Abstract
Broadly neutralizing antibodies against highly variable pathogens have stimulated the design of novel vaccines and therapeutics. Here, we report on diverse camelid single-domain antibodies to influenza hemagglutinin from which we generated multi-domain antibodies with unprecedented breadth and potency. Multi-domain antibody MD3606 protects mice against influenza A and B infection when administered intravenously or expressed locally from a recombinant adeno-associated virus vector. Crystal and single-particle EM structures of these antibodies with hemagglutinins from influenza A and B viruses reveal binding to highly conserved epitopes. Collectively, our findings demonstrate that multi-domain antibodies targeting multiple epitopes exhibit enhanced virus cross-reactivity and potency. In combination with adeno-associated virus-mediated gene delivery, they may provide a groundbreaking new strategy to prevent infection with influenza virus and other highly variable pathogens.
Summary
Universal multi-domain influenza antibodies or Multi-domain antibodies provide universal protection against influenza A and B viruses
Seasonal influenza epidemics cause worldwide morbidity and mortality (1), whereas the vast reservoir of influenza A viruses in aquatic birds represents continual pandemic threats (2–4). Vaccines remain essential for influenza prevention, but their efficacy is significantly reduced in the elderly, who are at increased risk of influenza-related complications (3, 5, 6). Annual selection of vaccine strains presents many challenges and a poor match with circulating viruses can result in suboptimal effectiveness (7). Moreover, most vaccine-induced antibodies are directed against the highly variable head region of hemagglutinin (HA) and are strain-specific. However, broadly neutralizing antibodies (bnAbs) targeting influenza HA have been isolated and characterized (8). Several bnAbs have entered clinical trials as therapeutic agents, but their use in influenza prophylaxis remains elusive due to (i) incomplete coverage against circulating human influenza A and B viruses, which necessitates administration of a bnAb cocktail, and (ii) the need for multiple, high-dose injections for protection throughout the entire influenza season. High serum bnAb levels are required because of poor distribution to the upper airways. Here, we present an alternative strategy for long-lasting protection based on single-domain Abs (sdAbs) (9) with influenza A or B reactivity that were linked together into a multi-domain Ab (MDAb) and expressed at the nasopharyngeal mucosa through intranasal administration of a recombinant adeno-associated virus (AAV) vector encoding the MDAb transgene (10, 11).
To obtain broadly neutralizing sdAbs, llamas were immunized with influenza vaccine and H7 and H2 recombinant HA (rHA) (12). HA cross-reactive sdAbs were isolated from the sdAb (VHH) repertoires of the immunized llamas by phage display using various cross-selection strategies on rHAs from different influenza subtypes. We isolated two influenza A (SD36 and SD38) and two influenza B (SD83 and SD84) sdAbs, and analyzed their in vitro neutralizing activity (Fig. 1). SD36 potently neutralized influenza A group 2 (H3, H4, H7 and H10), but not group 1 (H1, H2, and H5) viruses, whereas SD38 potently neutralized group 1 (H1, H2 and H5) and some group 2 (H3, H7 and H10) viruses, albeit with lower potency. SD84 and SD83 neutralized representative viruses from both influenza B lineages.
Fig. 1. In vitro neutralization of influenza A and B viruses by individual and genetically fused sdAbs.
In vitro potencies of SD36, SD38, SD83 and SD84 and genetically fused sdAbs SD38-SD36 and SD83-SD84 against selected influenza A and B viruses. Both SD38-SD36 and SD83-SD84 are statistically more potent (*: p-value <0.05) compared to each of their individual components (comparisons shown by the brackets) (see SI Materials and Methods). Data are representative of at least three independent experiments performed in quadruplicates.
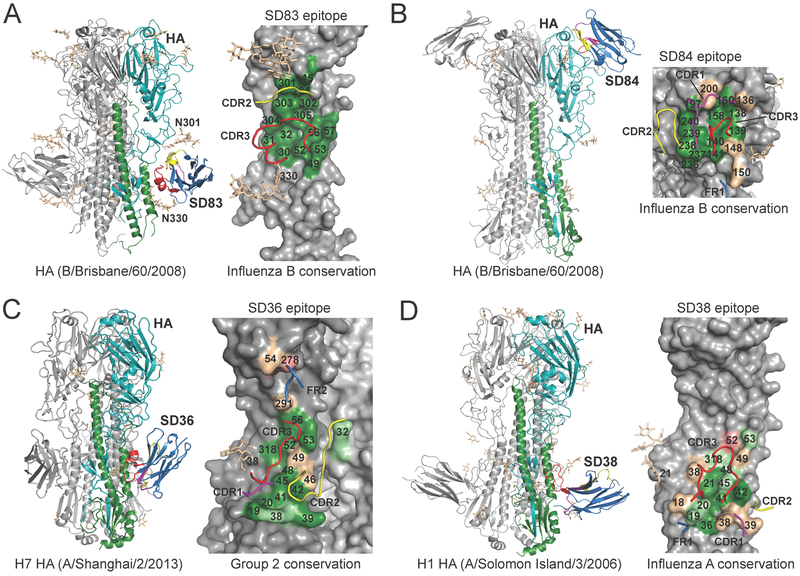
To elucidate the molecular basis for the broad HA recognition, we determined crystal structures of SD38 and SD83 to 2.0-Å resolution, SD36, SD38, and SD83 with different HAs to 2.2–2.8-Å, humanized SD84 (SD84h) to 0.94-Å, and with HA (B/Brisbane/60/08) and bnAb CR9114 to 4.1 Å (13) (Fig. 2, table S1). A cryo-EM reconstruction of SD84 with B/Massachusetts/02/12 HA and CR9114 was determined to 7.1-Å resolution (fig. S1). All sdAbs except SD84 recognized the HA stem with three sdAbs bound per trimer (Fig. 2). SD83 contacted conserved residues in the fusion subdomain (Fig. 2A) with CDR2 and CDR3 framing the epitope consisting of HA1 residues 30–32, K45, D291, N301 and P305, the HA2 A-helix, and N-linked glycans at N301 and N330 (Fig. 2A). The SD83 epitope was highly conserved with all contact residues being >99% identical in influenza B viruses (Fig. 2A and table S2). SD83 buried a relatively small surface area (588 Å2) on HA, which potentially presented fewer possibilities for escape. In contrast, SD84 bound a conserved epitope in the HA head around the receptor-binding site (Fig. 2B and table S2). SD36 and SD38 also recognized conserved epitopes that partially overlap influenza A stem epitopes of bnAbs CR9114, CR6261, and FI6v3, and to lesser extent CR8043 and CR8020 (13–16). SD36 CDR2 and CDR3 contacted the HA2 A-helix, HA1 N291, T318, and HA1 R32 of the adjacent protomer (Fig. 2C, fig. S2). CDR1 and CDR3 interacted with HA2 19–20, and FR2 with HA1 K54 and E278. In SD38, the 17-residue CDR3 dominated and bound to a shallow groove formed by the HA2 A-helix, HA2 18–21, and HA1 H38 and T318 (Fig. 2D, figs. S2 and S3) (17).
Fig. 2. Crystal structures of sdAbs in complex with HAs and conservation of their epitopes.
(A) Crystal structure of SD83 with influenza B HA (B/Brisbane/60/08) at 2.2-Å resolution. One HA-SD83 protomer of the trimeric complex is colored with HA1 in green, HA2 in cyan, and SD83 in blue (left). CDR1 is colored in magenta, CDR2 in yellow, and CDR3 in red. The other HA protomers are colored in gray. N-linked glycans are shown in beige in stick representation. The epitope of SD83 is mapped onto the influenza B HA and colored by conservation across influenza B HAs (right). Dark green, over 95% conserved; light green, 75%–95% conserved; wheat, 50%–75% conserved and salmon, 35%–50% conserved. Only the CDR loops involved in the interaction are shown. (B) Crystal structure of SD84h with influenza B HA (B/Brisbane/60/08) as well as with CR9114 Fab at 4.1-Å resolution (left). Epitope of SD84h mapped onto the influenza B HA colored by conservation across influenza B HAs (right). For clarity, CR9114 Fab, which binds to the conserved HA stem region, is not shown. (C) Crystal structure of SD36 with H7 HA (A/Shanghai/2/13) at 2.65-Å resolution (left). Epitope of SD36 mapped onto the H7 HA colored by conservation across influenza A group 2 HAs (right). (D) Crystal structure of SD38 with H1 HA (A/Solomon Islands/3/06) at 2.8-Å resolution (left). Epitope of SD38 mapped onto the H1 HA colored by conservation across influenza A HAs (right).
Thus, SD38 and SD36 made extensive contacts to the A-helix and other highly conserved residues (tables S3 and S4). However, 33% of H3 viruses had a D46N polymorphism and were not neutralized by SD36 (table S5). N46 would disrupt a salt bridge and hydrogen bond network with SD36 (fig. S4). SD36 bound H1 HA (A/Brisbane/59/07) with 290 nM KD (table S6), but did not neutralize the corresponding virus in vitro (IC50 >1 μM). As all group 1 viruses were glycosylated at HA1 N289, steric hindrance may have lowered the SD36 affinity below the neutralization threshold, whereas SD38 avoided this glycan (table S6). HA2 V18 and L38 on the SD38 epitope periphery were the least conserved across H1 strains (table S3, Fig. 2D), but natural mutations Q38 or I18/Q38 did not affect neutralization (table S7).
We next rationalized that linking different sdAbs could generate MDAbs with increased potency and breadth. Thus, MDAbs were engineered by genetically fusing individual sdAbs with peptide linkers and then linking to human IgG1-Fc (Fig. 3). SD38–SD36 was generally more potent with broader cross-reactivity than SD36 and SD38 alone, especially against H3 viruses (Fig. 1). Notably, SD38–SD36 neutralized D46N-carrying H3N2 viruses A/Panama/2007/99 and A/Wisconsin/67/05, neither of which was neutralized by SD36 or SD38, thereby demonstrating a synergistic effect (table S8). SD83–SD84 also neutralized influenza B viruses much more potently, especially B/Lee/40 (Fig. 1). Notably, MD2407 (SD38–SD36–SD83–SD84) and MD3606 (MD2407 fused to human IgG1-Fc) neutralized all A (H1-H12, H14) and B viruses tested except for one avian H12 virus (18), and had much greater breadth and potency compared to the individual sdAbs, or CR9114 (Fig. 3 and table S9). MD3606 also strongly bound avian H13, H15, H16, and bat H17, H18 HAs (table S6).
Fig. 3. In vitro neutralization of influenza A and B viruses by pan-influenza MDAbs MD2407 and MD3606 versus CR9114.
In vitro potencies of MD2407 (left), MD3606 (middle), and CR9114 (right) against a panel of 60 influenza A group 1 (blue), A group 2 (green), and B (red) viruses. Influenza strains tested together with their IC50 values are compiled in table S9. Both MD3606 and MD2407 are statistically more potent (p-value <0.05) than CR9114 (see SI Materials and Methods). Data are representative of at least three independent experiments performed in quadruplicate.
Depending on their epitopes, known bnAbs inhibit influenza infection by blocking viral attachment, HA proteolytic activation, membrane fusion, or viral egress (19–21). The HA head-binding SD84 and both MDAbs inhibited hemagglutination by influenza B (B/Florida/04/06-MA) virus (table S10). All three stem-binders and both MDAbs blocked the low-pH HA rearrangements required for membrane fusion (fig. S5). Unlike CR8020, the sdAbs or MDAbs could not prevent HA proteolytic activation, reflecting their epitope location further up the HA stem (figs. S2 and S6). In contrast to SD83 and SD84, MD2407 and MD3606 inhibited egress of B/Malaysia/2506/04 virus (fig. S7), similar to some other bnAbs (21, 22).
A shift from monovalency (Fab) to bivalency (IgG) can considerably increase the binding and neutralization breadth of influenza A antibodies, mainly to the HA head (23–25). We extended this concept with our MDAbs that targeted different HA conserved epitopes, resulting in greatly increased potency and unparalleled neutralization breadth. From our crystal and EM structures, we were able to exclude simultaneous binding of the MDAbs to epitopes on the same HA trimer (fig. S8A and table S11) (26). MDAbs could also increase avidity through cross-linking adjacent HA trimers on the viral surface. Intercross-linked HA complexes (85–96 Å) were indeed visible in negative-stain EM (fig. S8B) and, if this holds for HA trimers on the viral surface, it could partially explain the increased potency of MD2407 (and MD3606) versus their individual sdAb components.
To assess in vivo protection, we compared the prophylactic efficacy of MD3606 with CR9114 and CR8071 in BALB/c mice challenged with H1N1, H3N2, H7N9 and B viruses using intravenous (i.v.) administration (Figs. 4, A to D, figs. S9 and S10). MD3606 at 1.7 mg/kg completely protected mice from a lethal dose of mouse-adapted (MA) H1N1 virus (A/Puerto Rico/8/34-MA) and was superior to 5 mg/kg CR9114. MD3606 at 5 mg/kg provided complete protection from H3N2 virus (A/Hong Kong/1/68-MA) infection, whereas at 1.7 mg/kg, 7/8 mice survived with MD3606, compared to 5/8 with CR9114. With an H7N9 virus (A/Anhui/1/13), mice were completely protected with 5 mg/kg MD3606 versus 15 mg/kg for CR9114. Finally, at 1 mg/kg, MD3606 protected all mice from influenza B (B/Florida/4/06-MA) infection and was superior to CR9114 and CR8071 (12.5% and 50% survival). MD3606 administered one day before challenge with H1N1 or B virus also resulted in a dose-dependent reduction in lung viral load (fig. S11).
Fig. 4. Prophylactic efficacy of recombinant and AAV-expressed MD3606 in mice challenged with influenza virus.
Survival curves of BALB/c mice (n = 8 per group) treated i.v. with the indicated doses of recombinant MD3606 1 day before challenge with a lethal dose (25 LD50) of (A) H1N1 (A/Puerto Rico/8/34-MA), (B) H3N2 (A/Hong Kong/1/68-MA), (C) H7N9 (A/Anhui/1/13), or (D) B (B/Florida/4/06-MA) virus. Survival curves of BALB/c mice (n = 5–8 per group) treated intranasally with the indicated doses of AAV9.MD3606h vector (expressed as genome copies (GC) per mouse) 7 days before challenge with a lethal dose (5 LD50) of (E) H1N1 (A/Puerto Rico/8/34-MA), (F) H3N2 (A/Hong Kong/1/68-MA), or (G) B (B/Lee/40-MA) virus. *Treatment significantly different from vehicle or naïve control (p-value <0.05) (see SI Materials and Methods).
Antibody-dependent cellular cytotoxicity (ADCC) can also contribute to in vivo bnAb efficacy (27). In an ADCC reporter assay, MD3606 activated FcγRIIIa similar to CR9114 (fig. S12). MD3606 with a murine IgG2a-Fc protected all mice from lethality with influenza B virus (B/Florida/4/06-MA) at 1 mg/kg (i.v.) similar to MD3606 with human IgG1-Fc (fig. S13). An hIgG1-LALA Fc mutant, which decreases binding to human and mouse FcγRs, showed 37.5% survival at 1 mg/kg (i.v.), but 100% survival at 5 mg/kg. MD3606 with a mIgG2aσ-Fc domain lacking FcγR binding (28) provided full protection at 5 mg/kg, but none at 1 mg/kg. These FcγR-mediated effector functions will most likely extend to influenza A viruses, as binding of MD3606 to cells expressing H3 HA (A/Wisconsin/67/05) induced ADCC in vitro (fig. S12).
We then evaluated the prophylactic efficacy of a recombinant AAV9 vector encoding humanized MD3606 (29) (AAV9.MD3606h) in mice challenged with mouse-adapted influenza H1N1, H3N2, and B viruses (Fig. 4E–G, fig. S14). AAV9.MD3606h was administered intranasally 7 days before influenza challenge at vector doses ranging from 4×107 to 5×109 genome copies (GC) per mouse. 5×109 GC completely protected against lethal challenge with H1N1 virus (A/Puerto Rico/8/34-MA), whereas 7/8 mice survived with 1×109 GC. Mice challenged with H3N2 (A/Hong Kong/1/68-MA) and B (B/Lee/40-MA) viruses were fully protected by intranasal administration of 5×108 GC (lowest dose tested) and 1×109 GC, respectively. AAV9.MD3606h also conferred protection against H1N1 virus when administered 35 days before challenge (fig. S15). Pre-existing serum-circulating AAV9-specific neutralizing antibodies did not affect the efficacy of AAV9.MD3606h (fig. S16).
Limitations of seasonal influenza vaccines together with the constant threat of a new influenza pandemic have spurred the search for new influenza prevention strategies. One such strategy involves passive immunization by AAV-mediated delivery of genes encoding protective bnAbs (11). In preclinical mouse models, intranasal delivery of AAV9-encoding bnAb FI6 provided protection to H1N1 infection three days after vector administration (11). Notably, old and immunodeficient mice were also protected from a lethal H1N1 dose (10). Mucosal expression of the AAV9 transgene is durable [>9 months in mice (30) and 4 months in rhesus macaques (11)] and AAV9 vectors can be re-administered in the airway without loss of efficiency (30). To be clinically useful, AAV-encoded bnAbs should neutralize both influenza A and B viruses, but all bnAbs to date lack sufficient influenza A and B cross-reactivity; the limited AAV packaging capacity (<5 kb) also precludes expression of two individual bnAbs or a bispecific bnAb from a single vector. Here, we used an alternative antibody platform based on camelid-derived sdAbs to create two highly potent MDAbs, MD3606 and MD2407, with near-universal activity against influenza A and B, both of which can be expressed from a single AAV vector. Fc-containing MD3606 administered i.v. was more effective than state-of-the-art bnAb CR9114 against seasonal and pandemic influenza viruses, with a significant effect (p<0.05) on survival in H7N9 and B models (table S12). Intranasal delivery of AAV9.MD3606h provided full protection in mice at doses as low as 5×108 GC. If the above preclinical findings translate to humans, an annual intranasal administration of AAV9.MD3606h may provide passive protection for the entire influenza season, and would be of particular benefit to the elderly and other high-risk groups. The rapid onset of protection together with the unprecedented cross-reactivity of MD3606 to avian influenza strains also offers the possibility of using this approach as a prophylactic immediately upon onset of an influenza pandemic, providing significant advantages over vaccination.
Supplementary Material
ACKNOWLEDGEMENTS
We thank N. van Dijk for performing label-free binding assays, W. Yu for protein purification, H. Tien for automated crystal screening, and the staff of the Gene Therapy Program for their invaluable assistance with AAV-directed gene transfer studies.
Funding: This work is supported in part by NIH grants R56 AI117675 and R56 AI127371 (to I.A.W.) and Theme-based Research Scheme, Research Grants Council of the Hong Kong (ref. T11–705/14N). X-ray diffraction data were collected at the Advanced Photon Source (APS) beamlines 23ID-B, 23ID-D and 17-ID, and at the Stanford Synchrotron Radiation Lightsource (SSRL) BL12–2. Use of the APS was supported by the U.S. Department of Energy (DOE), Basic Energy Sciences, Office of Science, under contract no. DE-AC02–06CH11357. GM/CA CAT is funded in whole or in part with federal funds from the National Cancer Institute (Y1-CO-1020) and the NIGMS (Y1-GM-1104). Use of the SSRL, SLAC National Accelerator Laboratory, is supported by the U.S. DOE, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02–76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS, NIAID or NIH.
Footnotes
Competing interests: Janssen Vaccines & Prevention B.V. has a pending patent application (WO/2016/124768) relating to certain molecules described in this manuscript.
Data and materials availability: Coordinates and structure factors are deposited in the Protein Data Bank as entries 6FYU, 6FYT, 6CK8, 6FYW, 6FYS, 6CNW, and 6CNV. The EM reconstructions have been deposited in the Electron Microscopy Data Bank under accession code EMD-9029. This is publication 29558 from The Scripps Research Institute.
REFERENCES AND NOTES
- 1.World Health Organization (WHO), http://www.who.int/mediacentre/factsheets/fs211/en.
- 2.Liu J et al. , Highly pathogenic H5N1 influenza virus infection in migratory birds. Science 309, 1206 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Chen H et al. , Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature 436, 191–192 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Gao R et al. , Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med 368, 1888–1897 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Osterholm MT, Kelley NS, Sommer A, Belongia EA, Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12, 36–44 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Beyer WE et al. , Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine 31, 6030–6033 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Xie H et al. , H3N2 mismatch of 2014–15 northern hemisphere influenza vaccines and head-to-head comparison between human and ferret antisera derived antigenic maps. Sci. Rep 5, 15279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu NC, Wilson IA, A Perspective on the Structural and Functional Constraints for Immune Evasion: Insights from Influenza Virus. J. Mol. Biol 429, 2694–2709 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krah S et al. , Single-domain antibodies for biomedical applications. Immunopharmacol. Immunotoxicol 38, 21–28 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Adam VS et al. , Adeno-associated virus 9-mediated airway expression of antibody protects old and immunodeficient mice against influenza virus. Clin. Vaccine Immunol 21, 1528–1533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limberis MP et al. , Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza. Sci. Transl. Med 5, 187ra72 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.See supplementary materials on Science Online.
- 13.Dreyfus C et al. , Highly conserved protective epitopes on influenza B viruses. Science 337, 1343–1348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corti D et al. , A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Ekiert DC et al. , Antibody recognition of a highly conserved influenza virus epitope. Science 324, 246–251 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sui J et al. , Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol 16, 265–273 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CDR1 and CDR2 also made minor contacts to the A-helix.
- 18.H12 HA has more mutations in the SD38 epitope relative to other HA subtypes, including at HA1 318(Ile), HA2 48(Met), 49(Gln), and 52(Leu) that are not present in other subtypes (table S3). However, MD2407 binds H12 HA in biolayer interferometry (BLI) experiments, but with fast on and fast off rates and a KD value >550 nM for the first of two sites in a 2:1 binding model, which is higher than for other subtypes (data not shown). Further studies will be needed to find out which H12 HA mutation(s) are responsible for the lack of neutralization and if these resistance mutations can emerge in HAs of human influenza viruses (e.g. H1N1 or H3N2) upon treatment with MD3606.
- 19.Julien JP, Lee PS, Wilson IA, Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol. Rev 250, 180–198 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laursen NS, Wilson IA, Broadly neutralizing antibodies against influenza viruses. Antiviral. Res 98, 476–483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandenburg B et al. , Mechanisms of hemagglutinin targeted influenza virus neutralization. PLoS One 8, e80034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan GS et al. , Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza A virus hemagglutinin. J. Virol 88, 13580–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee PS et al. , Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc. Natl. Acad. Sci. U.S.A 109, 17040–17045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hultberg A et al. , Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS One 6, e17665 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekiert DC et al. , Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489, 526–532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The ten-residue linkers in MD2407 were too short to allow SD36 and SD38, or SD84 and SD83, to bind the same HA trimer (fig. S8A). Furthermore, the potency of SD38–SD36 with an 18-residue linker was indistinguishable from constructs with 38- or 60-residue linkers, which should be more permissive for intra-trimer binding (table S11).
- 27.DiLillo DJ, Tan GS, Palese P, Ravetch JV, Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat. Med 20, 143–151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vafa O et al. , An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 65, 114–126 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Humanization of the four sdAb components of MD3606 (SD36, SD38, SD83 and SD84) is described in patent application WO 2016/124768. The humanized sdAbs share 92–98% framework identity with their closest human VH germline sequences. Wild-type and humanized MD3606 were virtually indistinguishable in in vitro neutralization assays using different influenza A and B viruses.
- 30.Limberis MP, Wilson JM, Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. U.S.A 103, 12993–12998 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer RA et al. , The human antibody repertoire specific for rabies virus glycoprotein as selected from immune libraries. Eur. J. Immunol 35, 2131–2145 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Kramer RA et al. , A novel helper phage that improves phage display selection efficiency by preventing the amplification of phages without recombinant protein. Nucleic Acids Res 31, e59 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Throsby M et al. , Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3, e3942 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekiert DC et al. , A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333, 843–850 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ, Likelihood-enhanced fast translation functions. Acta Crystallogr. D Biol. Crystallogr 61, 458–464 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Murshudov GN, Vagin AA, Dodson EJ, Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr 53, 240–255 (1997). [DOI] [PubMed] [Google Scholar]
- 37.Emsley P, Lohkamp B, Scott WG, Cowtan K, Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afonine PV et al. , Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr 68, 352–367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabsch W, XDS. Acta Crystallogr. D Biol. Crystallogr 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carragher B et al. , Leginon: an automated system for acquisition of images from vitreous ice specimens. J. Struct. Biol 132, 33–45 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Lander GC et al. , Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol 166, 95–102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogura T, Iwasaki K, Sato C, Topology representing network enables highly accurate classification of protein images taken by cryo electron-microscope without masking. J. Struct. Biol 143, 185–200 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Yang Z, Fang J, Chittuluru J, Asturias FJ, Penczek PA, Iterative stable alignment and clustering of 2D transmission electron microscope images. Structure 20, 237–247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang G et al. , EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol 157, 38–46 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Hohn M et al. , SPARX, a new environment for Cryo-EM image processing. J. Struct. Biol 157, 47–55 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Roseman AM, FindEM-a fast, efficient program for automatic selection of particles from electron micrographs. J. Struct. Biol 145, 91–99 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Scheres SH, A Bayesian view on cryo-EM structure determination. J. Mol. Biol 415, 406–418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettersen EF et al. , UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Sanner MF, Olson AJ, Spehner JC, Reduced surface: an efficient way to compute molecular surfaces. Biopolymers 38, 305–320 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM, Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis 199, 381–90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popp MW, Site-specific labeling of proteins via sortase: protocols for the molecular biologist. Methods Mol. Biol 1266, 185–198 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Chen VB et al. , MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr 66, 12–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.