Abstract
Purpose
In a recent randomized, open-label trial (ECHELON-1), brentuximab vedotin (BV) combined with doxorubicin, vinblastine, and dacarbazine (AVD+BV) decreased the risk of progression in adults diagnosed with stage III or IV Hodgkin lymphoma (HL) compared with standard bleomycin-containing chemotherapy (doxorubicin, bleomycin, vinblastine, and dacarbazine [ABVD]). However, the cost effectiveness of incorporating BV (US$6,970 per 50-mg vial) into the first-line setting is unknown.
Patients and Methods
We constructed a Markov decision-analytic model to measure the costs and clinical outcomes for AVD+BV compared with ABVD as first-line therapy in a cohort of patients with stage III or IV HL. Transition probabilities were estimated from ECHELON-1 by fitting parametric survival distributions. Lifetime direct health care costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs) were calculated for AVD+BV compared with ABVD from a US payer perspective. Our model was also used to estimate BV price reductions that would achieve more favorable cost effectiveness under indication-specific pricing.
Results
AVD+BV was associated with an improvement of 0.56 QALYs compared with treatment with standard ABVD. However, incorporating BV into first-line therapy led to significantly higher lifetime health care costs ($361,137 v $184,291), causing the ICER for AVD+BV to be $317,254 per QALY. If indication-specific pricing were implemented, acquisition costs for BV used in the first-line setting would need to be reduced by 56% to 73% for ICERs of $150,000 to $100,000 per QALY, respectively.
Conclusion
Substituting BV for bleomycin during first-line therapy for stage III or IV HL is unlikely to be cost effective under current drug pricing. Should indication-specific pricing be implemented, significant price reductions for BV used in the first-line setting would be needed to reduce ICERs to more widely acceptable values.
INTRODUCTION
The chemotherapy regimen combining doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) is the most common first-line treatment of Hodgkin lymphoma (HL).1,2 The dominant role of ABVD in treatment of HL is supported by trials demonstrating manageable toxicities, clear efficacy, and reduced risk of secondary malignancies and infertility when compared with alternative regimens.3-5 However, bleomycin-associated pulmonary toxicity and treatment-related deaths remain a significant concern with ABVD. In fact, recent large-scale clinical efforts have focused on reducing or eliminating the use of bleomycin altogether from first-line treatment.6-8
Brentuximab vedotin (BV), an antibody-drug conjugate targeting CD30, is highly efficacious as a single agent for relapsed and refractory HL.9 Recent trials have incorporated BV into earlier lines of therapy,10,11 including ECHELON-1,8 a large randomized open-label study comparing BV combined with doxorubicin, vinblastine, and dacarbazine (AVD+BV) to standard ABVD for patients with newly diagnosed stage III or IV HL. After a median follow-up time of 24.6 months, there was no difference in overall survival between the two arms.8 However, AVD+BV was associated with a 23% reduction in the risk of progression, death, or incomplete response to first-line therapy leading to subsequent anticancer treatment (modified progression-free survival [PFS]).8 At 2 years, this translated into an absolute reduction in disease progression of 4.9%. Acute toxicities were also significantly different between treatment arms; febrile neutropenia and overall grade 3 or greater toxicities were more common with AVD+BV, but severe pulmonary toxicities more likely with ABVD (7% v 3% for AVD+BV).8
Along with clear differences in acute toxicities, combining BV with first-line chemotherapy adds significant drug-related expenses. Dosed at 1.2 mg/kg and available only in 50-mg single-use vials (US$6,970 per 50-mg vial),12 BV is associated with greater drug-related expenses compared with ABVD. However, it is unknown whether the fewer treatment failures associated with AVD+BV could reduce downstream health care expenditures and improve quality of life compared with ABVD. In this study, we use a Markov decision-analytic model to assess the cost effectiveness of AVD+BV compared with ABVD in patients with newly diagnosed stage III or IV HL.
PATIENTS AND METHODS
Patients and Intervention
Our baseline sample was constructed to mirror the ECHELON-1 trial.8 The age of our patient cohort was 36 years, and all individuals had stage III or IV disease. Individuals entered our model with newly diagnosed HL and received either standard ABVD or AVD+BV every 2 weeks for maximum of 12 doses. Primary prophylaxis with myeloid growth factor support was administered to patients receiving AVD+BV per recommendations from the ECHELON-1 investigators.8 Patients receiving ABVD did not receive growth factor support given its established low risk of febrile neutropenia and potential risk of increased pulmonary toxicity when administrated with bleomycin.13-15 Because there are no published data regarding the risk of febrile neutropenia and hospitalizations in the setting of AVD+BV with universal primary prophylaxis, we were conservative and assumed identical rates compared with standard ABVD.
Model Construction
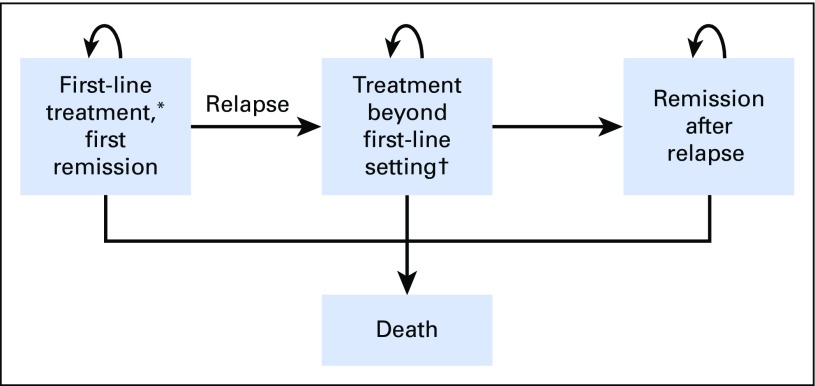
We created a Markov model to compare health care costs and clinical outcomes associated with AVD+BV versus ABVD when treating patients with stage III or IV HL. As displayed in Figure 1, both active therapy and remission transition states were used to capture first-line therapy through death (Data Supplement). Transition-state cycles were 3 months in duration, and a lifetime horizon was used to calculate direct health care costs and utilities. Our cost-effectiveness analysis was conducted from a US payer perspective, using a standard rate of 3% annually to discount future costs and benefits.16 The primary outputs of the model were used to calculate the incremental cost for AVD+BV compared with ABVD in 2017 US dollars for an additional quality-adjusted life-year (QALY) gained (incremental cost-effectiveness ratio [ICER]). We assumed a willingness-to-pay threshold of $150,000 per QALY gained.17 The model was constructed using TreeAge Pro (TreeAge Software, Williamstown, MA), and additional statistical analyses were performed using R (www.R-project.org).
Fig 1.
Markov model. (*) Includes patients treated with radiation alone for a modified progression event. (†) Health states representing treatment beyond first-line setting, including salvage chemotherapy, autologous stem-cell transplantation, post-transplantation consolidation with brentuximab vedotin, salvage with brentuximab vedotin, and therapy beyond brentuximab vedotin.
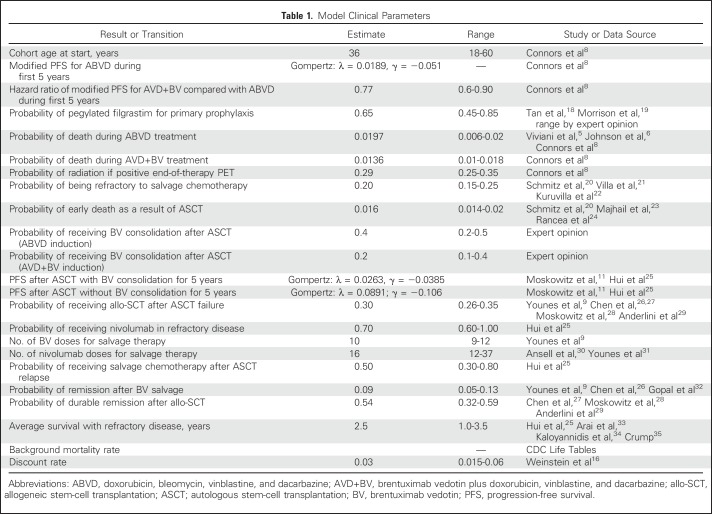
Transition Probabilities
Base-case estimates and ranges for clinical probabilities are listed in Table 1. We estimated rates of HL progression after first-line treatment out to 5 years using standard extrapolation methods.36-38 Because ECHELON-1 used a unique modified PFS, we reconstructed individual patient-level data from the independent review committee modified PFS curve and at-risk tables, published at a median observation time of 24.6 months.8 Individual patient-level data for the ABVD arm were best fit with a Gompertz distribution. Our predicted 5-years modified PFS from the ABVD arm of ECHELON-1 was 70.3%, comparable to 5-year PFS from contemporary clinical trials using ABVD with longer follow-up.39-41 Using the hazard ratio reported in ECHELON-1 (hazard ratio, 0.77), we then derived transition probabilities for patients receiving AVD+BV, with the 5-year modified PFS for the AVD+BV arm in ECHELON-1 predicted to be 76.2%. Mirroring the modified PFS reported in ECHELON-1, most patients with incomplete response to first-line therapy (ie, positive end-of-therapy positron emission tomography [PET]) received salvage chemotherapy, with the remaining patients successfully treated with radiation alone. The use of PET was similar between our treatment arms; response on interim PET was not used to alter first-line therapy.8 Patients in first remission beyond 5 years experienced age-adjusted mortality from other causes on the basis of US Life Tables available from the Centers for Disease Control and Prevention.
Table 1.
Model Clinical Parameters
In addition to ECHELON-1 data, we incorporated recently published studies to derive transition probabilities in individuals who experienced relapse after first-line therapy. This included the randomized trial establishing BV as consolidation therapy after autologous stem-cell transplantation (ASCT),11 long-term follow-up data for BV monotherapy for relapsed or refractory disease,32 and separate reports supporting the use of agents that inhibit programed cell death protein 1.30,31,42 Overall, health states after ASCT were similar to our prior model used to assess the cost effectiveness of BV in the post-transplantation setting.25
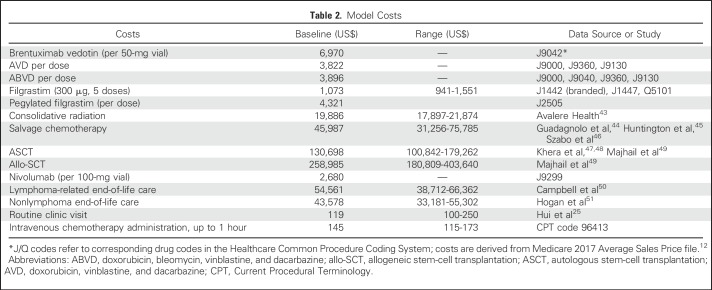
Costs
Baseline direct medical costs were derived from the 2017 Medicare fee schedule and relevant peer-reviewed medical literature (Table 2). All costs from literature were converted to 2017 US dollars using the Medical Care component of the Consumer Price Index. Similar to prior work,52,53 drug acquisition costs were derived from Centers for Medicare and Medicaid Services average sales price, taking into account rebates and discounts privately negotiated between manufacturers and payers.54 Our drug cost calculations assumed patients weighed 70 kg but accounted for drug wastage by rounding up to the next full single-use vial size available for each dose administered.55,56 Because BV is only available in 50-mg single-use vials, BV costs in the first-line setting were based on two single-use vials per dose (0.9 to 1.2 mg/kg). Further, patients experiencing progression in the first two cycles of our model (ie, within 6 months) experienced early discontinuation of their induction therapy and corresponding growth factor (AVD+BV arm). Costs for salvage cytotoxic chemotherapy and stem-cell transplantation were based on values used in previous studies and reflect paid amounts of adjudicated claims for inpatient and outpatient services.44,47-49 The cost of routine monitoring included office visits and routine laboratory tests. End-of-life health care costs were estimated from published data on the cost of care in the last year of life for patients with cancer compared with the general Medicare population.50,51
Table 2.
Model Costs
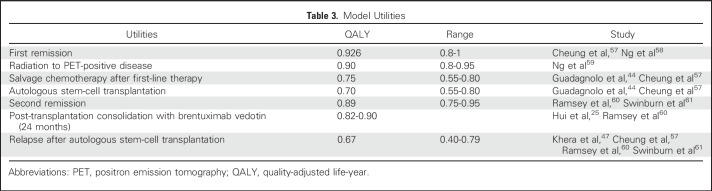
Utilities
Baseline clinical utilities for various health states were based on published literature (Table 3). Because quality-of-life data comparing AVD+BV to ABVD are not currently available, we were conservative and assumed similar baseline utilities regardless of first-line regimen. However, our model did incorporate treatment-related mortality reported by ECHELON-1 (1.36% for AVD+BV and 1.97% for ABVD). Using methods previously described,25 we also used quality-of-life data published from the randomized study comparing BV to placebo for consolidation after ASCT to inform our clinical utilities in the post-transplantation setting.60
Table 3.
Model Utilities
Sensitivity Analysis
We performed a series of sensitivity analyses to evaluate the robustness of our conclusions. We varied the value of model parameters one at a time during one-way sensitivity analysis to examine the individual effects on the ICER. We also performed scenario analyses to investigate the impact of primary prophylaxis with myeloid growth factor on the cost effectiveness of AVD+BV. During probabilistic sensitivity analysis (PSA), we performed 10,000 Monte Carlo simulations, each time randomly sampling from the distributions of model inputs. Clinical probabilities and health utilities were represented by β distributions, whereas costs were represented by γ distributions.
Finally, we performed a sensitivity analysis accommodating indication-specific pricing, where the price of BV used in combination with chemotherapy in the first-line setting varied from the cost of BV monotherapy used at relapse. Although the current price of BV may not be cost effective in some relapsed settings,25 we assumed BV monotherapy would remain at its current price when calculating reductions in drug acquisition costs required for BV to become cost effective in the first-line setting.
RESULTS
Baseline Analysis
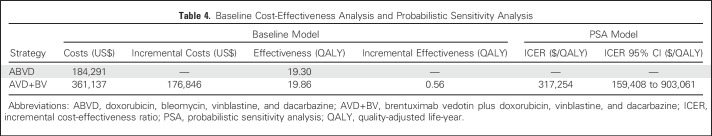
Results of the baseline cost-effectiveness analysis are listed in Table 4. AVD+BV led to significantly higher health care costs compared with standard treatment with ABVD ($361,137 v $184,291, respectively), with an incremental cost of $176,846. After applying quality-of-life adjustment and future discounting, AVD+BV was associated with an improvement of 0.56 QALYs compared with standard treatment with ABVD (19.86 vs. 19.3 QALYs, respectively). Therefore, the ICER for AVD+BV versus ABVD was estimated at $317,254 per QALY.
Table 4.
Baseline Cost-Effectiveness Analysis and Probabilistic Sensitivity Analysis
Sensitivity Analyses
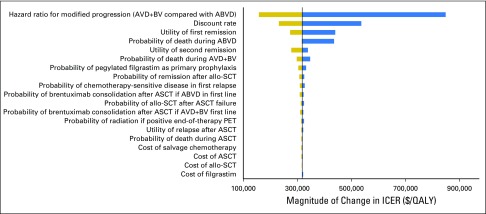
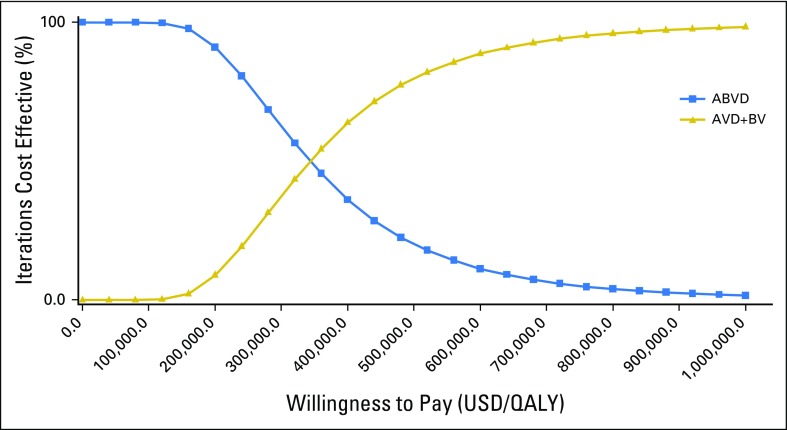
The results of one-way sensitivity analyses are presented in Figure 2. The model was most sensitive to the modified PFS hazard ratio for AVD+BV compared with ABVD, with ICERs ranging from $156,505 per QALY to $848,576 per QALY when varying the hazard ratio between 0.6 and 0.90. Additional parameters with significant contribution to model conclusions were the discount rate, utility of remission states, and the probability of using pegylated filgrastim rather than filgrastim for primary prophylaxis during AVD+BV. For example, varying the probability of receiving the more expensive pegylated filgrastim between 0.45 and 0.85 caused the ICER of AVD+BV to increase from $302,843 per QALY to $331,665 per QALY. If growth factor support during AVD+BV was exclusively limited to the least costly filgrastim, the ICER for AVD+BV compared with ABVD was reduced to $270,419 per QALY. Furthermore, if no growth factor support was required for AVD+BV, the ICER became $249,640 per QALY. All ICERs in our one-way sensitivity analyses remained greater than $150,000 per QALY across broad ranges for each model parameter. Furthermore, only nine of 10,000 iterations during our Monte Carlo simulation produced ICERs less than $100,000. In total, 95% of iterations during our PSA produced ICERs for AVD+BV compared with ABVD between $159,408 per QALY and $903,061 per QALY (Table 4). Cost-effectiveness acceptability curves are presented in Figure 3; the distribution for our PFS hazard ratio used during PSA is provided in the Data Supplement.
Fig 2.
One-way sensitivity analysis. (*) Model parameters not presented produced less than a $5,000 per quality-adjusted life-year (QALY) change when evaluated over their entire range. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; allo-SCT, allogeneic stem-cell transplantation; ASCT, autologous stem-cell transplantation; AVD+BV, brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine; ICER, incremental cost-effectiveness ratio; PET, positron emission tomography.
Fig 3.
Cost-effectiveness acceptability curves. Results of the probabilistic sensitivity analysis based on 10,000 iterations of the Markov model. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; AVD+BV, brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine; QALY, quality-adjusted life-year; USD, US dollars.
We also considered a scenario where indication-specific pricing was implemented. Here, BV used as monotherapy in the salvage and post-ASCT settings remained at the current price of $6,970 per 50-mg vial, but BV combined with chemotherapy in the first-line setting was discounted. Our model estimates that reductions in the acquisition costs for BV used in the first-line setting of 56% and 73% would translate into ICERs of $150,000 per QALY and $100,000 per QALY, respectively (Data Supplement).
Modeled Clinical Outcomes
In addition to calculating the incremental costs and utilities associated with AVD+BV compared with ABVD, we used our model to estimate long-term clinical outcomes. Although many patients experiencing progression after first-line therapy are successfully treated with salvage therapy in our model, some succumb to treatment- and lymphoma-related deaths, with nonfuture discounted survival favoring AVD+BV by an average of 1.34 years (39.04 years for AVD+BV v 37.7 years for ABVD). Model-derived 10-year outcomes are provided in the Data Supplement. As expected, the AVD+BV cohort required less use of salvage chemotherapy and ASCT compared with patients treated with ABVD. Here, use of AVD+BV led to a nearly 4% absolute reduction in salvage chemotherapy (22.8% for AVD+BV v 26.6% for ABVD) and a 3% absolute reduction in need for ASCT (18.3% for AVD+BV v 21.3% for ABVD).
DISCUSSION
ECHELON-1, a large randomized open-label trial, was recently reported comparing standard ABVD to AVD+BV in the first-line setting for stage III or IV HL.8 At a median follow-up of 24.6 months, investigators found fewer first-line treatment failures in the AVD+BV arm.8 However, the reduction in treatment failures comes at a cost, with AVD+BV carrying greater grade ≥ 3 acute toxicities and considerable drug-related expenses when compared with standard ABVD. Our model did not find that higher drug-related expenses of AVD+BV were offset substantially by lower relapse costs or improved quality of life, with AVD+BV compared with ABVD producing an ICER of $317,254 per QALY.
Although decision-analytic models are subject to inherent limitations related to the data available to populate the model, our study has important strengths. First, our model was based on results from a large randomized trial directly comparing AVD+BV to ABVD.8 Second, our model incorporates contemporary data to reflect recent advances in the treatment and outcomes of individuals with HL, including the use of BV for consolidation after transplantation and use of novel immunotherapies in relapsed disease.11,30-32,42
Our analysis also accounts for drug wastage by calculating drug costs on the basis of the number of single-use vials used rather than actual dose administered. Prior cost-effectiveness analyses for hematologic malignancies infrequently accounted for drug wastage in cost calculations,56 yet the economic impact can be substantial.62 In fact, not accounting for drug wastage of BV and nivolumab in our model for an average patient weighing 70 kg reduces the ICER from our base case of $317,254 per QALY down to $228,743 per QALY. Although scheduling patients on the same day to share drug vials has the potential to minimize wastage of high-cost therapies, opportunities are likely to be limited in the setting of advanced HL given its relatively low incidence. Further, safety concerns remain for vial sharing, with the Centers for Disease Control and Prevention stating that single-use vials should only be used for a single patient.63 Finally, we were conservative when populating our Markov model, selecting values for our base case parameters that favored BV when more than one reasonable value was available. For example, although ECHELON-1 reported a greater incidence of febrile neutropenia in the AVD+BV arm (19% v 8% for ABVD),8 we chose to accept that primary prophylaxis with myeloid growth factor support would reduce the incidence of febrile neutropenia in patients receiving AVD+BV to that of standard ABVD. However, it is important to note that even if primary prophylaxis for AVD+BV reduced the incidence of febrile neutropenia below that of standard ABVD, our model conclusions would not significantly change.
Although our study has multiple strengths, there are several limitations to consider. First, approaches for treating HL are evolving and now include PET-adapted therapy.6,64 Similar to ECHELON-1, our model does not include changes to first-line therapy on the basis of interim PET response. The recently reported RATHL (Response-Adjusted Therapy for Advanced Hodgkin Lymphoma) trial randomly assigned patients with negative interim PET after two cycles of ABVD to the standard six cycles of ABVD or to doxorubicin, vinblastine, and dacarbazine (AVD) for cycles 3 to 6.6 Although this noninferiority trial crossed its prespecified margin, outcomes remained excellent in the AVD arm; consequently, many clinicians have adopted this approach given the reduction in serious pulmonary toxicity.65 If the efficacy of the two arms in the RATHL trial is assumed to be equivalent, the improved adverse effect profile from the study arm (ABVD for two cycles followed by AVD for four cycles if interim PET is negative) would likely further reduce the cost effectiveness of AVD+BV compared with the non–PET-adapted ABVD used in our ECHELON-1–derived model.
Although data from randomized clinical trials informed much of our cost-effectiveness model, uncertainly exists concerning post-ASCT relapse as a result of recent clinical advancements with relatively short-term follow-up.30,31,42 Similar to our prior cost-effectiveness analysis of BV in post-ASCT consolidation,25 our current model incorporates salvage chemotherapy, BV monotherapy, programed cell death protein 1 blockade, and allogeneic stem-cell transplantation in the relapsed or refractory setting. Although we used multiple sources for our health care costs and included broad ranges during sensitivity analysis, future analyses may benefit from using real-world cost and clinical effectiveness data.66 However, our model is most influenced by parameters informing health transition states before late refractory disease, and model conclusions are robust despite current uncertainties concerning the durability of modern treatment advances for relapsed or refractory HL.
Finally, our cost-effectiveness analysis considers only direct health care expenditures. Future economic benefits resulting from improved survival in this relatively younger population of patients could be considerable. However, long-term survivors of HL also have greater incidence of serious comorbidities, including cardiac and secondary malignancies, and our use of age-matched US Life Tables to model long-term clinical outcomes in our HL cohort does not consider late inferior clinical outcomes reported in survivors of HL.67 Our model does assign lower quality of life during subsequent lines of therapy and second remission compared with durable remissions after first-line treatment. However, the ICER of AVD+BV compared with ABVD is likely to be lower than our base case prediction for a subset of patients in whom AVD+BV may offer greater safety (ie, baseline pulmonary dysfunction) or when toxicities related to subsequent lines of therapy (ie, infertility) are particularly important to a given patient.
In addition to assessing the cost effectiveness of AVD+BV compared with ABVD under current drug pricing in the United States, we used our model to estimate drug acquisition costs if indication-specific pricing were implemented. Here, BV used with chemotherapy in the first-line setting would be priced lower than BV used in the relapsed or refractory setting as a result of lower marginal clinical utility.68 Our model predicts that considerable price reduction (56% to 73%) to BV used in the first-line setting would be required to produce more widely acceptable ICERs ($100,000 to $150,000 per QALY). Although indication-specific pricing can lead to profit maximization and reduce consumer surplus,69 we and others would argue that profit maximization currently exists and present-day drug prices have little association with underlying clinical utility.70 Although administrative challenges would need to be overcome, indication-specific and value-based pricing in the United States offers the potential to better align drug prices to their utility and incentivize the development of highly effective therapies.71
Although BV is an active treatment for HL, incorporating this agent into first-line therapy for advanced HL is not a cost-effective strategy. Our study suggests that reductions in downstream health care costs and improvements in outcomes associated with AVD+BV do not offset up-front drug costs associated with BV and the need for myeloid growth factor. Some have hailed AVD+BV as the new standard for advanced-stage HL,8 whereas others are waiting for long-term data before recommending AVD+BV over ABVD.72 In the past, studies showing improvement in PFS alone did not dissuade clinicians from using ABVD, with most oncologists waiting for long-term toxicity and survival data of dose-escalated therapy.5 Only time will tell whether higher costs related to AVD+BV will be a similar deterrent for abandoning ABVD before long-term data are available.
Footnotes
Supported by the Lowe Endowment Fund, Grant No. T35HL007649 from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH), and Clinical and Translational Science Awards Grant No. KL2 TR001862 from the National Center for Advancing Translational Science, a component of the NIH.
Presented in part at the 54th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018.
The contents of this article are solely the responsibility of the authors and do not represent the official view of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Scott F. Huntington, Sapna A. Prasad
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cost-Effectiveness Analysis of Brentuximab Vedotin With Chemotherapy in Newly Diagnosed Stage III and IV Hodgkin Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Scott F. Huntington
Honoraria: Pharmacyclics
Consulting or Advisory Role: Celgene, Janssen, Bayer
Travel, Accommodations, Expenses: Celgene, Bayer
Gottfried von Keudell
Honoraria: Pharmacyclics
Consulting or Advisory Role: Pharmacyclics, Genentech, Bayer
Amy J. Davidoff
Honoraria: Celgene (I), Kyowa Hakko Kirin (I), Jazz Pharmaceuticals (I), Tolero Pharmaceuticals (I)
Consulting or Advisory Role: Celgene (I)
Research Funding: Boehringer Ingelheim (Inst), Celgene (Inst)
Other Relationship: PhRMA Foundation
Cary P. Gross
Research Funding: 21st Century Oncology, Johnson & Johnson, Pfizer
Travel, Accommodations, Expenses: Flatiron Health
Sapna A. Prasad
Honoraria: American Society of Health-System Pharmacists
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: Sandoz-Novartis (Inst)
REFERENCES
- 1.Hoppe RT, Advani RH, Ai WZ, et al. : Hodgkin Lymphoma Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15:608-638, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Eichenauer DA, Engert A, André M, et al. : Hodgkin’s lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:iii70-iii75, 2014. (suppl 3) [DOI] [PubMed] [Google Scholar]
- 3.Santoro A, Bonadonna G, Valagussa P, et al. : Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin’s disease: Superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol 5:27-37, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Canellos GP, Anderson JR, Propert KJ, et al. : Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med 327:1478-1484, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Viviani S, Zinzani PL, Rambaldi A, et al. : ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med 365:203-212, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Johnson P, Federico M, Kirkwood A, et al. : Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med 374:2419-2429, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behringer K, Goergen H, Hitz F, et al. : Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): An open-label, randomised, non-inferiority trial. Lancet 385:1418-1427, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Connors JM, Jurczak W, Straus DJ, et al. : Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med 378:331-344, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younes A, Gopal AK, Smith SE, et al. : Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 30:2183-2189, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes A, Connors JM, Park SI, et al. : Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: A phase 1, open-label, dose-escalation study. Lancet Oncol 14:1348-1356, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz CH, Nademanee A, Masszi T, et al. : Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 385:1853-1862, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services : Medicare Average Sales Price (ASP) file. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2017ASPFiles.html
- 13.Boleti E, Mead GM: ABVD for Hodgkin’s lymphoma: Full-dose chemotherapy without dose reductions or growth factors. Ann Oncol 18:376-380, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Evens AM, Cilley J, Ortiz T, et al. : G-CSF is not necessary to maintain over 99% dose-intensity with ABVD in the treatment of Hodgkin lymphoma: Low toxicity and excellent outcomes in a 10-year analysis. Br J Haematol 137:545-552, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Martin WG, Ristow KM, Habermann TM, et al. : Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol 23:7614-7620, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Weinstein MC, Siegel JE, Gold MR, et al. : Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 276:1253-1258, 1996 [PubMed] [Google Scholar]
- 17.Neumann PJ, Cohen JT, Weinstein MC: Updating cost-effectiveness: The curious resilience of the $50,000-per-QALY threshold. N Engl J Med 371:796-797, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Tan H, Tomic K, Hurley D, et al. : Comparative effectiveness of colony-stimulating factors for febrile neutropenia: A retrospective study. Curr Med Res Opin 27:79-86, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Morrison VA, Wong M, Hershman D, et al. : Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3-4 week chemotherapy regimens in community oncology practices. J Manag Care Pharm 13:337-348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz N, Pfistner B, Sextro M, et al. : Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: A randomised trial. Lancet 359:2065-2071, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Villa D, Seshadri T, Puig N, et al. : Second-line salvage chemotherapy for transplant-eligible patients with Hodgkin’s lymphoma resistant to platinum-containing first-line salvage chemotherapy. Haematologica 97:751-757, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuruvilla J, Keating A, Crump M: How I treat relapsed and refractory Hodgkin lymphoma. Blood 117:4208-4217, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Majhail NS, Weisdorf DJ, Defor TE, et al. : Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant 12:1065-1072, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Rancea M, Monsef I, von Tresckow B, et al. : High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database Syst Rev 6:CD009411, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hui L, von Keudell G, Wang R, et al. : Cost-effectiveness analysis of consolidation with brentuximab vedotin for high-risk Hodgkin lymphoma after autologous stem cell transplantation. Cancer 123:3763-3771, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen R, Gopal AK, Smith SE, et al. : Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 128:1562-1566, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R, Palmer JM, Tsai NC, et al. : Brentuximab vedotin is associated with improved progression-free survival after allogeneic transplantation for Hodgkin lymphoma. Biol Blood Marrow Transplant 20:1864-1868, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskowitz AJ, Perales MA, Kewalramani T, et al. : Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol 146:158-163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderlini P, Saliba RM, Ledesma C, et al. : Gemcitabine, fludarabine, and melphalan for reduced-intensity conditioning and allogeneic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 22:1333-1337, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansell SM, Lesokhin AM, Borrello I, et al. : PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372:311-319, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Younes A, Santoro A, Shipp M, et al. : Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 17:1283-1294, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopal AK, Chen R, Smith SE, et al. : Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood 125:1236-1243, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arai S, Fanale M, DeVos S, et al. : Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma 54:2531-2533, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Kaloyannidis P, Voutiadou G, Baltadakis I, et al. : Outcomes of Hodgkin’s lymphoma patients with relapse or progression following autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 18:451-457, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Crump M: Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology Am Soc Hematol Educ Program 326-333, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Diaby V, Adunlin G, Montero AJ: Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: A tutorial. Pharmacoeconomics 32:101-108, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Latimer NR: Survival analysis for economic evaluations alongside clinical trials: Extrapolation with patient-level data—Inconsistencies, limitations, and a practical guide. Med Decis Making 33:743-754, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Guyot P, Ades AE, Ouwens MJ, et al. : Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 12:9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merli F, Luminari S, Gobbi PG, et al. : Long-term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced Hodgkin lymphoma: A study by Fondazione Italiana Linfomi. J Clin Oncol 34:1175-1181, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Gordon LI, Hong F, Fisher RI, et al. : Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: An intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol 31:684-691, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carde P, Karrasch M, Fortpied C, et al. : Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, International Prognostic Score ≥ 3, high-risk Hodgkin lymphoma: First results of the phase III EORTC 20012 intergroup trial. J Clin Oncol 34:2028-2036, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Chen R, Zinzani PL, Fanale MA, et al. : Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 35:2125-2132, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avalere Health : Total cost of cancer care by site of service: Physician office vs outpatient hospital. https://www.communityoncology.org/pdfs/avalere-cost-of-cancer-care-study.pdf
- 44.Guadagnolo BA, Punglia RS, Kuntz KM, et al. : Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin’s disease. J Clin Oncol 24:4116-4122, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Huntington SF, Svoboda J, Doshi JA: Cost-effectiveness analysis of routine surveillance imaging of patients with diffuse large B-cell lymphoma in first remission. J Clin Oncol 33:1467-1474, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Szabo SM, Hirji I, Johnston KM, et al. : Treatment patterns and costs of care for patients with relapsed and refractory Hodgkin lymphoma treated with brentuximab vedotin in the United States: A retrospective cohort study. PLoS One 12:e0180261, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khera N, Emmert A, Storer BE, et al. : Costs of allogeneic hematopoietic cell transplantation using reduced intensity conditioning regimens. Oncologist 19:639-644, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khera N, Zeliadt SB, Lee SJ: Economics of hematopoietic cell transplantation. Blood 120:1545-1551, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Majhail NS, Mau L-W, Denzen EM, et al. : Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: A study using a large national private claims database. Bone Marrow Transplant 48:294-300, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell DE, Lynn J, Louis TA, et al. : Medicare program expenditures associated with hospice use. Ann Intern Med 140:269-277, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Hogan C, Lunney J, Gabel J, et al. : Medicare beneficiaries’ costs of care in the last year of life. Health Aff (Millwood) 20:188-195, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Shapiro CL, Moriarty JP, Dusetzina S, et al. : Cost-effectiveness analysis of monthly zoledronic acid, zoledronic acid every 3 months, and monthly denosumab in women with breast cancer and skeletal metastases: CALGB 70604 (Alliance). J Clin Oncol 35:3949-3955, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Q, Jain N, Ayer T, et al. : Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol 35:166-174, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bach PB: Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med 360:626-633, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Hornberger J, Chien R, Friedmann M, et al. : Cost-effectiveness of rituximab as maintenance therapy in patients with follicular non-Hodgkin lymphoma after responding to first-line rituximab plus chemotherapy. Leuk Lymphoma 53:2371-2377, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Lien K, Cheung MC, Chan KK: Adjusting for drug wastage in economic evaluations of new therapies for hematologic malignancies: A systematic review. J Oncol Pract 12:e369-e379, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Cheung MC, Prica A, Graczyk J, et al. : Granulocyte colony-stimulating factor in secondary prophylaxis for advanced-stage Hodgkin lymphoma treated with ABVD chemotherapy: A cost-effectiveness analysis. Leuk Lymphoma 57:1865-1875, 2016 [DOI] [PubMed] [Google Scholar]
- 58.Ng AK, Weeks JC, Mauch PM, et al. : Laparotomy versus no laparotomy in the management of early-stage, favorable-prognosis Hodgkin’s disease: A decision analysis. J Clin Oncol 17:241-252, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Ng AK, Kuntz KM, Mauch PM, et al. : Costs and effectiveness of staging and treatment options in early-stage Hodgkin’s disease. Int J Radiat Oncol Biol Phys 50:979-989, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Ramsey SD, Nademanee A, Masszi T, et al. : Quality of life results from a phase 3 study of brentuximab vedotin consolidation following autologous haematopoietic stem cell transplant for persons with Hodgkin lymphoma. Br J Haematol 175:860-867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swinburn P, Shingler S, Acaster S, et al. : Health utilities in relation to treatment response and adverse events in relapsed/refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk Lymphoma 56:1839-1845, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Bach PB, Conti RM, Muller RJ, et al. : Overspending driven by oversized single dose vials of cancer drugs. BMJ 352:i788, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention : Protect patients against preventable harm from improper use of single-dose/single-use vials. https://www.cdc.gov/injectionsafety/CDCposition-SingleUseVial.html
- 64.Amitai I, Gurion R, Vidal L, et al. : PET-adapted therapy for advanced Hodgkin lymphoma: Systematic review. Acta Oncol 57:765-772, 2018 [DOI] [PubMed] [Google Scholar]
- 65.Bartlett NL: Fine-tuning the treatment of Hodgkin’s lymphoma. N Engl J Med 374:2490-2492, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Garrison LP, Jr, Neumann PJ, Erickson P, et al. : Using real-world data for coverage and payment decisions: The ISPOR Real-World Data Task Force report. Value Health 10:326-335, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Schaapveld M, Aleman BM, van Eggermond AM, et al. : Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med 373:2499-2511, 2015 [DOI] [PubMed] [Google Scholar]
- 68.Pearson S, Dreitlein B, Henshall C: Indication-specific pricing of pharmaceuticals in the United States healthcare system. https://icer-review.org/wp-content/uploads/2016/03/Final-Report-2015-ICER-Policy-Summit-on-Indication-specific-Pricing-March-2016_revised-icons.pdf [DOI] [PubMed]
- 69.Chandra A, Garthwaite C: The economics of indication-based drug pricing. N Engl J Med 377:103-106, 2017 [DOI] [PubMed] [Google Scholar]
- 70.Mailankody S, Prasad V: Five years of cancer drug approvals: Innovation, efficacy, and costs. JAMA Oncol 1:539-540, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Bach PB: Indication-specific pricing for cancer drugs. JAMA 312:1629-1630, 2014 [DOI] [PubMed] [Google Scholar]
- 72.Armitage JO, Abutalib SA: ECHELON-1: A commendable study, but questions remain. http://www.ascopost.com/issues/january-25-2018/echelon-1-a-commendable-study-but-questions-remain/