Abstract
Aim
No studies have been published on the relationship between marital status and outcomes in small intestinal cancers. The present study was conducted to explore the influence of marital status on small intestinal adenocarcinoma survival based on the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
Data from eligible patients diagnosed with small intestinal adenocarcinoma between 2004 and 2015 were extracted from the SEER database. Patients were categorized into married group (including common law) and unmarried group (including single [never married], widowed, divorced, separated, and unmarried or domestic partner). The primary endpoints were 5-year overall survival (OS) and 5-year cancer-specific survival (CSS). A survival curve was generated by the Kaplan–Meier method, and the survival rate differences were estimated by a log-rank test. A multivariate Cox proportional hazard model was used to evaluate the independent risk factors for survival.
Results
A total of 6,747 small intestinal adenocarcinoma patients were enrolled, including 3,862 married and 2,885 unmarried patients. The 5-year OS and 5-year CSS were significantly greater in married patients than in unmarried patients (27.1 vs 18.8% for OS and 45.7 vs 39.3% for CSS, both P<0.001). After adjusting for age, insurance status, tumor primary site, TNM stage, tumor grade, tumor histology, and surgery, the multivariate Cox proportional hazards model showed that marriage is an independent protective factor for OS (HR =0.789, 95% CI: 0.745–0.836, P<0.001) and CSS (HR =0.794, 95% CI: 0.736–0.857, P<0.001).
Conclusion
Married small intestinal adenocarcinoma patients have better OS and CSS than unmarried patients. Psychological and economic supports from the spouses of married patients may contribute to improvements in survival.
Keywords: small bowel, adenocarcinomas, marriage, prognosis
Introduction
Small intestine cancers are uncommon malignancies of the digestive system. In 2018, an estimated 10,470 new small intestine cancer cases and 1,450 deaths will occur in USA.1 Adenocarcinoma, the second most common histological subtype, accounts for 36.9% of small intestine cancers.2 Other common histological subtypes included carcinoids, lymphomas, and stromal tumors.2–5
Due to the relative rarity of small intestinal cancers, few large-scale population-based studies have been conducted. The Surveillance, Epidemiology, and End Results (SEER) database includes almost all cancer incidence, prevalence, tumor pathology, and demographic data, such as age, gender, ethnicity, marital status, and prognosis.6 Some known prognostic factors, such as race, tumor location, TNM stage, and number of retrieved lymph nodes, have been proposed by studies based on the SEER database.2,7–9 The protective effect of marriage on survival was inconsistent. Marital status was reported to be associated with survival in some cancers, such as renal cell carcinoma,10 head and neck cancers,11 squamous cell carcinoma of the penis,12 and rectal cancer.13 However, other studies could not find any protective effect of marriage on patients’ outcome.14,15 Previous study by Kato et al16 found that single females showed increased risks for small intestine cancers. However, no study has been published to evaluate the relationship between marital status and outcome in small intestinal cancers. Therefore, the present study was conducted to explore the influence of marital status on small intestinal adenocarcinoma survival based on the SEER database.
Methods
Patients
The SEER database (http://seer.cancer.gov) is the largest publicly available dataset and consists of 18 tumor registries coverinĝ26% of the US population. The de-identified data held by the SEER database were authorized to obtain in May 2018. The following inclusion criteria were adopted: 1) tumor histological type: mucinous adenocarcinoma (SEER histology codes 8480 and 8481), signet ring cell (SEER histology code 8490), and other carcinoma adenocarcinoma (SEER histology codes 8140, 8144, 8210, 8211, 8220, 8221, 8255, 8260, 8261, 8262, 8263, 8574, and 8576); 2) marital status known; and 3) survival data known.
Tumor location was categorized by the following SEER cancer site codes: C17.0-duodenum, C17.1-jejunum, C17.2-ileum, C17.3-Meckel’s diverticulum, C17.8-overlapping lesion of small intestine, and c17.9-small intestine, not otherwise specified (NOS). Race classifications were “white” (race/ethnicity code, 1), “black” (race/ethnicity code, 2), and “others” (the remaining codes). The American Joint Committee on Cancer (AJCC) seventh TNM staging system was used.
Statistical analysis
Comparisons of categorical variables between married and unmarried patients were performed using the chi-squared test, and continuous variables were compared using the Student’s t-test. The primary endpoints were 5-year overall survival (OS) and 5-year cancer-specific survival (CSS). Survival curves were generated by the Kaplan–Meier method, and survival rate differences were estimated by a log-rank test. Variables with statistically significant differences were entered into a multivariate Cox proportional hazard model. HRs with 95% CIs were calculated for all the prognostic factors. Statistical analyses were performed using SPSS 23.0 (IBM Corporation, Armonk, NY, USA). P<0.05 was considered to be statistically significant.
Results
Patient baseline characteristics
A total of 6,747 small intestinal adenocarcinoma patients diagnosed between 2004 and 2015 were enrolled, including 3,656 (54.2%) male and 3,091 (45.8%) female patients. Of these patients, the mean age of the whole population was 67.78±13.81 years (mean ± SD). Therefore, patients were divided into the following two groups according to age: <68 and ≥68 years. According to the SEER coding program, the marital status of patients with small intestinal adenocarcinoma was divided into married (including common law, N=3,862) and unmarried (including single [never married], widowed, divorced, separated, and unmarried or domestic partner, N=2,885).
Clinicopathological features of married and unmarried patients
As shown in Table 1, male patients were more prevalent in the married group than in the unmarried group (65.2 vs 39.5%, P<0.001). The mean age of married patients was significantly younger than that of unmarried patients (66.47±12.76 vs 69.54±14.93, P<0.001). Additionally, more Caucasians and patients with insurance/any Medicaid were found in the married group than in the unmarried group (all P<0.001). For the TNM stage, unmarried patients had a higher percentage of stage IV disease than married patients. More married patients received surgery than unmarried patients (64.0 vs 55.9%, P<0.001). No significant differences were found between the married and unmarried groups regarding tumor primary site, histology, or distant metastasis (M stage).
Table 1.
Comparison of clinicopathological features between married and unmarried patients with small intestinal adenocarcinoma
| Characteristics | Unmarried N=2,885, n (%) | Married N=3,862, n (%) | P |
|---|---|---|---|
| Gender | <0.001 | ||
| Male | 1,139 (39.5) | 2,517 (65.2) | |
| Female | 1,746 (60.5) | 1,345 (34.8) | |
| Age (years), mean ± SD | 69.54±14.93 | 66.47±12.76 | <0.001 |
| Race | <0.001 | ||
| White | 2,040 (70.7) | 3,095 (80.1) | |
| Black | 673 (23.3) | 474 (12.3) | |
| Othersa | 172 (6.0) | 293 (7.6) | |
| Insurance | <0.001 | ||
| Insured/Medicaid | 2,138 (74.1) | 2,891 (74.9) | |
| Uninsured | 93 (3.2) | 64 (1.7) | |
| Unknown | 654 (22.7) | 907 (23.4) | |
| Tumor primary site | 0.066 | ||
| Duodenum | 1,671 (57.9) | 654 (58.7) | |
| Jejunum | 374 (13.0) | 574 (14.9) | |
| Ileum | 370 (12.8) | 475 (12.3) | |
| Meckel’s diverticulum | 5 (0.2) | 4 (0.1) | |
| Overlapping lesion of small intestine | 38 (1.3) | 43 (1.1) | |
| Small intestine, NOS | 427 (14.8) | 498 (12.9) | |
| AJCC seventh TNM stage | 0.001 | ||
| I | 191 (6.6) | 244 (6.3) | |
| II | 362 (12.5) | 489 (12.7) | |
| III | 305 (10.6) | 544 (14.1) | |
| IV | 524 (18.2) | 665 (17.2) | |
| Unknown/NA/blank(s) | 1,503 (52.1) | 1,920 (49.7) | |
| AJCC seventh T stage | 0.008 | ||
| T0 | 7 (0.2) | 6 (0.2) | |
| T1 | 221 (7.7) | 278 (7.2) | |
| T2 | 65 (2.3) | 119 (3.1) | |
| T3 | 371 (12.9) | 599 (15.5) | |
| T4 | 564 (19.5) | 750 (19.4) | |
| TX/unknown/NA/blank(s) | 1,657 (57.4) | 2,110 (54.6) | |
| AJCC seventh N stage | 0.047 | ||
| N0 | 845 (29.3) | 1,082 (28.0) | |
| N1 | 362 (12.5) | 557 (14.4) | |
| N2 | 173 (6.0) | 266 (6.9) | |
| NX/unknown/NA/blank(s) | 1,505 (52.2) | 1,957 (50.7) | |
| AJCC seventh M stage | 0.328 | ||
| M0 | 1,024 (35.5) | 1,433 (37.1) | |
| M1 | 526 (18.2) | 666 (17.2) | |
| Blank(s) | 1,335 (46.3) | 1,763 (45.6) | |
| Grade | 0.012 | ||
| Well differentiated | 215 (7.5) | 292 (7.6) | |
| Moderately differentiated | 1,174 (40.7) | 1,629 (42.2) | |
| Poorly differentiated | 848 (29.4) | 1,209 (31.3) | |
| Undifferentiated/anaplastic | 34 (1.2) | 40 (1.0) | |
| Unknown | 614 (21.3) | 692 (17.9) | |
| Histology | 0.676 | ||
| Other adenocarcinoma | 2,542 (88.1) | 3,407 (88.2) | |
| Mucinous adenocarcinoma | 234 (8.1) | 297 (7.7) | |
| Signet ring cell | 109 (3.8) | 158 (4.1) | |
| Surgery | <0.001 | ||
| Yes | 1,612 (55.9) | 2,472 (64.0) | |
| No | 1,257 (43.6) | 1,367 (35.4) | |
| Unknown | 16 (0.6) | 23 (0.6) |
Note:
Including American Indian/AK native and Asian/Pacific Islander (N=448) and unknown race (N=17).
Abbreviations: AJCC, American Joint Committee on Cancer; NOS, not otherwise specified; NA, not applicable.
Influence of marital status on small intestinal adenocarcinoma OS
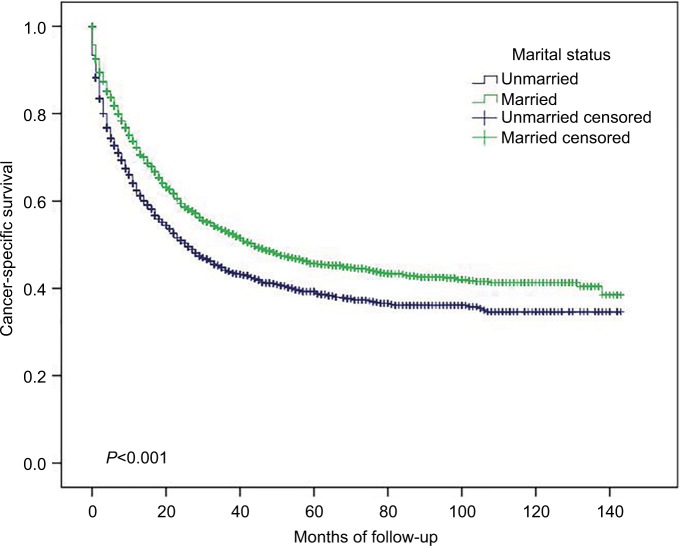
As shown in Table 2, the 5-year OS for the whole population was 23.6%. The 5-year OS was significantly longer in married patients than in unmarried patients (27.1 vs 18.8%, P<0.001, Figure 1). Younger patients (age at diagnosis <68 years) had a significantly better 5-year OS than older patients (30.7 vs 16.1%, P<0.001). Surprisingly, the 5-year OS of patients without insurance was significantly better than that of patients with insurance/Medicaid (32.4 vs 23.0%, P<0.021). Compared with other tumor sites, tumors located in the duodenum and Meckel’s diverticulum had the lowest 5-year OS at 18.2 and 0%, respectively (P<0.001). For the histology subtypes, signet ring cells had worse 5-year OS than mucinous adenocarcinoma and other adenocarcinoma (12.0 vs 27.3 and 23.8%, respectively, P<0.001). Unevent fully, the 5-year OS of well-differentiated and moderately differentiated small intestinal adenocarcinoma was better than that of poorly differentiated and undifferentiated/anaplastic adenocarcinoma (35.1 and 29.8% vs 21.6 and 14.7%, respectively, P<0.001). A total of 4,084 of the 6,747 (60.5%) patients received surgery, and the 5-year OS was better in patients undergoing surgery than in patients without surgery (36.7 vs 2.9%, P<0.001). Finally, TNM stage was significantly correlated with OS. The 5-year OS was 38.1, 41.0, 29.8, and 4.6% for stages I, II, III, and IV, respectively (P<0.001).
Table 2.
Univariate and multivariate analyses of small intestinal adenocarcinoma OS
| Characteristics | 5-Year OS (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Log-rank χ2 test | P | HR (95% CI) | P | ||
| Marital status | 121.810 | <0.001 | <0.001 | ||
| Unmarried | 18.8 | Reference | |||
| Married | 27.1 | 0.789 (0.745–0.836) | |||
| Gender | 0.023 | 0.879 | |||
| Male | 23.6 | ||||
| Female | 23.6 | ||||
| Age (years) | 387.401 | <0.001 | <0.001 | ||
| <68 | 30.7 | Reference | |||
| ≥68 | 16.1 | 1.515 (1.426–1.610) | |||
| Race | 0.421 | 0.810 | |||
| White | 24.0 | ||||
| Black | 21.7 | ||||
| Othersa | 23.3 | ||||
| Insurance | 7.762 | 0.021 | 0.703 | ||
| Insured/Medicaid | 23.0 | Reference | |||
| Uninsured | 32.4 | 0.914 (0.740–1.128) | |||
| Unknown | 24.0 | 0.998 (0.926–1.076) | |||
| Tumor primary site | 325.506 | <0.001 | <0.001 | ||
| Duodenum | 18.2 | Reference | |||
| Jejunum | 38.6 | 0.829 (0.751–0.914) | |||
| Ileum | 34.0 | 0.982 (0.887–1.088) | |||
| Meckel’s diverticulum | 0 | 1.632 (0.730–3.646) | |||
| Overlapping lesion of small intestine | 28.0 | 0.967 (0.732–1.276) | |||
| Small intestine, NOS | 21.3 | 1.218 (1.114–1.331) | |||
| AJCC seventh TNM stage | 626.818 | <0.001 | <0.001 | ||
| I | 38.1 | Reference | |||
| II | 41.0 | 1.011 (0.852–1.200 | |||
| III | 29.8 | 1.276 (1.080–1.508) | |||
| IV | 4.6 | 1.923 (1.652–2.238) | |||
| Unknown/NA/blank(s) | 22.1 | 1.460 (1.264–1.686) | |||
| Grade | 399.771 | <0.001 | <0.001 | ||
| Well differentiated | 35.1 | Reference | |||
| Moderately differentiated | 29.8 | 1.096 (0.969–1.239) | |||
| Poorly differentiated | 21.6 | 1.438 (1.268–1.631) | |||
| Undifferentiated/anaplastic | 14.7 | 1.731 (1.296–2.312) | |||
| Unknown | 0.9 | 1.102 (0.966–1.257) | |||
| Histology | 28.093 | <0.001 | 0.002 | ||
| Other adenocarcinoma | 23.8 | Reference | |||
| Mucinous adenocarcinoma | 27.3 | 1.042 (0.933–1.164) | |||
| Signet ring cell | 12.0 | 1.287 (1.117–1.483) | |||
| Surgery | 2,161.604 | <0.001 | <0.001 | ||
| Yes | 36.7 | Reference | |||
| No | 2.9 | 3.222 (2.983–3.480) | |||
| Unknown | 23.8 | 1.731 (1.118–2.520) | |||
Note:
Including American Indian/AK native and Asian/Pacific Islander (N=448) and unknown race (N=17).
Abbreviations: AJCC, American Joint Committee on Cancer; NOS, not otherwise specified; OS, overall survival; NA, not applicable.
Figure 1.
Overall survival in small intestinal adenocarcinoma according to marital status.
Influence of marital status on small intestinal adenocarcinoma CSS
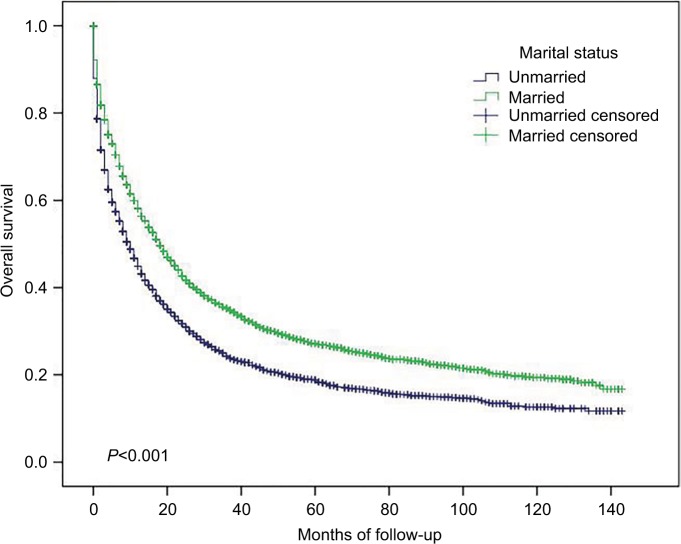
As shown in Table 3, the 5-year CSS for the whole population was 43.0%. The 5-year CSS was significantly greater in married patients than in unmarried patients (45.7 vs 39.3%, P<0.001, Figure 2). Patients whose age at diagnosis were <68 years had significantly better 5-year CSS than older patients (46.7 vs 39.5%, P<0.001). Gender, race, and insurance had no influence on 5-year CSS. Tumors located in the duodenum and Meckel’s diverticulum had lower 5-year CSS than tumors located in the jejunum, ileum, and small intestine NOS (35.6 and 0% vs 53.5, 57.0, and 57.0%, respectively, P<0.001). For histology subtypes, signet ring cells had worse 5-year CSS than mucinous adenocarcinoma and other adenocarcinoma (24.6 vs 48.6 and 43.3%, respectively, P<0.001). Additionally, the 5-year CSS of well-differentiated and moderately differentiated small intestinal adenocarcinoma was better than that of poorly differentiated and undifferentiated/anaplastic adenocarcinoma (61.9 and 51.3% vs 38.1 and 34.4%, respectively, P<0.001). Patients undergoing surgery had better 5-year CSS than those without surgery (57.2 vs 10.9%, P<0.001). Finally, TNM stage was significantly correlated with CSS. The 5-year CSS was 68.3, 65.4, 44.5, and 11.1%, respectively, for stages I, II, III, and IV (P<0.001).
Table 3.
Univariate and multivariate analyses of small intestinal adenocarcinoma CSS
| Characteristics | 5-Year CSS (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Log-rank χ2 test | P | HR (95% CI) | P | ||
| Marital status | 55.054 | <0.001 | <0.001 | ||
| Unmarried | 39.3 | Reference | |||
| Married | 45.7 | 0.794 (0.736–0.857) | |||
| Gender | 1.392 | 0.238 | |||
| Male | 43.3 | ||||
| Female | 42.7 | ||||
| Age (years) | 63.046 | <0.001 | <0.001 | ||
| <68 | 46.7 | Reference | |||
| ≥68 | 39.5 | 1.161 (1.075–1.254) | |||
| Race | 2.885 | 0.236 | |||
| White | 44.1 | ||||
| Black | 38.6 | ||||
| Othersa | 41.8 | ||||
| Insurance | 0.861 | 0.650 | |||
| Insured/Medicaid | 42.6 | ||||
| Uninsured | 43.6 | ||||
| Unknown | 43.6 | ||||
| Tumor primary site | 240.334 | <0.001 | 0.014 | ||
| Duodenum | 35.6 | Reference | |||
| Jejunum | 53.5 | 0.840 (0.741–0.951) | |||
| Ileum | 57.0 | 0.851 (0.740–0.978) | |||
| Meckel’s diverticulum | 0 | 2.207 (0.913–5.333) | |||
| Overlapping lesion of small intestine | 46.5 | 0.897 (0.624–1.287) | |||
| Small intestine, NOS | 48.7 | 0.967 (0.853–1.096) | |||
| AJCC seventh TNM stage | 689.047 | <0.001 | <0.001 | ||
| I | 68.3 | Reference | |||
| II | 65.4 | 1.144 (0.880–1.489) | |||
| III | 44.5 | 1.887 (1.475–2.413) | |||
| IV | 11.1 | 3.191 (2.540–4.008) | |||
| Unknown/NA/blank(s) | 42.4 | 1.995 (1.604–2.482) | |||
| Grade | 360.890 | <0.001 | <0.001 | ||
| Well differentiated | 61.9 | Reference | |||
| Moderately differentiated | 51.3 | 1.194 (1.002–1.423) | |||
| Poorly differentiated | 38.1 | 1.749 (1.464–2.090) | |||
| Undifferentiated/anaplastic | 34.4 | 2.030 (1.387–2.971) | |||
| Unknown | 22.0 | 1.290 (1.072–1.552) | |||
| Histology | 24.234 | <0.001 | 0.010 | ||
| Other adenocarcinoma | 43.3 | Reference | |||
| Mucinous adenocarcinoma | 48.6 | 1.070 (0.924–1.240) | |||
| Signet ring cell | 24.6 | 1.312 (1.096–1.571) | |||
| Surgery | 1,546.559 | <0.001 | <0.001 | ||
| Yes | 57.2 | Reference | |||
| No | 10.9 | 3.472 (3.138–3.841) | |||
| Unknown | 44.7 | 1.321 (0.745–2.341) | |||
Note:
Including American Indian/AK native and Asian/Pacific Islander (N=448) and unknown race (N=17).
Abbreviations: AJCC, American Joint Committee on Cancer; CSS, cancer-specific survival; NOS, not otherwise specified.
Figure 2.
Cancer-specific survival in small intestinal adenocarcinoma according to marital status.
Multivariate analysis
Variables that had a significant association with survival in univariate analysis (P<0.05) were enrolled in the multivariate Cox proportional hazards model. As shown in Tables 2 and 3, marital status, age, tumor primary site, TNM stage, grade, histology, and surgery were independent prognostic factors for OS and CSS. Compared with unmarried patients, married patients had an HR of 0.789 (95% CI: 0.745–0.836, P<0.001, Table 2) for OS and 0.794 (95% CI: 0.736–0.857, P<0.001, Table 3) for CSS.
Subgroup analysis
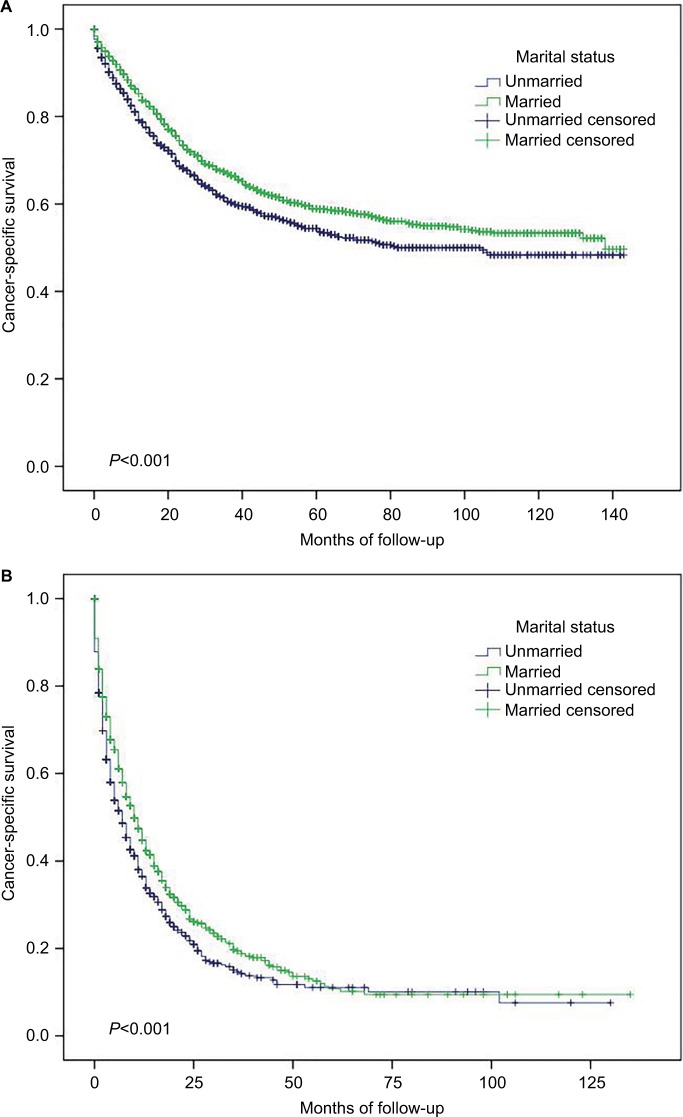
As shown in Table 4, widowed patients had worse survival (5-year CSS 36.6%) than married (5-year CSS 45.7%), never married (5-year CSS 39.9%), divorced (5-year CSS 43.1%), and separated (5-year CSS 47.7%) patients (similar results were found regarding OS, data not shown). For TNM stages I, II, III, and IV patients, married patients had better OS and CSS than unmarried patients (P<0.001, data not shown). Married patients had better CSS than unmarried patients no matter whether they received surgery or not (Figure 3, similar results were found regarding OS, data not shown).
Table 4.
Marital status and small intestinal adenocarcinoma CSS
| Marital status | n (%) | 5-Year CSS (%) | Cox univariate analysis | |
|---|---|---|---|---|
| HR (95% CI) | P | |||
| Married (including common law) | 3,862 (57.2) | 45.7 | Reference | |
| Unmarried | ||||
| Single (never married) | 1,040 (15.4) | 39.9 | 1.229 (1.107–1.366) | <0.001 |
| Widowed | 1,197 (17.7) | 36.6 | 1.521 (1.376–1.682) | <0.001 |
| Divorced | 583 (8.6) | 43.1 | 1.185 (1.035–1.356) | 0.014 |
| Separated | 61 (0.9) | 47.7 | 1.094 (0.737–1.652) | 0.655 |
| Unmarried or domestic partner | 4 (0.1) | 0 | 1.835 (0.459–7.347) | 0.391 |
Abbreviation: CSS, cancer-specific survival.
Figure 3.
Subgroup analysis of cancer-specific survival in small intestinal adenocarcinoma according to marital status.
Notes: (A) Surgery, P<0.001. (B) No surgery, P<0.001.
Discussion
To the best of our knowledge, this study is the first to examine whether marital status has an effect on survival in small intestine adenocarcinoma. Marriage has a protective effect on the outcome in small intestine adenocarcinoma, which is consistent with recent reports.10–13 Married patients have better 5-year OS and CSS than unmarried patients, including single (never married), widowed, divorced, separated, and unmarried or domestic partner patients. After adjusting for age, insurance status, tumor primary site, TNM stage, tumor grade, tumor histology, and surgery, the multivariate Cox proportional hazards model showed that marital status was an independent prognostic factor for OS and CSS in small intestine adenocarcinoma. After stratification by TNM stage or surgery, we still found that married patients had better OS and CSS than unmarried patients.
Previous studies demonstrated that African American/black patients have later tumor stages and worse outcomes.17,18 The present study shows that there are more Caucasian/white patients than African American/black and other ethnicity patients in the married group. However, we could not find any OS or CSS differences between white, black, and other ethnicity patients in univariate analysis. Thus, race is not a prognostic factor for small intestine adenocarcinoma and outcome differences between married and unmarried patients could not be explained by race disparities. Studies found that a greater percentage of uninsured patients were unmarried, which predicted poorer outcomes than those of patients with Medicaid or insurance in renal cell carcinoma and other cancers.10,19,20 Surprisingly, the present study found an adverse association between insurance and OS in small intestine adenocarcinoma. Patients with insurance/Medicaid had lower 5-year OS than those without insurance, although no differences were found for CSS.
Unmarried patients, lacking spousal care and support, usually live with chronic psychological distress and unhealthy lifestyle habits, such as smoking and alcohol abuse, resulting in disease progression and undertreatment.21–23 Unmarried patients also tend to have latter stages of disease.13 Hershman et al24 reported that unmarried patients were more likely to delay the initiation of adjuvant chemotherapy after breast cancer surgery, leading to higher mortality. Our results are in line with those of previous studies, as we found a higher percentage of stage IV disease in unmarried patients than in married patients and that unmarried patients were less likely to receive surgery than married patients (55.9 vs 66.0%, P<0.001). Encouragement and economic supports from the spouses of the married patients contributed to their compliance with surgery and adjuvant therapy, which may partly account for the discrepancies.25,26
Our study should be interpreted with caution due to the limited nature of the SEER database. First, some clinicopathological features, such as tumor grade, TNM stage, and surgery status, had missing data. Second, lifestyle, socioeconomic status, and adjuvant therapy were not included in the database. Finally, the database recorded marital status only at diagnosis and not thereafter. All these restrictions may have led to an unreliable conclusion.
Despite the above limitations, our study indicates that marital status is an independent prognostic factor in small intestine adenocarcinoma. Married patients have better OS and CSS than unmarried patients. Psychological and economic supports from the spouses of married patients may contribute to the improved survival.
Conclusion
Married small intestinal adenocarcinoma patients have better OS and CSS than unmarried patients. Psychological and economic supports from the spouses of married patients may contribute to improvements in survival.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of Guangdong Province, China (2017A030310194), Science and Technology Planning Project of Guangdong Province, China (2016A020216008), and Administration of Traditional Chinese Medicine of Guangdong Province, China (20151162).
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 3.Talamonti MS, Goetz LH, Rao S, Joehl RJ. Primary cancers of the small bowel: analysis of prognostic factors and results of surgical management. Arch Surg. 2002;137(5):564–570. doi: 10.1001/archsurg.137.5.564. discussion 570–571. [DOI] [PubMed] [Google Scholar]
- 4.Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small bowel cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control. 2005;16(7):781–787. doi: 10.1007/s10552-005-3635-6. [DOI] [PubMed] [Google Scholar]
- 5.Chow JS, Chen CC, Ahsan H, Neugut AI. A population-based study of the incidence of malignant small bowel tumours: SEER, 1973-1990. Int J Epidemiol. 1996;25(4):722–728. doi: 10.1093/ije/25.4.722. [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute [homepage on the Internet] Surveillance, epidemiology, and end results program. 2018. [Accessed May 6, 2018]. Available from: http://seer.cancer.gov/registries/
- 7.Wilhelm A, Müller SA, Steffen T, Schmied BM, Beutner U, Warschkow R. Patients with Adenocarcinoma of the Small Intestine with 9 or More Regional Lymph Nodes Retrieved Have a Higher Rate of Positive Lymph Nodes and Improved Survival. J Gastrointest Surg. 2016;20(2):401–410. doi: 10.1007/s11605-015-2994-x. [DOI] [PubMed] [Google Scholar]
- 8.Nicholl MB, Ahuja V, Conway WC, Vu VD, Sim MS, Singh G. Small bowel adenocarcinoma: understaged and undertreated? Ann Surg Oncol. 2010;17(10):2728–2732. doi: 10.1245/s10434-010-1109-x. [DOI] [PubMed] [Google Scholar]
- 9.Overman MJ, Hu CY, Wolff RA, Chang GJ. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116(23):5374–5382. doi: 10.1002/cncr.25324. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Zhu MX, Qi SH. Marital status and survival in patients with renal cell carcinoma. Medicine. 2018;97(16):e0385. doi: 10.1097/MD.0000000000010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273–1278. doi: 10.1002/cncr.29171. [DOI] [PubMed] [Google Scholar]
- 12.Thuret R, Sun M, Budaus L, et al. A population-based analysis of the effect of marital status on overall and cancer-specific mortality in patients with squamous cell carcinoma of the penis. Cancer Causes Control. 2013;24(1):71–79. doi: 10.1007/s10552-012-0091-y. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Cao W, Zheng C, Hu W, Liu C. Marital status and survival in patients with rectal cancer: An analysis of the Surveillance, Epidemiology and End Results (SEER) database. Cancer Epidemiol. 2018;54:119–124. doi: 10.1016/j.canep.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Johansen C, Schou G, Soll-Johanning H, Mellemgaard A, Lynge E. Influence of marital status on survival from colon and rectal cancer in Denmark. Br J Cancer. 1996;74(6):985–988. doi: 10.1038/bjc.1996.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rendall MS, Weden MM, Favreault MM, Waldron H. The protective effect of marriage for survival: a review and update. Demography. 2011;48(2):481–506. doi: 10.1007/s13524-011-0032-5. [DOI] [PubMed] [Google Scholar]
- 16.Kato I, Tominaga S, Terao C. An epidemiological study on marital status and cancer incidence. Jpn J Cancer Res. 1989;80(4):306–311. doi: 10.1111/j.1349-7006.1989.tb02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchioni M, Harmouch SS, Nazzani S, et al. Effect of African-American race on cancer specific mortality differs according to clear cell vs. non-clear cell histologic subtype in metastatic renal cell carcinoma. Cancer Epidemiol. 2018;54:112–118. doi: 10.1016/j.canep.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Wan JF, Yang LF, Shen YZ, et al. Sex, Race, and Age Disparities in the Improvement of Survival for Gastrointestinal Cancer over Time. Sci Rep. 2016;6:29655. doi: 10.1038/srep29655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry AM, Brunner AM, Zou T, et al. Association between insurance status at diagnosis and overall survival in chronic myeloid leukemia: A population-based study. Cancer. 2017;123(13):2561–2569. doi: 10.1002/cncr.30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu CD, Wang X, Habif DV, Jr, Ma CX, Johnson KJ. Breast cancer stage variation and survival in association with insurance status and sociodemographic factors in US women 18 to 64 years old. Cancer. 2017;123(16):3125–3131. doi: 10.1002/cncr.30722. [DOI] [PubMed] [Google Scholar]
- 21.Surman M, Janik ME. Stress and its molecular consequences in cancer progression. Postepy Hig Med Dosw (Online) 2017;71:485–499. doi: 10.5604/01.3001.0010.3830. [DOI] [PubMed] [Google Scholar]
- 22.Goldzweig G, Andritsch E, Hubert A, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol. 2010;21(4):877–883. doi: 10.1093/annonc/mdp398. [DOI] [PubMed] [Google Scholar]
- 23.Goldzweig G, Andritsch E, Hubert A, et al. How relevant is marital status and gender variables in coping with colorectal cancer? A sample of middle-aged and older cancer survivors. Psychooncology. 2009;18(8):866–874. doi: 10.1002/pon.1499. [DOI] [PubMed] [Google Scholar]
- 24.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006;99(3):313–321. doi: 10.1007/s10549-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 25.De Luca R, Dorangricchia P, Salerno L, Lo Coco G, Cicero G. The role of couples’ attachment styles in patients’ adjustment to cancer. Oncology. 2017;92(6):325–334. doi: 10.1159/000455956. [DOI] [PubMed] [Google Scholar]
- 26.Kanters AE, Morris AM, Abrahamse PH, Mody L, Suwanabol PA. The effect of peer support on colorectal cancer patients’ adherence to guideline-concordant multidisciplinary care. Dis Colon Rectum. 2018;61(7):1–823. doi: 10.1097/DCR.0000000000001067. [DOI] [PMC free article] [PubMed] [Google Scholar]