Abstract
Ischaemic heart disease is a leading cause of death worldwide. Injury to the heart is followed by loss of the damaged cardiomyocytes, which are replaced with fibrotic scar tissue. Depletion of cardiomyocytes results in decreased cardiac contraction, which leads to pathological cardiac dilatation, additional cardiomyocyte loss, and mechanical dysfunction, culminating in heart failure. This sequential reaction is defined as cardiac remodelling. Many therapies have focused on preventing the progressive process of cardiac remodelling to heart failure. However, after patients have developed end-stage heart failure, intervention is limited to heart transplantation. One of the main reasons for the dramatic injurious effect of cardiomyocyte loss is that the adult human heart has minimal regenerative capacity. In the past 2 decades, several strategies to repair the injured heart and improve heart function have been pursued, including cellular and noncellular therapies. In this Review, we discuss current therapeutic approaches for cardiac repair and regeneration, describing outcomes, limitations, and future prospects of preclinical and clinical trials of heart regeneration. Substantial progress has been made towards understanding the cellular and molecular mechanisms regulating heart regeneration, offering the potential to control cardiac remodelling and redirect the adult heart to a regenerative state.
Ischaemic heart disease is the leading cause of death worldwide, accounting for 9 million deaths per year1. Many of these patients not only undergo the acute phase of myocardial infarction (MI) but also develop progressive heart failure derived from ventricular dysfunction caused by the ischaemic conditions, defined as ischaemic cardiomyopathy. After MI, the damaged myocardium is replaced by fibrotic scar tissue owing to the minimal regenerative capacity of cardiomyocytes in the adult human heart. The presence of scar tissue in the heart results in loss of pump function and circulatory deficiency. Subsequently, the injured heart follows a remodelling process that results in further fibrosis, loss of myocardium, cardiac dysfunction, and dilatation, eventually leading to fatal heart failure2.
Treatment of ischaemic heart disease has focused on protecting the heart from progression to heart failure3. For example, revascularization by thrombolysis, cardiac intervention, and bypass surgery serve to improve blood supply and can salvage the injured ischaemic myocardium. Pharmacological approaches that slow or reverse cardiac remodelling, such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor-neprilysin inhibitors, β-blockers, and mineralocorticoid-receptor antagonists, have decreased heart failure mortality4–7. In contrast to these cardioprotective therapies that target the remodelling process in the failing heart, limited therapies are available for the advanced remodelled heart at end-stage heart failure. Mechanical support therapies, such as left ventricular assist devices (LVADs) and cardiac resynchronization therapy, show beneficial outcomes in patients with end-stage heart failure, but heart transplantation remains the only current solution to renew the impaired heart8,9. However, pragmatically, heart transplantation is not realistic as a standard therapy because of the lack of donors worldwide and the surgical complexities10.
To protect the failing heart, scientists have recently focused on approaches to promote heart regeneration. Initial approaches, the so-called first-generation cell- based therapies, involved transplanting noncardiac cells because researchers could not obtain adequate numbers of functional cardiomyocytes to replace the lost myocardium11. Initial cell candidates included skeletal myoblasts, which were expected to contribute to cardiac contraction, and bone marrow-derived cells and mesenchymal stem cells, which showed cardiogenic potential in vitro12–14. The next generation of cell-based therapy used resident cardiac cells with stem cell-like characteristics. These cardiac-derived cells were expandable and demonstrated multipotency, differentiating into various cell types of the heart in vitro15. Another approach to heart regeneration involved the generation of functional cardiomyocytes in vitro that then were transplanted into the injured heart. Preclinical studies used pluripotent stem cells, which can reliably differentiate into functional cardiomyocytes in vitro16.
Alternative cell-free approaches for heart regeneration have targeted cardiac resident cells. For example, a reprogramming approach focused on converting cardiac fibroblasts to a cardiomyocyte fate17,18. Inducing proliferation of the remaining endogenous cardiomyocytes is another approach to repair the heart19. Additionally, the fibrotic response after myocardial injury has also been targeted to block cardiac remodelling20. Deciphering and harnessing the molecular mechanisms regulating the transient regenerative capacity of the neonatal mammalian heart might also provide insights into regenerating the adult mammalian heart21,22.
Treating patients with ischaemic heart disease is the ultimate goal of therapeutic approaches for cardiac regeneration11. Despite the enthusiasm and effort invested in many clinical trials of heart repair and regeneration, to date, no effective approaches are available to regenerate the damaged human heart. With an eye on future clinical trials, we focus this Review on the advances in regenerative therapies that have clinical potential for the treatment of ischaemic heart disease. The objective of this Review is to present a comprehensive overview of therapeutic approaches for cardiac regeneration and repair. However, we do not include information on therapeutic approaches targeting the inflammatory process and the epicardium for heart repair, as these topics are reviewed elsewhere in this Focus Issue23,24.
Regenerative capacity of the heart
Cardiomyocytes are considered to be in a terminally differentiated state. Nevertheless, cardiomyocyte cell cycle activity and proliferative capacity differ in species and life stages. Cardiomyocytes from certain animals, such as axolotls, frogs, newts, and zebrafish, retain lifelong capacity to proliferate25,26. By contrast, cardiomyocytes from adult mammals are permanently quiescent. However, upon injury, the heart of a mouse aged 1 day can regenerate over a period of 3 weeks, whereas the injured heart of a mouse aged ≥7 days cannot repopulate lost cardiomyocytes22 (FIG. 1). These studies define a transitional period in the first week after birth when the mouse heart loses its regenerative capacity. Unsurprisingly, this regenerative time window correlates with the period after birth when mammalian cardiomyocytes withdraw from the cell cycle27. Genetic lineage tracing showed that the majority of cardiomyocytes in the recovered area of hearts from mice aged 1 day were derived from pre-existing cardiomyocytes22. These findings offer an opportunity to study the molecular mechanisms whereby the mammalian heart transitions from a regenerative to a non-regenerative organ.
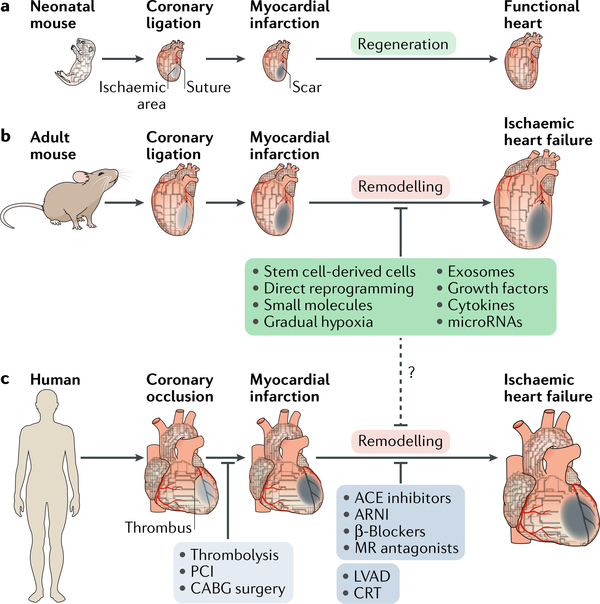
Fig. 1 |. Response and therapeutic approaches to myocardial injury.
a | Response tomyocardial injury differs between developmental stages in mice. Neonatal mice (aged <1 week) are capable of regenerating the heart, with functional recovery after injury.This regenerative capacity is lost postnatally after the first week. b,c | In adult mice and humans, the default response to myocardial injury is fibrosis, where the infarct necrotic tissue is replaced with a fibrotic scar, causing loss of cardiac contractility. The damaged adult heart enters a negative loop of cardiac remodelling that progresses to heart failure. In humans, the current goal of clinical therapies is either to salvage the ischaemic myocardium by early revascularization (light grey box) or to prevent cardiac remodelling with drug therapy and electromechanical support (dark grey box). Accumulating evidence in preclinical studies demonstrates promising outcomes with therapeutic approaches aimed at heart regeneration (green box), although these new approaches have clinical translational problems. The dashed line indicates potential clinical therapeutic approaches. ACE, angiotensin- converting enzyme; ARNI, angiotensin receptor–neprilysin inhibitor ; CRT, cardiac resynchronization therapy; LVAD, left ventricular assist device; MR, mineralocorticoid receptor ; PCI, percutaneous coronary intervention.
Shifting the focus to humans, a case report of a newborn baby demonstrating functional cardiac recovery after MI suggests a degree of regenerative capacity in the human neonatal heart28. Furthermore, accumulating evidence shows that cardiomyocyte turnover occurs in the adult mammalian heart, including in humans29. However, the turnover of cardiomyocytes is obviously insufficient to restore the contractile function of an injured human heart, which can lose up to 1 billion cardiomyocytes after an MI30. Therefore, establishing cardiac regenerative therapies is an important step towards repairing the damaged heart in patients with heart disease.
Cell-based therapies for cardiac repair
Noncardiomyocytes.
Noncardiac cells, which include skeletal myoblasts, bone marrow-derived cells, and mesenchymal stem cells (MSCs), have been the primary source for cell-based therapies in heart failure. The first cells used were skeletal myoblasts, which were expected to remuscularize the injured heart and restore the contractile function. In animal models, transplanted skeletal myoblasts survived and differentiated into a myogenic lineage, and the treatment improved the ejection fraction in both ischaemic and nonischaemic cardiomyopathies31–34. Initial clinical trials showed positive effects, with transplanted skeletal myoblasts leading to improved heart function in patients with ischaemic cardiomyopathy12,35. Unfortunately, long-term follow-up studies did not show beneficial effects. Moreover, adverse effects such as arrhythmogenesis occurred owing to the inability of skeletal myoblasts to integrate electromechanically with surrounding cardiomyocytes36. These poor outcomes ruled out the use of skeletal myoblasts in further clinical studies.
The next cells used for cardiac regeneration therapy were unselected bone marrow-derived mononuclear cells, which became widely tested in clinical studies for heart disease37. This approach was supported by studies demonstrating a beneficial contribution of bone marrow-derived mononuclear cells to cardiac repair in animal models after acute MI38,39. Early clinical trials, such as the BOOST trial13 and the REPAIR-AMI trial40, showed that transplantation of bone marrow-derived mononuclear cells conferred some beneficial effects in patients with acute MI by improving the ejection fraction. Unfortunately, multiple clinical trials that included larger patient populations and well-randomized and double-blinded settings did not reproduce these earlier results41–43. Much controversy remains, and no definite conclusion has been reached on the potential efficacy of cardiac regenerative therapies that are based on bone marrow-derived mononuclear cells.
MSCs are a subpopulation of stromal cells that can be isolated from various tissues, such as bone marrow or adipose tissue, and in vitro studies have shown the capacity of MSCs for self-renewal and multipotent differentiation into adipocytes, chondrocytes, hepatocytes, osteoblasts, neurons, and skeletal muscle cells44. MSCs can also differentiate into cardiomyocytes in the presence of the DNA methyltransferase inhibitor 5-azacytidine or by co-culture with primary cardiomyocytes45–47. Several preclinical studies showed that transplanting MSCs into the injured heart after MI led to improved cardiac function, although the mechanism was not understood48,49. However, in clinical trials such as the POSEIDON14 and MSC-HF50 trials, transplantation of MSCs led to only modest benefits for patients with ischaemic cardiomyopathy. Moreover, during the past decade, inconsistent reports in animal studies suggest that MSCs have minimal to no cardiomyogenic potential51,52.
Therefore, noncardiomyocyte-based cell therapies have not shown consistent positive results in the treatment of heart disease. Nevertheless, these clinical trials have effectively established a standard of operation in evaluating the safety and efficacy of cell-based regeneration therapies in heart failure.
Cardiac-derived cells.
Studies cast doubt on the cardiomyogenic potential of bone marrow-derived mononuclear cells and MSCs; therefore, interest shifted to harvesting cardiac stem cells (CSCs) for repairing the failing heart. CSCs are defined as resident heart cells that show clonogenic, self-renewal, and multipotent capacity. CSCs can differentiate into at least three major cardiac cell types: cardiomyocytes, smooth muscle cells, and endothelial cells53. Therefore, CSCs were expected to be an effective and potent cell source for replacing lost tissue in the damaged heart. Different populations of CSCs have been defined by cell surface markers such as mast/stem cell growth factor receptor KIT, stem cell antigen 1 (SCA1; also known as LY6A), and insulin gene enhancer protein ISL1 (also known as islet 1)15,54,55. Cardiospheres, a mixed population of CSCs (mainly Bassat, E. et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184 (2017). CSCs) that are isolated from cell cultures of mouse and human heart explants, are another cardiac-derived cell source for heart repair53. Indeed, preclinical trials showed some beneficial effects of cardiac-derived cell transplantation in animal models of MI56,57 (TABLE 1). However, clinical trials were initiated with limited understanding of how these cells contribute to heart regeneration58.
Table 1 |.
Cardiac regenerative therapies in preclinical studies
| Therapeutic source | Animal model and age | Cohort (n) | Disease model | Delivery method | Cardiac function recovery versus control; assessment method | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| Cardiac stem cells | |||||||
| Autologous CDCs (1 X 107) | • Pig • Adult | • C = 11 • T = 10 | • IHF • MI | • Intramyocardial • 4 weeks after MI | • LVEF improvement (~7%) at 8 weeks • Ventriculography | LacZ+ CDCs detected in the peri-infarct zone with the use of cardiac marker expression | 56 |
| Autologous KIT+ CSCs (5 X 105) | • Pig • 8–10 weeks | • C = 10 • T = 11 | • IHF • IR 90 min | • Intracoronary • 3–4 months after IR | • LVEF improvement (8–10%) at 8 weeks • Echocardiography | EGFP-labelled CSC- derived cells detected in the infarct zone with the use of cardiac marker expression | 57 |
| Pluripotent stem cells | |||||||
| Human ESC- CMs (~1 X 109) | • Macaque • 5–14 years | • C = 2 • T=4 | • IHF • IR 90 min | • Intramyocardial • 14 days after MI | • No significant change in LVEF • Echocardiography | • Proof-of-principle study • GFP-labelled human ESC-CMs detected in the peri-infarct zone • Electromechanical integration with host myocardium • All animals receiving cell transplants had ventricular arrhythmias | 69 |
| Allogeneic iPSC-CMs (4 X 108) | • Macaque • 4–5 years | • C = 5 • T = 5 | • IHF • MI | • Intramyocardial • 14 days after MI | • LVEF improvement (~10%) at 12 weeks • CT imaging | • GFP-labelled iPSC-CMs detected in the peri- infarct zone • Electromechanical integration with host myocardium • All animals receiving cell transplants had ventricular arrhythmias | 74 |
| Secretory factors | |||||||
| • NRG1-MPs • FGF1-MPs | • Pig • 12–24 months | • C = 6 • T (NRG1-MPs) = 5 • T (FGF1-MPs) = 6 | • IHF • IR 120 min | • Intramyocardial • 1 week after MI | • LVFS improvement (~9%) at 3 months • Echocardiography | • Increase in vascularization with NRG1-MP and FGF1-MP treatment • Reduced fibrosis with NRG1-MP treatment | 91 |
| • miR-199a-3p • miR-590–3p | • Mouse • 2 months | • C = 13 • T (miR-199a-3p) = 20 • T (miR-590–3p) = 13 | • IHF • MI | • Intramyocardial • After coronary ligation (with cationic lipid formulations) | • LVEF improvement (10–20%) at 8 weeks • Echocardiography | Increased numbers in the peri-infarct zone of CMs positive for the DNA synthesis marker EdU | 97 |
| Human CDC-derived exosomes (~16.5 X 1011) | • Pig • Adult | • C = 6 • T = 6 | • IHF • MI | • Intramyocardial • 4 weeks after MI | • LVEF improvement (~5%) at 1 month • MRI | • Reduced scar size • Increased numbers in the peri-infarct zone of CMs positive for the cell cycle active phase marker Ki67 | 109 |
| Direct cardiacreprogramming | |||||||
| Retroviral GMT | • Mouse • 2 months | • C = 9 • T = 9 | • IHF • MI | • Intramyocardial • After coronary ligation | • LVEF improvement (~10%) at 12 weeks • MRI | • Reduced scar size • Fibroblast lineage- traced cells (Fsp1-Cre or Postn-Cre) expressing cardiac markers in the peri-infarct zone | 135 |
| Retroviral GHMT | • Mouse • 8–10 weeks | • C = 9 • T = 10 | • IHF • MI | • Intramyocardial • After coronary ligation | • LVEF improvement (~25%) at 12 weeks • MRI | • Reduced scar size • Fibroblast lineage- traced cells (Tcf21 -Cre) expressing cardiac markers in the peri-infarct zone | 18 |
| Stimulation of endogenous cardiac repair | |||||||
| Agrin | • Mouse • 12 weeks | • C = 7 • T = 8 | • IHF • MI | • Intramyocardial • After coronary ligation | • LVEF improvement (~10%) at 5 weeks • Echocardiography | • Reduced scar size • Increased numbers in the peri-infarct zone of CMs positive for the proliferation markers Ki67, BrdU, and AURKB | 166 |
| Systemic | • Mouse | • C = 9 | • IHF | • Gradual hypoxia | • LVEF improvement | • Reduced scar size | 168 |
| exposure to hypoxia | • 2 months | • T = 9 | • MI | induction reaching 7% O2 level for 2 weeks • 1 week after MI | (~20%) at ~6 weeks • Echocardiography | • Increased numbers of BrdU+ CMs, PHH3+ CMs, and AURKB+ CMs, mainly in the MI remote zone | |
AURKB, aurora kinase B; BrdU, 5-bromodeoxyuridine; C, control; CDC, cardiosphere-derived cell; CM, cardiomyocyte; CSC, cardiac stem cell; EdU, 5-ethynyl-2’-deoxyuridine; EGFP, enhanced green fluorescent protein; ESC, embryonic stem cell; FGF1, fibroblast growth factor 1; GFP, green fluorescent protein; GMT, Gata4, Mef2c, and Tbx5; GHMT, Gata4, Hand2, Mef2c, and Tbx5; IHF, ischaemic heart failure; iPSC, induced pluripotent stem cell; IR, ischaemia-reperfusion; KIT, mast/stem cell growth factor receptor KIT; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; MI, myocardial infarction; MP, microparticle; NRG1, neuregulin 1; PHH3, phosphohistone H3; T, treatment.
Two clinical trials have been carried out using KIT+ CSCs and cardiosphere-derived cells. The first was the SCIPIO trial59,60, in which KIT+ CSCs were used to treat patients with ischaemic cardiomyopathy. This study showed a slight positive effect in patients treated with CSC therapies: left ventricular ejection fraction (LVEF) increased and infarct size decreased. However, because CSCs had shown low engraftment rates in preclinical studies, other researchers questioned the capacity of CSCs to form functional cardiomyocytes. Additionally, conflicting findings on the cardiomyogenic potential of endogenous KIT+ CSCs were reported61,62. A study based on lentiviral expression of Cre recombinase under the control of the Kit promoter, which has a pattern of expression restricted to KIT+ cells, showed that KIT+ CSCs were necessary and sufficient for heart regeneration after injury61. By contrast, another study using a mouse model in which the tamoxifen-inducible MerCreMer protein was targeted to the Kit locus, followed by cross-breeding with the R26-GFP reporter mouse line, showed a minimal contribution of KIT+ cells to heart regeneration after injury62. In addition, a concern was expressed about the integrity of certain data in the SCIPIO trial63. Despite the uncertainties of the benefit of CSC-based regeneration therapy, a second clinical trial, the CADUCEUS trial64, was initiated, in which a cardiosphere-derived cell population was transplanted into patients after MI. The results from this clinical trial showed a reduction in infarct size and an increase in viable myocardium, as shown by cardiac MRI at 6 months and 12 months after therapy. However, in the CADUCEUS trial, patients receiving the cell therapy did not show any improvement in left ventricular global function, indicating that, under current conditions, transplanting cardiosphere-derived cells is not an effective therapy to repair the heart64. Given the limited clinical data reported to date, additional studies are needed to determine the efficacy of CSCs in clinical regenerative therapy.
Pluripotent stem cells.
A major deficiency of the early clinical trials was that the transplanted stem cells had a limited capacity to differentiate into cardiomyocytes. Therefore, scientists were challenged to generate functional cardiomyocytes efficiently in vitro to enable transplantation of de novo cardiomyocytes to the injured heart. The first cell source studied was embryonic stem cells (ESCs) derived from the inner cell mass at the blastocyst stage of early embryos. ESCs are clonogenic, self-renewing, and pluripotent, thereby having the capacity to differentiate into all cell types of the three germ layers (endoderm, ectoderm, and mesoderm)65. Because ESCs are easily expandable and can differentiate into cardiomyocytes in vitro, these cells offer the opportunity to obtain sufficient amounts of cardiomyocytes for transplantation. Transplantation of ESC-derived cardiomyocytes into the injured heart in animal models of MI improved cardiac function despite low engraftment rates66,67. Remarkably, transplanted ESC-derived cardiomyocytes electromechanically coupled with resident cardiomyo- cytes in animal models, which was not the case with skeletal myoblasts. Nevertheless, complications associated with the ESC-derived cardiomyocyte therapy, such as arrhythmias, were detected in some species68,69 (TABLE 1). Moreover, using ESCs poses major issues for clinical application, such as risk of tumorigenesis and immune rejection. In addition, there is also broad ethical opposition to using human embryonic cells even for therapeutic use. Therefore, only one clinical trial using ESC-derived cardiomyocytes is ongoing70.
Fortuitously, Yamanaka and colleagues solved the ethical issues of using ESCs when they reported that mouse and human fibroblasts could be reprogrammed to an ESC-like pluripotent state, called induced pluripotent stem cells (iPSCs), by forced expression of four genes encoding transcription factors: 0CT3/0CT4 (also known as POU5F1), SOX2, KLF4, and MYC (referred to as OSKM factors)71,72. Not only did Yamanaka win the 2012 Nobel Prize in Physiology or Medicine for this discovery, but iPSCs also offered a new cell-based approach for heart repair, enabling autologous or allogeneic transplantation and avoiding the ethical concerns associated with ESCs. However, because autologous transplantation is considered unrealistic owing to the vast amount of work and high cost associated with this approach, preclinical studies are predominantly performed by transplantation of allogeneic or xenogeneic cells with administration of immunosuppressants to avoid rejection of the transplanted cells. Although an early study showed that transplantation of human iPSC- derived cardiomyocytes attenuated cardiac remodelling and improved LVEF in an immunosuppressed porcine MI model, most of the transplanted human iPSC- derived cardiomyocytes did not show long-term survival in the injured heart73. By contrast, in a 2016 study, transplanted allogeneic iPSC-derived cardiomyocytes survived up to 12 weeks after treatment in the infarcted heart of a macaque, with evidence of improved cardiac function74 (TABLE 1). A notable finding of this study is that the iPSC-derived cardiomyocytes showed long-term survival in the immunosuppressed macaque heart without any tumour formation. However, as predicted, the animals with cell transplants had a substantial incidence of ventricular tachycardia, which could be due to the immature state of the transplanted iPSC-derived cardiomyocytes.
Pluripotent stem cell-based approaches probably share similar problems with other cell-based approaches: inconsistencies in the reported engraftment rate and the risk of arrhythmia. Additionally, risk of tumorigenesis remains an underlying concern of pluripotent stem cell-based therapies and has been detected in immunodeficient mouse models75,76. However, improvements have been made in generating mature iPSC-derived cardiomyocytes with high purity and sufficient numbers, and these improvements are expected to enhance the cell retention rate by increasing the number of transplanted cells and to reduce the risk of tumorigenesis and arrhythmia associated with undifferentiated immature cells77,78. In addition, providing scaffolds for iPSC-derived cardiomyocytes, such as hydrogels or cell sheets, has been shown to improve therapeutic outcomes79,80. Regardless of the advancement in transplantation methods, before translating these therapies to clinical trials, studies in large-animal models are warranted to assess the efficacy and safety of iPSC-derived cardiomyocyte-based therapy.
Secretory factors for cardiac repair
In cell-based therapy, transplanted cells were expected to restore cardiac function by engrafting and differentiating into functional cardiomyocytes in vivo. Although this cell-based approach did provide modest cardioprotective benefits, paradoxically, the transplanted stem cells rarely differentiated into cardiomyocytes. To rationalize the beneficial effects observed, researchers reasoned that paracrine effects from growth factors, microRNAs (miRNAs), and exosomes secreted by the stem cells were responsible and potentially crucial for cardiac repair and regeneration81.
Growth factors to promote cardiac repair.
Growth factors are signalling molecules that contribute to multiple cellular processes. The growth factor neuregulin 1 (NRG1) and its receptors, receptor tyrosine- protein kinase ERBB2 and ERBB4, have a critical role in trabeculation and endocardial cushion formation during heart development82. Activation of the NRG1-ERBB2/ERBB4 signalling pathway in the injured adult mouse heart induces cardiomyocyte proliferation and improves cardiac function83,84 (FIG. 2). In a clinical trial in patients with heart failure, systemic delivery of human recombinant NRG1 prevented cardiac remodelling, as shown by decreases in end-diastolic volume and end-systolic volume compared with placebo85. However, the proliferative effect of NRG1 in cardio- myocytes in vivo has been challenged on the basis of findings in a mouse model of MI where NRG1 treatment did not increase cardiomyocyte DNA synthesis86. Therefore, additional studies are required to explain and confirm this beneficial outcome.
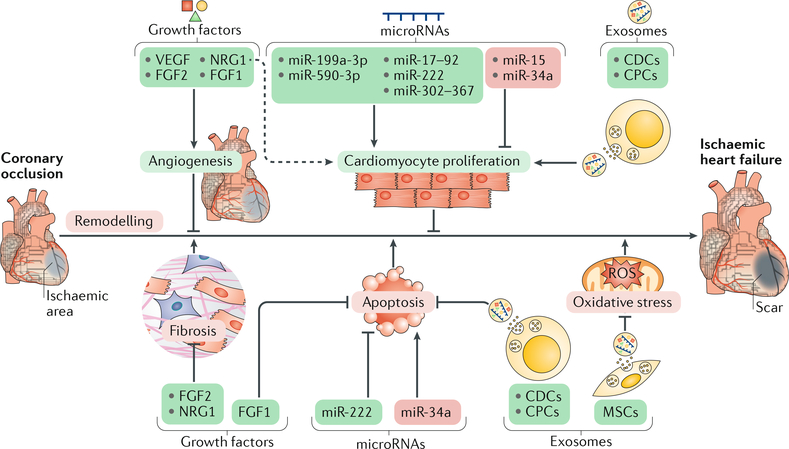
Fig. 2 |. Contributions of secretory factors to cardiac repair and regeneration.
A scheme of cardioprotective effects (green boxes) or cardiac remodelling effects (red boxes) by representative secretory factors (growth factors, microRNAs, and exosomes) is shown. Various secretory factors promote angiogenesis or cardiomyocyte proliferation, thereby promoting cardiac repair. Other secretory factors elicit cardioprotective effects by attenuating cardiac remodelling through inhibition of fibrosis, cardiomyocyte apoptosis, and oxidative stress. The dashed arrow indicates incompletely understood mechanisms. CDC, cardiosphere-derived cell; CPC, cardiac progenitor cell; FGF, fibroblast growth factor; MSC, mesenchymal stem cell; NRG1, neuregulin 1; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor.
Other growth factors that showed beneficial effects in preclinical studies did not realize this promise in clinical trials. For instance, administration of vascular endothelial growth factor A (VEGFA) — a well- known pro-angiogenic factor — improved regional coronary flow and restored cardiac function in an animal model of MI87, but this benefit was not observed in clinical trials88. Cardiac-specific overexpression in mice of the gene encoding another pro-angiogenic factor, fibroblast growth factor 2 (FGF2), decreased infarct size and improved cardiac function after MI compared with wild-type mice89. Furthermore, FGF2 depletion exacerbated cardiac dysfunction after ischaemia-reperfusion injury89. Despite these encouraging early results, treatment with FGF2 provided only minimal cardioprotective effects in patients with ischaemic heart disease90.
One possible explanation for these inconsistent findings is the inadequate amount of therapeutic factor available at the target sites. This low availability could be due to inefficient delivery of the therapeutic growth factors and/or to a short half-life of the growth factors. To enhance exposure of the injured heart to the therapeutic growth factors, scaffolds of biomaterials that enable sustained release of the factors to the target sites have been used and have shown improved cardioprotective effects. For instance, in a pig MI model, loading NRG1 and fibroblast growth factor 1 (FGF1) into microparticles provided sustained local release of the therapeutic factors, which improved left ventricular function associated with increased angiogenesis and reduced ventricular remodelling91 (TABLE 1). Additionally, improved gene- based therapeutics have also led to increased efficiency of delivery and expression of therapeutic factors, such as synthetic modified RNA, a method that has been used to express human VEGFA in the mouse heart after MI92. Treatment with this modified RNA led to an increase in VEGFA expression, induced vascular regeneration, and improved cardiac function and long-term survival compared with the use of DNA vectors92.
microRNAs in cardiac repair.
miRNAs have been implicated in paracrine signalling. miRNAs are highly conserved, single-strand, small non-coding RNAs that regulate gene expression post-transcriptionally by annealing with complementary sequences of mRNAs93. Individual miRNAs interfere with multiple target mRNAs, thereby controlling a variety of biological processes, including heart development and disease (reviewed elsewhere94,95). For example, miR-15 family members have been reported to control postnatal mitotic arrest in the mouse heart96. Cardiac overexpression of miR-195, a miR-15 family member, causes premature cardiomyocyte cell cycle arrest96. Moreover, inhibition of miR-15 family members with synthetic modified RNAs induced cardiomyocyte proliferation in vivo and improved cardiac function in the ischaemic adult mouse heart96 (FIG. 2).
Other studies have identified miRNAs that activate DNA replication in cardiomyocytes. An unbiased screen using a library of 875 human miRNAs detected multiple miRNAs that promote neonatal rat cardiomyocyte proliferation97. Among these miRNAs, cardiac overexpression of miR-199a or miR-590 in vivo induced proliferation of postnatal and adult cardiomyocytes and improved cardiac function and decreased fibrosis in the infarcted heart of adult mice (TABLE 1). Other miRNAs such as the miR-17–92 cluster, miR-214, miR-302–367, and the miR-222 cluster have been reported to contribute to cardiac repair in vivo in animal studies98–101. Therefore, the positive outcomes in preclinical studies demonstrate the therapeutic potential of miRNAs.
However, lessons from clinical trials of regenerative therapies based on growth factors caution us to consider a major challenge for the clinical translation of miRNA-based approaches: how can miRNAs be efficiently delivered to the target site? Similar to the studies on growth factors, providing a scaffold seems to be beneficial for continuous local delivery of miRNAs. As an example, miR-302 is reported to promote cardio- myocyte proliferation by inhibiting Hippo signalling components99. Use of a hydrogel scaffold to deliver miR-302 allowed the sustained, gradual release of the miRNA in mouse hearts, and a single injection of the hydrogel-miR-302 complex into the mouse heart after MI led to continuous cardiomyocyte proliferation and improved cardiac function102.
Exosomes in cardiac repair.
Exosomes provide a plausible therapeutic approach to cardiac repair. Exosomes are small extracellular vesicles (30–100 nm diameter) that are produced by cells and are characterized by the presence of specific surface markers such as CD9, CD63, and CD81 (REF103). Exosomes are released from cells by fusion of intracellular multivesicular bodies with the plasma membrane103. Exosomes are secreted by many cell types, including stem cells, and contain various cargos such as RNAs, lipids, or proteins. Exosomes can function as a vehicle for intercellular communication by carrying cell-specific mRNAs and miRNAs, and accumulating evidence supports a role for exosomes in cell-cell communication among cardiac cells104,105. For instance, after MI in mice, administration of murine cardiosphere-derived exosomes enriched in miR-451 inhibited cardiomyo- cyte apoptosis106 (FIG. 2). Administration of extracellular vesicles, predominantly comprising exosomes, derived from human CSCs also attenuated cardiac remodelling and improved cardiac function in preclinical studies of MI107–109 (TABLE 1). Delivery of MSC-derived exosomes into the mouse heart reduced oxidative stress and promoted survival of cardiomyocytes after ischaemia-reperfusion injury, thereby reducing infarct size and improving cardiac function110.
In addition to these cardioprotective properties, the capacity of exosomes to transport therapeutic factors supports the prospect of exosomes as a biological vehicle for clinical application. Interestingly, fusing the neuron-specific rabies viral glycoprotein peptide (RVG) with an exosomal membrane protein enabled targeted exosomal delivery of small interfering RNAs to knock down the expression of specific genes in the brain111. A cardiac-targeted exosome would be an attractive delivery vehicle for cardioprotective factors such as miRNAs or ligands because the use of exosomes could diminish the risk of activating the immune response associated with viral delivery systems112. Understanding the molecular mechanisms of exoso- mal biology will advance the therapeutic potential of exosomes in cardiac regenerative therapy.
Direct reprogramming for heart repair
Direct reprogramming of mouse fibroblasts into cardiomyocytes in vitro.
Two major issues arising from the use of iPSCs for heart repair and regeneration are the oncogenic potential of the remaining undifferentiated cells and the low engraftment rates of transplanted cells. An approach that could bypass these challenges is to convert resident cardiac cells directly into de novo cardiomyocytes. Fibroblasts constitute a large cell population of the heart, and considering that cardiac injury such as MI is followed by a fibrotic response, transdif- ferentiating cardiac fibroblasts to cardiomyocytes is an attractive possibility for repairing the heart and diminishing cardiac fibrosis113. To achieve this goal, a similar approach to that for establishing iPSCs has been taken, where multiple transcription factors related to cardiac development are combined and overexpressed in fibroblasts. Forced expression of a combination of three genes encoding cardiac transcription factors — Gata4, Mef2c, and Tbx5 (a cocktail referred to as GMT) — or of GMT factors plus Hand2 (referred to as GHMT) successfully reprogrammed murine fibroblasts into induced cardiomyocyte-like cells in vitro, and these cells expressed major cardiac genes and had cardiomyocyte characteristics (such as sarcomere structure, spontaneous intracellular calcium oscillations, and beating contractions) without going through a cardiac precursor state17,18 (FIG. 3). Remarkably, these reprogramming cocktails are sufficient to suppress fibrotic signalling and convert fibroblasts towards a cardiac fate. However, several hurdles need to be addressed before the clinical application of this approach, including inadequate reprogramming efficiency, lack of understanding of the molecular mechanisms, and the heterogeneous population of induced cardiomyocyte-like cells114,115.
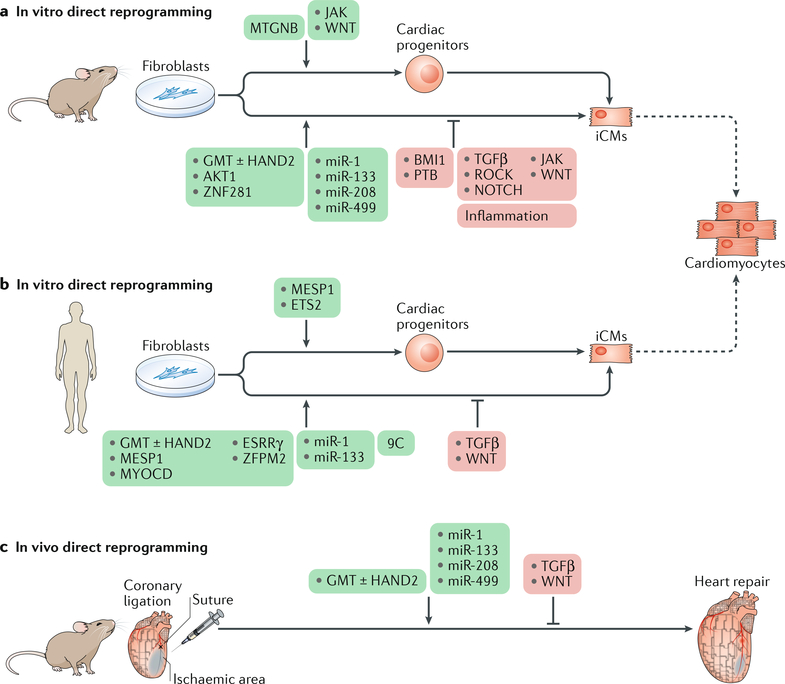
Fig. 3 |. Direct reprogramming of fibroblasts into cardiomyocytes.
a | Forced expression of cardiac transcription factors or myogenic microRNAs directly reprogrammes mouse fibroblasts to induced cardiomyocyte-like cells (iCMs) or cardiac progenitors. As iCMs are functionally and structurally different from endogenous cardiomyocytes, studies have aimed to improve the efficiency and quality of reprogrammed iCMs by adding factors (green box) or blocking the transdifferentiation barriers (red box) in vitro. b | In humans, the cardiac reprogramming factors are different from the factors used in mouse ceüs in vitro. However, inhibition of transforming growth factor-β (TGFβ) and WNT signalling pathways enhances reprogramming in both mouse and human cells. c | The reprogramming cocktails determined in vitro can reprogramme resident cardiac fibroblasts in mice in vivo. The dashed arrows indicate differentiation potential. 9C, CHIR99021, A83–01, BIX01294, AS8351, SC1, Y27632, OAC2, SU16F, and JNJ10198409; AKT1, RAC-α serine/threonine-protein kinase; BMI1, Polycomb complex protein BMI1; ESRRγ, oestrogen-related receptor-γ; GMT, transcription factor GATA4, myocyte-specific enhancer factor 2C (MEF2C), and T-box transcription factor TBX5; HAND2, heart and neural crest derivatives-expressed protein 2; JAK, Janus kinase; MESP1, mesoderm posterior protein 1; MTGNB, MESP1, TBX5, GATA4, homeobox protein NKX2–5, and BRG1-associated factor 60C (BAF60C; also known as SMARCD3); MYOCD, myocardin; PTB, polypyrimidine tract-binding protein 1; ROCK, RHO-associated protein kinase 1.
Many approaches have been implemented to improve cardiac reprogramming efficiency, including the addition of miRNAs and growth factors to the reprogramming cocktails and the modification of endogenous signalling pathways such as RAC-α serine/ threonine-protein kinase (AKT1), transforming growth factor-β (TGFβ), WNT, and Notch signalling116–122. Using an unbiased screen of human transcription factors, the zinc-finger protein ZNF281 was identified as an inducer of cardiac reprogramming123. Addition of ZNF281 to the reprogramming cocktail suppressed the expression of genes associated with the inflammatory response and modulated cardiac gene expression by interacting with the transcription factor GATA4 (REF123). In other studies, suppressing the expression of genes encoding certain factors such as the Polycomb complex protein BMI1 and the splicing factor polypyrimidine tract-binding protein 1 (PTB) enhanced cardiac reprogramming, suggesting that these factors act as barriers to cardiac repro- gramming124,125. However, reaching a consensus protocol for in vitro direct reprogramming of fibroblasts into cardiomyocytes is difficult because in these studies the cardiomyocyte markers, source of fibroblasts, and the time of analysis differ. Therefore, a precise comparison of the reprogramming efficiency between the different protocols should be performed.
Another strategy to reprogramme fibroblasts into cardiomyocytes has been the partial reprogramming of the cells into cardiac progenitor cells, bypassing a pluripotent state. Forced expression of a combination of five genes encoding early cardiac factors — Mespl, Gata4, Tbx5, Nkx2–5, and Baf60c (also known as SmarcdS) — reprogrammed murine fibroblasts into an expandable multipotent cardiac progenitor cell population126. These induced cardiac progenitor-like cells were transplanted into murine hearts after MI, where the cells differentiated into cardiomyocytes, endothelial cells, and smooth muscle cells, and improved survival. However, the drawbacks of transplanting cells diminish the advantages of direct reprogramming.
Direct reprogramming of human cells in vitro.
To translate reprogramming approaches to the clinic, efforts have focused on performing direct cardiac reprogramming in human cells. Previous studies reported that expression of a combination of the transcription factors protein c-ETS2 (ETS2) and mesoderm posterior protein 1 (MESP1) converted human dermal fibroblasts into cardiac progenitors that expressed early cardiac markers such as ISL1 and homeobox protein NKX2–5, which are not detected with direct reprogramming of mouse fibroblasts127. Interestingly, for direct reprogramming of human cells, GMT or GHMT reprogramming cocktails require additional factors such as myocar- din, MESP1, oestrogen-related receptor-γ (ESRRγ), and zinc-finger protein ZFPM2, or even miR-1 and/or miR-133, to successfully induce the conversion of human fibroblasts towards a cardiac fate128–130 (FIG. 3). Human reprogrammed cardiomyocyte-like cells showed a similar gene expression profile to cardiomyocytes and exhibited sarcomere structure and spontaneous calcium transients128–130. Moreover, transdifferentiation of human fibroblasts to cardiomyocyte-like cells has also been achieved with a virus-free method consisting of a pluripotent cocktail with a combination of nine chemicals, with up to 97% of the reprogrammed cells spontaneously beating131. These chemically treated human fibroblasts were transplanted into an immunodeficient mouse heart after MI, where the transplanted cells were eventually reprogrammed to cardiomyocyte-like cells that expressed cardiac genes and had a well-organized sarcomere structure. However, there was no evidence that the chemically reprogrammed cardiomyocyte-like cells were directly converted from fibroblasts without passing through a progenitor state.
Direct cardiac reprogramming in human cells is more challenging than in mouse cells, given the low reprogramming efficiency and the longer time needed for human cells to exhibit cardiomyocyte characteristics. This increased difficulty is consistent with other cellular reprogramming strategies132. For example, human iPSC reprogramming efficiency is less than that observed for mouse iPSCs and requires a longer time to reprogramme, even though the reprogramming cocktail contains the same factors for both cell types. Similarly, neuronal cell reprogramming with human cells requires additional factors and longer duration than neuronal cell reprogramming with mouse cells133. The difficulty in reprogramming human cells could be attributed to the difference in the epigenetic landscape between mouse and human fibroblasts, suggesting additional epigenetic barriers for reprogramming human cells134. Another feature to consider is that humans have a longer developmental time than mice, potentially contributing to the species differences in cell reprogramming. Interestingly, despite the requirement of different factors for direct cardiac reprogramming between species, crucial endogenous signalling pathways such as the TGFβ1 and WNT signalling pathways contribute similarly to cardiac reprogramming in both mouse and human cells120.
Direct reprogramming of fibroblasts into cardiomy- ocytes in vivo.
Direct reprogramming in vivo has been reported in mice with the use of GMT and GHMT reprogramming cocktails with retroviral delivery, which exclusively infects proliferating cells such as activated cardiac fibroblasts after myocardial injury18,135,136 (FIG. 3; TABLE 1). Lineage tracing of fibroblasts with the use of Fspl (also known as Sl00a4)-Cre and Myhó-MerCreMer mice demonstrated that these newly generated cardiomyocyte-like cells were derived from fibroblasts and not from cell fusion with endogenous cardiomyocytes. Interestingly, these in vivo- generated cardiomyocyte-like cells demonstrated similar characteristics to endogenous ventricular cardiomyo- cytes, such as their cardiac gene expression profile, sarcomere structure, contractility, calcium transients, and action potential; therefore, in vivo-generated cardiomyocyte-like cells seem to mimic endogenous cardiomyocytes better than in vitro-generated cardiomyocyte-like cells. Actually, this finding is not surprising considering that cardiomyocyte-like cells generated in vivo are exposed to a physiological microenvironment, including electromechanical stimulation and interactions with adjacent cells. In vivo-generated cardiomyocyte-like cells also form gap junctions with endogenous cardiomyocytes, increasing the potential for electromechanical coupling18. Similar results were reported with the use of a combination of muscle-specific miRNAs delivered with lentivirus to the murine heart122.
Although direct reprogramming in vivo improves cardiac function after MI in mice, the relatively low reprogramming efficiency compared with direct reprogramming in vitro suggests that other factors besides contractility of induced cardiomyocyte-like cells contribute to the therapeutic efficacy of in vivo reprogramming137. For example, a decrease in the size of the fibrotic tissue suggests that paracrine effects from induced cardiomyocyte-like cells or a direct contribution of the reprogramming factors suppress the fibrotic response. In addition, we cannot exclude the possibility that the reprogramming cocktail affects other cells in the heart, such as endothelial cells, immune cells, CSCs, and even pre-existing cardiomyocytes. This possibility raises concerns because direct cardiac reprogramming has been honed using fibroblasts; therefore, in other cell lineages, the reprogramming cocktail might induce incomplete reprogramming that could elicit unfavourable effects such as arrhythmias.
The positive outcomes observed in murine direct cardiac reprogramming in vivo give optimism to eventual clinical translation. However, several challenges remain to be addressed before clinical application, such as the low reprogramming efficiency, the immaturity and heterogeneity of induced cardiomyocyte-like cells, and the lack of a feasible delivery method for the reprogramming cocktail. In addition, in current studies, the induction of reprogramming is limited to a short period of time after coronary ligation. This short window of administration would restrict the therapeutic time window in a clinical setting. A 2018 study reported that direct cardiac reprogramming could be achieved in both mouse and human cells by delivering reprogramming factors with a Sendai virus vector, which is a non-segmented, negative-stranded RNA virus138. Sendai virus replicates only in the cytoplasm and does not integrate into the genome, suggesting a more feasible strategy for clinical application compared with delivery using retrovirus or lentivirus139.
Stimulating endogenous cardiac repair
Proliferation of endogenous cardiomyocytes.
To promote endogenous cardiomyocyte proliferation, initial approaches targeted universal cell cycle regulators such as cyclins, cyclin-dependent kinases (CDKs), and tumour suppressor genes. Although modulating these factors individually in adult cardiomyocytes did enable some proliferative activity140,141, a study published in 2018 reported that overexpression of a combination of cell cycle regulators increases the effect on cardiomyocyte proliferation and improves cardiac function after MI142 (FIG. 4). These findings suggest that specific factors involved in postnatal cardiomyocyte cell cycle regulation are also candidates to stimulate the proliferative capacity in adult cardiomyocytes. For example, the transcription factor homeobox protein MEIS1 is reported as a regulator of postnatal cardiomyocyte cell cycle arrest143. Cardiac-specific overexpression of Meisl in neonatal mice reduced cardiomyocyte proliferation and abolished heart regeneration. Conversely, genetic ablation of Meisl in neonatal mice extended the time window of postnatal cardiomyocyte proliferation and induced cardio- myocytes to re-enter the cell cycle in adult mice143. Gene expression analysis revealed that MEIS1 is required for transcriptional activation of the synergistic CDK inhibitors p15 (also known as CDK inhibitor 2B; encoded by Cdkn2b), p16 (also known as CDK inhibitor 2A; encoded by Cdkn2a), and p21(also known as CDK inhibitor 1A; encoded by Cdknla), which probably contribute to postnatal cardiomyocyte cell cycle withdrawal.
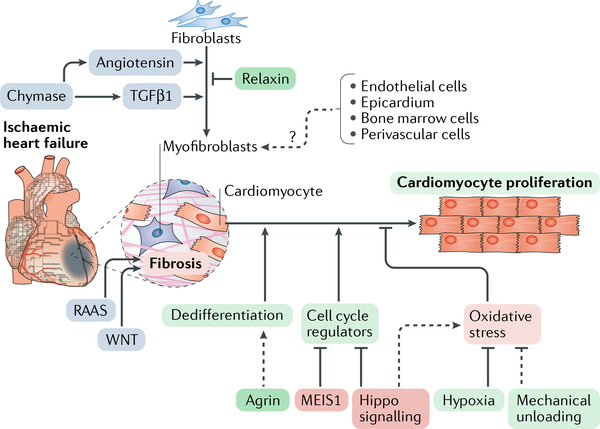
Fig. 4 |. Approaches to stimulate endogenous regenerative capacity for heart repair.
Approaches targeting endogenous cardiac regeneration (green boxes) involve activating the proliferation of endogenous cardiomyocytes and targeting the cardiac fibrotic response. Cardiomyocyte proliferation can be induced by overexpressing cell cycle-related genes or by inhibiting cell cycle suppressors such as the transcription factor homeobox protein MEIS1 or the Hippo signalling pathway (red boxes). Alternatively, approaches to mimic the neonatal cardiac environment by exposure to hypoxia or mechanical unloading or by providing the extracellular matrix protein agrin also evoke cardiomyocyte proliferation. Transforming growth factor-β1 (TGFβ1) and angiotensin have a pivotal role in inducing cardiac fibroblast differentiation into myofibroblasts during cardiac injury, thereby inducing cardiac fibrosis. Additionally, other cell lineages are proposed to transdifferentiate into myofibroblasts. The renin-angiotensin-aldosterone system (RAAS) and the WNT signalling pathway also contribute to cardiac fibrosis. These fibrotic processes (blue boxes) can be targeted to attenuate cardiac remodelling. The dashed arrows indicate incompletely understood mechanisms.
The Hippo signalling pathway is an evolutionarily conserved pathway shown to control cell proliferation and organ size144. This pathway is activated through phosphorylation of a series of factors, such as mammalian STE20-like protein kinase 1 (MST1; also known as STK4) and MST2 (also known as STK3), protein salvador homologue 1 (SAV1), and large tumour suppressor homologue 1 (LATS1) and LATS2, which together phosphorylate and inactivate the transcriptional co-activator Yes-associated protein 1 (YAP1). Phosphorylation of YAP1 blocks nuclear translocation and results in YAP1 being retained in the cytoplasm. In mice, similar phenotypes of heart enlargement owing to cardiomyocyte proliferation can be evoked by genetic ablation of Mstl, Mst2, Lats2, or Savl, or by activating YAP1 in cardio- myocytes19,145. Encouragingly, inhibiting the Hippo pathway in the heart after MI promotes cardiomyocyte proliferation and improves cardiac function in mice19,146 (FIG. 4). Accordingly, cell cycle-related genes were upregulated in the heart after Hippo signalling inhibition, but stress response genes, such as those associated with an antioxidant response, and mitochondrial quality control genes were also upregulated147,148. Because inhibiting the Hippo pathway is associated with the risk of off-target cell proliferation, antioxidant reagents have been tested and administered into the injured adult mouse heart as an alternative approach. Interestingly, administration of antioxidants induced cardiomyocyte proliferation and promoted heart repair, suggesting that targeting oxidative stress offers a therapeutic approach for heart regeneration147.
Targeting the fibrotic response for heart repair.
Cardiac fibroblasts are activated after myocardial injury, expressing genes associated with contractile responses and becoming myofibroblasts149. In the necrotic tissue after MI, myofibroblasts and inflammatory cells produce a secretome that leads to the formation of a reparative fibrotic scar (reviewed elsewhere150). The continuing activity of myofibroblasts produces fibrotic tissue, leading to progressive pathological cardiac remodelling. Multiple signalling pathways, such as the renin-angiotensin-aldosterone system and the TGFβ and WNT signalling pathways, contribute to this fibrotic process after injury20. Targeting these pathways and inhibiting the progressive phase of fibrosis has been shown to improve cardiac function by attenuating cardiac remodelling20,151–153 (FIG. 4). Inhibiting additional targets such as chymase, which is a serine protease that activates the renin-angiotensin-aldosterone system and TGFβ signalling, also prevents cardiac fibrosis and improves cardiac function after MI in rats154. The hormone relaxin inhibits TGFβ signalling-mediated fibroblast activation into myofibroblasts and thereby reduces cardiac fibrosis after MI155,156. However, the approach of targeting the fibrotic response for heart repair is not as straightforward as anticipated. One of the reasons is that the initial phase of the fibrotic response is considered a healing process because transient scar formation is also detected during heart regeneration after injury in both zebrafish and neonatal mice157.
At the healing site of the myocardial injury, angiotensin and TGFβ1 have a pivotal role in the secretome of myofibroblasts and inflammatory cells150. The macrophages at the injury site produce angiotensin II, which leads to the upregulation and secretion of TGFβ1. Macrophage-derived TGFβ1 induces fibroblast activation and differentiation into myofibroblasts, which also generate TGFβ1 (FIG. 4). TGFβ1 triggers myofibroblasts to produce matrix proteins, eventually forming fibrous tissue. Accumulating evidence from clinical trials shows that pharmacologically inhibiting the activity of angiotensin II attenuates cardiac remodelling158. However, blocking fibrosis by inhibiting TGFβ is not straightforward, as preclinical studies have shown that TGFβ treatment outcomes depend on the timing of intervention after the MI159. In mice, systemic inhibition of TGFβ in the first 24 h after MI increased mortality and impaired cardiac function, whereas TGFβ inhibition at a later phase attenuated cardiac hypertrophy and remodelling. These results support the concept that the initial fibrotic response after myocardial injury is a necessary phase for healing.
Additionally, nonfibroblast cells such as endothelial cells, epicardial cells, bone marrow-derived cells, and perivascular cells have been proposed to generate newly activated fibroblasts after MI160–163. For example, endothelial cells acquire a fibroblastic phenotype through endothelial-to-mesenchymal transition after myocardial injury160. Delivery of recombinant human bone morphogenetic protein 7 or pigment epithelium- derived factor attenuates myocardial fibrosis by inhibiting endothelial-to-mesenchymal transition after myocardial injury in animal studies160,164. These findings suggest endothelial cells as potent targets for heart repair. Nevertheless, additional lineage-tracing studies show that the contribution of nonfibroblasts to fibroblast activation is more limited than previously surmised149,165.
Further understanding of the fibrotic response after myocardial injury is necessary to fulfil the therapeutic potential of targeting this process for heart regeneration. Interestingly, a study reported that injecting agrin, an extracellular matrix protein that is enriched in the neonatal mouse heart, promoted heart regeneration after MI in adult mice, partly through cardiomyocyte dedifferentiation166 (TABLE 1). Therefore, a potential approach for heart regeneration lies in modulating, not inhibiting, the fibrotic response.
Future perspectives
New advances in basic researchfor heart regeneration.
The identification of a transient regenerative period in the heart of neonatal mice provided scientists with a new resource to study the mechanisms governing mammalian cardiac regeneration. In particular, a postnatal switch from glycolytic to oxidative metabolism and an increase in the oxygenation state of cardiomyocytes induce the production of reactive oxygen species (ROS) in mitochondria167. This increase in ROS levels leads to the activation of the DNA damage response (DDR) pathway, which induces postnatal cardiomyocyte cell cycle arrest167. Encouragingly, in this study, the postnatal cardiomyocyte proliferation window was extended by pharmacologically scavenging ROS or by inhibiting the DDR pathway, consistent with findings from another study147. Moreover, exposing adult mice to gradual, severe, systemic hypoxia led to decreases in ROS production and oxidative DNA damage, which reactivated cardiomyocyte mitosis and led to heart repair after MI168 (TABLE 1). In addition, an increase in cardiac mechanical load after birth has been proposed to activate cardiac mitochondrial biogenesis, thereby adapting the heart to the increase in energetic demand169. Remarkably, mechanical unloading after implantation of LVADs in the failing human heart caused a decrease in mitochondrial content and a reduction of the DDR, with signs of cardiomyocyte proliferation170. These findings suggest a novel therapeutic approach for heart regeneration whereby the proliferative capacity of cardiomyocytes that is silenced in the adult mammalian heart can be reawakened by environmental adjustments rather than by directly manipulating specific signalling pathways in cardiomyocytes.
A study analysing the transcriptome and the chromatin landscape in neonatal and adult mouse hearts also revealed that noncardiomyocytes activate a distinct injury-induced transcriptional programme in response to MI171. Another report demonstrated an apparent difference in the immune response after MI between newborn mice at day 1 and at week 2 (REF172). Additionally, depletion of macrophages abolished the early neonatal cardiac regenerative capacity, and this regenerative deficiency was associated with a defect in angiogenesis172. Future detailed analyses of the epigenetic landscape and the transcriptome in neonatal and adult mouse hearts will define the regulatory networks that contribute to heart regeneration.
Another novel approach to consider for the treatment of heart diseases that are caused by specific monogenic mutations is genome editing. Discovery and engineering of CRISPR technology has advanced the field by offering the possibility of genome editing targeted by only one guide RNA173·174. For example, Duchenne muscular dystrophy, an X-linked recessive monogenic disease caused by mutations in the dystrophin gene (DMD), is a severe progressive muscle disease that causes premature death usually in the mid-twenties owing to cardiac and respiratory failure175. In vivo genome editing with the CRISPR- Cas9 system restored muscle function by correcting the Dmd mutation in a mouse model of Duchenne muscular dystrophy in both the germline and the postnatal stage176–180. In a proof-of-principle study, genome editing technology also corrected mutations in human iPSC- derived cardiomyocytes from patients with Duchenne muscular dystrophy181–185. Corrected iPSC-derived cardiomyocytes had restored dystrophin expression and improved cardiomyocyte functional performance in vitro, such as improved contraction and suppression of arrhythmia (FIG. 5). Given the myriad mutations in cardiac structural and contractile protein genes that cause cardiomyopathy in humans, gene editing is an attractive means of eliminating such disease-causing mutations. Therefore, CRISPR technology has great potential for innovative therapies to treat heart disease186.
Fig. 5 |. Genome editing as a therapeutic approach to heart disease.
Genome editing offers the possibility of correcting mutations postnatally in congenital muscle diseases, such as Duchenne muscular dystrophy (DMD), to restore muscle function. DMD is caused by mutations in the dystrophin gene (DMD) that lead to abnormalities in the production of dystrophin protein and are associated with premature death owing to cardiac and respiratory failure. The CRISPR-Cas9 system has been successfully used to correct Dmd mutations and restore the expression of dystrophin in a mouse model of DMD. a | The panels show heart sections from a wild-type mouse, a DMD mouse model, and a CRISPR-Cas9-edited DMD mouse model. Tissues are immunostained with an antibody for dystrophin (green). b | The panels show induced pluripotent stem cell (iPSC)-derived cardiomyocytes from a healthy person (control) and from a patient with DMD, and iPSCs from a patient with DMD that were edited with CRISPR-Cas9 to correct the DMD mutation. Cells were immunostained with antibodies for dystrophin (green) and troponin I (red). Adapted with permission from REF180, AAAS.
Genome editing could potentially be adapted for heart regenerative therapies. Genetically engineered stem cells have been reported to have improved cardioprotective effects187,188. Priming stem cells with site-targeted modification with CRISPR technology before transplantation will reduce the risk of undesired insertional mutagenesis. This technology also opens new avenues to xenotransplantation. Xenografts could be genome-edited to silence unfavourable xenogeneic genes, which potentially could reduce the risk of immune rejection and infection after cell transplantation189,190. Additionally, CRISPR technology can be modified to regulate endogenous gene expression by using a cata- lytically inactive form of Cas9 (dCas9)191,192. It will be interesting to see whether direct cardiac reprogramming can be achieved by activating endogenous gene expression with the use of the CRISPR-dCas9 system, which has been successfully reported in the reprogramming of other cell lineages193,194. However, much work remains in addressing major issues before clinical application, such as off-target mutagenesis and unstable editing efficiency, as well as establishing feasible delivery methods and determining the therapeutic target population.
Challenges for clinical application.
How can we account for the disparity between the outcomes in preclinical studies of cardiac regeneration and the clinical trials of cardiac regeneration in humans? One explanation could be an insufficient delivery of therapeutic factors to the target sites, which prevents the therapeutic thresholds needed for cardiac regeneration from being reached when translated to clinical settings. To address this issue, biomaterials have attracted attention for their capacity to serve as a matrix to improve graft survival and behaviour, as well as to protect the therapeutic factors from degradation and to act as a reservoir for sustained local delivery of therapeutic factors. Biomaterial-based delivery systems thus provide a scaffold to improve the therapeutic effects of both cellular and noncellular approaches79,102,195–197. Furthermore, tissue-engineering technologies have allowed the building of myocardial patches and sheets and 3D heart tissues recapitulating in vitro the complex structure of heart tissue. Engineered heart tissues can be directly transplanted to the damaged heart and have been shown to improve therapeutic outcomes198–201. Meanwhile, the number of deliveries of the therapeutic factor might also be important, because repetitive delivery of stem cells was shown to improve therapeutic outcomes compared with a single delivery202. Therefore, optimizing the delivery protocol in preclinical settings is necessary to advance the potential of therapeutic factors to clinical trials.
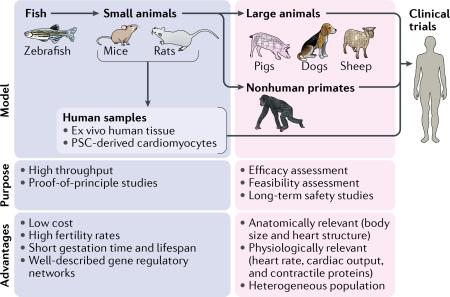
Another challenge in the process of clinical application of regenerative therapies is the limitations of current animal models (BOX 1). Typically, proof-of-principle studies are first performed in fish or rodent models and then repeated in larger animal models to test the feasibility, safety, and efficacy of the approach203. Although animal models undoubtedly have a pivotal role in translational medicine, differences between species, including the molecular basis of disease, anatomy, and physiology, might account for translational failures. In other words, no universally ideal animal model for preclinical studies is available, and for each purpose and use, an appropriate model should be selected. Additionally, preclinical studies tend to have weak statistical power because rigorously designed, large-scale studies require major financial investments. The lack of sufficient scientific rigour of clinical trials of cardiac regeneration stands as a major challenge for clinical application of cardiac regenerative therapies, because positive outcomes in initial clinical trials, which tended to be performed on a small scale, were often not reproduced in later, well-randomized, large-scale clinical trials. Altogether, study designs in preclinical settings require further refinements, and establishing a more feasible translational path could reduce the risk of clinical translational failure204.
Box 1 | Research models for translational medicine in heart regeneration
Representative research models in the flow of translational research in heart regeneration are shown in the figure.
Fish
Fish such as zebrafish are widely used model organisms in the early phase of translational research. High fertility rates, short gestation period, short lifespan, and low cost of maintenance make fish a suitable model organism for high-throughput studies using genetic models of heart disease206. However, fish have substantial differences compared with mammals in heart structure, having only two chambers and maintaining regenerative capacity throughout their lifetime. These properties make fish an invalid model to confirm efficacy of therapeutic approaches for human heart regeneration.
Small animals
Mammalian model organisms are phylogenetically, anatomically, and physiologically closer to humans, including the heart structure and the minimal regenerative capacity in the adult heart. Among mammals, rodents are one of the most popular model organisms to study heart regeneration. The short gestation period and lifespan of rodents, associated with well-understood molecular mechanisms of the cardiac regulatory networks, make these animal models suitable for proof-of-principle studies using genetically modified animals207. A fairly low cost of maintenance and the convenience of easy handling enable testing the reproducibility of regenerative approaches before proceeding to further studies. Nonetheless, rodents are substantially different from humans in many ways, including body size, cardiac output, and contractile proteins, and have a relatively homogenous genetic background; therefore, therapeutic approaches tested in rodent models require an additional confirmatory step before clinical application208.
Large animals
Large animals such as pigs, dogs, and sheep are considered to be the bridge between small-animal studies and clinical trials. Relatively similar to humans in body size and cardiac physiology, large-animal models enable testing the feasibility, efficacy, and long-term safety of therapeutic approaches for heart regeneration203. However, major disadvantages of large animals include the high cost of maintenance and the long life cycle compared with small animals. In addition, obtaining approval to use some species for research purposes might be difficult. These factors hinder the design of large-animal studies at a large scale with adequate numbers to confirm reproducibility.
Nonhuman primates
Nonhuman primates are distinct from other large-animal models because these animals are phylogenetically closer to humans and their immune system has been well studied in translational medicine209. These features give nonhuman primate models an important role in studies of allogeneic stem cell transplantation, which is considered to be one of the main therapeutic approaches for heart regeneration210.
Human samples
Alternative preclinical models involve the use of human ex vivo cultured cardiac tissues and human pluripotent stem cell (PSC)-derived cardiomyocytes, which theoretically should be suitable for performing in vitro proof-of-principle studies211.
Conclusions
In conclusion, new discoveries and promising preclinical outcomes in the field of cardiac regeneration give us optimism regarding the establishment of novel effective regenerative therapies for the failing human heart. However, considering the disappointing results of previous clinical trials of cardiac regenerative therapies, we must acknowledge and address the limitations of preclinical studies of cardiac regeneration and the difficulties of clinical translation. In addition, mammalian heart development is a complex and precisely spatiotemporally orchestrated process205. Therefore, repairing the human heart will probably require a combination of multiple therapeutic approaches.
Key points.
Preclinical outcomes of cardiac regenerative therapy approaches have not translated effectively to clinical trials.
Transplantation of induced pluripotent stem cell-derived cardiomyocytes for cardiac repair has encountered problems related to safety and low engraftment rates.
Cell-free-based approaches for heart repair and regeneration involve cardioprotective secretory factors or direct reprogramming of resident cardiac fibroblasts to cardiomyocyte-like cells.
Endogenous cardiomyocyte proliferation can be evoked by modulating cell cycle regulators, the Hippo signalling pathway, and the cardiac microenvironment.
Genome editing can correct underlying mutations causing heart disease in animals and offers a state-of-the-art therapeutic approach for cardiac repair.
The therapeutic potential of cardiac regeneration approaches can be improved by optimizing the delivery method of the therapeutic factors.
Left ventricular assist devices
(LVADs). Electromechanical devices to support circulation of a failing heart.
Cardiac resynchronization therapy
Therapy that uses an electromechanical device to resynchronize ventricular contraction in patients with heart failure.
Lineage tracing
A method to identify all progeny originating from a single cell.
MerCreMer
A fusion protein containing Cre recombinase flanked at both ends with a mutated murine oestrogen receptor (Mer) ligand binding domain. MerCreMer generates an inducible Cre recombinase activation system that can gain access to the nuclear compartment only with exposure to tamoxifen.
Hydrogels
Colloid gels composed of a network of hydrophilic polymer chains.
Paracrine effects
The effects on a cell that are induced by secreted factors from another cell
Synthetic modified RNA
Chemically synthesized RNA with changes to the chemical composition that alter function or stability of the RNA.
Xenotransplantation
Cell, tissue, or organ transplantation across different species.
Acknowledgements
The authors thank L. Amoasii and Y-L. Min (University of Texas Southwestern Medical Center, USA) and S. Tohyama (Keio University School of Medicine, Japan) for constructive scientific discussions, J. Cabrera (University of Texas Southwestern Medical Center, USA) for assistance with figures, and A. McKenzie (University of Texas Southwestern Medical Center, USA) for help with editing. Work in the authors’ laboratory is supported by grants from the NIH (AR-067294, HL-130253, HD-087351, and HL-138426), Fondation Leducq Transatlantic Networks of Excellence in Cardiovascular Research, and the Robert A. Welch Foundation (grant 1–0025 to E.N.O.). H.H. is supported by the Uehara Memorial Foundation Postdoctoral Fellowship and the Kanae Foreign Study Grant.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Cardiology thanks T. Eschenhagen and the other, anonymous reviewers for their contribution to the peer review of this work.
References
- 1.Roth GA et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 70, 1–25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn JN, Ferrari R & Sharpe N Cardiac remodeling — concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J. Am. Coll. Cardiol. 35, 569–582 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Sacks CA, Jarcho JA & Curfman GD Paradigm shifts in heart-failure therapy — a timeline. N. Engl. J. Med. 371,989–991 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Packer M et al. Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 344, 1651–1658 (2001). [DOI] [PubMed] [Google Scholar]
- 5.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 325, 293–302 (1991). [DOI] [PubMed] [Google Scholar]
- 6.Pitt B et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 341,709–717 (1999). [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371 , 993–1004 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Rose EA et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 345, 1435–1443 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Bristow MR et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 350, 2140–2150 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Yacoub M Cardiac donation after circulatory death: a time to reflect. Lancet 385, 2554–2556 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Behfar A, Crespo-Diaz R, Terzic A & Gersh BJ Cell therapy for cardiac repair — lessons from clinical trials. Nat. Rev. Cardiol. 11, 232–246 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Menasche P et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 117, 1189–1200 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Meyer GP et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST- elevation infarct regeneration) trial. Circulation 113, 1287–1294 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Hare JM et al. Comparison of allogeneic versus autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 308, 2369–2379 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beltrami AP et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Mummery CL et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ. Res. 111,344–358 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ieda M et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song K et al. Heart repair by reprogramming nonmyocytes with cardiac transcription factors. Nature 485, 599–604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin M et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl Acad. Sci. USA 110, 13839–13844 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gourdie RG, Dimmeler S & Kohl P Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat. Rev. DrugDiscov. 15, 620–638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin M, Olson EN & Bassel-Duby R Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 14, 529–541 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porrello ER et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forte E, Furtado M & Rosenthal N The interstitium in cardiac repair: role of the immune-stromal cell interplay. Nat. Rev. Cardiol. (in the press). [DOI] [PubMed] [Google Scholar]
- 24.Cao J & Poss KD The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. (in the press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poss KD, Wilson LG & Keating MT Heart regeneration in zebrafish. Science 298, 2188–2190 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Porrello ER & Olson EN A neonatal blueprint for cardiac regeneration. Stem Cell Res. 13, 556–570 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soonpaa MH, Kim KK, Pajak L, Franklin M & Field LJ Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 271 , H2183–H2189 (1996). [DOI] [PubMed] [Google Scholar]
- 28.Haubner BJ et al. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res. 118, 216–221 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Bergmann O et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laflamme MA & Murry CE Heart regeneration. Nature 473,326–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marelli D, Desrosiers C, el-Alfy M, Kao RL & Chiu RC Cell transplantation for myocardial repair: an experimental approach. Cell Transplant. 1, 383–390 (1992). [DOI] [PubMed] [Google Scholar]
- 32.Taylor DA et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat. Med. 4, 929–933 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Al Attar N et al. Long-term (1 year) functional and histological results of autologous skeletal muscle cells transplantation in rat. Cardiovasc. Res. 58, 148 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Durrani S, Konoplyannikov M, Ashraf M & Haider KH Skeletal myoblasts for cardiac repair. Regen Med. 5, 919–932 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Povsic TJ et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am. Heart J. 162, 654–662.e1 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Fouts K, Fernandes B, Mal N, Liu J & Laurita KR Electrophysiological consequence of skeletal myoblast transplantation in normal and infarcted canine myocardium. Heart Rhythm 3, 452–461 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Sanganalmath SK & Bolli R Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 113, 810–834 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orlic D et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Jackson KA et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 107, 1395–1402 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schachinger V et al. Intracoronary bone marrow- derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 355, 1210–1221 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Traverse JH et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA 308, 2380–2389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perin EC et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA 307, 1717–1726 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surder D et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation 127, 1968–1979 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Pittenger MF et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 147 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Planat-Benard V et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. 94, 223–229 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A & Papakonstantinou C In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact. Cardiovasc. Thorac. Surg 6, 593–597 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Li X et al. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J. Mol. Cell Cardiol. 42, 295–303 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Quevedo HC et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl Acad. Sci. USA 106, 14022–14027 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amado LC et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl Acad. Sci. USA 102, 11474–11479 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathiasen AB et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur. Heart J. 36, 1744–1753 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Dixon JA et al. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation 120, S220–229 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva GV et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111, 150–156 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Messina E et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 95, 911–921 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Oh H et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl Acad. Sci. USA 100, 12313–12318 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laugwitz KL et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433, 647–653 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnston PV et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 120, 1075–1083 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolli R et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 128, 122–131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leong YY, Ng WH, Ellison-Hughes GM & Tan JJ Cardiac stem cells for myocardial regeneration: they are not alone. Front. Cardiovasc. Med. 4, 47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bolli R et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Chugh AR et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126, S54–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellison GM et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154, 827–842 (2013). [DOI] [PubMed] [Google Scholar]
- 62.van Berlo JH et al. c-Kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.The Lancet E Expression of concern: the SCIPIO trial. Lancet 383, 1279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makkar RR et al. Intracoronary cardiosphere- derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murry CE & Keller G Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Mummery C et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107, 2733–2740 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Qiao H et al. Long-term improvement in postinfarct left ventricular global and regional contractile function is mediated by embryonic stem cell-derived cardiomyocytes. Circ. Cardiovasc. Imag. 4, 33–41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiba Y et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chong JJ et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.<W/>US National Library of Medicine ClinicalTrials.govhttps://clinicaltrials.gov/show/NCT02057900 (2014). [DOI] [PubMed]
- 71.Takahashi K & Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Takahashi K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131,861–872 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Kawamura M et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126, S29–S37 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Shiba Y et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Miura K et al. Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 27, 743–745 (2009). [DOI] [PubMed] [Google Scholar]
- 76.Sougawa N et al. Immunologic targeting of CD30 eliminates tumourigenic human pluripotent stem cells, allowing safer clinical application of hiPSC-based cell therapy. Sci. Rep 8, 3726 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tohyama S et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127–137 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Tohyama S et al. Efficient large-scale 2D culture system for human induced pluripotent stem cells and differentiated cardiomyocytes. Stem Cell Rep. 9, 1406–1414 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chow A et al. Human induced pluripotent stem cell-derived cardiomyocyte encapsulating bioactive hydrogels improve rat heart function post myocardial infarction. Stem Cell Rep. 9, 1415–1422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawamura M et al. Enhanced therapeutic effects of human iPS cell derived-cardiomyocyte by combined cell-sheets with omental flap technique in porcine ischemic cardiomyopathy model. Sci. Rep. 7, 8824 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen T S. et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 38, 215–224 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gassmann M et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378, 390–394 (1995). [DOI] [PubMed] [Google Scholar]
- 83.Bersell K, Arab S, Haring B & Kuhn B Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138, 257–270 (2009). [DOI] [PubMed] [Google Scholar]
- 84.D’Uva G et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 17, 627–638 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Gao R et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J. Am. Coll. Cardiol. 55, 1907–1914 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Reuter S, Soonpaa MH, Firulli AB, Chang AN & Field LJ Recombinant neuregulin 1 does not activate cardiomyocyte DNA synthesis in normal or infarcted adult mice. PLoS ONE 9, e115871 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harada K et al. Vascular endothelial growth factor administration in chronic myocardial ischemia. Am. J. Physiol. 270, H1791–H1802 (1996). [DOI] [PubMed] [Google Scholar]
- 88.Gyongyosi M et al. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation 112, I157–165 (2005). [DOI] [PubMed] [Google Scholar]
- 89.House SL et al. Cardiac-specific overexpression of fibroblast growth factor-2 protects against myocardial dysfunction and infarction in a murine model of low-flow ischemia. Circulation 108, 3140–3148 (2003). [DOI] [PubMed] [Google Scholar]
- 90.Simons M et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 105, 788–793 (2002). [DOI] [PubMed] [Google Scholar]
- 91.Garbayo E et al. Catheter-based Intramyocardial Injection of FGF1 or NRG 1 -loaded MPs Improves Cardiac Function in a Preclinical Model of Ischemia-Reperfusion. Sci. Rep 6, 25932 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zangi L et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 31,898–907 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartel DP MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu N & Olson EN MicroRNA regulatory networks in cardiovascular development. Dev. Cell 18, 510–525 (2010).. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Rooij E & Olson EN MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat. Rev. Drug Discov. 11, 860–872 (2012).. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Porrello ER et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl Acad. Sci. USA 110, 187–192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eulalio A et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492, 376–381 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Chen J et al. mir-17–92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ. Res. 112, 1557–1566 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian Y et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci. Transl Med 7, 279ra–38. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu X et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 21,584–595 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aurora AB et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca(2)(+) overload and cell death. J. Clin. Invest. 122, 1222–1232 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang LL et al. Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischaemic injury. Nat. Biomed. Engineer. 1, 983–992 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Colombo M, Raposo G & Thery C Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Valadi H et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Sluijter JP, Verhage V, Deddens JC, van den Akker F & Doevendans PA Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 102, 302–311 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Chen L et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/ reperfusion injury. Biochem. Biophys. Res. Commun. 431,566–571 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ibrahim AG, Cheng K & Marban E Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2, 606–619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barile L et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res 103, 530–541 (2014). [DOI] [PubMed] [Google Scholar]
- 109.Gallet R et al. Exosomes secreted by cardiosphere- derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J 38, 201–211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arslan F et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 10, 301–312 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Alvarez-Erviti L et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol 29, 341–345 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Kaufmann KB, Buning H, Galy A, Schambach A & Grez M Gene therapy on the move. EMBO Mol. Med 5, 1642–1661 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pinto AR et al. Revisiting cardiac cellular composition. Circ. Res 118, 400–409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sadahiro T, Yamanaka S & Ieda M Direct cardiac reprogramming: progress and challenges in basic biology and clinical applications. Circ. Res 116, 1378–1391 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Nam YJ et al. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development 141,4267–4278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou H, Dickson ME, Kim MS, Bassel-Duby R & Olson EN Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes. Proc. Natl Acad. Sci. USA 112, 11864–11869 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Muraoka N et al. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 33, 1565–1581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamakawa H et al. Fibroblast growth factors and vascular endothelial growth factor promote cardiac reprogramming under defined conditions. Stem Cell Rep. 5, 1128–1142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abad M et al. Notch inhibition enhances cardiac reprogramming by increasing MEF2C transcriptional activity. Stem Cell Rep. 8, 548–560 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mohamed TM et al. Chemical enhancement of in vitro and in vivo direct cardiac reprogramming. Circulation 135, 978–995 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao Y et al. High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat. Commun. 6, 8243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jayawardena TM et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 110, 1465–1473 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou H et al. ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression. Genes Dev. 31,1770–1783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou Y et al. Bmi1 is a key epigenetic barrier to direct cardiac reprogramming. Cell Stem Cell 18, 382–395 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Z et al. Single-cell transcriptomics reconstructs fate conversion from fibroblast to cardiomyocyte. Nature 551,100–104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lalit PA et al. Lineage reprogramming of fibroblasts into proliferative induced cardiac progenitor cells by defined factors. Cell Stem Cell 18, 354–367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Islas JF et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc. Natl Acad. Sci. USA 109, 13016–13021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fu JD et al. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 1, 235–247 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nam YJ et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl Acad. Sci. USA 110, 5588–5593 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wada R et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc. Natl Acad. Sci. USA 110, 12667–12672 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cao N et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science 352, 1216–1220 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Yang N, Ng YH, Pang ZP, Sudhof TC & Wernig M Induced neuronal cells: how to make and define a neuron. Cell Stem Cell 9, 517–525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pang ZP et al. Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou JX & Huang S Understanding gene circuits at cell-fate branch points for rational cell reprogramming. Trends Genet. 27, 55–62 (2011). [DOI] [PubMed] [Google Scholar]
- 135.Qian L et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Inagawa K et al. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ. Res. 111, 1147–1156 (2012). [DOI] [PubMed] [Google Scholar]
- 137.Nam YJ , Song K & Olson EN Heart repair by cardiac reprogramming. Nat. Med. 19, 413–415 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miyamoto K et al. Direct in vivo reprogramming with Sendai virus vectors improves cardiac function after myocardial infarction. Cell Stem Cell 22, 91–103.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Li HO et al. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J. Virol. 74, 6564–6569 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH & Field LJ Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ. Res. 96, 110–118 (2005). [DOI] [PubMed] [Google Scholar]
- 141.Chaudhry HW et al. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J. Biol. Chem. 279, 35858–35866 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Mohamed TMA et al. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell 173, 104–116.e12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mahmoud AI et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 497, 249–253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pan D The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Heallen T et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heallen T et al. Hippo signaling impedes adult heart regeneration. Development 140, 4683–4690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tao G et al. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature 534, 119–123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leach JP et al. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature 550, 260–264 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tallquist MD & Molkentin JD Redefining the identity of cardiac fibroblasts. Nat. Rev. Cardiol. 14, 484–491 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA & Gerling IC Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 10, 15–26 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Moon J et al. Blockade to pathological remodeling of infarcted heart tissue using a porcupine antagonist. Proc. Natl Acad. Sci. USA 114, 1649–1654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cittadini A et al. Aldosterone receptor blockade improves left ventricular remodeling and increases ventricular fibrillation threshold in experimental heart failure. Cardiovasc. Res. 58, 555–564 (2003). [DOI] [PubMed] [Google Scholar]
- 153.Duan J et al. Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J. 31,4 29–442 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kanemitsu H et al. Chymase inhibition prevents cardiac fibrosis and dysfunction after myocardial infarction in rats. Hypertens. Res. 29, 57–64 (2006). [DOI] [PubMed] [Google Scholar]
- 155.Sassoli C et al. Relaxin prevents cardiac fibroblastmyofibroblast transition via notch-1-mediated inhibition of TGF-beta/Smad3 signaling. PLoS ONE 8, e63896 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Samuel CS et al. Relaxin remodels fibrotic healing following myocardial infarction. Lab. Invest 91, 675–690 (2011). [DOI] [PubMed] [Google Scholar]
- 157.Chablais F & Jazwinska A The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development 139, 1921–1930 (2012). [DOI] [PubMed] [Google Scholar]
- 158.Pitt B et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial — the Losartan Heart Failure Survival Study ELITE II. Lancet 355, 1582–1587 (2000). [DOI] [PubMed] [Google Scholar]
- 159.Ikeuchi M et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc. Res. 64, 526–535 (2004). [DOI] [PubMed] [Google Scholar]
- 160.Zeisberg EM et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007). [DOI] [PubMed] [Google Scholar]
- 161.Zhou B et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Invest. 121,1894–1904 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mollmann H et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc. Res. 71, 661–671 (2006). [DOI] [PubMed] [Google Scholar]
- 163.Kramann R et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang H et al. Pigment epithelium-derived factor attenuates myocardial fibrosis via inhibiting Endothelial-to-Mesenchymal Transition in rats with acute myocardial infarction. Sci. Rep. 7, 41932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kanisicak O et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7, 12260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bassat E et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Puente BN et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakada Y et al. Hypoxia induces heart regeneration in adult mice. Nature 541,222–227 (2017). [DOI] [PubMed] [Google Scholar]
- 169.Piquereau J et al. Mitochondrial dynamics in the adult cardiomyocytes: which roles for a highly specialized cell? Front Physiol 4, 102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Canseco DC et al. Human ventricular unloading induces cardiomyocyte proliferation. J. Am. Coll. Cardiol. 65, 892–900 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Quaife-Ryan GA et al. Multicellular transcriptional analysis of mammalian heart regeneration. Circulation 136, 1123–1139 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aurora AB et al. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jinek M et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cong L et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guiraud S et al. The pathogenesis and therapy of muscular dystrophies. Annu. Rev. Genom. Hum. Genet. 16, 281–308 (2015). [DOI] [PubMed] [Google Scholar]
- 176.Long C et al. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 345, 1184–1188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Long C et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tabebordbar M et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351,407–411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nelson CE et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Amoasii L et al. Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci. Transl Med. 9, eaan8081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kazuki Y et al. Complete genetic correction of ips cells from Duchenne muscular dystrophy. Mol. Ther. 18, 386–393 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Young CS et al. A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell 18, 533–540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kyrychenko V et al. Functional correction of dystrophin actin binding domain mutations by genome editing. JCI Insight 2, e95918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang Y et al. CRISPR-Cpf1 correction of muscular dystrophy mutations in human cardiomyocytes and mice. Sci. Adv. 3, e1602814 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Long C et al. Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci. Adv. 4, eaap9004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Strong A & Musunuru K Genome editing in cardiovascular diseases. Nat Rev. Cardiol. 14, 11–20 (2017). [DOI] [PubMed] [Google Scholar]
- 187.McGinley LM et al. Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression. Hum. Gene Ther. 24, 840–851 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mohsin S et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J. Am. Coll. Cardiol. 60, 1278–1287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang YG & Sykes M Xenotransplantation: current status and a perspective on the future. Nat. Rev. Immunol. 7, 519–531 (2007). [DOI] [PubMed] [Google Scholar]
- 190.Yang L et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 350, 1101–1104 (2015). [DOI] [PubMed] [Google Scholar]
- 191.Qi LS et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gilbert LA et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Black JB et al. Targeted epigenetic remodeling of endogenous loci by CRISPR/Cas9-based transcriptional activators directly converts fibroblasts to neuronal cells. Cell Stem Cell 19, 406–414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chakraborty S et al. A CRISPR/Cas9-based system for reprogramming cell lineage specification. Stem Cell Rep. 3, 940–947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pascual-Gil S, Garbayo E, Diaz-Herraez P, Prosper F & Blanco-Prieto MJ Heart regeneration after myocardial infarction using synthetic biomaterials. J. Control. Release 203, 23–38 (2015). [DOI] [PubMed] [Google Scholar]
- 196.Oduk Y et al. VEGF nanoparticles repair heart after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 314, H278–H284 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Seif-Naraghi SB et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl Med. 5, 173ra–25. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Weinberger F et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl Med. 8, 363ra–148. (2016). [DOI] [PubMed] [Google Scholar]
- 199.Zimmermann WH et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 12, 452–458 (2006). [DOI] [PubMed] [Google Scholar]
- 200.Shudo Y et al. Novel regenerative therapy using cell-sheet covered with omentum flap delivers a huge number of cells in a porcine myocardial infarction model. J. Thorac. Cardiovasc. Surg. 142, 1188–1196 (2011). [DOI] [PubMed] [Google Scholar]
- 201.Riegler J et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 117, 720–730 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tokita Y et al. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ. Res. 119, 635–651 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Milani-Nejad N & Janssen PM Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 141, 235–249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chamuleau SAJ et al. Translational research in cardiovascular repair: a call for a paradigm shift. Circ. Res. 122, 310–318 (2018). [DOI] [PubMed] [Google Scholar]
- 205.Olson EN Gene regulatory networks in the evolution and development of the heart. Science 313, 1922–1927 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Major RJ & Poss KD Zebrafish heart regeneration as a model for cardiac tissue repair. Drug Discov. Today Dis. Models 4, 219–225 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rosenthal N & Brown S The mouse ascending: perspectives for human-disease models. Nat. Cell Biol. 9, 993–999 (2007). [DOI] [PubMed] [Google Scholar]
- 208.Gandolfi F et al. Large animal models for cardiac stem cell therapies. Theriogenology 75, 1416–1425 (2011). [DOI] [PubMed] [Google Scholar]
- 209.Daadi MM, Barberi T, Shi Q & Lanford RE Nonhuman primate models in translational regenerative medicine. Stem Cells Dev. 23 (Suppl. 1), 83–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chong JJ & Murry CE Cardiac regeneration using pluripotent stem cells — progression to large animal models. Stem Cell Res. 13, 654–665 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sayed N, Liu C & Wu JC Translation of human- induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J. Am. Coll. Cardiol. 67, 2161–2176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]