Abstract
Purpose
We conducted a randomized phase III trial to determine whether adjuvant chemotherapy improves survival in women with uterine leiomyosarcoma.
Methods
Women with uterus-confined, high-grade leiomyosarcoma who were confirmed disease free by imaging were randomly assigned to four cycles of gemcitabine plus docetaxel, followed by four cycles of doxorubicin, or to observation. All were followed for evidence of recurrence. The primary end point was overall survival (OS).
Results
With international collaboration, 38 of the targeted accrual of 216 patients were enrolled, after which the study was closed by the National Cancer Institute for accrual futility. Twenty patients were assigned to chemotherapy, 18 to observation. Among the 17 patients treated with at least one cycle of chemotherapy, grade 3 or 4 toxicities were observed in 47%; among the 18 patients assigned to observation, one had grade 3 hypertension. There were six deaths (chemotherapy, n = 5; observation, n = 1), all due to disease. The restricted mean survival time for OS was estimated as 34.3 months (95% CI, 25.3 to 43.3 months) in the chemotherapy arm and as 46.4 months (95% CI, 43.6 to 49.1 months) in the observation arm. There were eight recurrences in each arm. The restricted mean survival time for recurrence-free survival was estimated as 18.1 (95% CI, 14.2 to 22.0) months in the chemotherapy arm and as 14.6 months (95% CI, 10.3 to 19.0 months) in the observation arm. Neither survival outcome comparison was considered statistically robust, due to the small sample size.
Conclusion
Despite international collaboration to test the role of adjuvant chemotherapy in uterine-confined leiomyosarcoma, this study was closed for accrual futility. Although the sample size precludes robust statistical comparison, observed OS and recurrence-free survival data do not show superior outcomes with adjuvant chemotherapy.
INTRODUCTION
Patients with early-stage, high-grade uterine leiomyosarcoma (LMS) have a 50% to 70% chance of recurrence of disease.1 Neither adjuvant radiation nor adjuvant chemotherapy has been shown to improve survival outcomes in this high-risk disease. In a phase III randomized trial of adjuvant whole-pelvis radiation versus observation for International Federation of Gynecology and Obstetrics (FIGO) stage I and II uterine sarcomas, overall recurrence rates were approximately 50% in both arms of the study. Local recurrence rates did not differ between patients with LMS who were assigned to adjuvant radiation compared with those who were observed. The percentage of all patients who remained progression free at 3 years was 52%.2
Gemcitabine plus docetaxel, and doxorubicin-based regimens are active in women with metastatic LMS, achieving objective responses in 10% to 50% of patients.3-5 In a prospective phase II study of adjuvant gemcitabine plus docetaxel followed by doxorubicin for women with uterus-limited, high-grade LMS, the recurrence rate was 46%, with 57% of patients remaining disease free at 3 years.6 Because this 3-year disease-free survival might be superior to the survival outcomes among women who were observed, we sought to determine whether this adjuvant chemotherapy approach, using agents with efficacy in metastatic disease, could improve progression-free survival (PFS) and overall survival (OS) among women with uterus-limited, high-grade LMS.
There was consensus that this was a critical research question and that the ideal strategy to answer this question was a prospective randomized trial with observation as the control arm. The study was designed and conducted with international collaboration.
METHODS
Study Design and Objectives
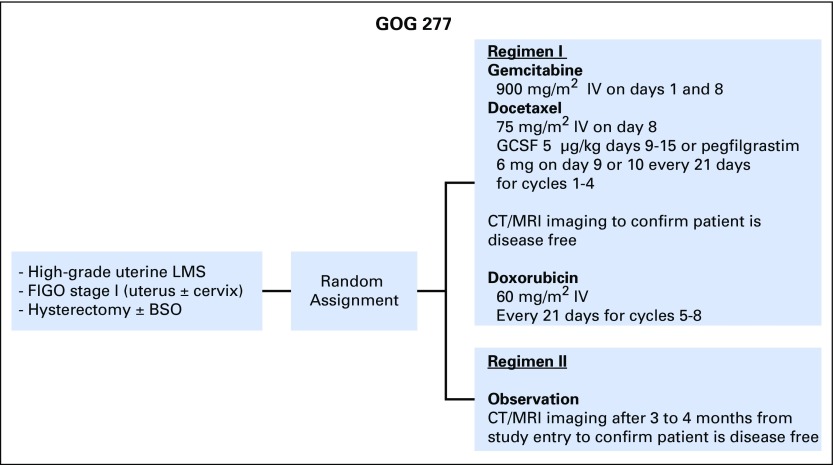
GOG-0277 was a two-arm, open-label, randomized phase III superiority trial of gemcitabine (NSC no. 613327) plus docetaxel (NSC no. 628503), followed by doxorubicin (NSC no. 123127) versus observation in women with uterus-limited, high-grade LMS. The study schema is shown in Fig 1. The chemotherapy arm included four cycles of gemcitabine and docetaxel. Patients who remained disease free as determined from computed tomography (CT) or magnetic resonance imaging (MRI) then received four cycles of doxorubicin. All patients were followed for recurrence by physical examination and CT scan of the chest, abdomen, and pelvis (or CT scan of the chest plus MRI of the abdomen and pelvis) every 4 months from study entry until 3 years out from the start of study treatment, then every 6 months for the next 2 years, for a maximum of 5 years of imaging.
Fig 1.
Study schema for GOG 0277, a randomized phase III trial of chemotherapy versus observation in uterus-limited, high-grade uterine LMS. BSO, bilateral salpingo-oophorectomy; CT, computed tomography; FIGO, International Federation of Gynecology and Obstetrics; GCSF, granulocyte-colony stimulating factor; IV, intravenous; LMS, leiomyosarcoma; MRI, magnetic resonance imaging.
The primary objective was to determine whether OS was superior among patients randomly assigned to treatment with multiagent chemotherapy compared with patients assigned to observation. The primary efficacy end point was OS, defined as the duration of time from study entry to time of death or the date of last contact. Safety end points include the frequency and severity of adverse events categorized and graded according to Common Terminology Criteria for Adverse Events, version 4. Secondary objectives included determining whether the assigned chemotherapy treatment regimen improves recurrence-free survival (RFS) compared with observation and exploring the effect of potential predictors of recurrence or death, including characteristics such as patient age and institution-reported tumor size, cervix involvement, and mitotic rate. RFS was defined as the duration of time from study entry to time of recurrence or death, whichever occurred first.
The study was open to patient accrual on June 4, 2012, in the United States through the Gynecologic Oncology Group (GOG) and outside the United States through the European Organization for the Research and Treatment of Cancer (EORTC) and Cancer Research United Kingdom (ClinicalTrial.gov identifier: NCT01533207). The study was opened at 701 international sites. Due to slow accrual, per the National Cancer Institute’s Cancer Therapy Evaluation Program (CTEP) Early Stopping Guidelines, the study was closed to patient entry effective September 20, 2016.
Patient Eligibility
Eligible patients were ≥ 18 years old with high-grade uterine LMS, FIGO 2009 stage I (ie, confined to the uterine corpus with or without involvement of the uterine cervix). Patients must have had a complete hysterectomy with or without bilateral salpingo-oophorectomy within 12 weeks of study enrollment. Patients who had an open morcellation hysterectomy procedure were required to have had an operative re-evaluation to confirm no residual disease. The enrolling institution must have rendered a histologic diagnosis of high-grade uterine LMS and, if a mitotic rate was provided, it must have been greater than or equal to five mitoses per 10 high-power fields.
All patients must have had no evidence of persistent or metastatic disease documented by a postresection CT scan of the chest, abdomen, and pelvis or by CT scan of the chest and MRI of the abdomen and pelvis performed within 4 weeks of study registration. Patients were required to have adequate bone marrow, renal, hepatic, and neurologic function; and Eastern Cooperative Oncology Group performance status of 0 or 1. Patients must not have received prior treatment with gemcitabine, docetaxel, or doxorubicin; and were not permitted to have had prior pelvic radiation. Patients with a known history of congestive heart failure or cardiac ejection fraction (EF) < 50% (or less than institutional normal limits) were not eligible. Although echocardiogram or other assessment of cardiac EF was not required before enrollment, for patients assigned to the chemotherapy arm, assessment of cardiac EF must have been done within 6 months of day 1 of treatment. Patients who were then determined to have a cardiac EF < 50% or below institutional normal were permitted to remain in the study but were not treated with doxorubicin.
Treatment Details
Patients assigned to the adjuvant chemotherapy arm received gemcitabine 900 mg/m2 over 90 minutes on days 1 and 8, plus docetaxel 75 mg/m2 on day 8, with granulocyte growth-factor support on day 9 (filgrastim or pegylated filgrastim). Each cycle was 21 days and patients were to receive four cycles. Standard dexamethasone premedication for docetaxel therapy and diuretic use for patients with fluid retention were recommended. Patients who remained disease free by imaging studies after cycle 4 were treated with doxorubicin 60 mg/m2 on day 1 of a 21-day cycle for four cycles. The use of granulocyte growth-factor support was optional during doxorubicin treatment. Up to one dose reduction was permitted for certain protocol-defined toxicities.
Statistical Design
The proposed primary analysis included comparing the distribution of time to death with censoring between the assigned treatment arms through hypothesis testing. The log-rank test was to be used to test the null hypothesis of independence between survival and randomized treatment. Assuming a one-sided α of 0.05 for controlling type I error for superiority and desired power of 80%, a hazard ratio (HR) of 1.6, comparing the control arm with the experimental arm, was deemed to be of clinical interest. There was also a proposed interim analysis of futility and superiority planned at 45% of information time. However, with only 38 of a planned 216 patients and six deaths out of a planned 115 events, hypothesis testing was not performed with these data. Additional exploratory analyses assessing the univariable and multivariable prognostic significance of baseline clinical, pathologic, or demographic factors such as patient age, tumor size, cervix involvement, and mitotic rate were also proposed to be carried out; exploratory analyses for risk factors were also abandoned because of limited events.
Survival analyses were performed to describe the relationship between treatment arm and the primary outcome of OS. All survival analyses assumed an intent-to-treat framework among eligible patients. Kaplan-Meier estimation was used to graphically characterize the relationship between treatment arm and the OS outcome. A plot of the log (survival) versus time was used to visually assess for departures from the proportional hazards (PHs) assumption required to estimate a valid HR using a Cox PHs model.7 The effect of treatment on OS estimated as an HR with a corresponding 95% Wald CI was estimated using a Cox PHs model. Because of potential issues with the PHs assumption, the restricted mean survival time (RMST) and 95% CI were estimated as well.8,9 RMST can be interpreted as the expected time spent event free for a future patient followed for the specified time. RMST for each regimen and the difference in RMSTs were estimated assuming a cutoff of 48 months.
These methods were repeated for the secondary end point of RFS. However, the cutoff for estimating RMST for RFS needed to be reduced to only 24 months because of the last event time reported for that end point. Most analyses were completed using SAS, version 9.4 (Cary, NC), except the RMST analysis, which was completed using the R program (https://www.r-project.org/).10
RESULTS
Study Accrual and Patient Characteristics
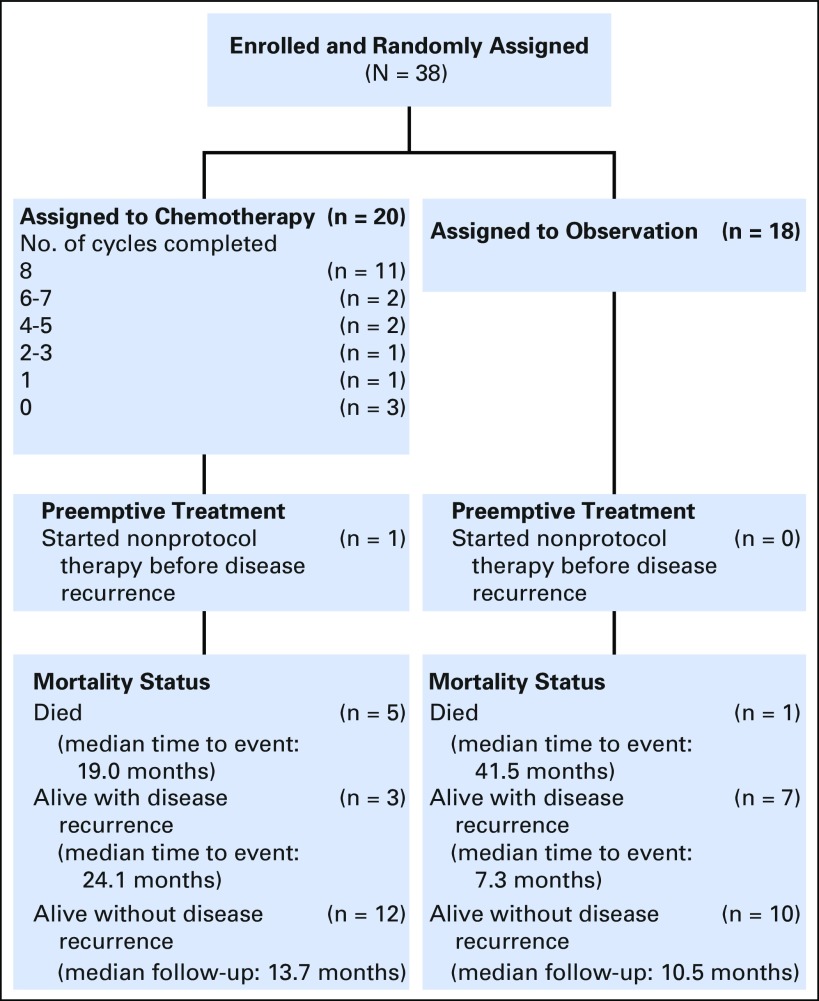
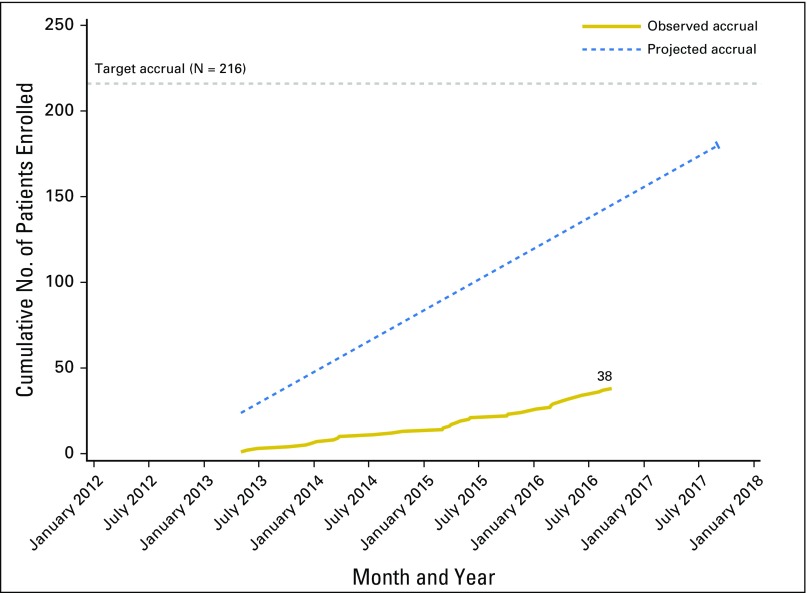
The study was opened at 701 international sites. The original accrual goal for the study was 216 patients, with an estimated accrual rate of three patients per month. The study was open to patient accrual on June 4, 2012, and closed to patient entry effective September 20, 2016, due to slow accrual. The observed versus projected accruals are shown in Appendix Figure A1 and the Data Supplement. At the time accrual was stopped, 38 patients were enrolled, approximately 17% of the target. Figure 2 is the CONSORT diagram showing patient assignment and treatment. Twenty patients were assigned to the adjuvant chemotherapy arm and 18 patients were assigned to observation. Three patients assigned to the chemotherapy arm never received chemotherapy treatment. Due to the low number of patients enrolled and, consequently, the low number of events, we report the study results using descriptive statistics rather than the originally proposed hypothesis tests for evaluating the study objectives.
Fig 2.
CONSORT diagram for GOG 0277, a randomized phase III trial.
Patient characteristics are detailed in Table 1. The study groups were similar in terms of patient age, race, ethnicity, and tumor stage. More than 75% of the patients accrued were in the United States. Among the 20 patients assigned to chemotherapy, 11 completed all eight planned cycles. Three patients were never treated, and six others stopped study treatment before completion of all eight cycles: One patient stopped because of disease progression during treatment, two refused further study treatment, two stopped because of treatment-related toxicity, and one stopped for other reasons.
Table 1.
Patient Characteristics (N = 38)

Adverse Events
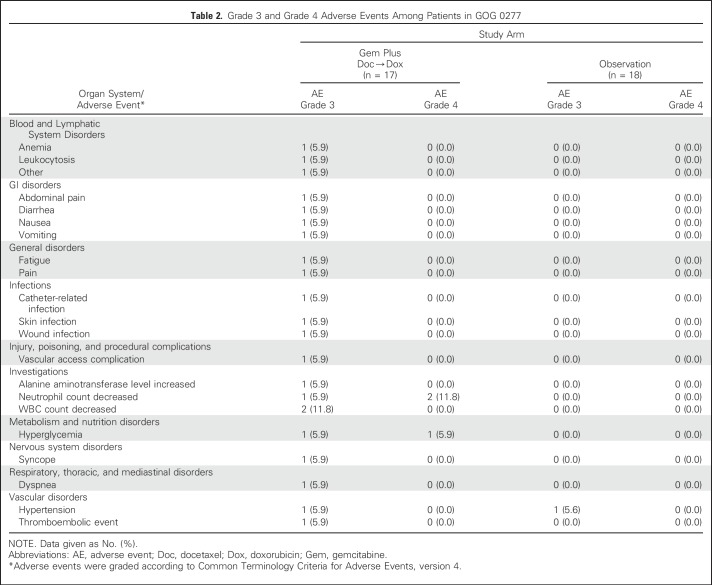
Thirty-five eligible patients (17 assigned to chemotherapy; 18 assigned to observation) received their assigned study treatment and were included in the assessment of adverse events. The three eligible patients who never initiated study treatment were excluded from the assessment of adverse events. There were no unexpected adverse events and no grade 5 toxicities. Among the patients assigned to chemotherapy, any type grade 3 toxicity was observed in eight patients (47%) and any grade 4 toxicity in three patients (17.6%). Details of the grade 3 and 4 events are shown in Table 2. Two of the grade 4 events were neutropenia and one was hyperglycemia. A single grade 3 toxicity (hypertension) was observed in one patient assigned to observation.
Table 2.
Grade 3 and Grade 4 Adverse Events Among Patients in GOG 0277
Survival Outcomes
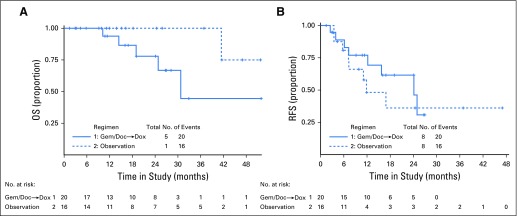
Thirty-eight patients were evaluable for OS (20 in the chemotherapy arm and 18 in the observation arm). There were six deaths over 51 months of follow-up, with five deaths in the chemotherapy arm and one death in the observation arm. All deaths were related to disease. Median follow-up of the whole cohort for OS was 19.1 months (95% CI, 13.8 to 26.9 months). Figure 3A displays OS curves for the chemotherapy arm versus observation. The median OS for patients in the chemotherapy arm was 30 months. There were too few events in the observation arm to estimate median OS in that arm. There were not enough data to assess whether the proportional hazards assumption held for OS. The ratio of the hazard of death relative to the observation arm was 6.63 (95% CI, 0.71 to 61.60); the 95% CI includes 1.0 but has an upper limit of 61.6, exemplifying the lack of precision in this estimate. Over 48 months, the RMST for OS in the chemotherapy arm was estimated to be 34.3 months (95% CI, 25.3 to 43.3 months), and the RMST for OS in the observation arm was estimated to be 46.4 months (95% CI, 43.6 to 49.1 months). The upper bound of the CI for the observation arm surpasses the cutoff of 48 months, because of the lack of data. The RMST for patients in the chemotherapy arm was 12.1 months fewer than the RMST for patients in the observation arm, (95% CI, −21.5 to −2.7 months).
Fig 3.
(A) Overall survival (OS) for adjuvant gemcitabine plus docetaxel followed by doxorubicin versus observation in GOG 0277. (B) Recurrence-free survival (RFS) for gemcitabine-docetaxel followed by doxorubicin versus observation in GOG 0277. Doc, docetaxel; Dox, doxorubicin; Gem, gemcitabine.
For the RFS end point, there were 16 recurrences or deaths over 47 months of follow-up, with eight events in each arm. Median follow-up of the whole cohort for RFS was 19.1 months (95% CI, 11.2 to 26.7 months). The sites of the 16 recurrences (local or pelvic v distant) are detailed in Table 3. There was no statistical difference in recurrence site distribution between the two study arms (P = .12).
Table 3.
Location of Recurrences by Study Treatment Arm (n = 16)
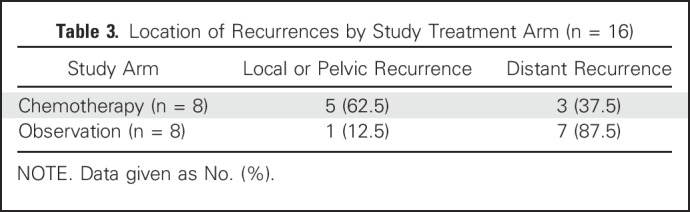
Figure 3B displays RFS survival curves for the chemotherapy treatment arm versus observation. In the chemotherapy arm, the median RFS estimate was 24 months. In the observation arm, the estimated median RFS was just less than 12 months. Graphical checks of the log (−log of survival) revealed that the PH assumption was potentially violated. The ratio of the hazard of recurrence relative to the observation arm was 0.71 (95% CI, 0.27 to 1.91). Over 24 months, the RMST for RFS in the chemotherapy arm was estimated to be 18.1 months (95% CI, 14.2 to 22.0 months), and the RMST for RFS in the observation arm was estimated to be 14.6 months (95% CI, 10.3 to 19.0 months). The difference in RMST comparing the chemotherapy arm with the observation arm was estimated as 3.4 months (95% CI, −2.4 to 9.3 months).
DISCUSSION
With international collaboration and consensus regarding the research question, we designed and opened a prospective randomized phase III trial to determine whether adjuvant chemotherapy using agents with established efficacy in metastatic disease could improve survival outcomes compared with observation in patients with completely resected, uterus-confined, high-grade LMS. Unfortunately, because of the accrual pace for this rare cancer, the study was closed before reaching its target accrual, in accordance with National Cancer Institute guidelines.
Although the small sample size and limited number of events preclude robust statistical comparisons, the descriptive data are of interest. Among the 17 patients treated with chemotherapy, there were no unexpected toxicities from these cytotoxic regimens; however, 11 patients (47%) experienced at least one grade 3 or 4 adverse event compared with one patient in the observation arm who experienced grade 3 hypertension. Overall survival appears to be worse by about 12 months among the patients assigned to chemotherapy compared with those assigned to observation. The 95% CI shows that OS could be worse with chemotherapy by as much as 21.5 months or as few as 2.7 months, and does not include the possibility that survival would be better with chemotherapy.
The RMST estimate for RFS was 18.1 months (95% CI, 14.2 to 22.0 months) in the chemotherapy arm and 14.6 months (95% CI, 10.3 to 19.0 months) in the observation arm, a difference of only 3.4 months (95% CI, −2.4 to 9.3 months). Although some may consider this outcome to favor chemotherapy, the small sample size and few events negatively affect the credibility of such a claim. In a study of adjuvant treatment of early-stage disease, which may be cured with surgery alone, a difference of 3.4 months in RFS is unlikely to be clinically meaningful. Furthermore, nearly half of patients treated with adjuvant chemotherapy would be expected to experience significant toxicity without a survival benefit.
Results of this study may be considered in the context of other randomized or single-arm prospective and case-control retrospective studies addressing adjuvant treatment of uterus-limited, high-grade LMS. The EORTC study of adjuvant pelvic radiation, which enrolled 92 patients with uterine carcinosarcoma, 99 with LMS, and 30 with endometrial stromal sarcoma, showed no benefit of radiation for OS or for RFS.2 A randomized trial of doxorubicin, ifosfamide, and cisplatin followed by pelvic radiation versus radiation alone for FIGO stage I, II, or III patients with uterine sarcomas of various histologies closed for failure to accrue. The 3-year disease-free survival was 55% in the chemotherapy plus radiation arm versus 41% with radiation alone (P = .048), but the OS was not statistically different (P = .41).11 The phase II trial of gemcitabine and docetaxel followed by doxorubicin had a 3-year PFS of 57%, which is similar to the 3-year PFS reported in the EORTC study of radiation versus observation.6 In a retrospective study that compared 33 patients with stage I uterine LMS who received adjuvant gemcitabine and docetaxel with 77 patients with stage I LMS who did not, there was no difference in OS or recurrence between the two cohorts.12 The results of these studies, and the data from this phase III trial support the recommendation for observation of women with uterus-limited, completely resected, high-grade LMS.13
Since the design of this study, there has been progress in the development of systemic chemotherapy for metastatic sarcomas. The addition of olaratumab to doxorubicin improved OS in patients with metastatic disease compared with single-agent doxorubicin in a randomized phase II trial.14 Trabectedin was compared with dacarbazine in patients with LMS or liposarcoma who had failed anthracycline treatment and was superior in terms of PFS, leading to its approval in these two sarcoma types.15 Neither of these regimens was associated with objective response rates as high as those achieved with gemcitabine plus docetaxel (doxorubicin plus olaratumab: 18%; trabectedin: 11%; gemcitabine plus docetaxel: 32%).16 It would require a prospective randomized trial to determine if moving any of these newer regimens to the adjuvant setting would improve survival outcomes. For now, the role of each of these regimens remains for treatment of metastatic disease.
The experience with the design, conduct, and accrual of this study merits discussion. Before protocol development, the research question and study design were discussed and refined with international collaboration, with support from the International Rare Cancer Initiative Gynecologic Sarcomas Group. Multiple research oversight committees insisted that the control arm be observation (rather than aromatase inhibition, or a shorter course of chemotherapy, for example). The fact that the study was opened at 701 sites attests to the importance of the research question for this population and the dedication of physicians who care for them. Unfortunately, despite the rarity of this diagnosis and the complexity of the international collaboration (the majority of the non-US sites opened more than a year after the study was activated by CTEP), the trial was held by the NCI CTEP to the same bar for accrual as phase III trials for common cancers comparing two active treatment regimens. It is hoped that future phase III trials testing critical questions for rare cancers may be permitted greater flexibility in accrual pace goals. In addition, patients and practitioners may have harbored biases for or against adjuvant chemotherapy, which may have hindered referrals for enrollment or made some patients reluctant to be randomly assigned.
In conclusion, despite premature closure, the results of the prospective study do not support the use of adjuvant gemcitabine plus docetaxel followed by doxorubicin for uterus-limited, high-grade LMS. Observation remains the standard of care in this setting.
ACKNOWLEDGMENT
The following NRG Oncology/Gynecologic Oncology Group member institutions participated in this study: European Organization for Research and Treatment of Cancer; University of Oklahoma Health Sciences Center; Roswell Park; University of Texas Southwestern Medical Center; University of California Medical Center at Irvine-Orange Campus; Washington University School of Medicine; Memorial Sloan Kettering Cancer Center; Ohio State University Comprehensive Cancer Center; Women and Infants Hospital; Duke University Medical Center; Abington Memorial Hospital; Rush University Medical Center; Fox Chase Cancer Center; Women’s Cancer Center of Nevada; University of Chicago; Froedtert and the Medical College of Wisconsin; University of Michigan Health System Cancer Center; Emory University School of Medicine; Maine Medical Center – Scarborough Campus; Kansas City Clinical Oncology Program; University of New Mexico; and John H. Stroger Jr. Hospital of Cook County.
Appendix
Fig A1.
Projected and actual accrual to GOG 0277.
Footnotes
Supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (Grant No. CA 27469), the Gynecologic Oncology Group Statistical and Data Center (Grant No. CA 37517), NRG Oncology (Grant No. 1 U10 CA180822), and NRG Operations (Grant No. U10CA180868); the Memorial Sloan Kettering Cancer Center Support (Grant No. P30 CA008748 to M.L.H.); and NETSARC, le Réseau de Référence en Pathologie des Sarcomes (RREPS), and Network for Bone Sarcoma and Rare Bone Tumors (ResOS) grants, Lyon Recherce Innovation Contre le Cancer (Grant No. INCa_INSERM_DGOS_12563), and European Rare Solid Cancer Reference Network (Grant No. SGA2 811490) to J.-Y.B.
Presented, in part, at the Annual Meeting, American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018.
AUTHOR CONTRIBUTIONS
Conception and design: Martee L. Hensley, Helen Hatcher, Anders Krarup-Hansen, Jean-Yves Blay, David S. Miller
Provision of study material or patients: Martee L. Hensley, Petronella B. Ottevanger, Katherine M. Moxley, Premal H. Thaker, David S. Millers
Collection and assembly of data: Martee L. Hensley, Petronella B. Ottevanger, Jean-Yves Blay, Cyril Fisher, Katherine M. Moxley, Jayanthi S. Lea, Premal H. Thaker, Katina Robison, David S. Miller
Data analysis and interpretation: Martee L. Hensley, Danielle Enserro, Petronella B. Ottevanger, Anders Krarup-Hansen, Jean-Yves Blay, Cyril Fisher, Shashikant B. Lele, Krishnansu S. Tewari, Premal H. Thaker, Oliver Zivanovic, David M. O’Malley, David S. Miller
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adjuvant Gemcitabine Plus Docetaxel Followed by Doxorubicin Versus Observation for High-Grade Uterine Leiomyosarcoma: A Phase III NRG Oncology/Gynecologic Oncology Group Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Martee L. Hensley
Employment: Sanofi (I)
Stock and Other Ownership Interests: Sanofi (I)
Consulting or Advisory Role: EMD Serono; Janssen; Tesaro; Eli Lilly; Onclive
Research Funding: Johnson & Johnson (Inst); Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: author, Up-to-Date
Danielle Enserro
No relationship to disclose
Helen Hatcher
Travel, Accommodations, Expenses: Pharmaar
Petronella B. Ottevanger
Research Funding: PharmaMar (Inst); Novartis (Inst)
Travel, Accommodations, Expenses: Roche; MSD
Anders Krarup-Hansen
No relationship to disclose
Jean-Yves Blay
Honoraria: Roche; Novartis; Bayer; GlaxoSmithKline; PharmaMar; Eli Lilly
Consulting or Advisory Role: Roche; Novartis; GlaxoSmithKline; Bayer; PharmaMar; Merck
Research Funding: GlaxoSmithKline (Inst); PharmaMar (Inst); Novartis (Inst); Bayer (Inst); Roche (Inst)
Other Relationship: Innate Pharma
Cyril Fisher
No relationship to disclose
Katherine M. Moxley
No relationship to disclose
Shashikant B. Lele
No relationship to disclose
Jayanthi S. Lea
Consulting or Advisory Role: Clovis Oncology
Krishnansu S. Tewari
Honoraria: Tesaro; Clovis Oncology
Consulting or Advisory Role: Roche
Speakers’ Bureau: Roche; AstraZeneca; Merck
Research Funding: AbbVie (Inst); Roche (Inst); Morphotek (Inst); Merck (Inst); Regeneron (Inst)
Travel, Accommodations, Expenses: Roche
Premal H. Thaker
Stock and Other Ownership Interests: Celsion; Stryker
Consulting or Advisory Role: Iovance Biotherapeutics; AbbVie/Stemcentrx; Clovis Oncology; Unleash Immunolytics; Celsion
Speakers’ Bureau: Merck; Tesaro
Research Funding: Merck (Inst)
Oliver Zivanovic
No relationship to disclose
David M. O’Malley
Honoraria: Clovis Oncology; Janssen Oncology; Ambry Genetics; AbbVie; AstraZeneca; GOG Foundation; Tesaro; Myriad Genetics; Amgen; Roche; Immunogen; OncoQuest; Novocure
Consulting or Advisory Role: Janssen Oncology; AstraZeneca; Clovis Oncology; Amgen; Tesaro; Novocure; Myriad Genetics; AbbVie; Roche; OncoQuest; Immunogen; GOG Foundation
Research Funding: Amgen (Inst), VentiRx (Inst); AstraZeneca (Inst); Roche (Inst); Regeneron (Inst); Immunogen (Inst); Janssen Research & Development (Inst); Clovis Oncology (Inst); EMD Serono (Inst); Ergomed (Inst); Ajinomoto (Inst); Immunogen (Inst); Cerulean Pharma (Inst); PharmaMar (Inst); Array BioPharma (Inst); Bristol-Myers Squibb (Inst); Agenus (Inst); Tesaro (Inst); TRACON Pharma (Inst); Stem CentRx (Inst)
Katina Robison
No relationship to disclose
David S. Miller
Consulting or Advisory Role: Genentech; Tesaro; Eisai; AstraZeneca; Guardant Health; Janssen Oncology; Alexion Pharmaceuticals
Speakers’ Bureau: Clovis Oncology; Genentech
Research Funding: US Biotest (Inst); Advenchen Laboratories (Inst); Millennium (Inst); Tesaro (Inst) Xenetic Biosciences (Inst)
REFERENCES
- 1.Gadducci A, Landoni F, Sartori E, et al. : Uterine leiomyosarcoma: Analysis of treatment failures and survival. Gynecol Oncol 62:25-32, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Reed NS, Mangioni C, Malmström H, et al. : Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer 44:808-818, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Hensley ML, Blessing JA, Degeest K, et al. : Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecol Oncol 109:323-328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hensley ML, Blessing JA, Mannel R, et al. : Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol Oncol 109:329-334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyman DM, Grisham RN, Hensley ML: Management of advanced uterine leiomyosarcoma. Curr Opin Oncol 26:422-427, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Hensley ML, Wathen JK, Maki RG, et al. : Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma: Results of a phase 2 trial (SARC 005). Cancer 119:1555-1561, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Cox DR: Regression models and life tables (with discussion). J R Stat Soc B 34:187-219, 1972 [Google Scholar]
- 8.Royston P, Parmar MK: The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med 30:2409-2421, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Uno H, Wittes J, Fu H, et al. : Alternatives to hazard ratios for comparing efficacy or safety of therapies in noninferiority studies. Ann Intern Med 163:127-134, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.R Core Team : R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org.
- 11.Pautier P, Floquet A, Gladieff L, et al. : A randomized clinical trial of adjuvant chemotherapy with doxorubicin, ifosfamide, and cisplatin followed by radiotherapy versus radiotherapy alone in patients with localized uterine sarcomas (SARCGYN study). A study of the French Sarcoma Group. Ann Oncol 24:1099-1104, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Littell RD, Tucker LY, Raine-Bennett T, et al. : Adjuvant gemcitabine-docetaxel chemotherapy for stage I uterine leiomyosarcoma: Trends and survival outcomes. Gynecol Oncol 147:11-17, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Hensley ML: Difficult choices in stage I uterine leiomyosarcoma – It’s okay to “stand there.” Gynecol Oncol 147:1-2, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Tap WD, Jones RL, Van Tine BA, et al. : Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: An open-label phase 1b and randomised phase 2 trial. Lancet 388:488-497, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensley ML, Patel SR, von Mehren M, et al. : Efficacy and safety of trabectedin or dacarbazine in patients with advanced uterine leiomyosarcoma after failure of anthracycline-based chemotherapy: Subgroup analysis of a phase 3, randomized clinical trial. Gynecol Oncol 146:531-537, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensley ML, Miller A, O’Malley DM, et al. : Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol 33:1180-1185, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]