Abstract
Background.
Alcohol and substance use disorders are important predictors for suicidal behavior. However, the role of individual substances as proximal risk factors for suicidal behavior and the mechanisms through which substance use affect risk are not entirely clear. We examine whether the frequency of substance use and whether biological markers in the HPA axis and inflammatory pathways are associated with clinical risk factors of suicidal behavior of aggression, impulsivity, hopelessness, and poor sleep.
Methods.
The sample consisted of psychiatric inpatients, aged 15–30 years, admitted for suicide attempt (n=38), suicidal ideation (n=40); and healthy controls (n=37). We measured hair cortisol concentrations, glucocorticoid receptor (GR) sensitivity, stimulated production of interleukin- or IL-6, C-Reactive Protein, and mRNA expression of GR, SKA2, FKBP5, TNF-α, and IL-1β.
Results.
Smoking was associated with increased aggression [β= 2.9, 95% CI (−0.03, 6), p=0.05], impulsivity [β= 3.1, 95% CI (1.6, 4.6), p<0.001], and poor sleep [β= 0.5, 95% CI (0.03, 0.95), p=0.04] even after controlling for demographics and group. Similarly, TNF-α mRNA was associated with impulsivity [β= 0.07, 95% CI (0.01, 0.1), p=0.02] and hopelessness [β= 0.03, 95% CI (0.004, 0.05), p=0.03]. Smoking tobacco (r=0.32, p<0.001) was positively associated with TNF-α mRNA.
Limitations.
Study limitations include the cross-sectional design, retrospective assessment, and relatively small sample size.
Conclusions.
Future longitudinal studies are needed to test whether inflammatory markers mediate the relationships between smoking, clinical risk factors, and suicidal behavior; and to examine whether smoking cessation could reduce the risk for suicidal behavior in at-risk patients.
Keywords: Substance Use, Smoking, Inflammation, HPA Axis, Imminent Risk, Suicidal Behavior
INTRODUCTION
Alcohol and substance use disorders are important risk factors for suicidal behavior (Darvishi et al., 2015; Poorolajal et al., 2015; Schneider, 2009). However, the role of individual substances as proximal risk factors for suicidal behavior and the mechanisms through which substance use affect risk are not entirely clear.
There are several studies that have shown the link between various substances of use and abuse with suicidal ideation and behavior. Alcohol use disorder and acute alcohol use have been demonstrated to increase the risk for suicidal behavior (Boenisch et al., 2010; Darvishi et al., 2015; Flensborg-Madsen et al., 2009; Sher et al., 2009; Wilcox et al., 2004). Smoking tobacco is also associated with increased risk for suicidal behavior and suicide (Berlin et al., 2015; Boden et al., 2008; Bohnert et al., 2014; Korhonen et al., 2017; Miller et al., 2000a, 2000b; Poorolajal and Darvishi, 2016). Similarly, stimulants, specifically cocaine/crack (Artenie et al., 2015); amphetamines (Artenie et al., 2015; Marshall et al., 2011; Youssef et al., 2016); and opioids, such as heroin and prescription opioid misuse, have been associated with suicidal ideation and behavior (Ashrafioun et al., 2017; Darke and Ross, 2002; Kazour et al., 2016; Roy, 2010; Wilcox et al., 2004). For example, heroin users are 14 times more likely to die by suicide compared to peers matched by age and sex (Darke and Ross, 2002).
The relationship between suicidal behavior and other substances is less clear. Some studies report marijuana use to be associated with increased risk for suicide attempts (Borowsky et al., 2001; Kung et al., 2003; Pedersen, 2008), whereas other studies did not find a significant association (Rylander et al., 2014). Two large population-based studies reported that lifetime use of psychedelics or hallucinogens is not linked to suicidal thoughts, plans, or attempts (Johansen and Krebs, 2015; Krebs and Johansen 2013); and a study showed that past month psychological distress, past year suicidal ideation, planning, and attempts were actually decreased in adults with a history of lifetime psychedelic use (Hendricks et al., 2015). LSD and other psychedelics have been reported to have protective effects by ways of increasing optimism, openness, positive mood, and sense of well-being beyond the acute drug-use period (Carhart-Harris et al., 2016; Griffiths et al., 2008). In the National Household Survey on Drug Abuse, adolescents with lifetime history of 3,4-Methylenedioxymethamphetamine (MDMA) use, commonly known as ecstasy, had twice the rates of suicide attempt in the past year compared to those who used other drugs and 9 times compared to those without a history of illicit drug use (Kim et al., 2011). On the other hand, MDMA has been shown to have clinically therapeutic benefits in the treatment of PTSD (Amoroso and Workman, 2016); and the US Food and Drug Administration has recently designated MDMA as a breakthrough therapy for the treatment of PTSD, leading the way for phase 3 trials (Knopf, 2016). However, there are drastic differences between therapeutic use in controlled studies vs. recreational use where the drug might be adulterated. Recreational drug users also typically use higher doses of the drug at a higher frequency and often show the concomitant use of other drugs (Sessa and Nutt, 2015).
The extant literature is limited in the retrospective assessment of lifetime use of drugs and in examining their association with lifetime or past 12 months suicidal ideation and attempts. In a meta-analysis of studies looking at proximal risk factors for suicide deaths, mood disorders and drug use disorders were associated with 7–9 times increased risk (Conner et al., 2017). In addition, substance use disorders were found to be important predictors of suicide attempts in the days and months preceding the attempt (Walsh, et al. 2017). Thus, these studies highlight the importance of examining the relationship of individual substances as proximal risk factors for suicidal behavior.
Extant studies are also limited in shedding light on the mechanisms through which various substances affect risk for suicidal behavior. Impulsivity, aggression, hopelessness, and poor sleep are well-established clinical risk factors for suicidal behavior (Beck et al., 1990; Bernert et al., 2015; Garrison et al., 1993; Giegling et al., 2009; Goldstein et al., 2008; Liu et al., 2017; Wolfe et al., 2017). Substances such as alcohol, smoking, MDMA, heroin, and cocaine have also been associated with an increase in these clinical risk factors. Marijuana has also been associated with increased impulsivity (Dougherty et al., 2013; Gruber et al., 2014; McDonald et al., 2003) and poor sleep (Bolla et al., 2008; Johnson and Breslau, 2001; Pacek et al., 2017). Paradoxically, marijuana might also be a useful treatment for insomnia (Babson et al., 2017). However, withdrawal from marijuana has been linked to aggression (Smith et al., 2013).
Substance use also affect biological markers in the HPA axis and inflammatory pathways. Alcohol, nicotine, and other substances have been linked to blunted or no cortisol responses to stress (Dai et al., 2007; Gianoulakis et al., 2003; Lovallo et al., 2000; Morris et al., 2016; Neves et al., 2017). However, other studies showed higher basal cortisol levels in heavy alcohol drinkers and those with alcohol dependence, cocaine and MDMA users, and patients with heroin dependence and are on long-term methadone maintenance treatment (Dai et al., 2007; Gianoulakis et al, 2003; Manetti et al., 2014; Parrott et al., 2014; Yang et al., 2016;). Alcohol use and abuse have been reported to be significantly and positively associated with interleukin- or IL-6 and C-Reactive Protein (CRP) whereas marijuana was negatively associated with IL-1β (Karoly et al., 2017; Ransome et al., 2017). Patients with cocaine use disorder, seeking treatment and abstinent, showed lower levels of several inflammatory markers (Mazaquiroga et al., 2017). Heroin injection drug users who are negative for HIV and Hepatitis C infections were found to display elevated levels of an array of inflammatory markers including Tumor Necrosis Factor- or TNF-α (Piepenbrink et al., 2016). We previously reported that suicide attempters, at the time of their admission for an attempt, are indistinguishable from inpatients admitted for suicidal ideation on clinical risk factors (Melhem et al., 2017). However, attempters were significantly different on several biological markers in the HPA axis and inflammatory pathways, specifically, hair cortisol concentrations, Glucocorticoid Receptor or GR mRNA, CRP, and TNF-α mRNA (Melhem et al., 2017).
In this study, we examine the relationships of various substances used by psychiatric inpatients with clinical risk factors of suicidal behavior of impulsivity, aggression, hopelessness, and poor sleep during an acute risk period for suicidal behavior; and with biological markers that differentiated attempters from those with ideation and other markers in the HPA axis and inflammatory pathways that have been previously implicated in suicidal behavior. We also examine whether the frequency of use of various substances in the 12 months preceding hospitalization and biological markers are associated clinical risk factors for suicidal behavior above and beyond suicide attempt status.
METHODS
Sample.
The sample consists of psychiatric inpatients, aged 15–30 years, admitted for suicide attempt (SA, n=38) and those admitted for suicidal ideation with no prior history of attempts (SI, n=40). Inpatients were recruited from Western Psychiatric Institute and Clinic (WPIC), University of Pittsburgh Medical Center (UPMC) and informed consent was obtained prior to participating in accordance with the University of Pittsburgh Institutional Review Board (IRB). Patients with impaired cognitive abilities to provide consent were excluded from the study. Healthy controls (n=37) with no personal or family history of psychiatric disorders or suicidal behavior were recruited from the University of Pittsburgh Clinical and Translational Science Institute (CTSI) Research Participant registry, which includes participants at points of routine clinical care at UPMC and from community outreach events. Inpatients and controls were excluded if they had no hair, had a chronic disease affecting HPA axis or inflammatory pathways, or received oral corticosteroid medication in the past year. Inpatients and controls were screened for symptoms of infection. Those who had an acute infection or received antibiotics in the past two weeks were screened again at least two weeks later, as long as inpatients were not discharged.
Assessment.
Suicidal behavior was measured using the Columbia-Suicide Severity Rating Scale (C-SSRS) with well-established psychometric properties to assesss suicidal ideation and behavior (Posner et al., 2011). Psychiatric diagnoses were obtained using the Family History-Research Diagnostic Criteria (Andreasen et al., 1986). The participants’ self-reported history of substance use over the past year was obtained using the Drug Use Screening Inventory-Revised (DUSI-R), which has been found to have high test-retest reliability (0.88–0.95) and to correctly identify adolescents and adults with substance use disorders (80%) (Kirisci et al., 1995; Siewert et al., 2004; Tarter and Kirisci, 1997). In our analyses, we examined substances that were used by at least 10% of the sample. The frequency of substance use per month over the past year was collected for each substance on the DUSI-R as an ordinal variable with 1= 0 times, 2=1–2 times, 3=3–9 times, 4=10–20 times, and 5=more than 20 times. We also examined substances used as binary (Yes/No) variables with 0 and 1–2 times coded as No and ≥3 times coded as Yes. Aggression was assessed using the Aggression Questionnaire, which has high internal consistency (0.89) and test-retest reliability (0.72–0.80) (Buss and Perry, 1992). Impulsivity was assessed using the Barratt Impulsiveness Scale, which has high internal consistency (Chronbach’s α=0.83) and test-retest reliability (0.83) (Barratt, 1965; Stanford et al., 2009). Sleep was assessed using the Pittsburgh Sleep Quality Index, which also has high internal consistency (Chronbach’s α=0.83), test-retest reliability, and differentiates good from poor sleepers with 89.6% sensitivity and 86.5% specificity (Buysse et al., 1989).Feelings of hopelessness were assessed using the Beck Hopelessness Scale with well-established psychometric properties of concurrent and construct validity (Beck et al., 1974). Higher scores on these measures indicate higher impulsivity, aggression, hopelessness, and poor sleep. Household income was measured using the Hollingshead index of social status (Hollingshead, 1975).
Biological markers.
We collected hair and blood samples from participants. The details about the biological assays were previously reported (Melhem et al., 2017). Hair Cortisol Concentrations (HCC): Hair cortisol was quantified in the 3 cm segment of hair closest to the scalp from a posterior vertex position and hair samples were processed following methods described by Laudenslager et al. (Laudenslager et al., 2011). We used an enzyme immunoassay run according to manufacturer’s instructions and cortisol levels are reported in pg/mg (Salimetrics, LLC, PA). All samples were run in duplicate. The average coefficient of variation (CV) was < 5% and the intra-plate CV was < 10%.
C-Reactive Protein.
CRP levels are reported in mg/dl and assays conducted using a CRPH reagent on a SYNCHRON LX System (Beckman Coulter, Inc., Brea, California).
Glucocorticoid receptor (GR) sensitivity and stimulated production of IL-6.
Whole blood samples were incubated with 50 ng/ml lipopolysaccharide from E.coli (LPS, serotype 026:B6, Sigma) with different levels of hydrocortisone and allowed to incubate for 18-h. The following hydrocortisone (H-0888, 5g, Sigma) concentrations were used: 0, 10−2, 10−3, 10−4, 10−5, and 10−6 M. We assessed corticosteroid suppression of cytokine (IL-6) production. GR sensitivity was determined using area under the curve, and stimulated production of IL-6 was obtained by measuring IL-6 in samples grown without hydrocortisone, which provided a measure of IL-6 derived solely from immune cells.
RNA analyses.
Blood samples using PAXgene Blood RNA Tubes were also collected and followed by RNA isolation and purification using PAXgene Blood RNA Kit. We used Illumina’s Human HT-12 v4 Expression BeadChip Kit. Glucocorticoid receptor (GR) mRNA α-isoform, GR mRNA β-isoform, SKA2 mRNA, FKBP5 mRNA, TNF-α mRNA, and IL-1β mRNA were examined as these have been previously implicated in suicidal behavior and all have passed quality control analysis.
Statistical Analysis.
We compared SA, SI, and controls on demographic and clinical correlates of suicidal behavior of aggression, impulsivity, hopelessness, and poor sleep. We also compared the groups on the frequency of substance use in the past year and substance use (Yes/No) using analysis of variance (ANOVA) and chi-square tests, respectively. When the group comparison was statistically significant, we conducted post-hoc pairwise comparisons in order to determine group differences and corrected for multiple comparisons using Bonferroni correction (α=0.05/3 pairwise comparisons=0.017). We then examined the relationship of each of the clinical correlates of suicidal behavior, i.e., aggression, impulsivity, hopelessness, and poor sleep with the frequency of use of substances using Pearson’s correlations. For these analyses, we also used a Bonferroni correction to correct the significance level since we are examining 4 clinical correlates as our outcomes of interest (α=0.05/4=0.01). We also examined the correlations of the frequency of each of the substances used with the biological markers using Pearson’s correlations.
Hierarchical linear regression analysis was conducted for each of the clinical correlates, as dependent variables, to examine whether the frequency of each of the substances used and biological markers were associated with clinical correlates even after controlling for group status (attempt vs. ideation vs. control), age, sex, race, and household income. We introduced substances that were significantly associated with the clinical correlate in the regression in a hierarchical order where the least illicit substances were introduced first (i.e., tobacco, alcohol, and marijuana) followed by the more illicit substances (i.e, cocaine/crack, amphetamines, painkillers, heroin, LSD, and MDMA); and then followed by biological markers significantly associated with the clinical correlate. Finally, we tested for interactions between the frequency of substance use and biological markers that were significant in the final model. All analyses were conducted including SA, SI, and healthy controls and were repeated using SA and SI groups only.
RESULTS
Characteristics of the sample.
The sample had a mean age of 22.8 ± 3.4, 57% males, and 82% White. SA, SI, and controls were similar with respect to age and racial distributions; however, SA and SI were significantly different on sex and household income (Table 1). SA and controls were similar on their sex distribution; however, SI were significantly more likely to be males compared to controls. SA and SI were also similar on household income and showed significantly different and lower household income compared to controls [4.8 ± 2.6 vs. 5.8 ± 3.1 vs. 8.1 ± 1.8, F=16.4, df (1,111), p<0.001]. SA and SI groups were similar in terms of primary and comorbid psychiatric diagnoses (Table 1). They were also similar, and both significantly different compared to healthy controls, on their scores of aggression [82.7 ± 22.8 vs. 81.8 ± 24.6 vs. 46.7 ± 12.1, F=39.5, df (2,114), p<0.001], impulsivity [79.7 ± 11 vs. 76.9 ± 13.6 vs. 55.4 ± 8.1, F=57.1, df (2,114), p<0.001], hopelessness [11.6 ± 5 vs. 11.1 ± 5.1 vs. 1.8 ± 1.9, F=66.1, df (2, 114), p<0.001], and sleep [10.5 ± 3.4 vs. 11.6 ± 3.7 vs. 3.2 ±1.9, F=79.6, df (2,111), p<0.001] (Table 1).
Table 1.
Demographic and clinical characteristics of suicide attempters, subjects with suicidal ideation, and healthy controls
| Suicide attempters (SA) | Subjects with suicidal ideation (SI) | Healthy controls | Test | df | P-value | |
|---|---|---|---|---|---|---|
| n=38 | n=40 | n=37 | ||||
| Demographics | ||||||
| Sex, % Males (n) | 55.3 (21 fa,b | 72.5 (29)b | 43.6 (17)a | χ2=6.84 | 2 | 0.03 |
| Race, % White (n) | 89.5 (34) | 75.0 (30) | 83.8 (31) | X2=2.9 | 2 | 0.24 |
| Age, Mean ± SD | 22.8±3.8 | 23.6±3.9 | 22.1±2.2 | F=1.8 | 2, 112 | 0.18 |
| Household income, Mean ± SD | 4.8±2.6a | 5.8±3.1a | 8.1±1.8b | F=15.8 | 1, 110 | <0.001 |
| Clinical correlates of suicidal behavior | ||||||
| Aggression, Mean ± SD | 82.7±22.8a | 81.8±24.6a | 46.7 (12.1)b | F=36.8 | 2, 112 | <0.001 |
| Impulsivity, Mean ± SD | 79.7±11.0a | 76.9±13.6a | 55.4± 8.1b | F=53.2 | 2, 112 | <0.001 |
| Hopelessness, Mean ± SD | 11.6± 5.0a | 11.1±5.1a | 1.8± 1.9b | F=61.9 | 2, 112 | <0.001 |
| Sleep, Mean ± SD | 10.5± 3.4a | 11.6±3.7a | 3.2± 1.9b | F=79.6 | 2, 111 | <0.001 |
| Psychiatric diagnoses | ||||||
| Major Depressive Disorder, N (%) | 50.0 (19) | 62.5 (25) | — | FET* | — | 0.58 |
| Bipolar disorder, N (%) | 42.1 (16) | 32.5 (13) | — | |||
| Psychotic disorders, N (%) | 0.0 (0) | 7.5 (3) | — | FET* | — | 0.24 |
| Anxiety disorders, N (%) | 71.1 (27) | 60.0 (24) | — | Χ2=1.05 | 1 | 0.31 |
| Posttraumatic Stress Disorder (PTSD), N (%) | 14 (36.8) | 16 (40.0) | — | Χ2=0.08 | 1 | 0.77 |
| Attention Deficit Hyperactivity Disorder (ADHD), N (%) | 8 (21.1) | 3 (7.5) | — | Χ2=2.95 | 1 | 0.09 |
| Eating disorders, N (%) | 2 (5.26) | 2 (5.0) | — | FET* | >0.99 | |
| Alcohol dependence, N (%) | 9 (23.7) | 6 (15.0) | — | Χ2=0.95 | 1 | 0.33 |
| Substance dependence, N (%) | 15 (39.5) | 17 (42.5) | — | Χ2=0.07 | 1 | 0.79 |
| Personality disorders, N (%) | 6 (15.8) | 2 (5.0) | — | FET* | — | 0.15 |
FET = Fisher’s Exact Test
Group comparisons on frequency of substance use.
The frequency of use for almost all substances—amphetamines, cocaine/crack, heroin, painkillers, MDMA, and marijuana were similar between SA and SI and were significantly different compared to controls (Table 2). The only exceptions were for smoking tobacco, alcohol, and LSD. SA showed higher frequency of smoking tobacco compared to SI and controls [4.55 ± 1.2 vs. 3.4 ±1.92 vs. 1.44 ±0.85, respectively, F=47.7, df (2,114), p<0.001]. SI and controls were also significantly different on smoking. SA also showed higher frequency of alcohol use compared to SI; however, controls were not statistically different than SA and SI [3.29 ± 1.27 vs. 2.6 ±1.28 vs. 3.26 ±1.09, df (2,114), p=0.02]. SA and SI were similar on the frequency of LSD use; however, only SA were significantly different than controls [1.53 ± 0.98 vs. 1.25 ±0.59 vs. 1.08 ±0.27, F=4.4, df (2, 114), p=0.015] (Table 2) Results were almost similar when examining group differences on presence or absence of substance use (Yes/No); however, SA and SI were significantly different on use of amphetamines and no differences between groups were found regarding LSD and MDMA. When looking at polysubstance use, SA and SI (86.6% vs. 77.5%) were significantly more likely to use 2 or more substances compared to controls (20.5%, χ2=45.5, df=4, p<0.001) (Table 2).
Table 2.
Frequency distributions of substance use in the past year in suicide attempters, subjects with suicidal ideation, and healthy controls
| Suicide attempters (SA) | Subjects with suicidal ideation (SI) | Healthy controls | Test | df | p-value | |
|---|---|---|---|---|---|---|
| n=38 | n=40 | n=39 | ||||
| Frequency of substance use in the past year | ||||||
| Mean±SD | Mean±SD | Mean±SD | ||||
| Alcohol | 3.29±1.27a | 2.60±1.28b | 3.22±1.11a,b | F= 3.7 | 2, 112 | 0.027 |
| Amphetamines | 2.39±1.53a | 1.93±1.35a | 1.10±0.39b | F=10.9 | 2, 112 | <0.001 |
| Cocaine/crack | 2.11±1.33a | 2.03±1.56a | 1.0 ±0.0b | F=10 | 2, 112 | <0.001 |
| Heroin | 2.55±1.83a | 2.70±1.96a | 1.0 ±0.0b | F=13.7 | 2, 112 | <0.001 |
| Painkillers | 2.32±1.42a | 2.38±1.66a | 1.18±0.51b | F= 9.9 | 2, 112 | <0.001 |
| LSD | 1.53±0.98a | 1.25±0.59 a,b | 1.08±0.28b | F= 4.1 | 2, 112 | 0.019 |
| MDMA | 1.53±0.83a | 1.50±0.75a | 1.03±0.16b | F= 6.9 | 2, 112 | 0.002 |
| Marijuana | 3.47±1.54a | 3.38±1.65a | 1.68±1.08b | F=18.3 | 2, 112 | <0.001 |
| Smoking tobacco | 4.55±1.20a | 3.4±1.92b | 1.43±0.87c | F=46.6 | 2, 112 | <0.001 |
| Substance use in the past year (Yes/No)* | ||||||
| % (n) | % (n) | % (n) | ||||
| Alcohol | 68.4 (26)a | 45.0 (18)a,b | 73 (29)a | X2= 7.4 | 2 | 0.024 |
| Amphetamines | 42.1 (16)a | 17.5 (7)b | 2.7 (1)b | X2=18.0 | 2 | <0.001 |
| Cocaine/crack | 31.6 (12)a | 27.5 (11)a | 0.0 (0)b | χ2=13.8 | 2 | 0.001 |
| Heroin | 39.5 (15)a | 42.5 (17)a | 0.0 (0)b | χ2=22.1 | 2 | <0.001 |
| Painkillers | 44.7 (17)a | 42.5 (17)a | 5.1 (2)b | χ2=17.1 | 2 | <0.001 |
| LSD | 7.9 (3) | 7.5 (3) | 0.0 (0) | FET** | 2 | 0.24 |
| MDMA | 10.5 (4)a | 15.0 (6)a | 0.0 (0)a | FET** | 2 | 0.06 |
| Marijuana | 68.4 (26)a | 65.0 (26)a | 13.5 (5)b | χ2=28.5 | 2 | <0.001 |
| Smoking tobacco | 89.5 (34)a | 62.5 (25)b | 5.4 (2)c | χ2=55.4 | 2 | <0.001 |
| Polysubstance use in the past year | ||||||
| 0 | 5.3 (2) | 15.0 (6) | 27.0 (10) | χ2=42.6 | 4 | <0.001 |
| 1 | 7.9 (3) | 7.5 (3) | 51.4 (19) | |||
| 2 or more | 86.8 (33) | 77.5 (31) | 21.6 (8) | |||
No=0 or 1–2 times; Yes > 3 times
FET = Fisher’s Exact Test
Relationship of the clinical correlates of suicidal behavior with the frequency of substance use.
Table 3 shows that each of aggression, impulsivity, hopelessness, and sleep were positively correlated with almost all substances, except for alcohol, when including all groups.
Table 3.
Pearson correlations (r) between clinical correlates of suicidal behavior of aggression, impulsivity, hopelessness, and sleep with the frequency of substance use in the past year
| Aggression | Impulsivity | Hopelessness | Sleep | |
|---|---|---|---|---|
| Including suicide attempters, subjects with suicidal ideation, and healthy controls | ||||
| Alcohol | 0.04 | 0.05 | −0.01 | −0.12 |
| Amphetamines | 0.45** | 0.45** | 0.24* | 0.35** |
| Crack/cocaine | 0.47** | 0.46** | 0.25* | 0.33** |
| Heroin | 0.51** | 0.48** | 0.30** | 0.37** |
| Painkillers | 0.41** | 0.42** | 0.29* | 0.41** |
| LSD | 0.19 | 0.29* | 0.14 | 0.18 |
| MDMA | 0.38** | 0.37** | 0.32** | 0.29* |
| Marijuana | 0.43** | 0.44** | 0.27* | 0.39** |
| Tobacco | 0.59** | 0.68** | 0.48** | 0.53** |
| Including suicide attempters and subjects with suicidal ideation only | ||||
| Alcohol | 0.19 | 0.18 | 0.14 | − 0.07 |
| Amphetamines | 0.31* | 0.30* | −0.05 | 0.12 |
| Crack/cocaine | 0.33* | 0.32* | −0.05 | 0.07 |
| Heroin | 0.35* | 0.29* | −0.04 | 0.16 |
| Painkillers | 0.25 | 0.22 | 0.02 | 0.21 |
| LSD | 0.09 | 0.23 | −0.01 | 0.05 |
| MDMA | 0.25 | 0.22 | 0.14 | 0.07 |
| Marijuana | 0.15 | 0.16 | −0.15 | 0.03 |
| Tobacco | 0.32* | 0.41** | 0.04 | 0.11 |
p<0.01
p<0.001
Correlations ranged between 0.25 and 0.68. Alcohol was not significantly associated with any of the clinical correlates of suicidal behavior. In addition, LSD was only significantly correlated with impulsivity (r=0.30, p<0.01). When looking at these correlations within the SA and SI groups only, we find the frequency of use of amphetamines, cocaine, heroin, and smoking to be significantly associated with aggression and impulsivity with correlations ranging between 0.29 and 0.41.
Relationship of frequency of substance use with biological measures.
Table 4 shows correlations between the frequency of substance use and biological measures including and excluding healthy controls. IL-1β mRNA was significantly and positively correlated with the frequency of almost all substances used in the past 12 months when including all groups, except for amphetamines and LSD, with correlations ranging between 0.21 and 0.27 (Table 4). Some of these correlations were no longer significant when excluding controls due to our reduced sample size. TNF-α mRNA was correlated with the frequency of use of cocaine/crack (r=0.22, p < 0.05), heroin (r=0.26, p < 0.01), painkillers (r=0.23, p < 0.05), MDMA (r=0.26, p < 0.01), and smoking tobacco (r=0.32, p < 0.001). However, none of these correlations were significant when excluding controls. FKBP5 mRNA was consistently correlated with heroin, painkillers, and MDMA use. HCC and GR sensitivity were not correlated with any of the substances including and excluding controls.
Table 4.
Pearson correlations (r) between biological markers and the frequency of substance use in the past year
| HCC | GR mRNA α-isoform | GR mRNA ß-isoform | SKA2 mRNA | FKBP5 mRNA | GR-Sensitivity | CRP | TNF- α mRNA | IL1ß mRNA | Stimulated production of IL-6 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Including suicide attempters, subjects with suicidal ideation, and healthy controls | ||||||||||
| Alcohol | 0.08 | 0.01 | −0.07 | −0.15 | 0.12 | 0.06 | 0.08 | 0.08 | 0.22* | −0.05 |
| Amphetamines | −0.06 | −0.17 | −0.01 | 0.06 | 0.06 | −0.004 | 0.18* | 0.17 | 0.13 | −0.12 |
| Cocaine/crack | −0.05 | −0.15 | 0.14 | 0.22* | 0.12 | −0.005 | 0.14 | 0.22* | 0.23* | −0.05 |
| Heroin | 0.15 | 0.01 | 0.18* | 0.21* | 0.19* | 0.07 | 0.09 | 0.26** | 0.26** | 0.005 |
| Painkillers | 0.03 | 0.07 | 0.12 | 0.12 | 0.23** | 0.12 | −0.05 | 0.23* | 0.27** | 0.01 |
| LSD | −0.01 | −0.01 | 0.03 | 0.04 | −0.002 | 0.16 | 0.16 | 0.17 | 0.11 | 0.08 |
| MDMA | −0.03 | −0.16 | 0.17 | 0.19* | 0.29** | 0.18 | 0.04 | 0.26** | 0.22* | 0.16 |
| Marijuana | −0.01 | −0.0003 | 0.10 | 0.002 | 0.08 | 0.09 | 0.02 | 0.12 | 0.21* | −0.05 |
| Smoking Tobacco | 0.09 | −0.09 | 0.03 | 0.18 | 0.10 | 0.07 | 0.11 | 0.32*** | 0.24** | 0.04 |
| Including suicide attempters and subjects with suicidal ideation only | ||||||||||
| Alcohol | 0.10 | 0.08 | −0.13 | −0.12 | 0.07 | 0.10 | 0.08 | 0.12 | 0.26* | 0.03 |
| Amphetamines | −0.07 | −0.22* | −0.12 | −0.04 | 0.15 | −0.10 | 0.21 | 0.05 | 0.08 | −0.23* |
| Cocaine/crack | −0.03 | −0.18 | 0.09 | 0.18 | 0.25* | −0.05 | 0.13 | 0.15 | 0.20 | −0.10 |
| Heroin | 0.21 | 0.03 | 0.14 | 0.16 | 0.26* | 0.05 | 0.07 | 0.18 | 0.24* | −0.04 |
| Painkillers | 0.04 | 0.09 | 0.01 | 0.02 | 0.26* | 0.07 | −0.10 | 0.13 | 0.25* | −0.04 |
| LSD | −0.03 | −0.03 | −0.04 | −0.02 | −0.04 | 0.11 | 0.19 | 0.10 | 0.09 | 0.04 |
| MDMA | −0.02 | −0.20 | 0.12 | 0.13 | 0.36** | 0.15 | 0.01 | 0.21 | 0.20 | 0.16 |
| Marijuana | −0.04 | −0.03 | −0.05 | −0.11 | 0.06 | 0.01 | 0.03 | −0.03 | 0.18 | −0.15 |
| Smoking Tobacco | 0.16 | −0.16 | −0.13 | 0.09 | 0.18 | −0.0004 | 0.19 | 0.19 | 0.19 | −0.06 |
p<0.05
p<0.01
p<0.001
Relationships of the clinical correlates of suicidal behavior with the frequency of substance use and biological markers controlling for group and demographic characteristics.
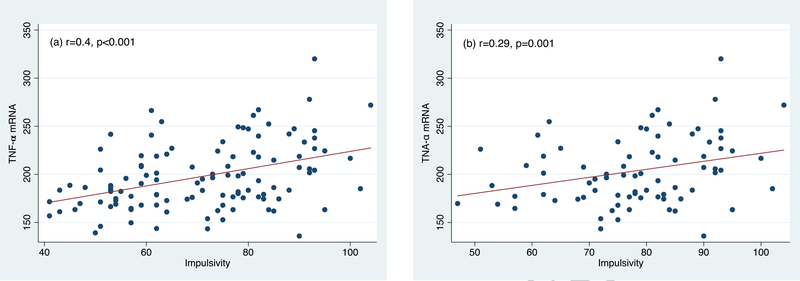
We have previously reported TNF-α mRNA to be significantly and positively correlated with impulsivity (r = 0.40, p < 0.001), hopelessness (r = 0.30, p < 0.01), and poor sleep (r = 0.31, p < 0.01) at α=0.01 (Melhem et al., 2017). We examined here whether the frequency of substance use in the past year and biological markers that were significantly associated with each of the clinical correlates of suicidal behavior at α=0.01 continued to be significant when controlling for group and demographic characteristics. Both SA and SI groups were significantly associated with each of aggression, impulsivity, hopelessness, and poor sleep (Table 5). Smoking tobacco was significantly associated with each of aggression [β= 2.9, 95% CI (−0.05, 6), p=0.05], impulsivity [β= 2.9, 95% CI (1.4, 4.4), p<0.001], and poor sleep [β= 0.5, 95% CI (0.03, 0.9), p=0.04] even after controlling for covariates. The frequency of use of heroin was also significantly associated increased aggression [β= 3.4, 95% CI (0.8, 6.0), p=0.01] (Table 5). TNF-α mRNA was significantly associated with increased impulsivity [β= 0.07, 95% CI (0.01, 0.1), p=0.01] (Figure 1) and hopelessness [β= 0.03, 95% CI (0.003, 0.05), p=0.03]. There was no significant interaction between smoking and TNF-α mRNA on impulsivity. Similar results were obtained when excluding controls except that the effect of TNF-α mRNA on impulsivity and that of smoking on sleep did not reach statistical significance given our reduced sample size. In addition, SA and SI were similar on aggression, impulsivity, and hopelessness; however, SA showed lower scores on sleep [β= −2.3, 95% CI (−4.3, −0.4), p=0.02], which reflects better sleep, compared to SI.
Table 5.
Regression analyses examining the relationships of the frequency of substance use and biological markers with clinical correlates of suicidal behavior controlling for age, sex, race, socioeconomic status, and group
| Aggression | Impulsivity | Hopelessness | Sleep | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
| Including suicide attempters, subjects with suicidal ideation, and healthy controls | ||||||||
| Age | 0.7 (−0.4, 1.9) | 0.23 | 0.07 (−0.5, 0.7) | 0.82 | −0.08 (−0.3, 0.2) | 0.57 | −0.13 (−0.32, 0.06) | 0.17 |
| Sex | 6.2 (−1.1, 13.6) | 0.09 | 1.8 (−2.1, 5.7) | 0.36 | −0.9 (−2.6, 0.8) | 0.29 | −1.2 (−2.4, 0.05) | 0.01 |
| Race | 8.5 (−1.6, 18.6) | 0.10 | −3.2 (−8.4, 1.9) | 0.22 | −0.4 (−2.6, 1.9) | 0.74 | −1.1 (−2.7, 0.54) | 0.07 |
| Household income | −1.1 (−2.6, 0.4) | 0.17 | −0.4 (−1.2, 0.4) | 0.30 | 0.4 (0.04, 0.7) | 0.03 | −0.1 (−0.4, 0.12) | 0.33 |
| Group* | ||||||||
| SA | 16.7 (4.0, 29.5) | 0.01 | 11.3 (4.6, 18) | 0.001 | 10.6 (8.3, 12.9) | <0.001 | 5.3 (3.2, 7.4) | <0.001 |
| SI | 18.0 (7.6, 28.4) | 0.001 | 13.5 (8.1, 19) | <0.001 | 10.0 (7.8, 12.1) | <0.001 | 7.6 (6.0, 9.3) | <0.001 |
| Smoking | 2.9 (−0.03, 6.0) | 0.05 | 3.1 (1.6, 4.6) | <0.001 | - | 0.5 (0.03, 0.95) | 0.04 | |
| Heroin | 3.4 (0.8, 6.0) | 0.01 | - | - | - | |||
| TNF- α mRNA | - | 0.07 (0.01, 0.1) | 0.02 | 0.03 (0.004, 0.05) | 0.03 | - | ||
| Including suicide attempters and subjects with suicidal ideation only | ||||||||
| Age | 0.8 (−0.7, 2.3) | 0.31 | 0.3 (−0.5, 1.1) | 0.43 | −0.04 (−0.4, 0.3) | 0.82 | −0.12 (−0.4, 0.13) | 0.33 |
| Sex | 4.3 (−6.7, 15.2) | 0.44 | 0.1 (−5.3, 5.5) | 0.97 | −2.1 (−4.5, 0.3) | 0.09 | −1.1 (−2.9, 0.6) | 0.20 |
| Race | 3.2 (−11.3, 17.7) | 0.66 | −5.0 (−12.2, 2.2) | 0.17 | −0.7 (−3.9, 2.5) | 0.65 | −1.1 (−3.5, 1.2) | 0.33 |
| Household income | −1.6 (−3.6, 0.4) | 0.11 | −0.3 (−1.3, 0.6) | 0.49 | 0.5 (0.04, 0.9) | 0.03 | −0.12 (−0.4, 0.2) | 0.45 |
| Group* | ||||||||
| SA vs. SI | −4.8 (−16.9, 7.3) | 0.43 | −2.2 (−8.2, 3.7) | 0.46 | 0.5 (−2, 3) | 0.69 | −2.3 (−4.3, −0.4) | 0.02 |
| Smoking | 4.3 (0.7, 8) | 0.02 | 2.9 (1.0, 4.7) | 0.003 | − | 0.5 (−0.09, 1.1) | 0.09 | |
| TNF- α mRNA | - | 0.07 (−0.01, 0.1) | 0.08 | 0.04 (0.005, 0.07) | 0.03 | - | ||
SA and SI are compared to Controls
Figure 1.
Correlations between impulsivity and TNF-?? mRNA. a) Including suicide attempters, subjects with suicidal ideation, and healthy controls; b) Including suicide attempters and subjects with suicidal ideation only
DISCUSSION
Of all substances, we found smoking was associated with higher levels of aggression, impulsivity, and poor sleep even after controlling for demographics and group. Similarly, TNF-α mRNA was associated with impulsivity and hopelessness even after controlling for demographics, group, and substances used. Similar results were obtained when including and excluding healthy controls.
We discuss these results in the context of the study’s strengths and limitations. Alcohol and substance use disorders are important predictors of suicidal behavior (Nock et al., 2010). However, most studies report on the relationship of lifetime or past 12 months substance use with lifetime or past 12 months suicidal ideation and behavior. This study examines the relationship of substance use in the past 12 months with HPA axis and inflammatory markers and clinical risk factors of suicidal behavior of aggression, impulsivity, hopelessness, and poor sleep in psychiatric inpatients at the time of their admission for suicide attempt or ideation. Another strength of our study design is the inclusion of psychiatric inpatients across the spectrum of psychopathology, which is more representative of the clinical population at risk for suicidal behavior. In addition, attempters and those with ideation were similar on primary and comorbid psychiatric diagnoses. There were several limitations including the cross-sectional design, retrospective assessment, and relatively small sample size. We relied on patient interviews to obtain information regarding the frequency of substance use in the past 12 months prior to admission rather than obtaining toxicology results at the time of admission. Thus, our study did not include objectives measures and subjective reporting of substance use at the time of admission or in the days prior to admission. However, toxicology results would only reflect acute levels of drugs and in suicide attempters, many of whom attempted through an overdose on medications and drugs, would not represent their chronic levels of exposure to these substances. Our study is also limited with the relatively small sample size and the low frequency of use of some substances (e.g., LSD, MDMA), which limits our power to examine their effects in this population. The sample size also limits our ability to examine the combined effects of polysubstance use. The prevalence of polysubstance use in the past year among inpatients is high and as such the relationships of substance use with biological and clinical measures may not reflect the individual effects of these substances.
While almost all substances were associated with clinical risk factors for suicidal behavior, smoking was the strongest correlate of aggression, impulsivity, and poor sleep. Suicide attempters showed higher rates of smoking compared to those with ideation and controls; and those with ideation also showed significantly higher rates of smoking compared to controls. These results are consistent with findings that people with mental illness are 2–3 times more likely to smoke compared to the general population (Prochaska et al., 2017). This increased prevalence in people with mental illness is attributed to several factors including disparities in access to tobacco cessation programs, lower socioeconomic status, and increased rates of stressful life events (Prochaska et al., 2017). Our results are also consistent with prior studies showing smoking to be associated with increased risk for suicidal behavior, suicide, and with increased aggression, impulsivity, and poor sleep (Bloom et al., 2013; Dakwar et al., 2011; Miller et al., 2000a, 2000b; Mitchell, 1999; Oquendo et al., 2004; Patterson et al., 2018; Poorolajal and Darvishi, 2016). Furthermore, aggression, impulsivity, and poor sleep are risk factors for suicidal behavior (Carli et al., 2010; Goldstein et al., 2008; Melhem et al., 2007; Nock and Kessler, 2006). These results suggest that smoking may increase risk for suicidal behavior through these clinical correlates; although bidirectional relationships are also reported (De Witt H, 2009; Pieters et al., 2015).
We also find the frequency of heroin use to be associated with aggression even after controlling for groups and demographic characteristics. Heroin use has long been identified as a risk factor for suicidal behavior and aggression has been previously reported to be a risk factor for suicide attempt among heroin users (Darke and Ross, 2002; Roy, 2010). Surprisingly, alcohol was the only substance that was not correlated with any of the clinical correlates of suicidal behavior. This could be attributed to the frequency of alcohol use in controls, which was similar to the frequency of alcohol use in SA and SI. Our sample of controls mostly consisted of college students where 36% of them consumed alcohol at a frequency of 10–20 times per month or more. However, these results remained unchanged when excluding healthy controls.
We find TNF-α mRNA to predict impulsivity and hopelessness even after controlling for group, demographics, and frequency of substances used that were significant in our models, specifically, smoking. Smoking was significantly and positively correlated with TNF-α mRNA, which is consistent with previous studies (O’Connor et al., 2009); however, there was no significant interaction between smoking and TNF-α mRNA in our sample. We have previously reported TNF-α mRNA to differentiate SA from SI and controls (Melhem et al., 2017). We were not able to test whether TNF-α mRNA mediates the relationships between substance use and clinical risk factors for suicidal behavior due to our cross-sectional design. Future longitudinal studies with large sample size are needed to test these mechanisms. These results are also consistent with several studies showing increased expression of TNF-α in people who died by suicide, and increased peripheral expression of inflammatory genes and markers of inflammation among subjects at risk for suicidal behavior (Hoyo-Becerra et al., 2013; Pandey et al., 2012; Tonelli et al., 2008). In a meta-analysis, TNF-α was robustly increased in patients with suicidal ideation and behavior (Black and Miller, 2015). Surprisingly, none of the substances used were correlated hair cortisol concentrations that previously differentiated suicide attempters (Melhem at al., 2017); and HCC was not associated with any of the clinical risk factors of suicidal behavior after controlling for demographics and group. However, we found FKBP5 mRNA to be positively correlated with the frequency of use of heroin, painkillers, and MDMA. Reduced hippocampal GR expression has been previously reported in brains of people who died by suicide and had a history of abuse (Labonte et al., 2012; McGowan et al., 2009). FKBP5 and SKA2 proteins are co-chaperones of the GR and regulate its unfolding and trafficking; and SKA2 and FKBP5 mRNA have been previously implicated in suicidal ideation and behavior (Guintivano et al., 2014; Niculescu et al., 2015; Pandey et al., 2016; Perez-Ortiz et al., 2013; Yin et al., 2016).
In conclusion, amongst licit and illicit substances, smoking tobacco has the strongest association with risk for suicidal behavior in psychiatric patients. Smoking may exert its effect on risk in this population through increased aggression, impulsivity, and poor sleep. Smoking is also associated with inflammatory markers and inflammatory markers are in turn associated with aggression, impulsivity, and poor sleep. Future longitudinal studies are needed to test whether inflammatory markers mediate the relationships between smoking, clinical risk factors, and suicidal behavior and to examine whether smoking cessation could reduce the risk for suicidal behavior in at-risk patients.
Acknowledgments
Role of the funding source
This study work was supported by an R21 grant (PI: Nadine M. Melhem) from the National Institute of Mental Health (NIMH) MH101208. NIMH did not participate in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest. All authors have no conflicts of interest to disclose except for Drs. David Brent and Antoine Douaihy. Dr. Brent receives royalties from Guilford Press, have or will receive royalties from the electronic self-rated version of the C-SSRS from ERT, Inc., is on the Editorial board of UpToDate, is a reviewer for Healthwise, is a consultant to McKeeson, is on the board of Klingenstein Foundation, and receives honoraria for presenting at Continuing Medical Education events. Dr. Douaihy received grant funding from pharmaceutical companies Orexo and Alkermes, royalties from Oxford University Press for 2 academic books, and royalties from PESI Publishing & Media for one academic book.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amoroso T, Workman M, 2016. Treating posttraumatic stress disorder with MDMA-assisted psychotherapy: A preliminary meta-analysis and comparison to prolonged exposure therapy. J. Psychopharmacol https://doi.org/10.1177/0269881116642542 [DOI] [PubMed] [Google Scholar]
- Andreasen NC, 1986. The Family History Approach to Diagnosis. Arch. Gen. Psychiatry 43, 421 https://doi.org/10.1001/archpsyc.1986.01800050019002 [DOI] [PubMed] [Google Scholar]
- Artenie AA, Bruneau J, Zang G, Lespérance F, Renaud J, Tremblay J, Jutras-Aswad D, 2015. Associations of substance use patterns with attempted suicide among persons who inject drugs: Can distinct use patterns play a role? Drug Alcohol Depend. 147, 208–214. https://doi.org/10.1016/j.drugalcdep.2014.11.011 [DOI] [PubMed] [Google Scholar]
- Ashrafioun L, Bishop TM, Conner KR, Pigeon WR, 2017. Frequency of prescription opioid misuse and suicidal ideation, planning, and attempts. J. Psychiatr. Res 92, 1–7. https://doi.org/10.1016/j.jpsychires.2017.03.011 [DOI] [PubMed] [Google Scholar]
- Baalash A, Fahmy M, Haggag W, Mohamed K, 2015. A study of the personality traits and the level of anxiety in suicidal polydrug users. Egypt. J. Psychiatry 36, 106 https://doi.org/10.4103/1110-1105.158119 [Google Scholar]
- Babson KA, Sottile J, Morabito D, 2017. Cannabis, Cannabinoids, and Sleep: a Review of the Literature. Curr. Psychiatry Rep 19 https://doi.org/10.1007/s11920-017-0775-9 [DOI] [PubMed] [Google Scholar]
- Barratt ES, 1965. Factor Analysis of Some Psychometric Measures of Impulsiveness and Anxiety. Psychol. Rep 16, 547–554. https://doi.org/10.2466/pr0.1965.16.2.547 [DOI] [PubMed] [Google Scholar]
- Beck AT, Brown G, Berchick RJ, Stewart BL, Steer RA, 2006. Relationship Between Hopelessness and Ultimate Suicide: A Replication With Psychiatric Outpatients. Focus (Madison). 4, 291–296. https://doi.org/10.1176/foc.4.2.291 [DOI] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L, 1974. The measurement of pessimism: The Hopelessness Scale. J. Consult. Clin. Psychol 42, 861–865. https://doi.org/10.1037/h0037562 [DOI] [PubMed] [Google Scholar]
- Berlin I, Hakes JK, Hu M-C, Covey LS, 2015. Tobacco Use and Suicide Attempt: Longitudinal Analysis with Retrospective Reports. PLOS ONE 10, e0122607 https://doi.org/10.1371/journal.pone.0122607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert RA, Kim JS, Iwata NG, Perlis ML, 2015. Sleep Disturbances as an Evidence-Based Suicide Risk Factor. Curr. Psychiatry Rep 17 https://doi.org/10.1007/s11920-0150554-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C, Miller BJ, 2015. Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biol. Psychiatry 78, 28–37. https://doi.org/10.1016/j.biopsych.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Bloom EL, Matsko SV, Cimino CR, 2013. The relationship between cigarette smoking and impulsivity: A review of personality, behavioral, and neurobiological assessment. Addict. Res. Theory 22, 386–397. https://doi.org/10.3109/16066359.2013.867432 [Google Scholar]
- Boenisch S, Bramesfeld A, Mergl R, Havers I, Althaus D, Lehfeld H, Niklewski G, Hegerl U, 2010. The role of alcohol use disorder and alcohol consumption in suicide attempts - A secondary analysis of 1921~suicide attempts. Eur. Psychiatry 25, 414–420. https://doi.org/10.1016/j.eurpsy.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Bohnert KM, Ilgen MA, McCarthy JF, Ignacio RV, Blow FC, Katz IR, 2013. Tobacco use disorder and the risk of suicide mortality. Addiction 109, 155–162. https://doi.org/10.1111/add.12381 [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, David PM, Verdejo-Garcia A, Benbrook AR, 2008. Sleep Disturbance in Heavy Marijuana Users. Sleep 31, 901–908. https://doi.org/10.1093/sleep/31.6.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky IW, Ireland M, Resnick MD, 2001. Adolescent Suicide Attempts: Risks and Protectors. Pediatrics 107, 485–493. https://doi.org/10.1542/peds.107.3.485 [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M, 1992. The Aggression Questionnaire. J. Pers. Soc. Psychol 63, 452–459. https://doi.org/10.1037//0022-3514.63.3.452 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Bolstridge M, Williams TM, Williams LT, Underwood R, Feilding A, Nutt DJ, 2016. The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol. Med 46, 1379–1390. https://doi.org/10.1017/s0033291715002901 [DOI] [PubMed] [Google Scholar]
- Carli V, Jovanović N, Podlešek A, Roy A, Rihmer Z, Maggi S, Marusic D, Cesaro C, Marusic A, Sarchiapone M, 2010. The role of impulsivity in self-mutilators, suicide ideators and suicide attempters - A study of 1265 male incarcerated individuals. J. Affect. Disord 123, 116–122. https://doi.org/10.1016/j.jad.2010.02.119 [DOI] [PubMed] [Google Scholar]
- Choi WS, Harris KJ, Okuyemi K, Ahluwalia JS, 2003. Predictors of smoking initiation among college-bound high school students. Ann. Behav. Med https://doi.org/10.1207/S15324796ABM2601_09 [DOI] [PubMed] [Google Scholar]
- Conner KR, Bridge JA, Davidson DJ, Pilcher C, Brent DA, 2017. Metaanalysis of Mood and Substance Use Disorders in Proximal Risk for Suicide Deaths. Suicide LifeThreatening Behav https://doi.org/10.1111/sltb.12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, Gianoulakis C, 2007. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology 32, 293–305. https://doi.org/10.1016/j.psyneuen.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Dakwar E, Popii M, Coccaro EF, 2011. Lifetime History of Cigarette Smoking Associated with Aggression and Impulsivity in Both Healthy and Personality Disorered Volunteers. J. Pers. Disord 25, 645–655. https://doi.org/10.1521/pedi.2011.25.5.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Ross J, 2002. Suicide among heroin users: rates, risk factors and methods. Addiction 97, 1383–1394. https://doi.org/10.1046/j.1360-0443.2002.00214.x [DOI] [PubMed] [Google Scholar]
- Darvishi N, Farhadi M, Haghtalab T, Poorolajal J, 2015. Alcohol-Related Risk of Suicidal Ideation, Suicide Attempt, and Completed Suicide: A Meta-Analysis. PLOS ONE 10, e0126870 https://doi.org/10.1371/journal.pone.0126870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, 2008. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol 14, 22–31. https://doi.org/10.1111/j.13691600.2008.00129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, Shannon EE, Acheson A, 2012. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology (Berl). 226, 307–319. https://doi.org/10.1007/s00213-012-2908-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellickson PL, Mcguigan KA, Klein DJ, 2001. Predictors of late-onset smoking and cessation over 10 years. J. Adolesc. Heal https://doi.org/10.1016/S1054-139X(00)00199-3 [DOI] [PubMed] [Google Scholar]
- Flensborg-Madsen T, Knop J, Mortensen EL, Becker U, Sher L, Grønbæk M, 2009. Alcohol use disorders increase the risk of completed suicide - Irrespective of other psychiatric disorders. A longitudinal cohort study. Psychiatry Res. 167, 123–130. https://doi.org/10.1016/j.psychres.2008.01.008 [DOI] [PubMed] [Google Scholar]
- Flay BR, d’Avernas JR, Best JA, Kersell MW, Ryan KB, 1983. Cigarette smoking: Why young people do it and ways of preventing it. Pediatr. Adolesc. Behav. Med 10, 132–183. [Google Scholar]
- Garrison CZ, McKeown RE, Valois RF, Vincent ML, 1993. Aggression, substance use, and suicidal behaviors in high school students. Am. J. Public Health 83, 179–184. https://doi.org/10.2105/ajph.83.2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Brown T, 2003. Effect of Chronic Alcohol Consumption on the Activity of the Hypothalamic-Pituitary-Adrenal Axis and Pituitary ??-Endorphin as a Function of Alcohol Intake, Age, and Gender. Alcohol. Clin. Exp. Res. 27, 410–423. https://doi.org/10.1097/01.alc.0000056614.96137.b8 [DOI] [PubMed] [Google Scholar]
- Giegling I, Olgiati P, Hartmann AM, Calati R, Möller H-J, Rujescu D, Serretti A, 2009. Personality and attempted suicide. Analysis of anger, aggression and impulsivity. J. Psychiatr. Res 43, 1262–1271. https://doi.org/10.1016/j.jpsychires.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Bridge JA, Brent DA, 2008. Sleep disturbance preceding completed suicide in adolescents. J. Consult. Clin. Psychol 76, 84–91. https://doi.org/10.1037/0022006x.76.1.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, Johnson MW, McCann UD, Jesse R, 2008. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J. Psychopharmacol 22, 621–632. https://doi.org/10.1177/0269881108094300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenç A, Lukas SE, 2013. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology (Berl). 231, 1455–1465. https://doi.org/10.1007/s00213-013-3326-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guintivano J, Brown T, Newcomer A, Jones M, Cox O, Maher BS, Eaton WW, Payne JL, Wilcox HC, Kaminsky ZA, 2014. Identification and Replication of a Combined Epigenetic and Genetic Biomarker Predicting Suicide and Suicidal Behaviors. Am. J. Psychiatry 171, 1287–1296. https://doi.org/10.1176/appi.ajp.2014.14010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Thorne CB, Clark CB, Coombs DW, Johnson MW, 2015. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J. Psychopharmacol 29, 280–288. https://doi.org/10.1177/0269881114565653 [DOI] [PubMed] [Google Scholar]
- Hollingshead A, 1975. Four factor index of social status. Yale J. Sociol [Google Scholar]
- Hoyo-Becerra C, Huebener A, Trippler M, Lutterbeck M, Liu ZJ, Truebner K, Bajanowski T, Gerken G, Hermann DM, Schlaak JF, 2013. Concomitant Interferon Alpha Stimulation and TLR3 Activation Induces Neuronal Expression of DepressionRelated Genes That Are Elevated in the Brain of Suicidal Persons. PLoS ONE 8, e83149 https://doi.org/10.1371/journal.pone.0083149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen P-Ø, Krebs TS, 2015. Psychedelics not linked to mental health problems or suicidal behavior: A population study. J. Psychopharmacol 29, 270–279. https://doi.org/10.1177/0269881114568039 [DOI] [PubMed] [Google Scholar]
- Johnson EO, Breslau N, 2001. Sleep problems and substance use in adolescence. Drug Alcohol Depend. 64, 1–7. https://doi.org/10.1016/s0376-8716(00)00222-2 [DOI] [PubMed] [Google Scholar]
- Karoly HC, Bidwell LC, Mueller RL, Hutchison KE, 2018. Investigating the Relationships Between Alcohol Consumption, Cannabis Use, and Circulating Cytokines: A Preliminary Analysis. Alcohol. Clin. Exp. Res 42, 531–539. https://doi.org/10.1111/acer.13592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazour F, Soufia M, Rohayem J, Richa S, 2015. Suicide Risk of Heroin Dependent Subjects in Lebanon. Community Ment. Health J 52, 589–596. https://doi.org/10.1007/s10597-015-9952-7 [DOI] [PubMed] [Google Scholar]
- Kim J, Fan B, Liu X, Kerner N, Wu P, 2011. Ecstasy Use and Suicidal Behavior among Adolescents: Findings from a National Survey. Suicide Life-Threatening Behav. 41, 435–444. https://doi.org/10.1111/j.1943-278x.2011.00043.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Mezzich A, Tarter R, 1995. Norms and sensitivity of the adolescent version of the drug use screening inventory. Addict. Behav 20, 149–157. https://doi.org/10.1016/03064603(94)00058-1 [DOI] [PubMed] [Google Scholar]
- Knopf A, 2016. FDA approves MDMA Phase 3 trials for PTSD. Alcohol. Drug Abus. Wkly 28, 5 https://doi.org/10.1002/adaw.30788 [Google Scholar]
- Korhonen T, Sihvola E, Latvala A, Dick DM, Pulkkinen L, Nurnberger J, Rose RJ, Kaprio J, 2018. Early-onset tobacco use and suicide-related behavior - A prospective study from adolescence to young adulthood. Addict. Behav 79, 32–38. https://doi.org/10.1016/j.addbeh.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs TS, Johansen P-Ø, 2013. Psychedelics and Mental Health: A Population Study. PLoS ONE 8, e63972 https://doi.org/10.1371/journal.pone.0063972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H-C, Pearson JL, Liu X, 2003. Risk factors for male and female suicide decedents ages 15?64 in the United States. Soc. Psychiatry Psychiatr. Epidemiol 38, 419–426. https://doi.org/10.1007/s00127-003-0656-x [DOI] [PubMed] [Google Scholar]
- Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, Turecki G, 2012. Differential Glucocorticoid Receptor Exon 1B, 1C, and 1H Expression and Methylation in Suicide Completers with a History of Childhood Abuse. Biol. Psychiatry 72, 41–48. https://doi.org/10.1016/j.biopsych.2012.01.034 [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Jorgensen MJ, Grzywa R, Fairbanks LA, 2011. A novelty seeking phenotype is related to chronic hypothalamic-pituitary-adrenal activity reflected by hair cortisol. Physiol. Behav. 104, 291–295. https://doi.org/10.1016/j.physbeh.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Niculescu H, Levey DF, Ayalew M, Palmer L, Gavrin LM, Jain N, Winiger E, Bhosrekar S, Shankar G, Radel M, Bellanger E, Duckworth H, Olesek K, Vergo J, Schweitzer R, Yard M, Ballew A, Shekhar A, Sandusky GE, Schork NJ, Kurian SM, Salomon DR, Niculescu AB, 2013. Discovery and validation of blood biomarkers for suicidality. Mol. Psychiatry 18, 1249–1264. https://doi.org/10.1038/mp.2013.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Trout ZM, Hernandez EM, Cheek SM, Gerlus N, 2017. A behavioral and cognitive neuroscience perspective on impulsivity, suicide, and non-suicidal self-injury: Meta-analysis and recommendations for future research. Neurosci. Biobehav. Rev 83, 440–450. https://doi.org/10.1016/j.neubiorev.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ, 2000. Blunted Stress Cortisol Response in Abstinent Alcoholic and Polysubstance-Abusing Men. Alcohol. Clin. Exp. Res 24, 651–658. https://doi.org/10.1111/j.1530-0277.2000.tb02036.x [PubMed] [Google Scholar]
- Manetti L, Cavagnini F, Martino E, Ambrogio A, 2014. Effects of cocaine on the hypothalamic-pituitary-adrenal axis. J. Endocrinol. Invest 37, 701–708. https://doi.org/10.1007/s40618-014-0091-8 [DOI] [PubMed] [Google Scholar]
- Marshall BDL, Galea S, Wood E, Kerr T, 2011. Injection methamphetamine use is associated with an increased risk of attempted suicide: A prospective cohort study. Drug Alcohol Depend. 119, 134–137. https://doi.org/10.1016/j.drugalcdep.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maza-Quiroga R, Garc’\ia-Marchena N, Romero-Sanchiz P, Barrios V, Pedraz M, Serrano A, Nogueira-Arjona R, Ruiz JJ, Soria M, Campos R, Chowen JA, Argente J, Torrens M, López-Gallardo M, Marco EM, de Fonseca FR, Pavón FJ, Araos P, 2017. Evaluation of plasma cytokines in patients with cocaine use disorders in abstinence identifies transforming growth factor alpha (TGFα) as a potential biomarker of consumption and dual diagnosis. PeerJ 5, e3926 https://doi.org/10.7717/peerj.3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H, 2003. Effects of THC on Behavioral Measures of Impulsivity in Humans. Neuropsychopharmacology 28, 1356–1365. https://doi.org/10.1038/sj.npp.1300176 [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D\textquotesingleAlessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ, 2009. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci 12, 342–348. https://doi.org/10.1038/nn.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem NM, Brent DA, Ziegler M, Iyengar S, Kolko D, Oquendo M, Birmaher B, Burke A, Zelazny J, Stanley B, Mann JJ, 2007. Familial Pathways to Early-Onset Suicidal Behavior: Familial and Individual Antecedents of Suicidal Behavior. Am. J. Psychiatry 164, 1364–1370. https://doi.org/10.1176/appi.ajp.2007.06091522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem NM, Munroe S, Marsland A, Gray K, Brent D, Porta G, Douaihy A, Laudenslager ML, DePietro F, Diler R, Driscoll H, Gopalan P, 2017. Blunted HPA axis activity prior to suicide attempt and increased inflammation in attempters. Psychoneuroendocrinology 77, 284–294. https://doi.org/10.1016/j.psyneuen.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Hemenway D, Bell NS, Yore MM, Amoroso PJ, 2000a. Cigarette Smoking and Suicide: A Prospective Study of 300, 000 Male Active-duty Army Soldiers. Am. J. Epidemiol 151, 1060–1063. https://doi.org/10.1093/oxfordjournals.aje.a010148 [DOI] [PubMed] [Google Scholar]
- Miller M, Hemenway D, Rimm E, 2000b. Cigarettes and suicide: a prospective study of 50,000 men. Am. J. Public Health 90, 768–73. https://doi.org/10.2105/AJPH.90.5.768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, 1999. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl). 146, 455–464. https://doi.org/10.1007/pl00005491 [DOI] [PubMed] [Google Scholar]
- Morris MC, Mielock AS, Rao U, 2016. Salivary stress biomarkers of recent nicotine use and dependence. Am. J. Drug Alcohol Abuse 42, 640–648. https://doi.org/10.1080/00952990.2016.1202263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi S, Battle A, Zhu X, Potash JB, Weissman MM, Shi J, Beckman K, Haudenschild C, McCormick C, Mei R, Gameroff MJ, Gindes H, Adams P, Goes FS, Mondimore FM, MacKinnon DF, Notes L, Schweizer B, Furman D, Montgomery SB, Urban AE, Koller D, Levinson DF, 2013. Type I interferon signaling genes in recurrent major depression: increased expression detected by wholeblood RNA sequencing. Mol. Psychiatry 19, 1267–1274. https://doi.org/10.1038/mp.2013.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves CDC, Lacerda ACR, Lima LP, Lage VKS, Balthazar CH, Leite HR, Mendonça VA, 2017. Different levels of brain-derived neurotrophic factor and cortisol in healthy heavy smokers. Brazilian J. Med. Biol. Res 50 https://doi.org/10.1590/1414431×20176424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu AB, Levey DF, Phalen PL, Le-Niculescu H, Dainton HD, Jain N, Belanger E, James A, George S, Weber H, Graham DL, Schweitzer R, Ladd TB, Learman R, Niculescu EM, Vanipenta NP, Khan FN, Mullen J, Shankar G, Cook S, Humbert C, Ballew A, Yard M, Gelbart T, Shekhar A, Schork NJ, Kurian SM, Sandusky GE, Salomon DR, 2015. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol. Psychiatry 20, 1266–1285. https://doi.org/10.1038/mp.2015.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Hwang I, Sampson NA, Kessler RC, 2009. Mental disorders, comorbidity and suicidal behavior: Results from the National Comorbidity Survey Replication. Mol. Psychiatry 15, 868–876. https://doi.org/10.1038/mp.2009.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Kessler RC, 2006. Prevalence of and risk factors for suicide attempts versus suicide gestures: Analysis of the National Comorbidity Survey. J. Abnorm. Psychol 115, 616–623. https://doi.org/10.1037/0021-843x.115.3.616 [DOI] [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR, 2009. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain. Behav. Immun 23, 887–897. https://doi.org/10.1016/j.bbi.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ, 2004. Prospective Study of Clinical Predictors of Suicidal Acts After a Major Depressive Episode in Patients With Major Depressive Disorder or Bipolar Disorder. Am. J. Psychiatry 161, 1433–1441. https://doi.org/10.1176/appi.ajp.161.8.1433 [DOI] [PubMed] [Google Scholar]
- Pacek LR, Herrmann ES, Smith MT, Vandrey R, 2017. Sleep continuity, architecture and quality among treatment-seeking cannabis users: An in-home, unattended polysomnographic study. Exp. Clin. Psychopharmacol 25, 295–302. https://doi.org/10.1037/pha0000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y, 2012. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J. Psychiatr. Res 46, 57–63. https://doi.org/10.1016/j.jpsychires.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Zhang H, Bhaumik R, Ren X, 2016. The Expression of the Suicide-Associated Gene SKA2 Is Decreased in the Prefrontal Cortex of Suicide Victims but Not of Nonsuicidal Patients. Int. J. Neuropsychopharmacol 19, pyw015 https://doi.org/10.1093/ijnp/pyw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Montgomery C, Wetherell MA, Downey LA, Stough C, Scholey AB, 2014. MDMA, cortisol, and heightened stress in recreational ecstasy users. Behav. Pharmacol 1 https://doi.org/10.1097/fbp.0000000000000060 [DOI] [PubMed] [Google Scholar]
- Patterson F, Grandner MA, Loranzo A, Satti A, Ma G, 2018. Transitioning from adequate to inadequate sleep duration associated with higher smoking rate and greater nicotine dependence in a population sample. Addict. Behav 77, 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W, 2008. Does cannabis use lead to depression and suicidal behaviours? A population-based longitudinal study. Acta Psychiatr. Scand 118, 395–403. https://doi.org/10.1111/j.1600-0447.2008.01259.x [DOI] [PubMed] [Google Scholar]
- Perez-Ortiz JM, Garcia-Gutierrez MS, Navarrete F, Giner S, Manzanares J, 2013. Gene and protein alterations of FKBP5 and glucocorticoid receptor in the amygdala of suicide victims. Psychoneuroendocrinology 38, 1251–1258. [DOI] [PubMed] [Google Scholar]
- Piepenbrink MS, Samuel M, Zheng B, Carter B, Fucile C, Bunce C, Kiebala M, Khan AA, Thakar J, Maggirwar SB, Morse D, Rosenberg AF, Haughey NJ, Valenti W, Keefer MC, Kobie JJ, 2016. Humoral Dysregulation Associated with Increased Systemic Inflammation among Injection Heroin Users. PLOS ONE 11, e0158641 https://doi.org/10.1371/journal.pone.0158641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters S, Burk JM, Van der Vorst H, Dahl RE, Wiers RW, Engels RCME, 2015. Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. J. Youth Adolescence 44, 379–388. [DOI] [PubMed] [Google Scholar]
- Poorolajal J, Darvishi N, 2016. Smoking and Suicide: A Meta-Analysis. PLOS ONE 11, e0156348 https://doi.org/10.1371/journal.pone.0156348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorolajal J, Haghtalab T, Farhadi M, Darvishi N, 2015. Substance use disorder and risk of suicidal ideation, suicide attempt and suicide death: a meta-analysis. J. Public Health (Bangkok) 38, e282–e291. https://doi.org/10.1093/pubmed/fdv148 [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ, 2011. The Columbia-Suicide Severity Rating Scale: Initial Validity and Internal Consistency Findings From Three Multisite Studies With Adolescents and Adults. Am. J. Psychiatry 168, 1266–1277. https://doi.org/10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Das S, Young-Wolff KC, 2017. Smoking, Mental Illness, and Public Health. Annu Rev Public Health 38, 165–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransome Y, Slopen N, Karlsson O, Williams DR, 2017. Elevated inflammation in association with alcohol abuse among Blacks but not Whites: results from the MIDUS biomarker study. J. Behav. Med https://doi.org/10.1007/s10865-017-9905-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, 2010. Risk Factors for Attempting Suicide in Heroin Addicts. Suicide Life-Threatening Behav. 40, 416–420. https://doi.org/10.1521/suli.2010.40.4.416 [DOI] [PubMed] [Google Scholar]
- Schneider B, 2009. Substance Use Disorders and Risk for Completed Suicide. Arch. Suicide Res 13, 303–316. https://doi.org/10.1080/13811110903263191 [DOI] [PubMed] [Google Scholar]
- Sessa B, Nutt D, 2015. Making a medicine out of MDMA. Br. J. Psychiatry 206, 4–6. https://doi.org/10.1192/bjp.bp.114.152751 [DOI] [PubMed] [Google Scholar]
- Sher L, Oquendo MA, Richardson-Vejlgaard R, Makhija NM, Posner K, Mann JJ, Stanley BH, 2009. Effect of acute alcohol use on the lethality of suicide attempts in patients with mood disorders. J. Psychiatr. Res. 43, 901–905. https://doi.org/10.1016/j.jpsychires.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewert EA, Stallings MC, Hewitt JK, 2004. Factor structure and concurrent validity of the Drug Use Screening Inventory in a community adolescent sample. Addict. Behav 29, 627–638. https://doi.org/10.1016/j.addbeh.2003.08.027 [DOI] [PubMed] [Google Scholar]
- Smith PH, Homish GG, Leonard KE, Collins RL, 2013. Marijuana withdrawal and aggression among a representative sample of U.S. marijuana users. Drug Alcohol Depend 132, 63–68. https://doi.org/10.1016/j.drugalcdep.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag H, Wittchen H-U, Hofler M, Kessler RC, Stein MB, 2000. Are social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? Eur. Psychiatry https://doi.org/10.1016/S09249338(00)00209-1 [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH, 2009. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences 47, 385–395. [Google Scholar]
- Tarter RE, Kirisci L, 1997. The Drug Use Screening Inventory for Adults: Psychometric Structure and Discriminative Sensitivity. Am. J. Drug Alcohol Abuse 23, 207–219. https://doi.org/10.3109/00952999709040942 [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Möller H-J, Chen HH, Postolache TT, 2008. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr. Scand 117, 198–206. https://doi.org/10.1111/j.1600-0447.2007.01128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CG, Ribeiro JD, Franklin JC, 2017. Predicting Risk of Suicide Attempts Over Time Through Machine Learning. Clin. Psychol. Sci 5, 457–469. https://doi.org/10.1177/2167702617691560 [Google Scholar]
- Wee CC, Rigotti N. a, Davis RB, Phillips RS, 2001. Relationship between smoking and weight control efforts among adults in the united states. Arch. Intern. Med https://doi.org/10.1001/archinte.161.4.546 [DOI] [PubMed] [Google Scholar]
- Wilcox HC, Conner KR, Caine ED, 2004. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 76, S11–S19. https://doi.org/10.1016/j.drugalcdep.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Wolfe KL, Nakonezny PA, Owen VJ, Rial KV, Moorehead AP, Kennard BD, Emslie GJ, 2017. Hopelessness as a Predictor of Suicide Ideation in Depressed Male and Female Adolescent Youth. Suicide Life-Threatening Behav. https://doi.org/10.1111/sltb.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li J, Xu G, Zhang J, Chen Z, Lu Z, Deng H, 2016. Elevated Hair Cortisol Levels among Heroin Addicts on Current Methadone Maintenance Compared to Controls. PLOS ONE 11, e0150729 https://doi.org/10.1371/journal.pone.0150729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Galfalvy H, Pantazatos SP, Huang Y, Rosoklija GB, Dwork AJ, Burke A, Arango V, Oquendo MA, Mann JJ, 2016. Glucocorticoid receptor-related genes: genotype and brain gene expression relationships to suicide and major depressive disorder. Depress. Anxiety 33, 531–540. https://doi.org/10.1002/da.22499 [DOI] [PMC free article] [PubMed] [Google Scholar]