Abstract
Background
There is convincing evidence that high physical activity lowers the risk of colon cancer; however, the underlying biological mechanisms remain largely unknown. We aimed to determine the extent to which body fatness and biomarkers of various biologically plausible pathways account for the association between physical activity and colon cancer.
Methods
We conducted a nested case-control study in a cohort of 519,978 men and women aged 25 to 70 years followed from 1992 to 2003. A total of 713 incident colon cancer cases were matched using risk-set sampling to 713 controls on age, sex, study center, fasting status, and hormonal therapy use. The amount of total physical activity during the past year was expressed in metabolic equivalent of task [MET]-hours/week. Anthropometric measurements and blood samples were collected at study baseline.
Results
High physical activity was associated with a lower risk of colon cancer (Relative Risk ≥91 MET-hours/week versus < 91 MET-hours/week = 0.75 [95% Confidence Interval (CI): 0.57 to 0.96]. In mediation analyses, this association was accounted for by waist circumference [proportion explained effect (PEE) = 17%; CI: 4% to 52%] and the biomarkers soluble leptin receptor (sOB-R) [PEE = 15%; 95% CI: 1% to 50%] and 5-hydroxyvitamin D (25[OH]D) [PEE = 30%; 95% CI: 12% to 88%]. In combination, these factors explained 45% (95% CI: 20% to 125%) of the association. Beyond waist circumference, sOB-R and 25[OH]D additionally explained 10% (95% CI: 1%; 56%) and 23% (95% CI: 6%; 111%) of the association, respectively.
Conclusion(s)
Promoting physical activity, particularly outdoors, and maintaining metabolic health and adequate vitamin D levels could represent a promising strategy for colon cancer prevention.
Accumulating evidence has emerged suggesting an association between physical activity and lower risk of colon cancer [1–4]. This association has been observed in both men and women, at various ages and across various physical activity exposures [e.g. occupational and leisure time activity] [5]. In line with this research, data from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort suggested a 22% lower risk of colon cancer among the most active participants compared with the most inactive ones [6].
Despite accumulated evidence, the underlying biological mechanisms for this association remain unclear. Physical activity can be associated with weight reduction and thereby may be implicated in a number of beneficial actions such as favorable modulation of hyperinsulinemia, chronic low-grade inflammation and abnormal adipokine secretion [7]. However, pathways other than weight control per se may also account for this association [8], including changes in endogenous hormone levels and growth factors [9], and possibly changes in immune function [10]. Moderate physical activity has also been considered an important factor in reducing oxidative stress [11, 12]. Low plasma 25-hydroxy-vitamin D (25[OH]D) concentrations have been associated with a higher risk of colon cancer, and physical activity (i.e. outdoor physical activity) might therefore also reduce colon cancer risk by improving vitamin D status through sun exposure [13, 14].
So far, there have been no studies to evaluate mediating factors on the observed association between physical activity and colon cancer. Such knowledge may aid in gaining insights on causality, as well as in providing research base for formulating targeted cancer prevention strategies. We therefore explored to what extent body fatness, as well as biomarkers of various biological pathways may account for the observed inverse association between physical activity and colon cancer using data from the EPIC cohort.
Methods
Study population
EPIC is a prospective cohort study including 366,521 women and 153,457 men aged 25 to 70 years from 23 study centers in 10 European countries [15, 16]. At enrollment, between 1992 and 2000, participants gave written informed consent, provided blood samples, underwent anthropometric measurements, and completed questionnaires on socio-demographic and lifestyle characteristics [16],[17]. Ethics approval was obtained from the ethics review board of the International Agency for Research on Cancer (Lyon, France) and the local review boards pertaining to the participating institutions.
Nested case-control study
The present analyses are based on a nested case-control study within the EPIC cohort comprising 713 incident colon cancer cases and 713 controls. Incident cancer cases were identified through record linkage with regional cancer registries or based on a combination of methods, including health insurance records, cancer and pathology registries, and active follow-up through study subjects and their next-of-kin. Closure dates for the present study ranged from December 1999 to June 2003 for centers using registry data, and from June 2000 to December 2002 for centers using active follow-up procedures. First incident colon cancers were defined following the 10th Revision of the International Statistical Classification of Diseases, Injury and Causes of Death (C18.0–C18.7). For each case, one control participant was chosen at random among risk sets consisting of all cohort members free of cancer (except non-melanoma skin cancer) at the time of diagnosis of the index case. Matching characteristics were study center, sex, age, time at blood collection, fasting status; and among women, menopausal status and current hormonal therapy use.
Assessment of physical activity
A description of the physical activity ascertainment used in the EPIC study and questionnaire validity assessment has been provided in detail elsewhere [6, 18]. The EPIC questionnaire assesses past-year physical activity in occupational, leisure and household domains. For recreational and household activities, participants reported the duration of activities during a typical week in the past year, in summer and winter season. Household activities included housework, home repair, gardening and stair climbing. Recreational activities included walking, cycling and sports activities. A metabolic equivalent of task (MET) value – was assigned to each reported activity according to the Compendium of Physical Activities [19]. As previously described, the mean numbers of hours per week of summer and winter household and recreational activities were estimated and then multiplied by the appropriate MET values to obtain MET-hours per week of activity [6]. The assigned MET values (using the EPIC data manual guidelines) were 3.0 for walking and housework, 4.0 for gardening, 4.5 for home repair (do-it-yourself work), 6.0 for cycling and sports, and 8.0 for stair climbing. In the previous EPIC analysis none of the different types of physical activity accounted for the inverse association of total physical activity with colon cancer risk [6], therefore total physical activity was considered as main exposure variable in mediation analyses.
Biomarker assessment
In the EPIC study, blood samples were collected from about 65% of women and 93% of men, and were processed, aliquoted into heat-sealed straws, and stored in liquid nitrogen freezers [-196°C][20]. Biomarkers in the following pathways were evaluated: metabolic dysfunction [glycated hemoglobin (HbA1c), C-peptide], insulin growth factors [IGF-1, IGFBP 1,IGFBP2, IGFBP3], adipokine secretion [adiponectin, high molecular weight (HMW)-adiponectin, non-HMW-adiponectin, leptin, soluble leptin receptor (sOB-R)], lipid metabolism [high density lipoprotein (HDL)-cholesterol, triglycerides, cholesterol, low-density lipoprotein (LDL)-cholesterol], oxidative stress [reactive oxygen metabolites (ROM), ferritic reduced ability of plasma (FRAP)], vitamin D metabolism [25[OH]D, parathyroid hormone (PTH)], inflammation and immune response [C-reactive protein (CRP), neopterin]. The blood collection and processing protocols [16] and detailed measurements of all biomarkers included in the analysis have been described elsewhere [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. For part of the biomarkers missing values have been detected (Supplementary Table 1), however there were no essential differences in the main study characteristics of participants with and without missing data. In order to preserve statistical power of the mediation analyses we imputed these values with respective sex and case control status specific mean values (simplified imputation). Multiple imputation conducted under the assumption that the values are missing at random and complete case analyses yielded similar results as simplified imputation.
Statistical analysis
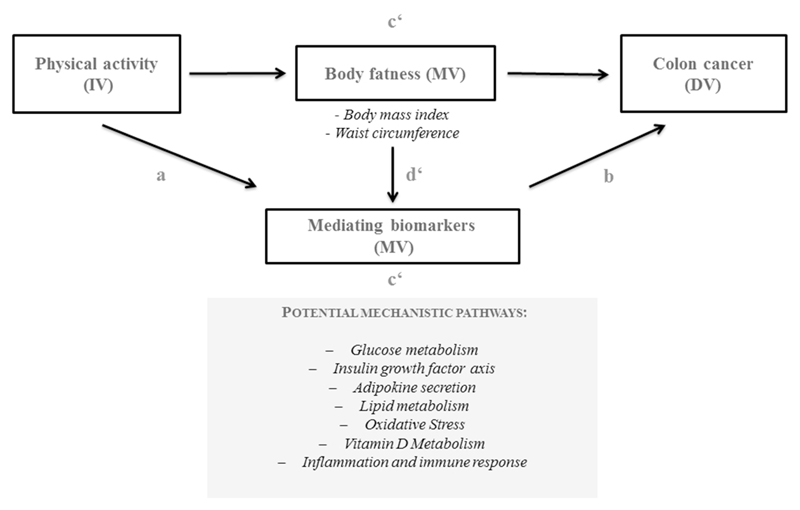
The potential mediating factors were selected following the formal criteria of Baron and Kenny [1986] [32, 33] namely these were biomarkers associated with physical activity as well as with the risk of colon cancer after adjusting for physical activity. Figure 1 illustrates a path diagram as a model for depicting a basic causal chain involved in mediation. This model assumes a three-variable system such that there are two paths feeding into the outcome variable: the direct impact of the independent variable [IV (physical activity)] on the dependent disease variable [DV (colon cancer)] and the indirect path from the IV to the DV via the mediating variables [MV-s]. In order to assess mediation we applied the causal steps approach as previously described [32, 33]. To facilitate the interpretation of the mediation analyses the IV was considered in two levels: ‘high vs low physical activity’ using an arbitrary cut point denoting lowest risk estimate within the 75th percentile of the MET variable distribution.
Figure 1.
Schematic presentation of suggested mediating role of body fatness and biomarkers of various biological pathways as explanatory mechanisms of the association between high physical activity and colon cancer risk
Causal path diagram for the potential indirect effects (c') of the independent variable (IV) - physical activity - on the dependent variable (DV) - colon cancer - through the hypothesized mediator variables (MV) - body fatness and biomarkers of various biological pathways
The mediation effect of biomarkers beyond body fatness (d') was evaluated by calculating proportion of effect explained by each of the biomarkers in a model that included both physical activity and adiposity measures (body mass index and waist circumference).
In Step 1, the association between IV and DV was evaluated using multivariable-adjusted conditional logistic regression model taking into account matching factors: age, sex, study centre, follow-up time since blood collection, time of the day at blood collection and fasting status; in women further matching by menopausal status, phase of menstrual cycle at blood collection and in postmenopausal women hormonal replacement therapy use. The model is further adjusted for education (no school degree or primary school, technical or professional school, secondary school, university degree, or unknown), smoking status (never, past, current, or unknown), alcohol intake (continuous), fruit and vegetable intake (g/day), fish and shellfish intake (g/day), fibre intake (g/day), red and processed meat intake (g/day) and high physical activity (>91 MET-hours per week of total activity).
In Step 2, the cross-sectional associations between MV-s (continuous) and IV (dischotomous) among controls were evaluated using Point-biserial correlation analysis adjusted for age at study recruitment, sex, education and lifestyle factors as covariates from the multivariable-adjusted model.
In Step 3, associations of MV-s and DV were evaluated in the multivariable-adjusted model with additional adjustment for IV. Linear trends were evaluated using restricted cubic spline regression models and potential non-linearity of the associations was evaluated using a likelihood ratio test.
In Step 4, mediation effects of MV-s were estimated as proportion of explained effect (PEE) calculated by adding one at a time individual MV-s to the multivariable-adjusted model using the difference of coefficients method [34, 35]. This approach is based on the formula %ßindirect effect = [(β - β1)/β]*100, in which the effect (β) is the multivariable-adjusted log relative risk of the association between IV and DV and the effect (β1) denotes the same model additionally including the MV. Finally, the combined mediation effect of important mediators was evaluated. In order to evaluate significance of the observed effect changes, we calculated corresponding 95% CIs based on Fieller’s theorem [36].
In sensitivity analyses, we repeated the main multivariable-adjusted analysis after excluding cases diagnosed in the first 2 years of study follow up, participants with type 2 diabetesand with reported cardio-vascular problem at study baseline, non-fasting participants, and dietary supplement users. In additional analyses aimed to test for potential exposure-mediator interaction, we included a cross-product term of variables for physical activity and biomarkers in the multivariable adjusted model for colon cancer and evaluated statistical interaction on the multiplicative scale. All statistical analyses were performed using Statistical Analysis System (SAS), Version 9.2, software (SAS Institute, Inc., Cary, North Carolina).
Results
The baseline characteristics of incident colon cancer cases and their corresponding controls are presented in Table 1. On average, colon cancer cases had lower levels of total physical activity and higher BMI and waist circumference compared to controls. They also had higher alcohol intake, HbA1c, triglycerides, ROM, FRAP, PTH, and CRP; lower non-HMW-adiponectin, sOB-R, HDL-cholesterol, 25[OH]D, and neopterin. The age- and sex-adjusted correlations among the biomarkers are presented in Supplementary Table 2.
Table 1. Baseline characteristics of incident colon cancer cases and matched controls.
| Characteristics | Colon Cancer | Pdifferencea | |
|---|---|---|---|
| Cases (n=713) | Controls (n=713) | ||
| Socio-demographic characteristics | |||
| Female sex, %b | 45.4 | 45.4 | 0.89 |
| Age, years b | 58.6 | 58.6 | |
| University degree, % | 16.7 | 17.5 | .46 |
| Postmenopausal status, % b | 11.7 | 11.7 | .50 |
| Lifestyle and dietary factors | |||
| Current smoking, % | 22.8 | 21.0 | .26 |
| Baseline alcohol intake, g/day, median (IQR) | 7.8 (0.94-20.7) | 7.3 (1.5-18.2) | .02 |
| Hormonal therapy in postmenopausal women, % b | 7.5 | 8.6 | .50 |
| Fibre intake, g/day, median (IQR) | 22.3 (17.4-27.8) | 22.1 (18.5-26.9) | .68 |
| Fruits and vegetables intake, g/day, median (IQR) | 379.1 (241.7-542.2) | 398.3 (255.7-558.3) | .15 |
| Fish and shellfish, g/day, median (IQR) | 25.3 (13.2-43.8) | 28.2 (13.6-49.0) | .37 |
| Red meat intake, g/day, median (IQR) | 44.3 (24.2-73.8) | 46.3 (24.3-73.9) | .90 |
| Processed meat intake, g/day, median (IQR) | 25.1 (13.1-41.1) | 23.6 (12.6-41.5) | .26 |
| Physical activity | |||
| Total physical activity, MET-hours/week, median (IQR) | 81.0 (4.1-116.5) | 83.6 (49.8-114.5) | .02 |
| High physical activityc, % | 34.6 | 39.5 | 0.04 |
| Adiposity status | |||
| BMI, kg/m2, mean (SD) | 26.8 (4.4) | 26.2 (4.0) | .05 |
| Waist circumference, cm, mean (SD) | 90.2 (12.9) | 87.9 (11.8) | <.0001 |
| Biomarkers | |||
| Glucose metabolism | |||
| HbA1c, %, median (IQR) | 5.9 (5.6-6.0) | 5.8 (5.6-5.8) | .0007 |
| C-peptide, ng/mL, median (IQR) | 4.8 (3.1-5.4) | 4.6 (3.3-4.6) | .26 |
| Insulin growth factor axis | |||
| IGF-1, mg/L, median (IQR) | 213.6 (174.0-243.2) | 211.8 (189.7-221.3) | .43 |
| IGFBP1, ng/mL, median (IQR) | 14.9 (5.9-15.5) | 16.5 (10.6-16.5) | .32 |
| IGFBP2, ng/mL, median (IQR) | 440.8 (274.5-444.8) | 437.9 (356.8-437.9) | .56 |
| IGFBP3, ng/mL, median (IQR) | 4285.0 (3986.9-4558.7) | 4244.3 (4232.4-4277.1) | .76 |
| Adipokine secretion | |||
| Adiponectin, μg/mL, median (IQR) | 6.8 (4.8-9.3) | 6.8 (5.0-9.4) | .12 |
| HMW-adiponectin, μg/mL, median (IQR) | 3.5 (2.2-5.2) | 3.5 (2.2-5.2) | .95 |
| Non-HMW-adiponectin, μg/mL, median (IQR) | 3.2 (2.5-4.1) | 3.4 (2.6-4.2) | <.0001 |
| Leptin, ng/mL, median (IQR) | 9.7 (4.9-18.5) | 8.9 (4.8-18.7) | .25 |
| sOB-R, ng/mL, median (IQR) | 20.2 (16.1-24.1) | 21.2 (17.4-26.3) | <.0001 |
| Lipid metabolism | |||
| HDL-cholesterol, mmol/L, median (IQR) | 1.37 (1.11-1.67) | 1.42 (1.18-1.73) | .001 |
| Triglycerides, mmol/L, median (IQR) | 1.42 (1.01-2.15) | 1.40 (0.96-2.00) | .05 |
| Cholesterol, mmol/L, median (IQR) | 6.28 (5.5-7.1) | 6.40 (5.6-7.2) | 0.07 |
| LDL-cholesterol, mmol/L, median (IQR) | 4.2 (3.5-4.8) | 4.2 (3.6-4.9) | 0.84 |
| Oxidative stress | |||
| ROM, U/mL, median (IQR) | 400.0 (350.0-450.0) | 382.5 (336.0-428.0) | <.0001 |
| FRAP, μmol/L, median (IQR) | 1046.0 (868.0-1223.0) | 1018.0 (860.0-1188.0) | .05 |
| Vitamin D metabolism | |||
| 25[OH]D, nmol/L, median (IQR) | 53.8 (39.2-71.3) | 58.0 (43.6-78.2) | .0001 |
| PTH, pg/mL, median (IQR) | 31.1 (19.1-49.6) | 30.4 (19.8-43.1) | .07 |
| Inflammation and immune response | |||
| CRP, mg/L, median (IQR) | 3.3 (1.3-5.2) | 2.6 (1.2-4.3) | .01 |
| Neopterin, nmol/L, median (IQR) | 19.2 (11.3-24.5) | 20.9 (17.0-22.1) | <.0001 |
P-values for difference between cases and controls were determined by Student’s paired t-test for variables expressed as means; by Wilcoxon’s signed rank test for variables expressed as medians, by Mc Nemar’s test and Bowker's test of symmetry for variables expressed as percentages.
Sex, age, postmenopausal status and hormonal therapy use were among the matching criteria.
High physical activity is defined as >91 MET-hours per week of total activity using a 75th percentile of the variable distribution in the controls as cut-point.
Abbreviations: N, number; IQR, interquartile range; BMI, body mass index; SD, standard deviation; CRP, C-reactive protein; HbA1c, glycated haemoglobin; IGF-1, insulin growth factor; IGFBP, insulin-like growth factor-binding protein; HMW-adiponectin, high-molecular weight adiponectin; sOB-R, soluble leptin receptor; HDL-C, high density lipoprotein-cholesterol; LDL-cholesterol, low density lipoprotein cholesterol; ROM, Reactive oxygen metabolites; FRAP, Ferric reducing ability of plasma; PTH, Parathyroid hormone; 25[OH]D, 25-hydroxy-vitamin D.
In a multivariable-adjusted model, high physical activity was associated with a lower risk of colon cancer compared to low-medium physical activity (cut point of MET variable distribution at 75th percentile, RR = 0.75 (95% CI: 0.57-0.96; P = 0.0001 [Mediation analysis Step 1].
Table 2 presents the Point-biserial correlations of high physical activity with potentially mediating factors and among controls [Mediation analysis Step 2]. After adjustment for age, sex, education and lifestyle factors, high physical activity was inversely correlated with waist circumference, C-peptide, PTH, CRP and neopterin, whereas positive correlations were observed with IGFBP-1, sOB-R, LDL-cholesterol and 25[OH]D. Overall, the correlation coefficients were of weak to moderate magnitude.
Table 2. Point-biserial correlation coefficients (rpb-s)a and 95% confidence intervals for high physical activityb and individual mediating factors (incl. measures of adiposity and biomarkers of various pathways) among controls (n=713).
| Mediating factors | High Physical Activity | ||
|---|---|---|---|
| rpb | 95%CI | P-value | |
| Adiposity status | |||
| BMI, kg/m2 | -0.06 | -0.14 to 0.00 | 0.06 |
| Waist circumference, cm | -0.20 | -0.27 to 0.13 | <0.0001 |
| Glucose metabolism | |||
| HbA1c, % | -0.04 | -0.11 to 0.03 | 0.26 |
| C-peptide, ng/mL | -0.11 | -0.11 to -0.03 | 0.003 |
| Insulin growth factor axis | |||
| IGF1, mg/L | -0.008 | -0.08 to 0.06 | 0.81 |
| IGFBP1, ng/mL | 0.11 | 0.03 to 0.18 | 0.003 |
| IGFBP2, ng/mL | 0.05 | -0.02 to 0.12 | 0.15 |
| IGFBP3, ng/mL | 0.02 | -0.05 to 0.09 | 0.55 |
| Adipokine secretion | |||
| Adiponectin, μg/mL | -0.01 | -0.08 to 0.06 | 0.73 |
| HMW-adiponectin, μg/mL | -0.01 | -0.08 to 0.06 | 0.74 |
| Non-HMW-adiponectin, μg/mL | 0.02 | -0.04 to 0.10 | 0.47 |
| Leptin, ng/mL | -0.03 | -0.11 to 0.03 | 0.33 |
| sOB-R, ng/mL | 0.10 | 0.02 to 0.16 | 0.02 |
| Lipid metabolism | |||
| HDL-cholesterol, mmol/L | -0.05 | -0.11 to 0.03 | 0.32 |
| Triglycerides, mmol/L | 0.02 | -0.04 to 0.10 | 0.48 |
| Cholesterol, mmol/L | 0.03 | -0.03 to 0.11 | 0.31 |
| LDL-cholesterol, mmol/L | 0.08 | 0.01 to 0.16 | 0.02 |
| Oxidative stress | |||
| ROM, U/m L | 0.03 | -0.03 to 0.11 | 0.29 |
| FRAP, μmol/L | 0.04 | -0.02 to 0.12 | 0.21 |
| Vitamin D metabolism | |||
| 25[OH]D, nmol/L | 0.10 | 0.01 to 0.16 | 0.01 |
| PTH, pg/mL | -0.09 | -0.15 to -0.01 | 0.02 |
| Inflammation and immune response | |||
| CRP, mg/L | -0.08 | -0.15 to -0.007 | 0.03 |
| Neopterin, nmol/L | -0.06 | -0.10 to 0.00 | 0.05 |
Adjusted for age at study recruitment, sex, education (no school degree or primary school, technical or professional school, secondary school, university degree, or unknown), smoking status (never, past, current, or unknown), alcohol intake (continuous), fruit and vegetable intake (g/day), fish and shellfish intake (g/day), fibre intake (g/day), red and processed meat intake (g/day),
High physical activity is defined as >91 MET-hours per week of total activity using a 75th percentile of the variable distribution in the controls as cut-point.
Abbreviations: MET, metabolic equivalent of task; BMI, body mass index; CRP, C-reactive protein; HbA1c, glycated haemoglobin; IGF-1, insulin growth factor; IGFBP, insulin-like growth factor-binding protein HMW-adiponectin, high-molecular weight adiponectin; sOB-R, soluble leptin receptor; HDL-C, high density lipoprotein-cholesterol; LDL-cholesterol, low density lipoprotein cholesterol; ROM, reactive oxygen metabolites; FRAP, ferric reducing ability of plasma; PTH, parathyroid hormone; 25[OH]D, 25-hydroxy-vitamin D.
Table 3 presents the multivariable-adjusted relative risks of colon cancer associated with individual mediating factors additionally adjusted for physical activity [Mediation analysis Step 3]. Corresponding to previously published data, most of the investigated biomarkers were associated with risk of colon cancer in the expected direction. For neopterin, a non-linear risk association was observed.
Table 3. Multivariable-adjusted incidence rate ratiosa and 95% confidence intervals for the association between individual mediating factors (incl. measures of adiposity and biomarkers of various pathways) and risk of colon cancer.
| Biomarkers | IRR | 95% CI | P-value |
|---|---|---|---|
| Adiposity status | |||
| High BMI, kg/m2b | 1.40 | 1.04 to 1.87 | .01 |
| High waist circumference, cmc | 1.53 | 1.20 to 1.95 | .005 |
| Metabolic dysfunction | |||
| HbA1c, % | 3.77 | 1.47 to 9.64 | .004 |
| C-peptide, ng/mL | 1.03 | 0.85 to 1.24 | .74 |
| Insulin growth factor axis | |||
| IGF-1, mg/L | 0.97 | 0.73 to 1.27 | .87 |
| IGFBP, ng/mL | 0.79 | 0.71 to 0.88 | <.0001 |
| IGFBP2, ng/mL | 0.91 | 0.79 to 1.06 | .15 |
| IGFBP3, ng/mL | 1.07 | 0.64 to 1.80 | .66 |
| Adipokine secretion | |||
| Adiponectin, μg/mL | 0.86 | 0.71 to 1.02 | .09 |
| HMW-adiponectin, μg/mL | 0.99 | 0.96 to 1.02 | .60 |
| Non-HMW-adiponectin, μg/mL | 0.65 | 0.52 to 0.82 | 0.0002 |
| Leptin, ng/mL | 1.09 | 0.98 to 1.20 | .10 |
| sOB-R, ng/mL | 0.57 | 0.46 to 0.73 | <.0001 |
| Lipid metabolism | |||
| HDL-cholesterol, mmol/L | 0.64 | 0.48 to 0.85 | .002 |
| Triglycerides, mmol/L | 1.13 | 0.97 to 1.31 | .10 |
| Cholesterol, mmol/L | 0.82 | 0.54 to 1.25 | .27 |
| LDL-cholesterol, mmol/L | 0.90 | 0.67 to 1.21 | .39 |
| Oxidative stress | |||
| ROM, U/mL | 2.47 | 1.59 to 3.84 | <.0001 |
| FRAP, μmol/L | 1.26 | 0.88 to 1.80 | .20 |
| Vitamin D metabolism | |||
| 25[OH]D, nmol/L | 0.71 | 0.60 to 0.85 | 0.0002 |
| PTH, pg/mL | 1.00 | 0.99 to 1.01 | .15 |
| Inflammation and immune response | |||
| CRP, mg/L | 1.07 | 1.00 to 1.15 | .06 |
| Neopterin, nmol/Ld | - | - | - |
The multivariable-adjusted model in conditional logistic regression takes into account matching factors: age, sex, study centre, follow-up time since blood collection, time of the day at blood collection and fasting status; in women further matching by menopausal status, phase of menstrual cycle at blood collection and in postmenopausal women hormonal replacement therapy use. The model is further adjusted for education (no school degree or primary school, technical or professional school, secondary school, university degree, or unknown), smoking status (never, past, current, or unknown), alcohol intake (continuous), fruit and vegetable intake (g/day), fish and shellfish intake (g/day), fibre intake (g/day), red and processed meat intake (g/day) and high physical activity (>91 MET-hours per week of total activity).
Defined based on BMI≥30kg/m2.
Defined based on waist circumference ≥94 in men and ≥80 cm according to established cut-points for European populations.
Data examination revealed a non-linear association for neopterin and colon cancer in a restricted cubic spline regression model (P non-linearity <0.0001) which precludes continuous data modeling.
Abbreviations: IRR, incidence rate ratio; CI, confidence intervals; CRP, C-reactive protein; HbA1c, glycated haemoglobin; IGF-1, insulin growth factor; IGFBP, insulin-like growth factor-binding protein; HMW-adiponectin, high-molecular weight adiponectin; sOB-R, soluble leptin receptor; HDL-C, high density lipoprotein-cholesterol; LDL-cholesterol, low density lipoprotein cholesterol; ROM, reactive oxygen metabolites; FRAP, ferric reducing ability of plasma; PTH, parthyroid hormone; parathyroid hormone; 25[OH]D, 25-hydroxy-vitamin D.
Table 4 presents the results from the analyses aimed to quantify mediating effects of individual biomarkers [Mediation analysis Step 4]. These analyses revealed that the observed association between physical activity and colon cancer was accounted for by waist circumference [PEE = 17% (95% CI: 4%; 52%)], and the biomarkers 25[OH]D [PEE = 30% (95% CI: 12%; 88%)] and sOB-R [PEE = 15% (95% CI: 1%; 50%)]. In combination, these factors explained 45% (95% CI: 20%; 125%) of the association. We further tested whether biomarkers statistically explain the association beyond waist circumference. In these analyses, sOB-R, 25[OH]D and neopterin additionally explained 10% (95% CI: 1%; 56%) and 23% (95% CI: 6%; 111%) of the association, respectively.
Table 4. Incidence rate ratios (95% confidence intervals) for colon cancer risk associated with high physical activitya and proportion explained effect after adjustment for individual biomarkers.
| Biomarkers | IRR | 95% CIc | ß | PEEd | 95% CIe |
|---|---|---|---|---|---|
| Multivariable modelb | 0.75 | 0.57 to 0.96 | -0.29 | ||
| Adiposity status | |||||
| BMI, kg/m2 | 0.76 | 0.59 to 0.98 | -0.28 | 6 | -2 to 22 |
| Waist circumference, cm | 0.78 | 0.60 to 1.02 | -0.24 | 17 | 4 to 52 |
| Metabolic dysfunction | |||||
| HbA1c, % | 0.75 | 0.58 to 0.97 | -0.27 | 5 | -6 to 24 |
| C-peptide, ng/mL | 0.75 | 0.58 to 0.97 | -0.28 | 1 | -4 to 8 |
| Insulin growth factor axis | |||||
| IGF-1, mg/L | 0.75 | 0.57 to 0.96 | -0.27 | 4 | -2 to 14 |
| IGFBP1, ng/mL | 0.76 | 0.58 to 0.98 | -0.27 | 6 | -3 to 24 |
| IGFBP2, ng/mL | 0.75 | 0.57 to 0.96 | -0.29 | 2 | -11 to 6 |
| IGFBP3, ng/mL | 0.75 | 0.58 to 0.97 | -0.29 | 1 | -11 to 20 |
| Adipokine secretion | |||||
| Adiponectin, μg/mL | 0.75 | 0.58 to 0.96 | -0.29 | 0 | -6 to 8 |
| HMW-adiponectin, μg/mL | 0.75 | 0.58 to 0.96 | -0.29 | 0 | -2 to 2 |
| Non-HMW-adiponectin, μg/mL | 0.75 | 0.57 to 0.96 | -0.29 | 0 | -13 to 18 |
| Leptin, ng/mL | 0.75 | 0.58 to 0.98 | -0.28 | 6 | -1 to 20 |
| sOB-R, ng/mL | 0.77 | 0.60 to 1.01 | -0.25 | 15 | 1 to 50 |
| Lipid metabolism | |||||
| HDL-cholesterol, mmol/L | 0.76 | 0.58 to 0.98 | -0.27 | 7 | -3 to 28 |
| Triglycerides, mmol/L | 0.75 | 0.58 to 0.96 | -0.29 | 0 | -9 to 6 |
| Cholesterol, mmol/L | 0.75 | 0.58 to 0.96 | -0.29 | 0 | -9 to 6 |
| LDL-cholesterol, mmol/L | 0.75 | 0.58 to 0.96 | -0.29 | 0 | -9 to 6 |
| Oxidative stress | |||||
| ROM, U/mL | 0.74 | 0.57 to 0.96 | -0.30 | 0 | -19 to 15 |
| FRAP, μmol/L | 0.74 | 0.57 to 0.96 | -0.29 | 0 | -10 to 5 |
| Vitamin D metabolism | |||||
| 25[OH]D, nmol/L | 0.81 | 0.62 to 1.05 | -0.20 | 30 | 12 to 88 |
| PTH, pg/mL | 0.75 | 0.58 to 0.97 | -0.28 | 4 | -1 to 15 |
| Inflammation and immune response | |||||
| CRP, mg/L | 0.75 | 0.58 to 0.97 | -0.28 | 3 | -2 to11 |
| Neopterin, nmol/L | 0.68 | 0.52 to 0.88 | -0.38 | -30 | -91 to -8 |
High physical activity is defined as >91 MET-hours per week of total activity using a 75th percentile of the variable distribution in the controls as cut-point.
The multivariable-adjusted model in conditional logistic regression takes into account matching factors: age, sex, study centre, follow-up time since blood collection, time of the day at blood collection and fasting status; in women further matching by menopausal status, phase of menstrual cycle at blood collection and in postmenopausal women hormonal replacement therapy use. The model is further adjusted for education (no school degree or primary school, technical or professional school, secondary school, university degree, or unknown), smoking status (never, past, current, or unknown), alcohol intake (continuous), fruit and vegetable intake (g/day), fish and shellfish intake (g/day), fibre intake (g/day), red and processed meat intake (g/day).
The β-coefficient (regression coefficient) is the natural log of the relative risk estimate.
The proportion explained effect (PEE) denotes percent change in the regression coefficients with adjustment for each additional biomarker compared with the multivariable-adjusted model.
The corresponding 95% confidence interval (CI) of PEE was calculated based on the Fieller’s theorem.
Abbreviations: IRR, incidence rate ratio; CI, confidence interval; PEE, proportion explained effect; BMI, body mass index; CRP, C-reactive protein; HbA1c, glycated haemoglobin; IGF-1, insulin growth factor; IGFBP, insulin-like growth factor-binding protein; sOB-R, soluble leptin receptor; HDL-C, high density lipoprotein-cholesterol; LDL-cholesterol, low density lipoprotein cholesterol; ROM, reactive oxygen metabolites; FRAP, ferric reducing ability of plasma; PTH, parathyroid hormone; 25[OH]D, 25-hydroxy-vitamin D.
We additionally evaluated whether reported dietary vitamin D intakes could explain the association beyond circulating concentrations of 25[OH]D, however adding this variable to the model did not virtually change effect estimates [PEE =1% (95% CI: -11; 3)]. Further, a pronounced mediation effect was observed for the immune response biomarker neopterin, albeit influencing risk estimates in the opposite direction [PEE = -30%]. Beyond waist circumference neopterin further explained 34% of the association. In order to understand the opposing mediation effect for neopterin, we evaluated the mediating effects of very low and very high neopterin levels defined based on the lowest and highest deciles of neopterin distribution in controls. When the respective variables were added to the multivariable adjusted model, we observed a negative mediation effect for the lowest neopterin level [PEE =-35% (95% CI: -119; - 1)], whereas a positive mediation effect for the highest level [PEE =29% (95% CI: -1; 100)].
In additional analyses aimed to evaluate potential exposure-mediator interactions, no statistically significant interaction on the multiplicative scale was revealed between physical activity and biomarkers in relation to colon cancer risk. In sensitivity analyses, no substantial differences in main results were observed after excluding people diagnosed with cancer during the first two years of study follow-up, participants with diagnosed diabetes or with reported cardio-vascular problem at study baseline, non-fasting participants and dietary supplement users (data not shown).
Discussion
These data suggest that the inverse association between physical activity and colon cancer risk could be accounted for by the potential beneficial actions of physical activity on reducing abdominal fatness and modulating metabolic and immune status. Furthermore, induced vitamin D synthesis could play an essential role as an explanatory mechanism. To our knowledge, this is the first attempt to evaluate the role of various potentially mediating factors on the association between physical activity and colon cancer, thereby bridging mechanistic insights with epidemiological evidence.
Numerous studies have examined associations between physical activity and cancer risk confirming reduced risks for colon cancer [5, 37]. The beneficial effects of physical activity in controlling weight have been most commonly suggested explanation for this association [38]. However, our data challenged this common notion putting forward more specific explanatory mechanisms.
In our analyses the association between physical activity and colon cancer risk changed little when adjusted for body mass index. In contrast, a mediating role has been revealed for waist circumference. Despite highly correlated, BMI and waist circumference represent different obesity phenotypes related to body composition and body fat distribution and have been differentially associated with colon cancer. Epidemiological studies, including EPIC data, consistently revealed a prominent role of waist circumference as compared to BMI for the risk of colon cancer [38, 39]. Waist circumference is an indicator for abdominal obesity closely associated with insulin resistance. In this context, regular physical activity was particularly suggested to reduce waist circumference and induce insulin sensitivity independent of changes in body weight [40–42]. For example, an intervention trial among sedentary individuals reported that physical activity without weight loss increased insulin sensitivity and favorably modulated other metabolic features [40]. Randomized control trials further demonstrated that weight loss induced by increased daily physical activity without caloric restriction substantially reduce abdominal obesity and insulin resistance in obese men [42] and women [43].. The specific effects of physical activity in reducing central obesity was further demonstrated in a a recent meta-analysis of 13 clinical trials [44]. A favorable role of physical activity on various metabolic features was also supported by two meta-analyses of prospective cohort studies [45, 46]. However, whether additional metabolic factors play a special role in the link between physical activity and colon cancer beyond abdominal obesity per se is less studied. We found this to be the case for sOB-R.
sOb-R binds to leptin in human blood and modulates its bioavailability depending on certain metabolic conditions [47], [48]. Our previous work has shown that lower sOB-R concentrations are associated with a higher risk of colon cancer independent of obesity [27], as well as suggested sOB-R to be an important statistical mediator of the association of colon cancer with waist circumference and weight gain in adult life [49]. Of note, no such links have been revealed for BMI and leptin [27, 49]. Here, we further report on a cross-sectional positive association between sOB-R and physical activity; however, more research is needed to identify specific physical activity interventions on circulating sOB-R and understand how physical activity may influence the interplay between adipose tissue hormones and cancer.
Remarkably, our work further highlighted the role of vitamin D as an important mediator of the association between physical activity and colon cancer, acting beyond observed mediating effects of waist circumference. Previous research has highlighted the relationship between vitamin D levels and sun exposure during outdoor physical activity [13]. At the same time, leisure-time physical activity was recently suggested to play a particularly important role in colorectal cancer in a study pooling data on 1.44 million people from the United States and Europe [50]. Since the major source of vitamin D in human circulation is exposure to natural sunlight, herewith we speculate that the observed mediating role of vitamin D in the risk for colon cancer may be due to increased levels of outdoor recreational activity [51]. However, in the EPIC cohort, none of the different types of physical activity considered (occupational, household or recreational) independently explained the inverse association with colon cancer[6]. More sensitive analyses are therefore needed to elucidate the relationship between different types of outdoor physical activity, vitamin D status and colon cancer risk. In mechanistic studies, vitamin D was suggested to exert a number of anti-carcinogenic effects, including decreasing cell proliferation and enhancing cell differentiation, inhibiting the growth of new blood vessels, and exerting anti-inflammatory and immune-regulatory properties [51, 52]. Although more research is needed, promoting physical activity, especially outdoors, and assuring adequate vitamin D levels seem to represent promising strategies for colon cancer prevention.
Finally, our data also suggested that neopterin - a biomarker of cell-mediated immunity - mediates the association between physical activity and colon cancer in an opposing manner. This statistical phenomenon known as “inconsistent mediation” might reflect subgroups within the study population for whom the mediating effects are in opposite directions [53]. We previously reported a J-shaped relationship between neopterin and colon cancer risk, with both very low (immune suppression) and very high (immune activation) neopterin concentrations associating with a higher risk of colon cancer [31]. A similar J-shaped association was suggested for physical activity and immune response in intervention studies, such that exhaustive physical activity was shown to transiently suppress immune activity, whereas physical activity at moderate intensity was shown to enhance immune function [12]. Thus, it may be speculated that extreme physical activity levels could rather lead to suppressed immune activity, and thereby an increased risk of colon cancer. Unfortunately, intensity of physical activity was not elaborately assessed in our questionnaires. The interpretation of these data is further complicated by the complex and insufficient evidence on the role of immunity and physical activity in colon cancer. More detailed analyses are needed to evaluate the complex interplay between immunity and cancer risk taking into account intensity, duration and type of physical activity.
Our results should be interpreted in the context of inherent assumptions of statistical mediation analysis. These analyses were introduced in psychological and social sciences and further adapted in epidemiology within the framework of causal analysis. Under valid causal assumptions based on scientific knowledge, mediation analysis addresses the questions of how and why exposure and outcomes of interest are related. However, inherent limitations of mediation analysis approach such as bias that may arise from the validity of mediator, potential for uncontrolled confounding, lack of statistical power or insufficient sample size and exposure-mediator interaction in the model may influence study conclusions. Our study also has other limitations that should be considered. Physical activity was assessed based on self-reported data. However, given the prospective study design, errors in physical activity are likely to be non-differential with respect to subsequent colon cancer status. We measured biomarkers at a single time point, which may not reflect habitual long-term levels. Nevertheless, in reproducibility studies, evaluated biomarkers have proven to be relatively stable over time. Furthermore, laboratory assessment method and assay performance could introduce measurement error in evaluated biomarkers and hereby influenced observed mediation effects. However, we evaluated biomarkers measured with established bioassays with low analytical variation. The presence of mediator–exposure interaction can also influence the results [54]; however in our data no interactions were observed for physical activity and biomarkers in relation to colon cancer risk.It should be also noted that the observed mediation effect sizes were relatively small. This may not be surprising due to the moderate magnitude of the main association. Similarly, weak to moderate correlations were observed for physical activity with biomarkers of interest. Future studies are needed to replicate these data and quantify mediation effect sizes in other populations. The presence of mediator–exposure interaction can also influence the results [54]; however in our data no significant interaction was observed for physical activity and biomarkers in relation to colon cancer risk.
Finally, it should be acknowledged that the mediators quantified in our analyses may merely be statistical intermediates not essentially on the same causal pathway as physical activity. In this regard, biological plausibility rather than merely statistical evaluation should be leading in the interpretation of mediation analysis findings.
The main strengths of our study include the prospective design, long duration of follow-up, detailed information on physical activity and a number of potential confounders, the wide range of measured biomarkers addressing several potentially causal pathways, and the inclusion of diverse population groups across Europe.
In conclusion, our findings provide important lines of evidence in understanding the links between physical activity and risk of colon cancer and suggest that this association could be at least partially explained by the beneficial effects of physical activity on reducing abdominal adiposity, and influences on biomarkers of metabolic dysfunction, immunity and vitamin D metabolism. Promoting physical activity, particularly outdoors, and maintaining metabolic health and adequate vitamin D levels could therefore represent a promising strategy for colon cancer prevention.
Supplementary Material
Key messages.
The inverse association between physical activity and colon cancer risk could be partially explained by specific properties of physical activity on reducing abdominal fatness and modulating biomarkers of metabolic dysfunction, immunity and vitamin D metabolism.
The study provides valid means for utilizing mediation analysis approach in molecular epidemiology research study setting aimed to provide a mechanistic understanding of lifestyle-disease associations.
Despite methodological challenge facing the mediation analysis approach, the study demonstrates that well-phenotyped biomarker studies are able to provide novel insights on causality and strengthen the evidence-base for the formulation of targeted disease prevention strategies.
The practical means of the study highlight the promotion of physical activity, particularly outdoors, and maintaining metabolic health and adequate vitamin D levels as promising targets for colon cancer prevention.
Funding
This work was supported by the German Research Foundation (DFG) Grant AL 1784/3-1 which has funded the research position of Dr. Aleksandrova for the time of study conduct and analysis The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l'Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM; France); Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); Hellenic Health Foundation (Greece); Italian Association for Research on Cancer (AIRC) and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); ERC-2009-AdG 232997 and Nordforsk, Nordic Centre of Excellence programme on Food, Nutrition and Health (Norway); Health Research Fund (FIS), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Scientific Council and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK, Medical Research Council (United Kingdom).
Notes
None of the funders/sponsors had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors disclose no potential conflicts of interest related to this study.
We would like to express special thanks to Ellen Kohlsdorf (EPIC-Potsdam, Germany) and Bertrand Hemon (IARC-France) for their work on data management and technical assistance. We thank all participants in the EPIC study for their outstanding cooperation. Persons named in the acknowledgement have confirmed their agreement.
Data sharing: For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php
References
- 1.Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2012;104(20):1548–61. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- 2.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. British journal of cancer. 2009;100(4):611–6. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang D, Wu Y, Jiang W, Jiang X. Re: Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2013;105(10):746–7. doi: 10.1093/jnci/djt068. [DOI] [PubMed] [Google Scholar]
- 4.de Vries E, Soerjomataram I, Lemmens VE, Coebergh JW, Barendregt JJ, Oenema A, et al. Lifestyle changes and reduction of colon cancer incidence in Europe: A scenario study of physical activity promotion and weight reduction. European journal of cancer. 2010;46(14):2605–16. doi: 10.1016/j.ejca.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. Bmj. 2016;354:i3857. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedenreich C, Norat T, Steindorf K, Boutron-Ruault MC, Pischon T, Mazuir M, et al. Physical activity and risk of colon and rectal cancers: the European prospective investigation into cancer and nutrition. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(12):2398–407. doi: 10.1158/1055-9965.EPI-06-0595. [DOI] [PubMed] [Google Scholar]
- 7.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. The Journal of nutrition. 2002;132(11 Suppl):3456S–64S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 8.McTiernan A. Mechanisms linking physical activity with cancer. Nature reviews Cancer. 2008;8(3):205–11. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 9.Nindl BC. Insulin-like growth factor-I, physical activity, and control of cellular anabolism. Medicine and science in sports and exercise. 2010;42(1):35–8. doi: 10.1249/MSS.0b013e3181b07c39. [DOI] [PubMed] [Google Scholar]
- 10.Chin SH, Kahathuduwa CN, Binks M. Physical activity and obesity: what we know and what we need to know. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2016 doi: 10.1111/obr.12460. [DOI] [PubMed] [Google Scholar]
- 11.Huang CJ, Zourdos MC, Jo E, Ormsbee MJ. Influence of physical activity and nutrition on obesity-related immune function. TheScientificWorldJournal. 2013;2013 doi: 10.1155/2013/752071. 752071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romeo J, Warnberg J, Pozo T, Marcos A. Physical activity, immunity and infection. The Proceedings of the Nutrition Society. 2010;69(3):390–9. doi: 10.1017/S0029665110001795. [DOI] [PubMed] [Google Scholar]
- 13.Hibler EA, Sardo Molmenti CL, Dai Q, Kohler LN, Warren Anderson S, Jurutka PW, et al. Physical activity, sedentary behavior, and vitamin D metabolites. Bone. 2016;83:248–55. doi: 10.1016/j.bone.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanner M, Richard A, Martin B, Linseisen J, Rohrmann S. Associations between objective and self-reported physical activity and vitamin D serum levels in the US population. Cancer causes & control : CCC. 2015;26(6):881–91. doi: 10.1007/s10552-015-0563-y. [DOI] [PubMed] [Google Scholar]
- 15.Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(Suppl 1):S6–14. doi: 10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 16.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–24. doi: 10.1079/PHN2002394. S1368980002001350 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Haftenberger M, Lahmann PH, Panico S, Gonzalez CA, Seidell JC, Boeing H, et al. Overweight, obesity and fat distribution in 50- to 64-year-old participants in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002;5(6B):1147–62. doi: 10.1079/PHN2002396. [DOI] [PubMed] [Google Scholar]
- 18.Haftenberger M, Schuit AJ, Tormo MJ, Boeing H, Wareham N, Bueno-de-Mesquita HB, et al. Physical activity of subjects aged 50-64 years involved in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public health nutrition. 2002;5(6B):1163–76. doi: 10.1079/PHN2002397. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Medicine and science in sports and exercise. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 20.Bingham S, Riboli E. Diet and cancer--the European Prospective Investigation into Cancer and Nutrition. Nature reviews Cancer. 2004;4(3):206–15. doi: 10.1038/nrc1298. [DOI] [PubMed] [Google Scholar]
- 21.Rinaldi S, Rohrmann S, Jenab M, Biessy C, Sieri S, Palli D, et al. Glycosylated hemoglobin and risk of colorectal cancer in men and women, the European prospective investigation into cancer and nutrition. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(11):3108–15. doi: 10.1158/1055-9965.EPI-08-0495. [DOI] [PubMed] [Google Scholar]
- 22.Jenab M, Riboli E, Cleveland RJ, Norat T, Rinaldi S, Nieters A, et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. International journal of cancer Journal international du cancer. 2007;121(2):368–76. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 23.Rinaldi S, Cleveland R, Norat T, Biessy C, Rohrmann S, Linseisen J, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. International journal of cancer Journal international du cancer. 2010;126(7):1702–15. doi: 10.1002/ijc.24927. [DOI] [PubMed] [Google Scholar]
- 24.Aleksandrova K, Jenab M, Boeing H, Jansen E, Bueno-de-Mesquita HB, Rinaldi S, et al. Circulating C-reactive protein concentrations and risks of colon and rectal cancer: a nested case-control study within the European Prospective Investigation into Cancer and Nutrition. American journal of epidemiology. 2010;172(4):407–18. doi: 10.1093/aje/kwq135. [DOI] [PubMed] [Google Scholar]
- 25.Aleksandrova K, Boeing H, Jenab M, Bueno-de-Mesquita HB, Jansen E, van Duijnhoven FJ, et al. Total and high-molecular weight adiponectin and risk of colorectal cancer: the European Prospective Investigation into Cancer and Nutrition Study. Carcinogenesis. 2012;33(6):1211–8. doi: 10.1093/carcin/bgs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2011;60(8):1094–102. doi: 10.1136/gut.2010.225011. [DOI] [PubMed] [Google Scholar]
- 27.Aleksandrova K, Boeing H, Jenab M, Bueno-de-Mesquita HB, Jansen E, van Duijnhoven FJ, et al. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer research. 2012;72(20):5328–37. doi: 10.1158/0008-5472.CAN-12-0465. [DOI] [PubMed] [Google Scholar]
- 28.Leufkens AM, van Duijnhoven FJ, Woudt SH, Siersema PD, Jenab M, Jansen EH, et al. Biomarkers of oxidative stress and risk of developing colorectal cancer: a cohort-nested case-control study in the European Prospective Investigation Into Cancer and Nutrition. American journal of epidemiology. 2012;175(7):653–63. doi: 10.1093/aje/kwr418. [DOI] [PubMed] [Google Scholar]
- 29.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. Bmj. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedirko V, Riboli E, Bueno-de-Mesquita HB, Rinaldi S, Pischon T, Norat T, et al. Prediagnostic circulating parathyroid hormone concentration and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(5):767–78. doi: 10.1158/1055-9965.EPI-10-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aleksandrova K, Chuang SC, Boeing H, Zuo H, Tell GS, Pischon T, et al. A prospective study of the immune system activation biomarker neopterin and colorectal cancer risk. Journal of the National Cancer Institute. 2015;107(4) doi: 10.1093/jnci/djv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 33.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual review of psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11(2):167–78. doi: 10.1002/sim.4780110204. [DOI] [PubMed] [Google Scholar]
- 36.Fieller E. The biological standardization of insulin. J Roy Stat Soc. 1940;7(Supplement):1–15. [Google Scholar]
- 37.Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(7):1018–28. doi: 10.1158/1055-9965.EPI-16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleksandrova K, Nimptsch K, Pischon T. Influence of Obesity and Related Metabolic Alterations on Colorectal Cancer Risk. Current nutrition reports. 2013;2(1):1–9. doi: 10.1007/s13668-012-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aleksandrova K, Nimptsch K, Pischon T. Obesity and colorectal cancer. Frontiers in bioscience. 2013;5:61–77. doi: 10.2741/e596. [DOI] [PubMed] [Google Scholar]
- 40.Duncan GE, Perri MG, Theriaque DW, Hutson AD, Eckel RH, Stacpoole PW. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes care. 2003;26(3):557–62. doi: 10.2337/diacare.26.3.557. [DOI] [PubMed] [Google Scholar]
- 41.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. Jama. 2001;286(10):1218–27. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 42.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Annals of internal medicine. 2000;133(2):92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 43.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obesity research. 2004;12(5):789–98. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 44.Lee G, Choi HY, Yang SJ. Effects of Dietary and Physical Activity Interventions on Metabolic Syndrome: A Meta-analysis. Journal of Korean Academy of Nursing. 2015;45(4):483–94. doi: 10.4040/jkan.2015.45.4.483. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, Liu X. Leisure-time physical activity and the risk of metabolic syndrome: meta-analysis. European journal of medical research. 2014;19:22. doi: 10.1186/2047-783X-19-22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.He D, Xi B, Xue J, Huai P, Zhang M, Li J. Association between leisure time physical activity and metabolic syndrome: a meta-analysis of prospective cohort studies. Endocrine. 2014;46(2):231–40. doi: 10.1007/s12020-013-0110-0. [DOI] [PubMed] [Google Scholar]
- 47.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochemical and biophysical research communications. 2001;283(4):982–8. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 48.Schaab M, Kratzsch J. The soluble leptin receptor. Best practice & research Clinical endocrinology & metabolism. 2015;29(5):661–70. doi: 10.1016/j.beem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Aleksandrova K, Drogan D, Boeing H, Jenab M, Bas Bueno-de-Mesquita H, Jansen E, et al. Adiposity, mediating biomarkers and risk of colon cancer in the European prospective investigation into cancer and nutrition study. International journal of cancer. 2014;134(3):612–21. doi: 10.1002/ijc.28368. [DOI] [PubMed] [Google Scholar]
- 50.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA internal medicine. 2016;176(6):816–25. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair R, Maseeh A. Vitamin D: The “ sunshine” vitamin. Journal of Pharmacology & Pharmacotherapeutics. 2012;3(2):118–26. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konijeti GG, Arora P, Boylan MR, Song Y, Huang S, Harrell F, et al. Vitamin D Supplementation Modulates T Cell-Mediated Immunity in Humans: Results from a Randomized Control Trial. The Journal of clinical endocrinology and metabolism. 2016;101(2):533–8. doi: 10.1210/jc.2015-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prevention science : the official journal of the Society for Prevention Research. 2000;1(4):173–81. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.VanderWeele TJ, Shrier I. Sufficient Cause Representation of the Four-way Decomposition for Mediation and Interaction. Epidemiology. 2016;27(5):e32–3. doi: 10.1097/EDE.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.