Abstract
Purpose
The aim of this study was to evaluate the cost-effectiveness of ribociclib compared to palbociclib, both in combination with letrozole, in the first-line treatment of postmenopausal women with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) advanced or metastatic breast cancer (ABC) from the perspective of the Spanish National Health System (NHS).
Patients and methods
Disease progression was simulated with a partitioned survival model developed from the parameterization and extrapolation of survival curves of postmenopausal women with HR+/HER2− ABC from clinical trials with ribociclib or palbociclib, both in combination with letrozole. The model was structured on the basis of three health states (progression-free, progressed disease, and death), with a 1-month cycle length and inclusion of subsequent treatments administered for disease progression, over a time horizon of 15 years. Clinical, economic, and quality of life parameters were drawn from clinical trials and the literature. The use of resources and clinical practice in the Spanish setting was validated by a panel of experts. The Spanish NHS perspective was adopted, taking into account exclusively direct health costs from 2017 expressed in Euros. Drug prices used were the reported ex-factory prices. Uncertainty of the parameters and robustness of the results were evaluated using deterministic and probabilistic sensitivity analyses (2,000 iterations).
Results
This cost-effectiveness analysis showed a greater benefit (0.437 and 0.285 life-years gained [LYGs] and quality-adjusted life years [QALYs] gained, respectively) and a slightly higher cost (€439.86) for ribociclib+letrozole compared to palbociclib+letrozole. The resulting incremental cost-effectiveness and cost-utility ratios were €1,007.69 per LYG and €1,543.62 per QALY gained, respectively. The results of the multiple sensitivity analyses showed limited dispersion of the outcomes, thus corroborating their robustness.
Conclusion
From the NHS perspective, considering the most commonly established willingness-to-pay thresholds in the Spanish setting, ribociclib+letrozole would represent a cost-effective therapeutic option compared to palbociclib+letrozole in the first-line treatment of HR+/HER2− ABC in postmenopausal women.
Keywords: economic evaluation, CDK4/6 inhibitors, breast cancer, payers’ perspective
Introduction
Currently, breast cancer is the second most common cancer in the world and the most common cancer in women. In Spain, it ranks fourth in incidence in the general population. In 2015, 27,747 patients were diagnosed with this disease, accounting for 11.2% of the total number of tumors diagnosed in Spain.1 About 5% of patients have metastatic disease at the time of diagnosis, and it is estimated that 20%–25% of patients diagnosed with early stage disease will eventually experience metastatic relapse.2
In terms of mortality, breast cancer is the leading cause of cancer-related deaths among women in developed countries,1,3,4 and Spain is no exception: in 2015, 6,213 deaths due to this disease were recorded.1
Despite the available treatments, locally advanced or metastatic breast cancer (ABC) is considered to be essentially incurable.5,6 The two main therapeutic goals are to improve survival and optimize patients’ quality of life.6 The management of ABC is complex, and the participation of a multidisciplinary team is essential.5,6
In patients with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2−) disease, hormone therapy (HT) is the treatment of choice in the first-line treatment, even in the presence of visceral disease, unless visceral crisis occurs.5,6
Good results were observed during the clinical development of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, a new class of innovative medications for the treatment of HR+/HER2− ABC, which includes palbociclib and ribociclib.7,8 This has prompted the most recent international clinical guidelines to list these drugs as therapeutic candidates, in combination with aromatase inhibitors (AIs), in the first-line treatment of patients with HR+/HER2− ABC.5,9 CDK4/6 inhibitors in combination with AIs have shown not only a manageable safety profile10 but also improved clinical response and progression-free survival (PFS).11
To address the long-term economic challenges associated with therapeutic innovations, the uncertainty regarding the relative value of the different therapeutic options, and budgetary constraints faced by the Spanish National Health System (NHS); this study was designed to evaluate the cost-effectiveness of ribociclib vs palbociclib, both in combination with letrozole, as the first-line treatment in postmenopausal women with HR+/HER2− ABC from the perspective of the Spanish NHS.
Patients and methods
Study design
A targeted non-systematic literature review was performed to retrieve pharmacoeconomic assessments, studies on cost and use of resources associated with ABC, and to obtain the main parameters for the pharmacoeconomic model. A panel of four clinical experts in oncology and one hospital pharmacy in the Spanish setting participated in the validation of the model assumptions through a structured questionnaire with with 149 total queries presented as matrix, closed-ended, and opened-ended questions. Individual responses were analyzed, gathered, and presented to reach a consensus through a face-to-face meeting.
Patients
The study population was a hypothetical cohort of postmenopausal women receiving the first-line treatment for HR+/HER2− ABC. On the basis of pivotal clinical trials of CDK4/6 inhibitors (MONALEESA-2 and PALOMA-2),8,12 average age was 62 years, with an estimated mean body surface of 1.7 m2.13,14
Therapeutic options
Currently, ribociclib and palbociclib are the only CDK4/6 inhibitors authorized for the treatment of HR+/HER2− ABC in Spain.15 Both drugs are dispensed via hospital pharmacies.16 The recommended daily dose of ribociclib and palbociclib is 600 mg once a day orally (po) and 125 mg once a day po, respectively. Both drugs should be administered for 21 consecutive days followed by 7 days without treatment. Letrozole is administered at a dose of 2.5 mg once a day po in concomitant treatment and monotherapy.17–19
The pharmacoeconomic model also included subsequent treatments attributed to disease progression. It was assumed that the subsequent treatments would remain independent of the CDK4/6 inhibitors used in the first-line treatment. The duration of subsequent treatment was obtained from the study by Macalalad et al,20 which describes the usage patterns and durations in real clinical practice of the second- and third-line treatments of postmenopausal patients with HR+/HER2− ABC. The distribution of the chemotherapies, targeted therapies, and endocrine therapies administered as subsequent treatments was based on the clinical practice recommendations of the Spanish Society of Medical Oncology, described in the consensus document in the study by Gavilá et al.6 The treatments with higher degree of recommendation and levels of evidence were selected. The selection, duration, and distribution of the subsequent treatments were validated by the panel of clinical experts (Table 1).
Table 1.
Duration and distribution of subsequent treatments considered
| Subsequent treatments | Second line
|
Third line
|
||
|---|---|---|---|---|
| Distribution of treatments (%) | Duration of treatments (months) | Distribution of treatments (%) | Duration of treatments (months) | |
|
| ||||
| Endocrine therapy | 31.35 | 4.9 | 11.31 | 4.9 |
| Targeted therapy | 16.15 | 4.9 | 5.57 | 4.9 |
| Chemotherapy | 47.50 | 3.7 | 50.63 | 3.1 |
| No active treatment | 5.00 | – | 32.50 | – |
|
| ||||
| Total | 100 | – | 100 | – |
Type of analysis
The costs (monetary value) and effects (health benefit) of the therapeutic options were compared. Results of this analysis were expressed as an incremental cost-effectiveness ratio (ICER) and incremental cost-utility ratio (ICUR), estimated using the following formula:
Costribociclib+letrozole and Costpalbociclib+letrozole are the total direct healthcare costs of treating a postmenopausal patient with HR+/HER2− ABC in the first-line treatment with ribociclib+letrozole or palbociclib+letrozole, respectively. Effectivenessribociclib+letrozole and Effectivenesspalbociclib+letrozole are the clinical benefits expressed as life years (LYs) and quality-adjusted life years (QALYs) gained with each of the therapeutic options evaluated.
The analysis was performed from the perspective of the Spanish NHS. Similar to the study by Raphael et al,21 a time horizon of 15 years was set, which was sufficient to capture differences between the compared strategies in terms of costs and health outcomes. In accordance with Spanish guidelines, a discount rate of 3.0% was applied to both costs and health outcomes.22
Pharmacoeconomic model
A cohort-based partitioned survival model was developed to simulate the natural history of the disease, using three mutually exclusive health states: 1) progression-free (PF), which was in turn divided into two sub-states: PF with complete response (CR) or partial response (PR), or PF with stable disease (SD); 2) progressed disease (PD); and 3) death (Figure 1). The two health sub-states were included to capture the quality of life benefits associated with tumor response in ABC patients who remained PF.
Figure 1.
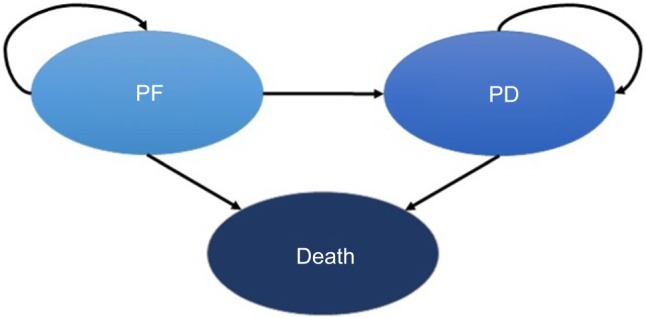
State diagram of the three health states considered.
Abbreviations: PD, progressed disease; PF, progression-free.
All patients in the hypothetical cohort of postmenopausal women with a confirmed diagnosis of HR+/HER2− ABC entered the model in a state of PF with SD, and the treatment was started immediately. Their health states could transition to PF with CR/PR, PD, or death. All transitions between health states were assumed to occur on a monthly basis (cycles determined in the model). A transition in the health state from PF with SD to PF with CR/PR was considered as an improvement in the patient’s quality of life. In contrast, a transition in the health state from PF to PD implies a diminished quality of life as a result of the disease progression, emotional burden, and clinical impact. The PD health state captures clinical outcomes experienced by ABC patients as they receive subsequent treatments after the first-line therapy. Consequently, after transitioning to the PD health state, patients could only transition, at some stage, to the death state.
Both CDK4/6 inhibitors have not been directly compared in head-to-head clinical trials, but clinical trials that evaluated the efficacy of both treatments used the same comparator: letrozole monotherapy. Accordingly, the letrozole monotherapy arm from the MONALEESA-2 study was established as the reference group to estimate survival curves for ribociclib+letrozole and palbociclib+letrozole. After the parameterization and extrapolation of the area under the curves of the PFS and overall survival (OS) for letrozole monotherapy in the MONALEESA-2 study, the hazard ratios (HRs) obtained from the comparisons of ribociclib+letrozole or palbociclib+letrozole vs letrozole monotherapy were applied (see clinical parameters below). In every cycle of the model, the proportion of patients in the PF state was obtained from the PFS curves (PPF=PPFS). The proportion of patients in the death state in every cycle was obtained from the OS curves (PDeath=1–POS). The difference between OS and PFS was used to establish the proportion of patients with PD (PPD=POS−PPFS).
This model was developed using Microsoft Excel® 2013.
Clinical parameters
Efficacy
PFS and OS for ribociclib+letrozole were obtained from the phase III MONALEESA-2 study (data cutoff, January 2, 2017).23 PFS parameters for palbociclib+letrozole were obtained from the phase III PALOMA-2 study (data cutoff, May 31, 2017),24 whereas the OS data were obtained from the phase II PALOMA-1 clinical trial (data cutoff, December 30, 2016)25 since the OS data have not been published in the PALOMA-2 trial. All of these studies assessed the efficacy and safety of both CDK4/6 inhibitors in combination with letrozole, compared with letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2− ABC and comprise the main sources of clinical evidence of both CDK4/6 inhibitors.26,27
As no head-to-head clinical trials were available, the individual results of the different clinical trials had to be taken into consideration. Matching-adjusted indirect comparison (MAIC) was used to match the individual data of the MONA-LEESA-2 study with those of PALOMA-1 and PALOMA-2 studies. This is a technique that allows two studies to be compared when the individual data of the patients from one of the studies are available (MONALEESA-2), but not from the other studies (PALOMA-1 and PALOMA-2). Thus, the individual patient-level data of the MONALEESA-2 study were adjusted and weighted to match with the published data from the PALOMA-1 and PALOMA-2 studies. MAIC was used to estimate the adjusted HR of ribociclib+letrozole vs letrozole monotherapy.28,29 The results obtained from the MAIC were considered for the base case, whereas the use of non-adjusted HR was considered as a sensitivity analysis (Table 2).
Table 2.
Efficacy, safety, and quality of life parameters considered
| Efficacy parameters | |||||||
|---|---|---|---|---|---|---|---|
| Progression-free survival | HR (vs LZE) | 95% CI | |||||
| Base case | |||||||
| Ribociclib+LZE (MAIC; MONALEESA-2; cutoff, January 2017)29 | 0.524 | 0.407–0.676 | |||||
| Palbociclib+LZE (PALOMA-2; cutoff, May 2017)24 | 0.563 | 0.461–0.687 | |||||
| Sensitivity analysis | |||||||
| Ribociclib+LZE (unmatched HR; MONALEESA-2; cutoff, January 2017)23 | 0.568 | 0.457–0.704 | |||||
| Overall survival | HR (vs LZE) | 95% CI | |||||
| Base case | |||||||
| Ribociclib+LZE (MAIC; MONALEESA-2; cutoff, January 2017)29 | 0.682 | 0.456–1.021 | |||||
| Palbociclib+LZE (PALOMA-1; cutoff, December 2016)25 | 0.897 | 0.623–1.294 | |||||
| Sensitivity analysis | |||||||
| Ribociclib+LZE (unmatched HR; MONALEESA-2; cutoff, January 2017)23 | 0.746 | 0.517–1.078 | |||||
| Response rates29 | OR (vs LZE) | 95% CI | |||||
| Ribociclib+LZE | 1.420 | 1.200–1.600 | |||||
| Palbociclib+LZE | 1.230 | 1.030–1.440 | |||||
| Safety parametersa | |||||||
| First-line treatment | Diarrhea (%) | Infection (%) | Nausea (%) | Febrile neutropenia (%) | Pulmonary embolism (%) | Vomiting (%) | |
| Ribociclib+LZE | 1.2026 | 4.1926 | 2.4026 | 1.2026 | 0.9026 | 3.5926 | |
| Palbociclib+LZE | 1.3527 | 5.1618 | 0.2327 | 1.8027 | 1.3527 | 0.4527 | |
| Quality of life | |||||||
| Health state | Mean | Standard error | |||||
| Progression-free state (PR/CR) | 0.8345 | 0.0068 | |||||
| Progression-free state (SD) | 0.8296 | 0.0063 | |||||
| Progressed disease | 0.5050 | 0.0505 | |||||
Note:
Only severe events (grade 3 or above) that met the following criteria were considered: AEs related to treatment and likely to result in hospitalization or expected to have a meaningful impact on patient’s well-being.
Abbreviations: AE, adverse event; CI, confidence interval; CR, complete response; HR, hazard ratio; LZE, letrozole; MAIC, matching-adjusted indirect comparison; OR, odds ratio; PR, partial response; SD, stable disease.
Since the follow-up period in the MONALEESA-2 study was shorter than the time horizon considered in the model, the respective PFS and OS curves for letrozole monotherapy from the MONALEESA-2 study (reference arm) had to be extrapolated using parametric adjustment. The following parametric distributions were tested: exponential, log-logistic, log-normal, Gompertz, gamma, generalized gamma, and Weibull. To determine the degree of adjustment of the parametric curves obtained from the Kaplan–Meier (KM) curves, the Akaike information criteria (AIC) and Bayesian information criteria (BIC) were used. According to both criteria, all tested distributions provided a similar goodness of fit to the KM curve values. However, in the period after the MONALEESA-2 study follow-up, the models generated highly variable extrapolations. For this reason, the selection of the parametric distribution was based fundamentally on the clinical plausibility of the data extrapolated to the long term. Taking into account the results of studies carried out with the first-line letrozole monotherapy in women with HR+/HER2− ABC,8,12,30–34 clinical experts agreed that it would be clinically plausible to see PFS rates of between 8%–10% and 1%–2% at 5 and 10 years, respectively. They also confirmed that it would be clinically plausible to observe OS rates of between 20%–30% and 1%–5% for letrozole monotherapy at 5 and 20 years, respectively. In accordance with the criteria for clinical plausibility and goodness of fit, the exponential distribution and Weibull distribution were used when modeling the KM curves of the letrozole monotherapy arm for PFS and OS, respectively (Table S1; Figures S1 and S2).29
Safety
The model included adverse events (AEs) associated with the first-line treatments. Only severe events (grade 3 or above) that met the following criteria were considered: AEs related to treatment and likely to result in hospitalization or expected to have a meaningful impact on patient’s well-being. As a result, the AEs included in the model were diarrhea, infection, nausea, febrile neutropenia, pulmonary embolism, and vomiting. The incidence of AEs was obtained from the phase III clinical trials (Table 2).18,26,27
Quality of life
Utility values were used to represent the impact on quality of life of each health state considered by the model, on a scale of 0 (death) to 1 (perfect health). QALYs associated with each of the therapeutic options evaluated were estimated by assigning the utility values to the health states.
The utility values for the PF state were obtained from the MONALEESA-2 study, quantified using the EQ-5D-5L quality of life questionnaire. The utility values for the PD state were obtained from the quality of life study by Lloyd et al35 conducted in 100 patients with ABC using the standard gamble method (Table 2).
Use of resources and costs
Direct health costs considered in the analysis included drug costs, administration costs, costs associated with monitoring, costs attributed to the patient’s health state, costs for the management of AEs, and costs of end-of-life care.
List ex-factory prices (EFPs) were used in the base case to estimate drug costs.16 It should be acknowledged that the reimbursed price of ribociclib and palbociclib are lower than the reported EFP, when charged to the Spanish NHS.16 Those reimbursed prices by the NHS are confidential and could not be used in this study (Table 3).
Table 3.
Economic parameters considered
| Drug costs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pharmaceutical form | EFP per package (€; 2017)a | Posologyc | Monthly cost (€; 2017)b | |||||||
| Ribociclib 200 mg, 63 tablets | 4,444.44 | Recommended starting dose: 600 mg/day for 21 days+7 days without treatment (3/1 scheme) | 4,831.34 | |||||||
| Ribociclib 200 mg, 42 tablets | 2,962.96 | After first dose reduction: 400 mg/day for 21 days+7 days without treatment (3/1 scheme) | 3,220.90 | |||||||
| Ribociclib 200 mg, 21 tablets | 1,481.48 | After second dose reduction: 200 mg/day for 21 days+7 days without treatment (3/1 scheme) | 1,610.45 | |||||||
| Palbociclib 125 mg, 21 capsules | 3,600.00 | Recommended starting dose: 125 mg/day for 21 days+7 days without treatment (3/1 scheme) | 3,913.39 | |||||||
| Palbociclib 100 mg, 21 capsules | 3,600.00 | After first dose reduction: 100 mg/day for 21 days+7 days without treatment (3/1 scheme) | 3,913.39 | |||||||
| Palbociclib 75 mg, 21 capsules | 3,600.00 | After second dose reduction: 75 mg/day for 21 days+7 days without treatment (3/1 scheme) | 3,913.39 | |||||||
| Letrozole GE 2.5 mg, 30 tablets | 59.00 | 2.5 mg/day | 59.86 | |||||||
| Administration costs | ||||||||||
| Type of administration | Unit cost (€; 2017)d | Frequencyc | ||||||||
| Parenteral administration | 211.67 | Per day of active treatment with parenteral chemotherapy, endocrine therapy, or targeted therapy | ||||||||
| Costs attributed to health state | ||||||||||
| Resource use | Unit cost (€; 2017)d | Patients (%) | Frequency (PF state) | Frequency (PD state) | ||||||
| General medicine visits | 22.59 | 50.00 | 1 visit/2 months | 1 visit/2 months | ||||||
| Outpatient visits (oncology) | 84.32 | 100.00 | 1 visit/month | 1 visit/month | ||||||
| Nursing visits | 26.73 | 25.00 | 1 visit/2 months | 1 visit/month | ||||||
| Computed tomography | 175.51 | 20.00 | 1 visit/3 months | 1 visit/3.5 months | ||||||
| Bone scintigraphy | 173.73 | 8.00 | 1 visit/6 months | 1 visit/6.5 months | ||||||
| Hospitalizations | 544.77 | 12.50 | – | 8 days | ||||||
| Monitoring costs | ||||||||||
| Resource use | Unit cost (€; 2017)d | Frequencyc Ribociclib+letrozole | Frequencyc Palbociclib+letrozole | |||||||
| Liver function tests | 22.67 | Baseline Every 2 weeks, cycles 1 and 2 Once, cycles 3, 4, 5, and 6 | – | |||||||
| Complete blood count | 4.16 | Baseline Every 2 weeks, cycles 1 and 2 Once, cycles 3, 4, 5, and 6 | Baseline Every 2 weeks, cycles 1 and 2 Once, remaining cycles. For patients without grade >2 neutropenia during first six cycles, monitoring every 3 months | |||||||
| Electrocardiogram | 38.83 | Baseline Every 2 weeks, cycle 1 Once, cycle 2 | – | |||||||
| Adverse event costs | ||||||||||
| Adverse event | Diarrhea | Infection | Nausea | Febrile neutropenia | Pulmonary embolism | Vomiting | ||||
| Unit cost (€; 2017) | 738.10e | 4,791.79f | 589.65e | 2,624.35e | 4,543.68d | 589.65e | ||||
| End-of-life care | ||||||||||
| Unit cost (€; 2017) | Patients (%) | Frequency | ||||||||
| Palliative care unit | 332.30f | 40.00 | 2–3 weeks until death (mean, 15.19 days) | |||||||
| Home care, doctor’s visits | 65.38d | 12.50 | 2–3 visits/week until death (mean, 8.74 visits) | |||||||
| Home care, nurse visits | 45.26d | 12.50 | 2–3 visits/week until death (mean, 8.74 visits) | |||||||
| Acute hospital unit | 540.81g | 35.00 | 1–2 weeks until death (mean, 9.01 days) | |||||||
Notes:
Reported EFP from the Spanish drug prices database.16
Estimated from the described posology. A mean body surface area of 1.7 m2 was considered.
Technical specifications of the respective pharmaceutical forms.15
Using average rates from the Spanish health costs database.37
Unit cost updated to 2017 Euros from the study by Alba et al.13
Unit cost updated to 2017 Euros from the study by Isla et al.36
Unit cost updated to 2017 Euros and estimated from NHS minimum basic data set.38
Abbreviations: EFP, ex-factory price; GE, generic equivalent; NHS, national health system; PD, progressed disease; PF, progression-free.
The use of resources in the management of ABC according to the clinical practice in Spain was obtained from the expert panel, allowing the cost estimation of the different health states considered in the model. Costs due to subsequent treatments (second- and third-line therapy) were considered in the PD state. The resources used during the month before patient’s death were also obtained from the expert panel. In both cases, resources associated with both health states and end-of-life care were considered to be independent from the CDK4/6 inhibitor used during the first-line treatment. The use of resources associated with the monitoring of both CDK4/6 inhibitors was based on the initial monitoring recommendations from the respective product information sheets.17,18 The costs of initial monitoring were applied in the first cycle of the model. For palbociclib+letrozole, according to the product information sheet of palbociclib, a follow-up monitoring was also taken into consideration after the sixth treatment cycle (Table 3).18
The unit costs assigned to intravenous administration, disease management, management of AEs, monitoring, and end-of-life care were obtained from databases and cost studies carried out from the perspective of the Spanish NHS.13,36–38 All costs were expressed in Euros for 2017. The cost attributed to each cycle of the model (1 month) in which patients remained in the PF health state during the time horizon of the analysis was €107.33. For patients in the PD health state, the cost of the cycle was €544.77, including the cost associated with treatments administered after the first-line treatment (€329.09 for each cycle). With regard to initial monitoring, the costs of patients treated in the first line with ribociclib and palbociclib, both in combination with letrozole, were €396.79 and €20.97, respectively. For palbociclib+letrozole, a subsequent monitoring cost of €3.23 per model cycle was also taken into account. The total costs attributed to AEs with CDK4/6 inhibitors were €317.24 and €369.93 for patients treated with ribociclib and palbociclib, respectively, both in combination with letrozole. The cost attributed to end-of-life care was €3,966.31 (Table 3).
The model considered dose reductions of CDK4/6 inhibitors during treatment, given that during their clinical development, a proportion of patients required dose modifications to alleviate AEs.8,39 For ribociclib, the dose reductions observed in the MONALEESA-2 trial (data cutoff, January 2, 2017) were applied in each cycle. No dose reductions had to be applied for palbociclib, since the prices of the three pharmaceutical forms authorized in the Spanish setting are equivalent (Table 3), so the drug cost attributed to an ABC patient receiving full dose of palbociclib is the same as for a patient receiving a reduced dose.
However, in the base case, a drug wastage cost was assumed for palbociclib, since whenever a patient with ABC needs to reduce her dose during the treatment cycle, a new drug package must be used (switch from 150 to 100 mg or from 100 to 75 mg). According to the results of the study by Li et al,40 who assessed the dosing patterns and economic implications of palbociclib in real clinical practice in the treatment of postmenopausal women with HR+/HER2− ABC, 10.3% of the patients were assumed to have experienced prescription fill overlap (average 11.1 days). In the case of ribociclib, each commercial package has a different drug price, but they all contain 200 mg tablets with an equivalent price per tablet, so it was considered acceptable to assume that dose modifications would not require a new package.
Sensitivity analyses
The uncertainty associated with the study variables and the robustness of the obtained results were evaluated using deterministic (univariate analysis) and probabilistic sensitivity analyses (PSAs).
Univariate sensitivity analysis
Alternative parametric distributions were tested for modeling letrozole monotherapy PFS and OS curves (reference arm). Base case parametric distributions were selected based on the best goodness of fit and clinical plausibility. Moreover, while maintaining the base case parametric distribution in the letrozole monotherapy PFS and OS curves, the use of alternative unmatched HRs was tested.
With regard to patient characteristics, the results of the analysis were evaluated after varying age by ±20%. For quality of life parameters, an analysis with a variation of ±20% of the utility values used was proposed. In addition, it was assessed to consider the utility values quantified by using the EQ-5D-5L quality of life questionnaire in the MONA-LEESA-2 trial for the PD health state.
With regard to economic parameters, given the uncertainty of the prices reimbursed by the Spanish NHS, a scenario was proposed involving price parity between ribociclib (package of 200 mg, 63 tablets) and palbociclib (package of 125 mg, 21 capsules). In addition, an analysis that did not include drug wastage attributed to the change in dose during palbociclib treatment was proposed. Variations of ±20% were applied to costs associated with monitoring, patients’ health states, drug costs of subsequent treatments, and costs of managing AEs.
With regard to structural parameters, discount rates per time preference of 0% and 5% were considered, both in costs and health effects, together with alternative time horizons (5, 10, 20, and 30 years).
Probabilistic sensitivity analysis
The PSA consists of simultaneously modifying all the parameters of the model according to an established distribution. Two thousand simulations were performed using the Monte-Carlo method.41 A beta distribution for utility and clinical parameters was applied (except for response and HRs, for which a log-normal distribution was used), whereas a gamma distribution was used for the economic parameters (except for end-of-life costs and costs of subsequent treatments, for which a log-normal distribution was used) (Table S2). The results of the probabilistic simulation were shown in an incremental cost-effectiveness plane and an acceptability curve.
Given the uncertainty of the prices reimbursed by the Spanish NHS as mentioned above, a PSA was proposed with price parity between ribociclib (package of 200 mg, 63 tablets) and palbociclib (package of 125 mg, 21 capsules), maintaining the price proportionality per package of the other ribociclib pharmaceutical forms depending on the number of tablets per package.
Results
Base case
The results of the base case analysis, which took into account the comparators’ list price, showed that the treatment of postmenopausal women with HR+/HER2− ABC, when the first-line ribociclib is used instead of palbociclib, both in combination with letrozole, involves an incremental cost of €439.86 together with an incremental effectiveness of 0.437 LYs and 0.285 QALYs. These outcomes resulted throughout the patient’s life from the first treatment for advanced disease, ie, time horizon of 15 years. Resulting ICER and ICUR were €1,007.69 per life-year gained (LYG) and €1,543.62 per QALY gained, respectively (Table 4). Considering the willingness-to-pay thresholds most commonly established in the Spanish setting (from €20,000 to €30,000/QALY gained),42–44 ribociclib+letrozole would be a cost-effective treatment option compared to palbociclib+letrozole in the first-line treatment of HR+/HER2− ABC in postmenopausal women.
Table 4.
Base case and univariate sensitivity analysis
| Base case results | |||
|---|---|---|---|
| Ribociclib + LZE | Palbociclib + LZE | Ribociclib + LZE vs palbociclib + LZE | |
| Cost results | |||
| First-line line treatment (€) | 90,923.49 | 91,895.72 | −972.23 |
| PF state (€) | 4,155.48 | 3,902.99 | 252.49 |
| PD state (€) | 1,798.47 | 1,474.23 | 324.24 |
| Subsequent treatments (€) | 4,925.20 | 3,975.62 | 949.58 |
| AEs (€) | 317.24 | 369.93 | −52.69 |
| End-of-life (€) | 3,458.24 | 3,519.78 | −61.55 |
| Total costs (€) | 105,578.12 | 105,138.26 | 439.86 |
| Health outcomes | |||
| LYG | 4.474 | 4.037 | 0.437 |
| QALY | 3.313 | 3.028 | 0.285 |
| Incremental ratios | |||
| ICER(€/LYG) | - | - | 1,007.69 |
| ICUR (€/QALY gained) | - | - | 1,543.62 |
| Univariate analysis results | |||||
|---|---|---|---|---|---|
| Scenario | Δ Cost (€) | Δ LY | Δ QALY | ICER (€/LYG) | ICUR (€/QALY) |
| Alternative parametric distributions | |||||
| PFS, Weibull distribution | 988.86 | 0.470 | 0.289 | 2,106.07 | 3,421.83 |
| PFS, gamma distribution | 870.70 | 0.460 | 0.288 | 1,892.13 | 3,022.53 |
| PFS, log-normal distribution | 103.74 | 0.424 | 0.302 | 244.65 | 343.39 |
| OS, Gompertz distribution | −468.08 | 0.260 | 0.196 | Ribociclib is dominant | Ribociclib is dominant |
| OS, gamma distribution | 1,945.13 | 0.738 | 0.437 | 2,634.84 | 4,447.75 |
| OS, log-logistic distribution | 2,532.82 | 0.873 | 0.505 | 2,901.96 | 5,012.69 |
| Unadjusted HR (MONALEESA-2; cutoff, January 2017) | |||||
| Ribociclib + LZE | 65.36 | 0.258 | 0.142 | 252.84 | 460.18 |
| PFS HR: 0.568 (0.457 - 0.704) | |||||
| OS HR: 0.746 (0.517 - 1.078) | |||||
| Patient characteristics | |||||
| Patients’ age (+20%) | 439.86 | 0.437 | 0.285 | 1,007.69 | 1,543.62 |
| Patients’ age (−20%) | 439.86 | 0.437 | 0.285 | 1,007.69 | 1,543.62 |
| Quality of life questionnaires | |||||
| Utility PF state (CR/PR) (+20%) | 439.86 | 0.437 | 0.315 | 1,007.69 | 1,396.30 |
| Utility PF state (CR/PR) (−20%) | 439.86 | 0.437 | 0.255 | 1,007.69 | 1,725.69 |
| Utility PF state (SD) (+20%) | 439.86 | 0.437 | 0.288 | 1,007.69 | 1,529.45 |
| Utility PF state (SD) (−20%) | 439.86 | 0.437 | 0.282 | 1,007.69 | 1,558.06 |
| Utility PD state (+20%) | 439.86 | 0.437 | 0.309 | 1,007.69 | 1,422.39 |
| Utility PD state (−20%) | 439.86 | 0.437 | 0.261 | 1,007.69 | 1,687.44 |
| Utility PF and PD states (MONALEESA-2) | 439.86 | 0.437 | 0.352 | 1,007.69 | 1,249.29 |
| Economic parameters | |||||
| Disregarding palbociclib wastage | 722.72 | 0.437 | 0.285 | 1,655.70 | 2,536.26 |
| Price parity between CDK4/6 inhibitors | −16,321.32 | 0.437 | 0.285 | Ribociclib is dominant | Ribociclib is dominant |
| Cost PF state (+20%) | 553.16 | 0.437 | 0.285 | 1,267.25 | 1,941.21 |
| Cost PF state (−20%) | 326.57 | 0.437 | 0.285 | 748.14 | 1,146.03 |
| Cost PD state (+20%) | 631.83 | 0.437 | 0.285 | 1,447.48 | 2,217.30 |
| Cost PD state (−20%) | 247.89 | 0.437 | 0.285 | 567.91 | 869.94 |
| Cost subsequent treatments (+20%) | 629.78 | 0.437 | 0.285 | 1,442.78 | 2,210.10 |
| Cost subsequent treatments (−20%) | 249.95 | 0.437 | 0.285 | 572.61 | 877.14 |
| Cost ribociclib monitoring (+20%) | 517.59 | 0.437 | 0.285 | 1,185.77 | 1,816.40 |
| Cost ribociclib monitoring (−20%) | 362.13 | 0.437 | 0.285 | 829.62 | 1,270.84 |
| Cost palbociclib monitoring (+20%) | 421.64 | 0.437 | 0.285 | 965.95 | 1,479.68 |
| Cost palbociclib monitoring (−20%) | 458.08 | 0.437 | 0.285 | 1,049.44 | 1,607.56 |
| Scenario | Δ Cost (€) | Δ LY | Δ QALY | ICER (€/LYG) | ICUR (€/QALY) |
| Cost AEs (+20%) | 429.32 | 0.437 | 0.285 | 983.55 | 1,506.64 |
| Cost AEs (−20%) | 450.40 | 0.437 | 0.285 | 1,031.83 | 1,580.60 |
| Structural parameters | |||||
| Discount rate (0%) | 467.44 | 0.502 | 0.329 | 931.00 | 1,421.02 |
| Discount rate (5%) | 432.00 | 0.400 | 0.260 | 1,080.89 | 1,658.60 |
| Time horizon: 5 years | −208.42 | 0.235 | 0.151 | Ribociclib is dominant | Ribociclib is dominant |
| Time horizon: 10 years | 395.04 | 0.411 | 0.264 | 960.34 | 1,496.27 |
| Time horizon: 20 years | 459.07 | 0.444 | 0.291 | 1,034.70 | 1,577.98 |
| Time horizon: 30 years | 465.91 | 0.446 | 0.293 | 1,044.63 | 1,590.89 |
Abbreviations: AEs, adverse events; CDK4/6, cyclin-dependent kinase 4 and 6; CR, complete response; HR, hazard ratio; ICER, incremental cost-effectiveness ratio; ICUR, incremental cost-utility ratio; LY, life years; LYG, life-years gained; LZE, letrozole; OS, overall survival; PD, progressed disease; PF, progression-free; PFS, progression-free survival;PR, partial response; QALY, quality-adjusted life years; SD, stable disease; Δ, differential.
Sensitivity analyses
Table 4 details the univariate sensitivity scenarios assessed. For each scenario, the main results are shown, expressed in incremental costs, LYs and QALYs, and the respective ICER and ICUR. Sensitivity scenarios that generated greater variability in the results of this analysis were listed in a decreasing order: price parity between the CDK4/6 inhibitors, alternative parametric distributions, and finally short-term time horizons (Table 4).
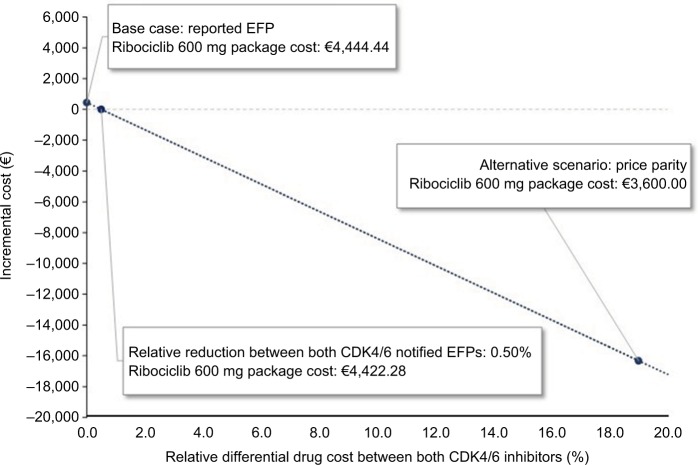
Due to the significant variability observed in the price parity scenario, an ad hoc sensitivity analysis was conducted to describe the impact of the differential drug cost between ribociclib and palbociclib, whereas the remaining parameters were not modified. As represented in Figure 2, the y-axis shows the resulting incremental cost, and the x-axis shows the relative differential drug cost between both CDK4/6 inhibitors. The extremes show the base case on one side, with the reported EFPs for both CDK4/6 inhibitors, and the scenario of price parity between both CDK4/6 inhibitors on the other side. With a relative reduction of >0.50% in the notified EFP for ribociclib (package of 63 tablets × 200 mg, <€4,422.28), the use of ribociclib+letrozole would represent a dominant alternative (greater effectiveness and lower costs) compared to palbociclib+letrozole.
Figure 2.
Ad hoc sensitivity analysis results.
Abbreviations: CDK4/6, cyclin-dependent kinase 4 and 6; EFP, ex-factory price.
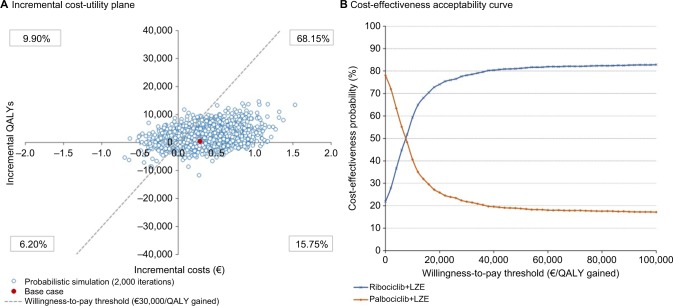
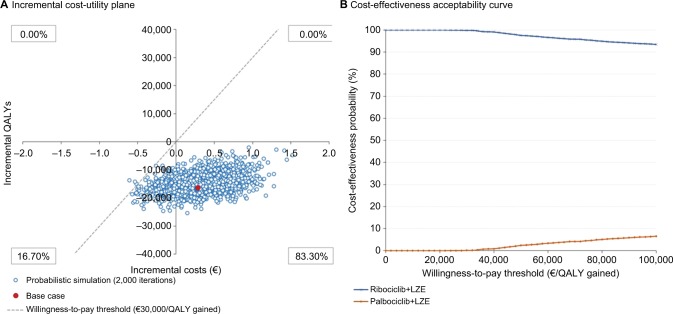
The results of the PSA are shown in Figure 3 (without price parity between both CDK4/6 inhibitors) and Figure 4 (with price parity between both CDK4/6 inhibitors) using an incremental cost-utility plane (Figures 3A and 4A) and an acceptability curve (Figures 3B and 4B).
Figure 3.
Probabilistic sensitivity analysis results (without price parity between the two CDK4/6 inhibitors), represented through an incremental cost-utility plane (A) and a cost-effectiveness acceptability curve (B)
Abbreviations: CDK4/6, cyclin-dependent kinase 4 and 6; LZE, letrozole; QALY, quality-adjusted life year.
Figure 4.
Probabilistic sensitivity analysis results (with price parity between the two CDK4/6 inhibitors), represented through an incremental cost-utility plane (A) and a cost-effectiveness acceptability curve (B)
Abbreviations: CDK4/6, cyclin-dependent kinase 4 and 6; LZE, letrozole; QALY, quality-adjusted life year.
Ribociclib+letrozole is a dominant option over palbociclib+letrozole in 15.75% of the simulated cases. In 68.15% of the simulations, ribociclib+letrozole provides greater effectiveness and results in higher costs than palbociclib+letrozole. In 6.20% of the simulations, it is a less effective and less costly option. Finally, in 9.90% of the PSA simulated cases, ribociclib+letrozole represents an option dominated by palbociclib+letrozole (Figure 3A). Considering the willingness-to-pay thresholds most commonly established in the Spanish setting (from €20,000 to €30,000/QALY gained),42–44 78.10% of the PSA simulations were found to be below this threshold (Figure 3B).
In the price parity scenario, ribociclib+letrozole is found to be a dominant therapeutic option over palbociclib+letrozole in 83.30% of the simulated cases. In 16.70% of the simulations, it is a less effective and less costly option. No simulated cases emerged in which the therapeutic option of ribociclib+letrozole is more costly than palbociclib+letrozole (Figure 4A). Considering the willingness-to-pay thresholds most commonly established in the Spanish setting (from €20,000 to €30,000/QALY gained),42–44 99.85% of the PSA simulations were found to be below this threshold (Figure 4B).
Discussion
In Spain, as in other developed countries,45 there has been a recent decline in breast cancer incidence rates, mainly attributed to the implementation of screening programs.46 However, breast cancer in Spain remains one of the most common neoplasms and the major cause of mortality and morbidity among women.1,46,47 Given the increasing health costs and the budgetary restrictions of the Spanish NHS, economic assessments may be a useful tool to quantify the use of resources derived from competing therapeutic alternatives in the management of diseases that incur high costs, such as HR+/HER2− ABC.
Economic assessments of innovative treatments for the first-line treatment of ABC can be found in the literature,48–55 together with findings published by health technology assessment agencies.56,57 However, this study represents the first economic evaluation that compares the use of different CDK4/6 inhibitors in the first-line treatment of ABC according to the Spanish healthcare setting.
The base case analysis showed that, without taking into account the confidential discounts applied to drugs reimbursed by the Spanish NHS, the use of ribociclib+letrozole vs palbociclib+letrozole in the first-line treatment of HR+/HER2− ABC in postmenopausal women would increase the clinical benefit with 0.437 LYGs and 0.285 QALYs gained, at a slight increase in costs of €439.86. This resulted in incremental cost-effectiveness and cost-utility ratios of €1,007.69 per LYG and €1,543.62 per QALY gained, respectively. Considering the willingness-to-pay thresholds most commonly established in the Spanish setting (from €20,000 to €30,000/QALY gained),42–44 ribociclib+letrozole would be a cost-effective treatment option compared to palbociclib+ letrozole in the first-line treatment of HR+/HER2− ABC in postmenopausal women. The sensitivity analyses conducted to evaluate the uncertainty of the variables and assumptions included in the model showed a low dispersion in results, corroborating the robustness of the base case findings.
This model has a number of limitations, some of which are inherent to pharmacoeconomic modeling, the structural rigidity of which makes it difficult to simulate precisely the real clinical progress of patients. Partitioned survival analysis models are conceptually similar to the state transition models. However, they differ in the way the state membership is determined. In state transition models, state membership is usually determined by the transition probability matrix. In the partitioned survival analysis approach, the state membership is determined from a set of non-mutually exclusive survival curves. Strengths of the partitioned survival analysis derive from the direct correspondence between frequently reported time-to-event endpoints, such as PFS and OS, and the survival functions used to estimate state membership. This promotes model comprehension, communication, and construction. Moreover, partitioned survival analysis models each survival curve as a function of time. This makes it straightforward to reflect any time-dependencies in the event rates, or treatment effects on event rates, of each survival curve. Thus, partitioned survival analysis generally provides accurate predictions for the within-trial period. In addition, partitioned survival analysis can be implemented using the individual patient data. The limitations of the partitioned survival analysis approach stem from its fundamental structural assumption that the survival endpoints are independent. The assumption that the modeled survival endpoints are structurally independent is potentially problematic as there are a number of dependencies between the survival endpoints. For the within-trial period, these dependencies are reflected in the data and therefore should be closely reflected in the partitioned survival analysis results. However, beyond the trial period analysis, dependencies between endpoints are ignored with potentially important implications for extrapolation. Some work has attempted to overcome this limitation via the development of statistical models for extrapolation. However, the proposed methods increase the role of subjectivity and only informally reflect the full set of available information. The lack of a link between clinical endpoints also limits the clinical plausibility from the generated extrapolations and limits the degree of scrutiny of sensitivity analyses. Therefore, assuming independence between endpoints may reduce the value of PSA as a means of quantifying decision uncertainty.58 In this analysis, long-term extrapolations had to be conducted given the limited follow-up period of the available clinical trials. For this reason, alternative parametric distributions were considered for long-term survival curve modeling. The results of this sensitivity analysis showed some variability from the base case results. However, in all scenarios tested, ribociclib was shown to improve clinical benefit and, considering the commonly established willingness-to-pay thresholds in Spain, proved to be at least a cost-effective treatment for the Spanish NHS.
One of the strengths of this study is the application of a previously published model.29 The methodology of this partitioned survival model has also been developed and validated in previous economic assessments submitted to health technology assessment agencies.56,57
Given the lack of randomized clinical trials comparing CDK4/6 inhibitors head-to-head, the treatments have been indirectly compared on the basis of their respective clinical trials. Baseline patient demographic, clinical characteristics, inclusion and exclusion criteria from clinical trials considered in this analysis were in detail presented and validated within the expert panel. Despite the mounting evidence regarding the variability between testing modalities and thresholds, patient HR+/HER2− qualification is similar between the assessed clinical trials: Among MONALEESA-2 eligibility criteria, HER2-negative breast cancer had to be defined as a negative in-situ hybridization test or an immunohistochemistry (IHC) status of 0, 1+, or 2+. If IHC status is 2+, a negative fluorescent, chromogenic, or silver in-situ hybridization assay was required by local laboratory testing.23 Likewise, in PALOMA-2 trial, HER2 status had to be determined by the US Food and Drug Administration approved assays. Commercial kits considered as acceptable for fluorescent, chromogenic, or silver in-situ hybridization assays are detailed in the PALOMA-2 protocol.24 Finally, in the PALOMA-1 trial, HR receptor status was determined by routine IHC, whereas HER2 status was assessed by either fluorescent in-situ hybridization or IHC.25 The MAIC technique was adopted in the base case analysis because it incorporates individual patient data and can be used to address a number of limitations related with indirect comparisons based solely on data aggregation. MAICs are useful for resolving significant differences in patient baseline characteristics, for resolving differences associated with the definition of clinical responses, for reducing the sensitivity of the mea surement of the effects, and for allowing the comparison of clinically relevant dosing schedules.59 By using MAIC from the ribociclib trial, patients were selected on the basis of inclusion/exclusion criteria specified in the palbociclib trials and were reweighted to match baseline characteristics reported for the palbociclib trials.28 However, as a sensitivity analysis, the results of using an indirect comparison without considering the individual patient data (ie, without the MAIC adjustment) were assessed. The results of this analysis reported a more favorable result for ribociclib, which combined with letrozole would represent a dominant therapeutic option for HR+/HER2− ABC postmenopausal women compared to palbociclib+letrozole. This represents a strength of the present analysis, as it indicates that considering the MAIC in the base case analysis provides not only methodological advantages but also a more conservative approach.
Another limitation is the use of data from the UK for health state utilities, but this is a common limitation in Spanish economic assessments, given the lack of national utility values, specifically in postmenopausal women with ABC. Moreover, ribociclib+letrozole remained cost-effective compared to palbociclib+letrozole in the scenario in which the results of the EQ-5D-5L quality of life questionnaire from the MONALEESA-2 study were used to estimate the utility values of the PD health state.
The use of resources and economic parameters validated by the Spanish expert panel were modified by ±20% to capture and simulate possible variations in clinical practice. The resulting sensitivity scenarios showed a low dispersion compared to the base case results. This supports the robustness of the analysis by suggesting that the results presented a low sensitivity to possible scenarios derived from the clinical variability.
The wastage associated with palbociclib, although it favors the results of the analysis, was based on experience in real clinical practice in postmenopausal women with HR+/HER2− ABC receiving palbociclib. As shown by the sensitivity analysis when wastage was not considered, the variation on incremental cost remained relatively limited, indicating that its inclusion or exclusion from the analysis does not alter the conclusion.
In contrast, the results and conclusion of this analysis were highly sensitive to variations in the cost of the CDK4/6 inhibitors evaluated. This, along with the uncertainty of the prices reimbursed by the Spanish NHS, supported the conduction of the price parity scenario between ribociclib and palbociclib, along with the ad hoc sensitivity scenario which describes the impact of varying the differential cost between both CDK4/6 inhibitors.
After reductions of >0.50% in the price of ribociclib, the first-line treatment of choice for postmenopausal women with HR+/HER2− ABC would be ribociclib+letrozole, since this would provide greater effectiveness together with economic savings. The degree of dominance of ribociclib over palbociclib, both in combination with letrozole, would be more marked in the scenario of price parity, in which the economic benefits of using a CDK4/6 inhibitor plus letrozole would result in savings of €16,321.32 per patient treated with ribociclib+letrozole compared to palbociclib+letrozole.
Conclusion
The results of this cost-effectiveness analysis show a greater benefit and a slightly higher cost for ribociclib vs palbociclib, both in combination with letrozole, in the first-line treatment of HR+/HER2− ABC in postmenopausal women. From the Spanish NHS perspective, taking into account the willingness-to-pay thresholds commonly established in the Spanish setting, ribociclib would represent a cost-effective alternative therapy compared to palbociclib. The limited dispersion of the sensitivity analyses demonstrates the robustness of the results of this appraisal. After deductions of >0.50% in the drug cost of ribociclib, the treatment of choice would be ribociclib+letrozole because this option would be dominant (greater effectiveness and lower costs) over palbociclib+letrozole. In scenarios of price parity between both CDK4/6 inhibitors, the economic benefits of using ribociclib+letrozole compared to palbociclib+letrozole would yield savings of €16,321.32 for each postmenopausal woman with HR+/HER2− ABC treated in first line with ribociclib+letrozole compared to palbociclib+letrozole.
Supplementary materials
Fit of the parametric distributions to the latest KM plots for PFS in MONALEESA-2 of letrozole monotherapy group (reference arm) with model extrapolation over a 20-year time horizon (240 months).
Abbreviations: KM, Kaplan–Meier; PFS, progression-free survival.
Fit of the parametric distributions to the latest KM plots for OS in MONALEESA-2 of letrozole monotherapy group (reference arm) with model extrapolation over a 20-year time horizon (240 months).
Abbreviations: KM, Kaplan–Meier; OS, overall survival.
Table S1.
Details on goodness of fit with the KM curves and survival rates of letrozole monotherapy from MONALEESA-2 study (reference arm)
| Parameterization of the PFS KM curves
| ||||
|---|---|---|---|---|
| Distribution | Goodness of fit
|
PFS rate (%)
|
||
| AIC | BIC | 5 years | 10 years | |
|
| ||||
| Exponential | 1,693.30 | 1,697.11 | 6.79 | 0.46 |
| Weibull | 1,689.87 | 1,697.49 | 3.93 | 0.07 |
| Gamma | 1,689.11 | 1,696.73 | 4.53 | 0.15 |
| Gompertz | 1,692.06 | 1,699.68 | 1.97 | 0.00 |
| Log-normal | 1,688.60 | 1,696.22 | 12.16 | 4.07 |
| Log-logistic | 1,692.66 | 1,700.29 | 12.19 | 4.90 |
|
| ||||
| Parameterization of the OS KM curves | ||||
|
| ||||
| Distribution |
Goodness of fit
|
OS rate (%)
|
||
| AIC | BIC | 5 years | 20 years | |
|
| ||||
| Exponential | 763.63 | 767.44 | 60 | 13.0 |
| Weibull | 733.69 | 741.31 | 26 | 0.0 |
| Gamma | 735.99 | 743.61 | 37 | 0.1 |
| Gompertz | 729.74 | 737.36 | 4 | 0.0 |
| Log-normal | 741.89 | 749.51 | 49 | 7.8 |
| Log-logistic | 735.52 | 743.14 | 38 | 2.8 |
Abbreviations: AIC, Akaike information criteria; BIC, Bayesian information criteria; KM, Kaplan–Meier; OS, overall survival; PFS, progression-free survival.
Table S2.
Detail of the economic parameters considered in the probabilistic sensitivity analysis\
| Name | Base case value (€; 2017) | Lower CI (€; 2017) | Higher CI (€; 2017) | Standard error | Distribution |
|---|---|---|---|---|---|
|
| |||||
| Health care costs | |||||
|
| |||||
| General medicine visits | 22.59 | 10.45 | 43.35 | 32.24 | Gamma |
| Outpatient visits (oncology) | 84.32 | 25.25 | 149.31 | 121.58 | Gamma |
| Nursing visits | 26.73 | 12.37 | 49.27 | 36.16 | Gamma |
| Hospitalizations | 544.77 | 435.82 | 653.72 | 213.55 | Gamma |
| Liver function tests | 22.67 | 4.95 | 63.19 | 57.08 | Gamma |
| Complete blood count | 4.16 | 2.90 | 5.30 | 2.35 | Gamma |
| Electrocardiogram | 38.83 | 8.04 | 183.90 | 172.34 | Gamma |
| Bone scintigraphy | 173.73 | 138.98 | 208.48 | 68.10 | Gamma |
| Computed tomography | 175.51 | 36.67 | 743.49 | 692.68 | Gamma |
| End-of-life cost | 3,966.31 | 3,569.68 | 4,362.94 | 396.63 | Log-normal |
| Subsequent treatment costs | 6,746.43 | 6,071.79 | 7,421.08 | 674.64 | Log-normal |
|
| |||||
| Adverse events cost | |||||
|
| |||||
| Diarrhea | 738.10 | 664.29 | 811.91 | 73.81 | Gamma |
| Infection | 4,791.79 | 4,312.61 | 5,270.97 | 479.18 | Gamma |
| Nausea | 589.65 | 530.68 | 648.61 | 58.96 | Gamma |
| Febrile neutropenia | 2,624.35 | 2,361.92 | 2,886.79 | 262.44 | Gamma |
| Pulmonary embolism | 4,543.68 | 4,089.31 | 4,998.05 | 454.37 | Gamma |
| Vomiting | 589.65 | 530.68 | 648.61 | 58.96 | Gamma |
Abbreviation: CI, confidence interval.
Acknowledgments
The authors would like to thank Dr Miguel Ángel Seguí Palmer, medical oncologist from Hospital de Sabadell-Consorcio Sanitario Parc Taulí de Sabadell (Barcelona) and directors’ board representative of the Spanish Society of Medical Oncology (SEOM); and Dr Emilio Alba, medical oncologist from Hospital Virgen de la Victoria (Málaga) and director of the Centro de Investigaciones Médico-Sanitarias (CIMES). Without their invaluable support and coordination, clinical expert panel validation of the model inputs and assumptions would not have been feasible.
Abbreviations
- ABC
advanced or metastatic breast cancer
- AE
adverse event
- AIC
Akaike information criteria
- AIs
aromatase inhibitors
- BIC
Bayesian information criteria
- CDK4/6
cyclin-dependent kinase 4 and 6
- CR
complete response
- EFP
exfactory price
- HER2−
human epidermal growth factor receptor 2-negative
- HR
hazard ratio
- HR+
hormone receptor-positive
- HT
hormone therapy
- ICER
incremental cost-effectiveness ratio
- ICUR
incremental cost-utility ratio
- KM
Kaplan–Meier
- LY
life-years
- LYG
life-years gained
- MAIC
matching-adjusted indirect comparison
- NHS
National Health System
- OS
overall survival
- PD
progressed disease
- PF
progression-free
- PFS
progression-free survival
- PR
partial response
- PSA
probabilistic sensitivity analysis
- QALY
quality-adjusted life years
- SD
stable disease
Footnotes
Disclosure
EG-C and EG-H are, respectively, employed by Basurto University Hospital and Hospital Universitario Gregorio Marañón. IM and AP-M are employees of Oblikue Consulting, an independent contract health economic organization that received consultancy fees from Novartis Farmacéutica, S.A. to conduct this research. JG is an employee of Novartis Farmacéutica, S.A., the marketing authorization holder for Kisqali® (ribociclib). The funding body was not involved in the study design, collection and interpretation of the data, or the decision to publish. The authors report no other conflicts of interest in this work.
Author contributions
- None of the material in the manuscript is included in another manuscript, has been published previously, or is currently under consideration for publication elsewhere.
- Has participated substantially in the work (conception and design of the study, or acquisition, analysis, and interpretation of data) taking public responsibility for the content of the paper and has critically revised and approved the final version of the manuscript.
- Agree to be accountable for all aspects of the study, ensuring that questions related to the accuracy or integrity of any part of the study are appropriately investigated and resolved.
- If requested, the authors will provide the data or will cooperate fully in obtaining and providing the data on which the manuscript is based for examination by the editors or their assignees.
References
- 1.SEOM Sociedad Española de Oncología Médica. Las Cifras del Cáncer en España, 2017 [The Cancer figures in Spain, 2017] 2017. [Accessed January 16, 2018]. Available from: https://www.seom.org/seomcms/images/stories/recursos/Las_cifras_del_cancer_en_Esp_2017.pdf. Spanish.
- 2.SEOM Sociedad Española de Oncología Médica. [Report of Ribociclib (Kisqali®) as initial endocrine treatment of HR+/HER2− ABC] 2018. [Accessed February 27, 2018]. Available from: https://seom.org/seomcms/images/stories/Informes_SEOM/IEV_Ribociclib.pdf. Spanish.
- 3.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3) Ann Oncol. 2017;28(12):3111–3133. doi: 10.1093/annonc/mdx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavilá J, Lopez-Tarruella S, Saura C, et al. SEOM clinical guidelines in metastatic breast cancer 2015. Clin Transl Oncol. 2015;17(12):946–955. doi: 10.1007/s12094-015-1476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner NC, Ro J, André F, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 8.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 9.Gradishar WJ, Anderson BO, Balassanian R, et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(3):310–320. doi: 10.6004/jnccn.2018.0012. [DOI] [PubMed] [Google Scholar]
- 10.Polk A, Kolmos IL, Kümler I, Nielsen DL. Specific CDK4/6 inhibition in breast cancer: a systematic review of current clinical evidence. ESMO Open. 2016;1(6):e000093. doi: 10.1136/esmoopen-2016-000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev. 2016;45:129–138. doi: 10.1016/j.ctrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med Overseas Ed. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 13.Alba E, Ciruelos E, López R, et al. Cost-utility analysis of nanoparticle albumin-bound paclitaxel versus paclitaxel in monotherapy in pretreated metastatic breast cancer in Spain. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):381–391. doi: 10.1586/erp.13.18. [DOI] [PubMed] [Google Scholar]
- 14.Seguí MÁ, Crespo C, Cortés J, et al. Genomic profile of breast cancer: cost-effectiveness analysis from the Spanish National Healthcare System perspective. Expert Rev Pharmacoecon Outcomes Res. 2014;14(6):889–899. doi: 10.1586/14737167.2014.957185. [DOI] [PubMed] [Google Scholar]
- 15.Ministry of Health, Social Services and Equality [webpage on the Internet] Centro de Información Online de Medicamentos de la Agencia Española de Medicamentos y Productos Sanitarios [Medicines Online Information Center of the Spanish Agency of Medicines and Health Products] [Accessed February 28, 2017]. Available from: https://www.aemps.gob.es/cima/publico/home.html.
- 16.Bot PLUS 20 [homepage on the Internet] Base de datos del Consejo General de Colegios Oficiales de Farmacéuticos [General Council of the Association of Official Pharmacists Database] [Accessed March 19, 2017]. Available from: https://botplusweb.portalfarma.com/ Spanish.
- 17.Kisqali® (ribociclib) film-coated tablets [summary of product characteristics] [Accessed February 28, 2017]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004213/WC500233997.pdf.
- 18.Ibrance® (palbociclib) hard capsules [summary of product characteristics] [Accessed February 28, 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003853/WC500217196.pdf.
- 19.Femara® (letrozole) film-coated tablets [summary of product characteristics] [Accessed February 28, 2018]. Available from: https://cima.aemps.es/cima/dochtml/ft/61628/FT_61628.html.
- 20.Macalalad AR, Hao Y, Lin PL, et al. Treatment patterns and duration in post-menopausal women with HR+/HER2− metastatic breast cancer in the US: a retrospective chart review in community oncology practices (2004–2010) Curr Med Res Opin. 2015;31(2):263–273. doi: 10.1185/03007995.2014.980885. [DOI] [PubMed] [Google Scholar]
- 21.Raphael J, Helou J, Pritchard KI, Naimark DM. Palbociclib in hormone receptor positive advanced breast cancer: A cost-utility analysis. Eur J Cancer. 2017;85:146–154. doi: 10.1016/j.ejca.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 22.López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias [A proposed guideline for economic evaluation of health technologies] Gac Sanit. 2010;24(2):154–170. doi: 10.1016/j.gaceta.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase 3 trial of first-line ribociclib + letrozole in hormone receptor-positive (HR+), HER2-negative (HER2−), advanced breast cancer (ABC) J Clin Oncol. 2017;35(15 Suppl):1038. [Google Scholar]
- 24.Rugo HS, Finn RS, Dieras V, et al. Abstract P5-21-03: Palbociclib (PAL) + letrozole (LET) as first-line therapy in estrogen receptor– positive (ER+)/human epidermal growth factor receptor 2–negative (HER2−) advanced breast cancer (ABC): Efficacy and safety updates with longer follow-up across patient subgroups. Cancer Res. 2018;78(4 Supplement):P5–21-03. [Google Scholar]
- 25.Finn RS, Crown J, Lang I, et al. Overall survival results from the randomized phase II study of palbociclib (P) in combination with letrozole (L) vs letrozole alone for frontline treatment of ER+/HER2– advanced breast cancer (PALOMA-1; TRIO-18. J Clin Oncol. 2017;35(15 Suppl):1001. [Google Scholar]
- 26.Committee for Medicinal Products for Human Use. European Medicines Agency EMA/CHMP/506968/2017 Ribociclib [European Public Assessment Report] 2017. [Accessed March 15, 2017]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004213/WC500233999.pdf.
- 27.Committee for Medicinal Products for Human Use European Medicines Agency. 2016. [Accessed March 15, 2017]. (EMA/652627/2016. Palbociclib [European Public Assessment Report]). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003853/WC500217198.pdf.
- 28.Forsythe A, Chandiwana D, Dolph M, Tremblay G, Monaco M. 254PMatching-adjusted indirect treatment comparison of ribociclib and palbociclib as first-line treatments for HR+, HER2− ABC. Ann Oncol. 2017;28(Suppl 5):254P. doi: 10.2147/CMAR.S163478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hettle R, Suri G, Mistry R, Chandiwana D, Lee A. Cost-Effectiveness of Ribociclib Plus Letrozole Versus Palbociclib Plus Letrozole for Postmenopausal Women with Hormone Receptor-Positive (HR+), Human Epidermal Growth Factor Receptor 2-Negative (HER2−) Advanced/Metastatic Breast Cancer from A UK National Health Service Perspective. Value Health. 2017;20(9):A433. [Google Scholar]
- 30.Dickler MN, Barry WT, Cirrincione CT, et al. Phase III Trial Evaluating Letrozole As First-Line Endocrine Therapy With or Without Bevacizumab for the Treatment of Postmenopausal Women With Hormone Receptor-Positive Advanced-Stage Breast Cancer: CALGB 40503 (Alliance) J Clin Oncol. 2016;34(22):2602–2609. doi: 10.1200/JCO.2015.66.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weide R, Feiten S, Friesenhahn V, et al. Metastatic breast cancer: prolongation of survival in routine care is restricted to hormone-receptor- and Her2-positive tumors. Springerplus. 2014;3(1):535. doi: 10.1186/2193-1801-3-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Cancer. 2004;100(1):44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 33.Tai P, Yu E, Vinh-Hung V, Cserni G, Vlastos G. Survival of patients with metastatic breast cancer: twenty-year data from two SEER registries. BMC Cancer. 2004;4:60. doi: 10.1186/1471-2407-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tevaarwerk AJ, Gray RJ, Schneider BP, et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer. 2013;119(6):1140–1148. doi: 10.1002/cncr.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–690. doi: 10.1038/sj.bjc.6603326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isla D, González-Rojas N, Nieves D, Brosa M, Finnern HW. Treatment patterns, use of resources, and costs of advanced non-small-cell lung cancer patients in Spain: results from a Delphi panel. Clin Transl Oncol. 2011;13(7):460–471. doi: 10.1007/s12094-011-0683-0. [DOI] [PubMed] [Google Scholar]
- 37.eSalud [homepage on the Internet] Base de Datos de Costes Sanitarios Españoles [Spanish Health Costs Database] Oblikue Consulting, S.L; 2007Available from: http://www.oblikue.com/bddcostes/Accessed June 21, 2017 [Google Scholar]
- 38.Conjunto Mínimo Básico de Datos al alta hospitalaria [Basic minimum dataset of hospital discharge] [homepage on the Internet] Ministerio de Sanidad, Servicios Sociales e Igualdad [Ministry of Health, Social Services and Equality] [Accessed June 22, 2017]. Available from: http://pestadistico.inteligenciadegestion.msssi.es.
- 39.Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 40.Li N, du EX, Chu L, et al. Real-world palbociclib dosing patterns and implications for drug costs in the treatment of HR+/HER2- metastatic breast cancer. Expert Opin Pharmacother. 2017;18(12):1167–1178. doi: 10.1080/14656566.2017.1351947. [DOI] [PubMed] [Google Scholar]
- 41.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 42.Sacristán JA, Oliva J, del Llano J, Prieto L, Pinto JL. ¿Qué es una tecnología sanitaria eficiente en España? [What is an efficient health technology in Spain?] Gac Sanit. 2002;16(4):334–343. doi: 10.1016/s0213-9111(02)71933-x. [DOI] [PubMed] [Google Scholar]
- 43.Ortega Eslava A, Marín Gil R, Fraga Fuentes MD, López-Briz E, Puigventós Latorre F. Guía de Evaluación Económica e Impacto Presu-puestario En Los Informes de Evaluación de Medicamentos [Economic Evaluation and Budgetary Impact Guidelines for Assessment Reports on Medicinal Products] Madrid: Sociedad Española de Farmacia Hospitalaria (SEFH); 2016. [Accessed May 22, 2017]. Available from: https://gruposdetrabajo.sefh.es/genesis/genesis/Documents/GUIA_EE_IP_GENESIS-SEFH_19_01_2017.pdf. [Google Scholar]
- 44.Vallejo-Torres L, Garcia-Lorenzo B, Garcia-Pérez L, Linertová R, Serrano-Aguilar P. Disponibilidad a Pagar de La Sociedad Española Por Un Año de Vida Ajustado Por Calidad [Spanish Society Willingness to- Pay for One Quality-Adjusted Life Year] Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad. Servicio de Evaluación del Servicio Canario de la Salud; 2017. [Accessed May 22, 2017]. Available from: http://www.redets.mscbs.gob.es/documentos/SESCS_2016_DAP_AVAC.pdf. [Google Scholar]
- 45.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez MJ, Payer T, de Angelis R, et al. Cancer incidence and mortality in Spain: estimates and projections for the period 1981–2012. Ann Oncol. 2010;21(Suppl 3):iii30–iii36. doi: 10.1093/annonc/mdq090. [DOI] [PubMed] [Google Scholar]
- 47.Fernández de Larrea-Baz N, Alvarez-Martín E, Morant-Ginestar C, et al. Burden of disease due to cancer in Spain. BMC Public Health. 2009;9(1):42. doi: 10.1186/1471-2458-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matter-Walstra K, Ruhstaller T, Klingbiel D, Schwenkglenks M, Dedes KJ. Palbociclib as a first-line treatment in oestrogen receptor-positive, HER2-negative, advanced breast cancer not cost-effective with current pricing: a health economic analysis of the Swiss Group for Clinical Cancer Research (SAKK) Breast Cancer Res Treat. 2016;158(1):51–57. doi: 10.1007/s10549-016-3822-z. [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharya K, Yang Y. A Cost-Effectiveness Analysis of Palbociclib and other Aromatase Inhibitors for Treatment of Advanced Breast Cancer. Value Health. 2016;19(3):A150. [Google Scholar]
- 50.Simons WR, Jones D, Buzdar A. Cost-effectiveness of anastrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer. Clin Ther. 2003;25(11):2972–2987. doi: 10.1016/s0149-2918(03)80348-x. [DOI] [PubMed] [Google Scholar]
- 51.Cressman S, Browman GP, Hoch JS, Kovacic L, Peacock SJ. A Time-Trend Economic Analysis of Cancer Drug Trials. Oncologist. 2015;20(7):729–736. doi: 10.1634/theoncologist.2014-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dranitsaris G, Verma S, Trudeau M. Cost utility analysis of first-line hormonal therapy in advanced breast cancer: comparison of two aromatase inhibitors to tamoxifen. Am J Clin Oncol. 2003;26(3):289–296. doi: 10.1097/01.COC.0000021042.55557.2B. [DOI] [PubMed] [Google Scholar]
- 53.Marchetti M, Caruggi M, Colombo G. Cost utility and budget impact of third-generation aromatase inhibitors for advanced breast cancer: a literature-based model analysis of costs in the Italian National Health Service. Clin Ther. 2004;26(9):1546–1561. doi: 10.1016/j.clinthera.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 54.das R, Cope S, Ouwens M, Turner P, Howlett M. Economic evaluation of fulvestrant 500 mg versus generic nonsteroidal aromatase inhibitors in patients with advanced breast cancer in the United Kingdom. Clin Ther. 2013;35(3):246–260. doi: 10.1016/j.clinthera.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Nerich V, Bazan F, Compagnat F, et al. First-line bevacizumab plus taxane-based chemotherapy for metastatic breast cancer: cost-minimisation analysis. Anticancer Res. 2012;32(8):3547–3552. [PubMed] [Google Scholar]
- 56.NICE National Institute for Health and Care Excellence [webpage on the Internet] Fulvestrant for the Treatment of Locally Advanced or Metastatic Breast Cancer. 2011. [Accessed March 15, 2017]. Available from: https://www.nice.org.uk/guidance/ta239.
- 57.NICE National Institute for Health and Care Excellence [webpage on the Internet] Everolimus with Exemestane for Treating Advanced Breast Cancer After Endocrine Therapy. 2013. [Accessed March 15, 2017]. Available from: https://www.nice.org.uk/guidance/TA421.
- 58.Woods B, Sideris E, Palmer S, Latimer N, Soares M. NICE: DSU Technical Support Document 19. Partitioned Survival Analysis for Decision Modelling in Health Care: A Critical Review. London: NICE Decision Support Unit; 2017. [Accessed September 10, 2018]. Available from: http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2017/06/Partitioned-Survival-Analysis-final-report.pdf. [Google Scholar]
- 59.Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947. doi: 10.1016/j.jval.2012.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fit of the parametric distributions to the latest KM plots for PFS in MONALEESA-2 of letrozole monotherapy group (reference arm) with model extrapolation over a 20-year time horizon (240 months).
Abbreviations: KM, Kaplan–Meier; PFS, progression-free survival.
Fit of the parametric distributions to the latest KM plots for OS in MONALEESA-2 of letrozole monotherapy group (reference arm) with model extrapolation over a 20-year time horizon (240 months).
Abbreviations: KM, Kaplan–Meier; OS, overall survival.
Table S1.
Details on goodness of fit with the KM curves and survival rates of letrozole monotherapy from MONALEESA-2 study (reference arm)
| Parameterization of the PFS KM curves
| ||||
|---|---|---|---|---|
| Distribution | Goodness of fit
|
PFS rate (%)
|
||
| AIC | BIC | 5 years | 10 years | |
|
| ||||
| Exponential | 1,693.30 | 1,697.11 | 6.79 | 0.46 |
| Weibull | 1,689.87 | 1,697.49 | 3.93 | 0.07 |
| Gamma | 1,689.11 | 1,696.73 | 4.53 | 0.15 |
| Gompertz | 1,692.06 | 1,699.68 | 1.97 | 0.00 |
| Log-normal | 1,688.60 | 1,696.22 | 12.16 | 4.07 |
| Log-logistic | 1,692.66 | 1,700.29 | 12.19 | 4.90 |
|
| ||||
| Parameterization of the OS KM curves | ||||
|
| ||||
| Distribution |
Goodness of fit
|
OS rate (%)
|
||
| AIC | BIC | 5 years | 20 years | |
|
| ||||
| Exponential | 763.63 | 767.44 | 60 | 13.0 |
| Weibull | 733.69 | 741.31 | 26 | 0.0 |
| Gamma | 735.99 | 743.61 | 37 | 0.1 |
| Gompertz | 729.74 | 737.36 | 4 | 0.0 |
| Log-normal | 741.89 | 749.51 | 49 | 7.8 |
| Log-logistic | 735.52 | 743.14 | 38 | 2.8 |
Abbreviations: AIC, Akaike information criteria; BIC, Bayesian information criteria; KM, Kaplan–Meier; OS, overall survival; PFS, progression-free survival.
Table S2.
Detail of the economic parameters considered in the probabilistic sensitivity analysis\
| Name | Base case value (€; 2017) | Lower CI (€; 2017) | Higher CI (€; 2017) | Standard error | Distribution |
|---|---|---|---|---|---|
|
| |||||
| Health care costs | |||||
|
| |||||
| General medicine visits | 22.59 | 10.45 | 43.35 | 32.24 | Gamma |
| Outpatient visits (oncology) | 84.32 | 25.25 | 149.31 | 121.58 | Gamma |
| Nursing visits | 26.73 | 12.37 | 49.27 | 36.16 | Gamma |
| Hospitalizations | 544.77 | 435.82 | 653.72 | 213.55 | Gamma |
| Liver function tests | 22.67 | 4.95 | 63.19 | 57.08 | Gamma |
| Complete blood count | 4.16 | 2.90 | 5.30 | 2.35 | Gamma |
| Electrocardiogram | 38.83 | 8.04 | 183.90 | 172.34 | Gamma |
| Bone scintigraphy | 173.73 | 138.98 | 208.48 | 68.10 | Gamma |
| Computed tomography | 175.51 | 36.67 | 743.49 | 692.68 | Gamma |
| End-of-life cost | 3,966.31 | 3,569.68 | 4,362.94 | 396.63 | Log-normal |
| Subsequent treatment costs | 6,746.43 | 6,071.79 | 7,421.08 | 674.64 | Log-normal |
|
| |||||
| Adverse events cost | |||||
|
| |||||
| Diarrhea | 738.10 | 664.29 | 811.91 | 73.81 | Gamma |
| Infection | 4,791.79 | 4,312.61 | 5,270.97 | 479.18 | Gamma |
| Nausea | 589.65 | 530.68 | 648.61 | 58.96 | Gamma |
| Febrile neutropenia | 2,624.35 | 2,361.92 | 2,886.79 | 262.44 | Gamma |
| Pulmonary embolism | 4,543.68 | 4,089.31 | 4,998.05 | 454.37 | Gamma |
| Vomiting | 589.65 | 530.68 | 648.61 | 58.96 | Gamma |
Abbreviation: CI, confidence interval.