicating that TCR75 cells in tolerant mice detected and responded to the cardiac allograft. However, the TCR75 cells failed to accumulate in tolerant mice, suggesting abortive proliferation (refs), as their percentages and total numbers remained similar to those observed in naïve mice, and were significantly lower than those in mice undergoing unmodified acute rejection (Figure 3D,E).
Figure 3: Low dose (LD) alloreactive T cells seeded during the maintenance phase of tolerance are well controlled.
A. Experimental design. B. Representative plots of CFSE dilution of 2.5 × 105 TCR75 cells adoptively transferred into naïve mice (Naïve+LD, n=3), or into mice undergoing acute rejection (AR+LD, n=3), or into tolerant mice (Tol+LD, n=3), at four days post-transfer in the spleen. C. Percentages of TCR75 cells expressing CD44hi in the spleen and heart graft 8 days post adoptive transfer. Percentages (D) and total numbers (E) of TCR75 cells recovered in the spleen and graft 8 days post adoptive transfer. Percentages of TCR75 cells expressing IFNγ and TNFα upon PMA/ionomycin restimulation (F) and PD-1 without restimulation (G) 8 days after adoptive transfer. C-G. Results were pooled from two or more independent experiments. Mean values were compared using one-way ANOVA with Bonferroni correction for pairwise comparisons or Student’s t test where appropriate. ns=not significant, *p<0.05, **p<0.01, ***p<0.001.
In addition to the lack of accumulation, lower percentages of TCR75 cells from tolerant hosts produced cytokines upon restimulation with PMA and ionomycin (Figure 3F), which suggested profound hyporesponsiveness or a reduced ability to fully differentiate into multi-functional effector cells, as restimulation with PMA and ionomycin bypasses proximal TCR signaling and would recover the function of anergic T cells. Phenotypically, despite similar proliferation and acquisition of CD44hi expression (Figure 3B,C), a higher proportion of TCR75 cells expressed the negative costimulatory molecule PD-1 when transferred into tolerant recipients than into rejecting mice (Figure 3G). Of note, the TCR75 cells themselves do not upregulate Foxp3 in a tolerant environment [data not shown and (21)] and therefore might be intrinsically controlled through PD-1/PD-L1 signals and/or extrinsically controlled through host regulatory cells. In sum, the tolerant environment did not prevent low-dose TCR75 cells from proliferating or becoming activated, but did result in a greater proportion of cells expressing PD-1 and in prevention of their accumulation and cytokine production.
Alloreactive T cells transferred in high numbers are controlled in the periphery but not in the graft
Because transfer of high numbers but not low numbers of TCR75 cells precipitated rejection in already tolerant recipients, we traced the fate of TCR75 cells when transferred at high numbers into tolerant or control animals. Surprisingly, numbers of TCR75 cells recovered from the spleens of tolerant mice 5 days after transfer were significantly lower than those from acutely rejecting recipients, and comparable to the numbers recovered from naïve mice (Figure 4A). Cytokine production by TCR75 cells from tolerant mice was dramatically reduced compared to TCR75 cells from acutely rejecting mice (Figure 4B), similar to the observation with low numbers of TCR75 cells (Figure 3F), and was increased relative to cells from naïve mice. Finally, the proportion of TCR75 cells expressing PD-1 was high and persistent in tolerant mice (Figure 4C) compared to TCR75 cells from acutely rejecting or naïve mice. Extrapolation of the data from Figures 3 and 4 suggests that the phenotype and function of TCR75 cells recovered from the spleen was unexpectedly similar in tolerant mice injected with low versus high numbers, despite tolerance being maintained versus lost, respectively.
Figure 4: HD alloreactive T cells are controlled in the periphery of tolerant mice but not in the graft.
A-C. TCR75 cells injected at high doses (2–4 × 106 cells, HD) into naïve mice or tolerant mice 60 days post transplantation and were recovered from spleens 5, 10, or 14 days later (n=2–4 mice per group per time point). A. Total numbers of TCR75 cells in the spleen. Percentages of splenic TCR75 cells expressing IFNγ and TNFα upon PMA/ionomycin restimulation (B) and PD-1 without restimulation (C) on the indicated days post adoptive transfer. Mean values for each time point were compared using two-way ANOVA with Bonferroni correction for pairwise comparisons ###p<0.001, between tolerant and naïve groups ***p<0.001, between tolerant and acute rejection groups. D-F. Graft-infiltrating cells were analyzed 7–14 days post adoptive transfer into tolerant mice (n=10–12 mice per group). D. Percentages and total numbers of Foxp3+ cells within TCR75 and endogenous CD4+ T cells in the graft. E. Total numbers of graft-infiltrating TCR75 cells and endogenous CD4+ and CD8+ T cells. F. Total numbers of IFNγ-producing graft-infiltrating TCR75 cells and endogenous CD4+ and CD8+ T cells. Results were pooled from three or more independent experiments. Mean values of total graft T cells were compared using one-way ANOVA with Bonferroni correction for pairwise comparisons *p<0.05, **p<0.01, ***p<0.001.
We hypothesized that the loss of tolerance with high dose TCR75 cell transfer was due to an increase in alloreactive T cells numbers in the allograft thereby overwhelming endogenous Tregs. We first investigated whether the percentages of Foxp3+ cells were decreased within the grafts of high dose transfer mice. However, this was not the case as Treg percentages in the grafts were similar whether tolerant mice received a low or high dose of TCR75 cells (Figure 4D). In contrast, the numbers of T cells that accumulated in the allografts, and specifically the numbers of IFNγ-producing T cells, were significantly greater in the tolerant mice receiving high dose TCR75 cells when compared to the other groups (Figure 4E,F). This suggests that despite an increase in intragraft Treg numbers (Figure 4D) to counter the increase in effector T cell numbers, resulting in equivalent Treg:Tconv ratios to the non-transfer group, intragraft effector T cells that accumulated following high dose TCR75 transfer were inadequately controlled.
Cell-intrinsic and cell-extrinsic mechanisms cooperate to control alloreactive T cells in a tolerant environment
Our data indicate that controlling the numbers of alloreactive T cells, to levels observed in naïve mice, is essential in order to maintain tolerance, and this may be achieved through cell-intrinsic and/or cell-extrinsic mechanisms. To investigate the role of these mechanisms, tolerant mice were treated with either anti-CD25 to deplete Tregs, anti-PD-L1 to block signals from PD-1/PD-L1, alone or in combination with an infusion of low dose (105) TCR75 cells, and graft survival was assessed (Figure 5A). PD-L1 has been shown to be the dominant ligand for PD-1 in transplantation settings (22,23) and blocking anti-PD-L1 antibodies have been shown to have similar effects as anti-PD-1 antibodies (24). Each treatment of Treg depletion, anti-PD-L1 administration or low-dose TCR75 transfer given alone or two treatments given in combination were insufficient to induce the loss of tolerance, and only the triple combination was sufficient to break tolerance in a majority of the mice (Figure 5B). We conclude that multiple mechanisms cooperate to maintain robust transplantation tolerance.
Figure 5: Maintenance of tolerance of primary grafts is dependent on controlling the size of the alloreactive T cell pool through regulatory T cells and signals from PD-L1.
A. C57BL/6 mice were transplanted with BALB/c hearts and treated with anti-CD154+DST. On day >45 post-transplantation, tolerant recipients were treated with individual or combined treatments to block pathways of peripheral tolerance. B. Graft survival following transfer of naïve TCR75 T cell (105), blockade of PD-L1, or administration of anti-CD25 alone or in combination. Graft rejection was significantly induced only when all 3 therapies were combined (triangles, p<0.05).
Discussion
Recent advances towards achieving transplantation tolerance in the clinic are heightening the need to better identify the mechanisms required for maintaining tolerance in a robust and durable manner. Reports that some tolerant patients acutely reject their graft, often following an infectious episode (25), suggest that tolerance in some individuals may not be sufficiently stable and robust to preserve life-long allograft acceptance. Anti-CD154 and DST, without bone marrow cell transplantation, can achieve a robust state of peripheral allograft tolerance that is resistant to many inflammatory challenges (1,16). To understand why tolerance is so robust in this model, we investigated the fate of transferred TCR-transgenic alloreactive CD4+ T cells in C57BL/6 mice treated with anti-CD154 and DST to induce tolerance to MHC- and non-MHC mismatched BALB/c cardiac grafts.
The seeding of low numbers of TCR75 T cells (103-105) at the time of transplantation permitted tolerance to develop normally, and revealed that clonal deletion of these cells was incomplete and that these cells persisted to day 60 post-transplantation at levels comparable to naïve, untransplanted mice. This partial deletion and persistence was similar to recent results by Chai et al. using TCR75 cells seeded in a model of single minor antigen-expressing skin transplantation (21) and to earlier results tracking TCR-Tg CD8+ cells in synchimeric recipients of allogeneic skin grafts (26,27). Alloreactive T cell apoptosis has been shown to be an important mechanism for tolerance induction with costimulation blockade (8); however, our data highlight that this process can be incomplete and tolerance of remaining T cells still needs to occur. Interestingly, whereas in the DST-immunized group there was a positive correlation between the number of TCR75 T cells seeded and the number of these cells recovered 60 days later (Spearman r=0.85, p=0.02), in the tolerant mice the same number of TCR75 cells were recovered at day 60 post-transplantation, irrespective of the dose of cells transferred suggesting strong constraints on the frequency of alloreactive T cells for the successful induction and maintenance of transplantation tolerance. These observations therefore complement and extend the well established notion that a high initial precursor frequency of naïve and memory alloreactive T cells presents a barrier to tolerance induction with costimulation blockade (6,24,28).
The long-term persistence of low numbers of TCR75 T cells adoptively transferred during the initiation of tolerance induction could be due to cell-intrinsic changes turned on only during the tolerance induction phase. To control for this possibility, we transferred low numbers of TCR75 T cells into tolerant mice at 60 days after transplantation. Similar to adoptive transfer in the tolerance induction phase, alloreactive T cells transferred during the maintenance phase of tolerance did not undergo complete deletion but were maintained at levels comparable to naïve untransplanted mice. However these TCR75 cells were only limited in their accumulation and function, but not in their proliferation or acquisition of the activation marker CD44. This observation is consistent with abortive proliferation that has been described during the tolerance induction phase, whereby T cells proliferate but then undergo apoptosis leading to a lack of net accumulation of these cells (6,21,29). In addition, this observation is also consistent with studies showing that CD4+ T cells proliferate when encountering self-antigens and yet become tolerant (30), and that adoptively transferred naïve CD8+ T cells into tolerant skin graft recipients have limited cytokine production and cytotoxicity without reduction in proliferation (31). Thus, the net constraints on non-tolerant alloreactive T cells introduced into a tolerant environment are similar for both CD4+ and CD8+ T cells, and provide mechanistic insights into infectious tolerance of new thymic emigrants into a tolerant host.
In the tolerant environment, the transferred TCR75 cells acquired sustained PD-1 upregulation in both the spleen and the graft. We have previously shown elevated PD-1 expression on intra-graft polyclonal regulatory T cells in long-term tolerant mice, compared to peripheral Tregs in the same mice or to Tregs from naïve mice (17). We also find PD-1 expressed on polyclonal effector T cells in tolerant allografts (M. Daniels et al., in preparation). The expression of PD-1 by T cells infiltrating the graft is not surprising, because its expression is dependent on antigen encounter and TCR stimulation (32); however the greater proportion of cells expressing this marker in tolerance than acute rejection in both the spleen and the graft may reveal a different quality or duration of T cell activation in these two environments. PD-1 expression on TCR75 cells in the spleen, albeit at lower levels than in the graft, suggests that there is persistent antigen presentation in the spleen or that these T cells are recirculating between the graft and the secondary lymphoid organs or both. Because TCR75 cells recognize a peptide from donor H-2Kd in an indirect manner, it is likely that there will be a continuous supply of cognate antigen for the lifespan of the graft. Whether T cells recognizing donor MHC II indirectly have fates distinct from those of TCR75 cells, because of different levels and duration of antigen expression, remains to be investigated (33). It is also unknown whether alloreactive T cells recognizing alloantigens directly would have the same fate in a tolerant environment as the TCR75 cells which use indirect recognition.
Precipitation of acute allograft rejection following adoptive transfer of high numbers of TCR75 cells into tolerant recipients correlated with increased intragraft accumulation of T cells and higher cytokine production when compared with transfer of low numbers of TCR75 cells. Interestingly, not only did greater numbers of TCR75 accumulate in these grafts, but endogenous T cell numbers were also elevated. Though the specificity of these endogenous T cells is unknown, this is reminiscent of recent reports indicating that TCR-mediated migration of alloreactive T cells into a transplant opens the door to chemokine-dependent migration of non-graft reactive effector T cells (34).
It was surprising to observe as high a percentage of Tregs in the grafts of tolerant animals that had received high doses of TCR75 cells and went on to reject their grafts, as in those of fully tolerant hosts. Indeed, it is generally thought that the ratio of intragraft Tregs to Teffector cells determines tolerance versus rejection. Because the specificity of the intragraft Tregs cannot be assessed in this model, it is possible that not all Tregs were alloreactive and could suppress the effector T cells in this setting. Moreover, effector and memory T cells have been shown to be more resistant to the suppressive effect of Tregs (35) perhaps explaining their ability to produce IFNγ and reject the established allografts.
Depletion of Tregs at the maintenance phase of tolerance in skin-transplanted mice treated with combined coreceptor/costimulation blockade has been shown to be sufficient to precipitate rejection (24,36) whereas it is not sufficient in the cardiac model of tolerance following costimulation blockade therapy (11). In our experiments, even in the context of 105 more alloreactive T cells, Treg depletion was unable to precipitate graft rejection, showing at least in this model of allograft tolerance, that T cell-intrinsic mechanisms of tolerance, such as through PD-1/PD-L1 interactions probably play a key role. Indeed only when adding anti-PD-L1 to Treg depletion in the context of an increased frequency of alloreactive T cells did graft rejection ensue. While we believe this is most likely due to blocking PD-L1 on antigen presenting cells and preventing it from ligating to PD-1 on the TCR75 cells and endogenous T effector cells, we cannot exclude the possibility that it also blocks PD-L1 signals to PD-1 on any remaining endogenous regulatory cells that escaped depletion with the anti-CD25 treatment.
Blocking T cell-intrinsic PD-1/PD-L1 signaling alone was also insufficient to cause rejection in mice made tolerant with anti-CD154 and DST. However, this same intervention has been shown to be effective at inducing cardiac allograft rejection in mice made tolerant with CTLA4-Ig (22), in mice made tolerant to islet allografts with anti-CD3 (37), and in synchimeric mice with long-term single-mismatch skin grafts following anti-CD154 and CTLA4-Ig treatment (24). It is currently unknown to what extent each peripheral tolerance mechanism is induced and maintained following different costimulation-blockade tolerance induction strategies, but this may suggest that anti-CD154 + DST induces more robust tolerance than CTLA4-Ig, at least at the doses used. Investigating differences in efficacy of T cell control with these two regimens is the subject of future research to identify additional pathways that can be targeted in patients receiving CTLA4-Ig to strengthen its ability to induce immunological hyporesponsiveness to an allograft.
It is also unknown to what extent each peripheral tolerance mechanism is engaged in other models of tolerance, such as in models of spontaneous transplantation tolerance. Kidney allografts can be spontaneously accepted between particular strain combinations of mice. One study found that Treg depletion alone was sufficient to break tolerance (38). This may suggest that the spontaneous tolerance in this model is less robust than that induced with anti-CD154+DST but future research should determine how many peripheral tolerance mechanisms are involved in this kidney transplant model.
Our data collectively show that a tolerant environment can constrain the numbers of alloreactive T cells, but it can be overwhelmed and allograft rejection can occur with increased frequency of alloreactive T cells, or when multiple mechanisms of peripheral tolerance are compromised. To reveal the importance for preserving low numbers of alloreactive T cells for tolerance maintenance, our model relied on the adoptive transfer of high numbers of TCR-Tg T cells. There are several limitations to using TCR-Tg T cells. The cells all have the same specificity and affinity, and are not able to reflect endogenous populations of differing affinities that would be able to compete with one another. While the specific affinity of TCR75 cells is not known, most TCR-Tg models are of high affinity T cells and may not be representative of endogenous polyclonal T cell responses. However, despite these known limitations, our results with this model may reveal an important underlying mechanism for tolerance maintenance. Indeed of the documented interventions that result in a loss of tolerance during the maintenance phase, almost all point to a loss of Tregs’ ability to control an increased endogenous alloreactive effector T cell pool. These interventions include Listeria infection, high dose IL-2, lymphodepletion, mast cell degranulation, agonistic CD40 mAb, and peptide immunization in the absence of persistent donor antigen (16,18,39–41). Of these documented interventions that result in a loss of tolerance during its maintenance phase, This interpretation is supported by our data showing that transfer of high numbers of alloreactive T cells alone is sufficient to precipitate rejection.
Further research into the kinetics and durability of each of the peripheral mechanisms of tolerance induced with costimulation blockade will help predict effective combinations of new or existing drugs in the clinic for tolerance protocols that simultaneously or sequentially induce multiple mechanisms of tolerance in patients. Having a better understanding of the mechanisms that are compromised when tolerance is lost in different settings may also lead to more targeted interventions that can either reinforce particular arms of the tolerance repertoire that may have been compromised by infections, or bolster the existing unaffected pathways.
Supplementary Material
Supplementary Figure 1: Gating of TCR75 cells adoptively transferred into mice at the time of tolerance induction. TCR75 cells were identified by co-expression of CD45.1 and Vβ8.3 60 days post-adoptive transfer into naïve mice, mice treated with DST, and mice treated with anti-CD154+ DST and a heart transplant. Non-enriched fractions were used to determine the percentages of TCR75 cells amongst CD4+ T cells, while CD45.1-enriched fractions were used to determine total numbers of TCR75 cells. Representative plots of n=3 mice per group per cell dose are shown.
Acknowledgements
M.L.M. was funded by AHA predoctoral fellowships (13PRE14550022 and 15PRE22180007), a Cardiovascular Pathophysiology and Biochemistry Training Grant (T32 HL07237), and an HHMI Med-into-Grad Program training grant (56006772). The work was also supported by NIAID P01AI-97113 to A.S.C. and M.-L.A.
Abbreviations:
- ANOVA
analysis of variance
- DMEM
Dulbecco modified Eagle medium
- DST
donor splenocyte transfusion
- HBSS
Hanks balanced salt solution
- HEPES
N-2-hydroxyethylpiperazine-N′−2-ethanesulfonic acid
- IFNγ
Interferon gamma
- n
number in group
- ns
not significant
- P
probability
- PBS
phosphate-buffered saline
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death ligand-1
- PMA
phorbol myristate acetate
- TNFα
Tumor necrosis factor alpha
- TCR-Tg
T cell receptor transgenic
- Tregs
regulatory T cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References:
- 1.Welsh RM, Markees TG, Woda BA, Daniels KA, Brehm MA, Mordes JP, et al. Virus-Induced Abrogation of Transplantation Tolerance Induced by Donor-Specific Transfusion and Anti-CD154 Antibody. J Virol. 2000. March 1;74(5):2210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed EB, Wang T, Daniels M, Alegre M-L, Chong AS. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant. 2011. May;11(5):936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T, Chen L, Ahmed E, Ma L, Yin D, Zhou P, et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008. May 1;180(9):5991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169(7):3686–93. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006. October;6(10):2282–91. [DOI] [PubMed] [Google Scholar]
- 6.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007. February 19;204(2):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004. January;10(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999. November;5(11):1303–7. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder G, Risch K, Kotsch K, Siepert A, Brock J, Nickel P, et al. FTY720 prevents anti-CD4 mAb-induced tolerance but cannot reverse established tolerance in a rat kidney transplantation model. Am J Transplant. 2004. June;4(6):863–71. [DOI] [PubMed] [Google Scholar]
- 10.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005. April 4;201(7):1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X, Sun W, Guo D, Cui Z, Zhu L, Lin L, et al. Cardiac allograft acceptance induced by blockade of CD40-CD40L costimulation is dependent on CD4+CD25+ regulatory T cells. Surgery. 2011. March;149(3):336–46. [DOI] [PubMed] [Google Scholar]
- 12.Lal G, Nakayama Y, Sethi A, Singh AK, Burrell BE, Kulkarni N, et al. Interleukin-10 From Marginal Zone Precursor B-Cell Subset Is Required for Costimulatory Blockade-Induced Transplantation Tolerance. Transplantation. 2015. September;99(9):1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu L-F, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006. August 31;442(7106):997–1002. [DOI] [PubMed] [Google Scholar]
- 14.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005. June 1;174(11):6648–56. [DOI] [PubMed] [Google Scholar]
- 15.Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008. September 1;112(5):2149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, et al. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009. October;9(10):2270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller ML, Daniels MD, Wang T, Chen J, Young J, Xu J, et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat Commun. 2015;6:7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang C-R, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010. July;10(7):1524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973. October;16(4):343–50. [DOI] [PubMed] [Google Scholar]
- 20.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. “Infectious” transplantation tolerance. Science. 1993. February 12;259(5097):974–7. [DOI] [PubMed] [Google Scholar]
- 21.Chai J-G, Ratnasothy K, Bucy RP, Noelle RJ, Lechler R, Lombardi G. Allospecific CD4(+) T cells retain effector function and are actively regulated by Treg cells in the context of transplantation tolerance. Eur J Immunol. 2015. July;45(7):2017–27. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. Journal of Immunology. 2007;179(8):5204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Han R, Hancock WW. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol. 2007. October;37(10):2983–90. [DOI] [PubMed] [Google Scholar]
- 24.Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, et al. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol. 2008. October 15;181(8):5313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brouard S, Pallier A, Renaudin K, Foucher Y, Danger R, Devys A, et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant. 2012. December;12(12):3296–307. [DOI] [PubMed] [Google Scholar]
- 26.Iwakoshi NN, Markees TG, Turgeon N, Thornley T, Cuthbert A, Leif J, et al. Skin allograft maintenance in a new synchimeric model system of tolerance. J Immunol. 2001. December 1;167(11):6623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of Allograft Recipients with Donor-Specific Transfusion and Anti-CD154 Antibody Leads to Deletion of Alloreactive CD8+ T Cells and Prolonged Graft Survival in a CTLA4-Dependent Manner. J Immunol. 2000. January 1;164(1):512–21. [DOI] [PubMed] [Google Scholar]
- 28.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003. June;111(12):1887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J Immunol. 2005. July 15;175(2):771–9. [DOI] [PubMed] [Google Scholar]
- 30.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000. January 15;164(2):649–55. [DOI] [PubMed] [Google Scholar]
- 31.Lin C-Y, Graca L, Cobbold SP, Waldmann H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat Immunol. 2002. December;3(12):1208–13. [DOI] [PubMed] [Google Scholar]
- 32.Bennett F, Luxenberg D, Ling V, Wang I-M, Marquette K, Lowe D, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003. January 15;170(2):711–8. [DOI] [PubMed] [Google Scholar]
- 33.Ali J, Harper I, Bolton E, Bradley JA, Pettigrew G. Recipient natural killer cell allorecognition of passenger donor lymphocytes and its effect on adaptive alloimmunity after transplantation. Lancet. 2015. February 26;385 Suppl 1:S18. [DOI] [PubMed] [Google Scholar]
- 34.Walch JM, Zeng Q, Li Q, Oberbarnscheidt MH, Hoffman RA, Williams AL, et al. Cognate antigen directs CD8+ T cell migration to vascularized transplants. J Clin Invest. 2013. June;123(6):2663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, et al. Allograft rejection mediated by memory T cells is resistant to regulation. PNAS. 2007. December 11;104(50):19954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kendal AR, Chen Y, Regateiro FS, Ma J, Adams E, Cobbold SP, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med. 2011. September 26;208(10):2043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baas M, Besançon A, Goncalves T, Valette F, Yagita H, Sawitzki B, et al. TGFβ-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della Pelle P, Benichou G, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011. April;178(4):1635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada Y, Nadazdin O, Boskovic S, Lee S, Zorn E, Smith RN, et al. Repeated Injections of IL-2 Break Renal Allograft Tolerance Induced via Mixed Hematopoietic Chimerism in Monkeys. American Journal of Transplantation. 2015. December 1;15(12):3055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iida S, Suzuki T, Tanabe K, Valujskikh A, Fairchild RL, Abe R. Transient lymphopenia breaks costimulatory blockade-based peripheral tolerance and initiates cardiac allograft rejection. Am J Transplant. 2013. September;13(9):2268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okumi M, Fishbein JM, Griesemer AD, Gianello PR, Hirakata A, Nobori S, et al. Role of persistence of antigen and indirect recognition in the maintenance of tolerance to renal allografts. Transplantation. 2008. January 27;85(2):270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Gating of TCR75 cells adoptively transferred into mice at the time of tolerance induction. TCR75 cells were identified by co-expression of CD45.1 and Vβ8.3 60 days post-adoptive transfer into naïve mice, mice treated with DST, and mice treated with anti-CD154+ DST and a heart transplant. Non-enriched fractions were used to determine the percentages of TCR75 cells amongst CD4+ T cells, while CD45.1-enriched fractions were used to determine total numbers of TCR75 cells. Representative plots of n=3 mice per group per cell dose are shown.