Abstract
Objectives
To assess paediatricians’ use of genetic testing for children with global developmental delay (GDD).
Study Design
We developed and piloted a questionnaire assessing the use of genetic tests in children with GDD and awareness of relevant guidelines. All practicing Quebec paediatricians were contacted. Paediatricians who did not evaluate children with GDD in their practice were excluded. Descriptive and statistical analyses were performed with SPSS.
Results
Of the 651 paediatricians, 225 answered (34.5%) and 141 were eligible. Only 31.9% were familiar with at least one guideline about genetic tests for the investigation of children with GDD, but 93.6% had ordered genetic testing for children with GDD (Fragile X testing [92.9%], karyotype [87.2%] and chromosomal microarray [63.8%]). Based on vignettes, 20.6% of participants would order genetic tests for isolated GDD and 95.0% for GDD with dysmorphic features and microcephaly. Only 56.7% ordered Fragile X testing for a girl with GDD and a known family history of Fragile X syndrome. Use of tests for isolated GDD was increased in presence of maternal pregnancy, compared with absence of pregnancy (44.7% and 27.7%, respectively). More participants would order genetic tests for a child with GDD and fetal exposure to alcohol (69.5%) than isolated GDD (20.6%).
Conclusions
Even though paediatricians often order genetic testing for children with GDD, practices and knowledge regarding testing are not optimal. As new and more complex genetic tests are developed, up-to-date training about the use of genetic tests for children with GDD needs to be integrated into paediatrics residency programs and continuous medical education.
Keywords: Bioethics, Chromosomal microarray, Clinical ethics, Fragile X, Genetic testing, Global developmental delay
Global developmental delay (GDD) affects 1% of children aged 0 to 4 years in Canada (1). GDD is defined as significant delay in at least two spheres of development, including language, motor development, socialization and adaptive functioning. It can be associated with other conditions (e.g., epilepsy, autism spectrum disorder, etc.) or physical features (e.g., microcephaly, dysmorphic features, etc.) (2–6). GDD is considered isolated when it is not associated with other conditions or physical features.
GDD is due to genetic causes in 17% to 47% of cases and to exogenous causes (teratogens, infections) in 18% to 44% of cases (2). Common genetic causes include chromosomal rearrangements and monogenic diseases. The evaluation of children with GDD is the topic of clinical practice guidelines in the USA (2–6). It is thought that identifying the underlying genetic cause of disease will improve clinical management through anticipatory guidance and better access to appropriate supportive services, and enable parents to make informed reproductive decisions about future pregnancies (7).
Recommendations about the use of genetic tests to investigate children with GDD have been issued by the American Academy of Pediatrics (AAP), the American Academy of Neurology (AAN) and the American College of Medical Genetics (ACMG) (Table 1) (2–6). They are based on expert consensus, relying on available evidence on test diagnostic yields. All guidelines agree on the use of molecular testing for Fragile X syndrome (thereafter mentioned as ‘Fragile X testing’) as a first-line test. Karyotype was considered the first-line test for chromosomal anomalies in children with GDD, but has been replaced by array comparative genomic hybridization (aCGH) in recent guidelines (2–6). Fluorescent in situ hybridization (FISH) for specific microdeletions were considered second-tier tests and have also now been replaced by aCGH. First-line testing (Fragile X testing and now aCGH) is the same for isolated and nonisolated GDD (2–6). For other tests, guidelines vary in their recommendations: the AAN recommends Rett Syndrome testing when clinical presentation is suggestive, and metabolic testing is considered second-tier testing by the AAP and the AAN (2,3).
Table 1.
Summary of recommended genetic investigations for children with global developmental delay: Guidelines available at the time of the study (2013–2014)
| Professional organization | AAP2 | AAN3* | ACMG4–6 |
|---|---|---|---|
| Year | 2006 | 2003 | 2005–2007 |
| Test | |||
| Karyotype | Yes* | Yes | Yes5 |
| aCGH | --* | --* | Yes6 |
| FISH for specific region | -- | -- | If suspected5 |
| Fragile X testing | Yes | Yes | Yes4 |
| Other single gene tests or gene panels | Testing for Rett syndrome may be considered in girls with moderate to severe GDD | -- | |
| Metabolic tests | As second tier, after karyotype and Fragile X testing | Not as first tier | -- |
AAP American Academy of Pediatrics; AAN American Academy of Neurology; ACMG American College of Medical Genetics; aCGH array comparative genomic hybridization; FISH fluorescence in situ hybridization; GDD global developmental delay.
*An evidence report was issued by the AAN in 2011 on genetic and metabolic testing on children with global developmental delay, focusing on the evidence of diagnostic yield of genetic tests, including aCGH. No formal recommendations were made in this evidence report, but the authors concluded that diagnostic yield should be considered in the choice of tests. This report was also endorsed by the AAP.
Despite available guidelines, there is little information about how such tests are used by providers, or about providers’ knowledge of guidelines. In Quebec, karyotype and Fragile X testing have been available for many years, and aCGH started being widely available to paediatricians in 2011.
The goal of this study is to assess paediatricians’ reported use of genetic tests for children with GDD and assess paediatricians’ knowledge of clinical practice guidelines about genetic testing for children with GDD.
METHODS
Participants
All paediatricians (N=651) in the province of Quebec (Canada) were invited to participate by mail. Names and addresses were obtained from publicly available contact information on the Collège des Médecins du Québec website. Paediatricians who reported not evaluating children with GDD as part of their practice were excluded.
Questionnaire
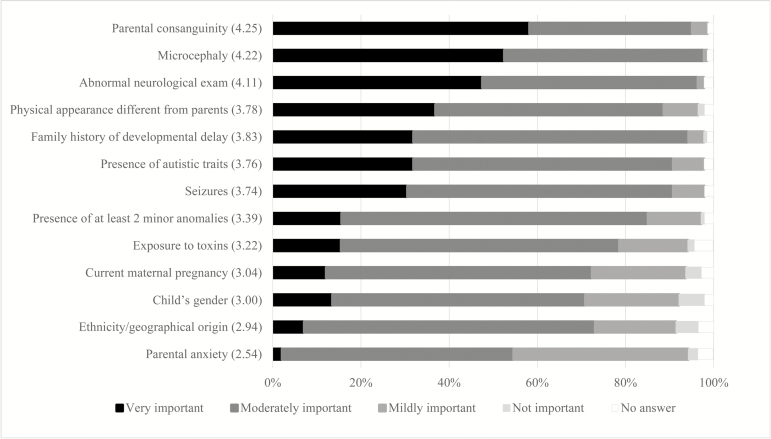
The questionnaire was developed based on a review of professional guidelines on genetic tests in children with GDD. The first version of the questionnaire was pilot-tested by one medical geneticist, two paediatric neurologists, one paediatrician specialized in neurodevelopment and three general paediatricians. Cognitive debriefing was done with each pilot-tester. Based on piloting and feedback, the questionnaire was finalized. It contains 42 questions: 11 on demographic information, 6 on participants’ use of genetic tests, 2 on participants’ perception of the utility of test results, 17 on 8 clinical vignettes, 2 on impact of patient characteristics on test use and 4 on awareness and use of guidelines. It also included a short description of aCGH. Questions were multiple-choice questions, except for questions on utility of test results and impact of patient characteristics for which we used 6-point Likert scales, to avoid neutral results. An option to write-in short answers was offered where appropriate. Vignettes were used to explore participants’ decision-making processes. They illustrate common clinical situations for which there are clear recommendations about testing, as well as situations for which recommendations are unclear. We also included vignettes on nongenetic causes (fetal exposure to alcohol) and contextual or psychosocial factors (parental anxiety, maternal pregnancy) to assess whether test use was influenced by factors that are not suggestive of a genetic etiology. All vignettes were followed by the same questions about the use of genetic tests, proposing the same list of genetic tests (Table 2). Finally, participants were asked to rate the importance of specific clinical characteristics on their decision to order a genetic test for a child with GDD using a 6-point Likert scale (from 0 ‘not important’ to 5 ‘very important’), without a specific clinical situation in mind. For ease of presentation, some categories were combined (1 to 2 and 3 to 4) (Figure 1).
Table 2.
Participants’ decision to order genetic tests based on clinical vignettes of children with global developmental delay (GDD) (n=141)
| Isolated GDD | GDD/behavioural problems | GDD/dysmorphic features and microcephaly | GDD/neurological abnormalities | GDD/family history of fragile X syndrome | Isolated GDD/ maternal pregnancy | Isolated GDD/ parental anxiety | GDD/fetal exposure to alcohol | |
|---|---|---|---|---|---|---|---|---|
| Would order at least one genetic test | 29 (20.6%) | 68 (48.2%) | 134 (95%) | 106 (75.2%) | 120(85.1%) | 63 (44.7%) | 39 (27.7%) | 98 (69.5%) |
| p* | -- | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.1644 | <0.001 |
| Tests the participant would have ordered | ||||||||
| Karyotype | 14 (9.9%) | 33 (23.4%) | 82 (58.2%) | 49 (34.8%) | 56 (39.7%) | 37 (26.2%) | 23(16.3%) | 58 (41.1%) |
| Fragile X testing | 5 (3.5%) | 34 (24.1%) | 24 (17.0%) | 8 (5.7%) | 80 (56.7%) | 19 (13.5%) | 11 (7.8%) | 16 (11.3%) |
| FISH | 1 (0.7%) | 10 (7.1%) | 18 (12.8%) | 8 (5.7%) | 6 (4.2%) | 6 (4.3%) | 3 (2.1%) | 9 (6.3%) |
| aCGH | 13 (9.2%) | 21 (22.7%) | 48 (34.0%) | 3 (16.3%) | 22 (15.6%) | 24 (17.0%) | 17 (12.1%) | 37 (26.2%) |
| Molecular test (other) | 9 (6.4%) | 29 (20.5%) | 39 (27.7%) | 67 (49.7%) | 91 (64.6%) | 23 (16.3%) | 11 (7.8%) | 25 (17.7%) |
Tests recommended by AAP, AAN and/or ACMG as first-line test in that situation are in bold.
Tests that may be considered, or recommended only if clinically suspected or in specific subgroups are in italics.
All columns indicate number and proportion of participants who ordered the test(s) (n [%]).
*P-value is result of chi-square test to compare proportion of participants who would order at least one test for a given vignette to the proportion who would order at least one test in isolated GDD (baseline).
Figure 1.
Importance of clinical characteristics influencing the decision to order a genetic test for a child with GDD (n=141). Clinical characteristics are ordered based on % of paediatricians who answered ‘very important’, from highest to lowest %. Average score on Likert scale (from 0—Not important to 5—Very important) is in parenthesis next to each clinical characteristic. Categories 1 to 2 were grouped into ‘mildly important’ and 3 to 4 into ‘moderately important’.
Protocol
This study was approved by the CHU Sainte-Justine Research Ethics Committee. Data collection was performed between December 2013 and March 2014. The survey was sent to all eligible physicians in December 2013 by mail with a preaddressed and prestamped return envelope, with an introduction letter explaining the study. Each survey had an identification number to keep track of responses. Nonresponders received two mailed reminders at 4-week intervals. Survey responses were anonymized.
Analysis
Statistical analyses were performed using SPSS version 24.0. Descriptive analyses were performed for all variables. Where appropriate, results were compared by age group, gender and practice setting using chi-square tests. For vignettes, the vignette on isolated GDD was used as the baseline for comparison with results for other vignettes, using chi-square tests to compare two proportions. Chi-square tests for independence were done to compare answers to questions with categorical variables (Likert scales) across gender, age groups, subspecialty (developmental paediatrics) and practice setting. Gender distribution and age groups were compared to publicly available demographic data from the Association des Pédiatres du Québec (8).
RESULTS
Two hundred and twenty-five questionnaires were returned, for a response rate of 34.5%. However, 84 questionnaires were excluded because participants did not see children with GDD. A total of 141 participants satisfied eligibility criteria, for a response rate of 29% (141 of 483) after exclusions.
Participant’s characteristics
Of 141 participants, 42% were men. Participants ranged in age from 30 to 39 (14.9%) to 40 to 49 (27.0%), 50 to 59 (26.2%) and over 60 (29.8%). They were general paediatricians (91.5%) or developmental paediatricians (8.5%). 45.4% practiced in an academic centre. Average years of experience were 21.2 years (SD 11.9). Compared to all Quebec paediatricians, gender distribution was not significantly different (P=0.09) (8). The average age of Quebec paediatricians was 50 years old (8), compared to our sample where 55% of respondents were over 50.
Reported use of genetic tests for children with GDD
The majority of participants (93.6%) reported having used genetic tests for children with GDD. Karyotype (92.9%), Fragile X testing (87.2%) and FISH (68.1%) for specific microdeletion syndromes (e.g., DiGeorge/VCF syndrome, Williams syndrome, etc.) were the three tests most frequently used. In addition, 35.5% reported having used molecular tests to look for specific genetic conditions, such as Rett syndrome. Most (63.8%) had used aCGH at least once and 88.7% had heard of aCGH.
When asked how they would use aCGH, 68.1% participants would order it for children with GDD and dysmorphic features, 28.4% would order aCGH for all children with GDD and 34.0% for a child with a family history of developmental delay.
When asked what motivates their use of genetic tests for children with GDD, participants reported that genetic testing helped clarify patients’ diagnosis (97%) and prognosis (96%), improved ability to provide reproductive genetic counselling (94%), increased access to rehabilitation services (84%), and decreased the number of subsequent medical investigations (71%). Gender, age, subspecialty (developmental paediatrics) and practice setting were not associated with participants’ answers.
Reported use of genetic tests for GDD in different clinical scenarios
Participants were asked about their use of genetic tests using eight vignettes describing clinical scenarios of children with GDD (Table 2). Only 20.6% would order genetic tests for isolated GDD, even though guidelines recommend karyotype and/or aCGH and fragile X testing in all cases of GDD. Participants were more likely to order genetic tests when additional features were present, such as microcephaly/dysmorphic features, associated neurological conditions, or a family history of fragile X syndrome. For a child with GDD with dysmorphic features and microcephaly, 5% of participants would not order a genetic test. Only 56.7% ordered Fragile X testing for a girl with GDD with a known family history of Fragile X syndrome in her maternal cousin. Use of tests for a child with isolated GDD was significantly higher in presence of maternal pregnancy (P<0.0001), but not parental anxiety (P=0.16). Participants order significantly more genetic tests for a child with GDD and a history of fetal exposure to alcohol than in the case of isolated GDD (P<0.0001). Gender, subspecialty (developmental paediatrics), age and practice setting were not associated with participants’ answers.
Clinical characteristics influencing the decision to order a genetic test
Based on average scores on the Likert scale, the three most important factors identified by participants were, in order of importance, parental consanguinity, microcephaly, abnormal neurological exam (Figure 1). Demographic factors were not associated with participants’ answers.
Knowledge of guidelines about the use of genetic tests for children with GDD
Only 31.9% of participants knew of at least one guideline. The AAP recommendations were the most well-known: 27% were familiar with AAP recommendations (2). Only 7.1% were familiar with the ACMG guideline on Fragile X syndrome (4) and 2.8% were familiar with the ACMG guideline on cytogenetic tests (5). Only 6.4% were familiar with the AAN recommendations (3). Developmental paediatricians were significantly more familiar with at least one guideline than general paediatricians (P=0.001). Developmental paediatricians were also more likely to be familiar with the ACMG guideline on Fragile X syndrome (P=0.014) and the ACMG guideline on cytogenetic tests (P<0.001) (4,5).
DISCUSSION
Paediatricians tended not to order genetic tests for all children with GDD, despite recommendations. This was especially true in the case of isolated GDD. Karyotype, Fragile X testing and aCGH are, as expected, the most frequently used genetic tests in children with GDD, but are not used as often as would be expected based on guidelines (Table 2) (2–6). Only 56.7% ordered Fragile X testing for a girl with GDD with a known family history of Fragile X syndrome. Recommendations for Fragile X testing are not based on gender: Fragile X syndrome can be as severe in girls, even though penetrance is not as high. It is the vignette with the highest use of Fragile X testing, but we expected a much higher use of Fragile X testing since the likelihood of the diagnosis is high. In the case of fetal exposure to alcohol, participants ordered genetic tests, presumably to rule out an underlying genetic cause. Still, we were surprised to observe that the use of genetic tests was over three times higher than for isolated GDD: we would have expected the use of genetic tests to be equal or lower than for isolated GDD with no risk factors.
Paediatricians’ use of tests was influenced by external factors, such as maternal pregnancy. It is understandable to pursue testing in this situation, but this suggests that children with GDD who are seen while their mother is pregnant may have better access to recommended genetic tests.
Paediatricians lacked knowledge of available guidelines. Less than a third of participants were familiar with AAP guidelines (2), even though it was the guideline with which participants were most familiar. Paediatricians may prefer to refer children with GDD to a developmental paediatrician, geneticist or neurologist for specialized assessment, instead of ordering genetic tests themselves (9,10). In that case, if access to these specialists is limited, children with GDD remain underinvestigated for genetic causes of their GDD.
A similar study with American paediatricians found that 98% initiated a diagnostic workup for GDD in an average year, but that 74% would refer to a specialist without testing (11). Only 11% ordered a genetic test themselves (aCGH, karyotype or targeted DNA testing), compared to 93.6% who ordered genetic tests in our study. Among those who did, reported use of karyotype, aCGH and other DNA-based tests were lower than in our study (39%, 19% and 4%, respectively). One possible explanation is that American paediatricians have better access to specialists and are therefore more likely to refer without testing, but only 5% reported referring to a geneticist. This suggests that, similarly to our study, children with GDD in the USA are under-investigated for genetic causes of GDD.
Guidelines issued may not be well disseminated to paediatricians, especially if issued by other specialties (e.g., neurologists, geneticists). Even when physicians are familiar with guidelines, their adherence is influenced by internal barriers, including lack of agreement with recommendations, lack of self-efficacy (confidence in ability to carry out recommendations) and lack of motivation (12–15). These are known barriers to the provision of genetic services by providers in general and paediatricians in particular (16,17). In our study, we confirmed a lack of awareness of guidelines. Other barriers are certainly present, including external barriers, such as time constraints and lack of resources, etc (12–15,18).
Recent reviews reinforce the importance of genetic testing in children with GDD (19–23). Part of genetic test underuse may be attributable to slow adoption of recommendations, but current recommendations have been available for over 5 years, emphasizing that physicians are slow to change their behaviour. Lack of (perceived) competence is a major issue, as genetic tests move into the genomic era (24,25). Ability to communicate genetic test results is becoming increasingly important for paediatricians, since many genetic conditions have a childhood onset (26).
The democratization of genetic tests has facilitated access to specialized tests such as aCGH for children with GDD (27–30). However, before using these tests, physicians need to have sufficient knowledge and expertise to provide appropriate counselling and interpret test results (10,31). It is increasingly clear that training about genetic test use should be part of the medical school curriculum (18). There is a need for continuing medical education on the use of genetic tests (32–34). Quality improvement efforts may be an effective way to improve adherence to recommendations (35).
The main limitation of this study is its response rate. Physicians are notoriously difficult to survey, and it was difficult for us to target specifically paediatricians who see children with GDD. We sent surveys to all paediatricians in the province, so we expect a proportion of nonresponders to have self-selected out because this topic was not of interest to them. For this reason, we expect that our observed response rate after exclusions (141 of 483, 29%) is an underestimate of the response rate of eligible paediatricians. A study of paediatricians and their use of genetic services obtained a similar response rate (29%) (16). Also, our participants are similar to Quebec paediatricians in general.
In summary, paediatricians underuse recommended genetic tests for children with GDD. As new and more complex genetic tests are developed, we suggest that up-to-date training about the use of genetic tests for children with GDD be integrated at all levels of training, from paediatrics residency curriculum to continuous medical education activities, to ensure that paediatricians in practice are knowledgeable about indications for testing, pretest counselling, and appropriate interpretation and management of results.
Funding Sources
This study was supported by the Canadian Institutes of Health Research through an Operating Grant - Priority Announcement: Knowledge Translation Priority Funding (Funding Reference Number 111415) obtained by AM Laberge. Isabelle Tremblay obtained salary support funding for her doctoral training through CHU Sainte-Justine, the Université de Montréal Faculty of Medicine, and the Fondation du Grand Défi Pierre Lavoie.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgements
We would like to thank Marie-Christine Haché for her contribution in the development and piloting of the questionnaire.
References
- 1. Cossette L, Duclos E. Statistics Canada. A Profile of Disability in Canada, 2001. <http://www.statcan.gc.ca/pub/89-577-x/89-577-x2001001-eng.pdf>. (Accessed November 10, 2015).
- 2. Moeschler JB, Shevell M; American Academy of Pediatrics Committee on Genetics Clinical genetic evaluation of the child with mental retardation or developmental delays. Pediatrics 2006;117:2304–16. [DOI] [PubMed] [Google Scholar]
- 3. Shevell M, Ashwal S, Donley D, et al. ; Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society Practice parameter: evaluation of the child with global developmental delay: report of the quality standards subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2003;60:367–80. [DOI] [PubMed] [Google Scholar]
- 4. Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: Diagnostic and carrier testing. Genet Med 2005;7:584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaffer LG; American College of Medical Genetics Professional Practice and Guidelines Committee American College of Medical Genetics Guideline on the cytogenetic evaluation of the individual with developmental delay or mental retardation. Genet Med 2005;7:650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manning M, Hudgins L. ACMG Practice Guidelines: Use of array-based technology in the practice of medical genetics. Genet Med 2007;9:650–3. [DOI] [PubMed] [Google Scholar]
- 7. Burke W, Zimmern RL, Kroese M. Defining purpose: A key step in genetic test evaluation. Genet Med 2007;9:675–81. [DOI] [PubMed] [Google Scholar]
- 8. Association des Pédiatries du Québec - Fédération des médecins spécialistes du Québec (https://www.fmsq.org/fr/la-fmsq/organisation/associations/association-des-pediatres-du-quebec). Using data from 2015 (Accessed November 10, 2015).
- 9. Committee on Genetics. Molecular genetic testing in pediatric practice: A subject review. Pediatrics 2000;106:1494–97. [DOI] [PubMed] [Google Scholar]
- 10. Ross LF, Ross LF, Saal HM, David KL, Anderson RR; American Academy of Pediatrics; American College of Medical Genetics and Genomics Technical report: Ethical and policy issues in genetic testing and screening of children. Genet Med 2013;15:234–45. [DOI] [PubMed] [Google Scholar]
- 11. Tarini BA, Zikmund-Fisher BJ, Saal HM, Edmondson L, Uhlmann WR. Primary care providers’ initial evaluation of children with global developmental delay: A clinical vignette study. J Pediatr 2015;167:1404–8.e1. [DOI] [PubMed] [Google Scholar]
- 12. Burgers JS, Grol RP, Zaat JO, Spies TH, van der Bij AK, Mokkink HG. Characteristics of effective clinical guidelines for general practice. Br J Gen Pract 2003;53:15–9. [PMC free article] [PubMed] [Google Scholar]
- 13. Cabana MD, Rand CS, Powe NR, et al. . Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–65. [DOI] [PubMed] [Google Scholar]
- 14. Foy R, MacLennan G, Grimshaw J, Penney G, Campbell M, Grol R. Attributes of clinical recommendations that influence change in practice following audit and feedback. J Clin Epidemiol 2002;55:717–22. [DOI] [PubMed] [Google Scholar]
- 15. Grol R, Dalhuijsen J, Thomas S, Veld C, Rutten G, Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: Observational study. BMJ 1998;317:858–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rinke ML, Mikat-Stevens N, Saul R, Driscoll A, Healy J, Tarini BA. Genetic services and attitudes in primary care pediatrics. Am J Med Genet A 2014;164A:449–55. [DOI] [PubMed] [Google Scholar]
- 17. Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: A systematic review of the literature. Genet Med 2015;17:169–76. [DOI] [PubMed] [Google Scholar]
- 18. Scott SD, Grimshaw J, Klassen TP, Nettel-Aguirre A, Johnson DW. Understanding implementation processes of clinical pathways and clinical practice guidelines in pediatric contexts: A study protocol. Implement Sci 2011;6:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Karnebeek CDM, Jansweijer MCE, Leenders AGE, Offringa M, Hennekam RCM. Diagnostic investigations in individuals with mental retardation: A systematic literature review of their usefulness. Eur J Hum Genet 2005;13:6–23. [DOI] [PubMed] [Google Scholar]
- 20. Moeschler JB. Genetic evaluation of intellectual disabilities. Semin Pediatr Neurol 2008;15:2–9. [DOI] [PubMed] [Google Scholar]
- 21. Michelson DJ, Shevell MI, Sherr EH, Moeschler JB, Gropman AL, Ashwal S. Evidence report: genetic and metabolic testing on children with global developmental delay: Report of the quality standards subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2011;77:1629–35. [DOI] [PubMed] [Google Scholar]
- 22. Flore LA, Milunsky JM. Updates in the genetic evaluation of the child with global developmental delay or intellectual disability. Semin Pediatr Neurol 2012;19:173–80. [DOI] [PubMed] [Google Scholar]
- 23. Moeschler JB, Shevell M; Committee on Genetics Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics 2014;134:e903–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowdin S, Gilbert A, Bedoukian E, et al. . Recommendations for the integration of genomics into clinical practice. Genet Med 2016;18:1075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manolio TA, Chisholm RL, Ozenberger B, et al. . Implementing genomic medicine in the clinic: The future is here. Genet Med 2013;15:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosas-Blum E, Shirsat P, Leiner M. Communicating genetic information: A difficult challenge for future pediatricians. BMC Med Educ 2007;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manning M, Hudgins L; Professional Practice and Guidelines Committee Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med 2010;12:742–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dawson AJ, Riordan D, Tomiuk M, et al. . Cytogenetic microarrays in Manitoba patients with developmental delay. Clin Genet 2009;75:498–500. [DOI] [PubMed] [Google Scholar]
- 29. Saam J, Gudgeon J, Aston E, Brothman AR. How physicians use array comparative genomic hybridization results to guide patient management in children with developmental delay. Genet Med 2008;10:181–6. [DOI] [PubMed] [Google Scholar]
- 30. Shur N, Gunn S, Feit L, Oh AK, Yatchmink Y, Abuelo D. The role of new genetic technology in investigating autism and developmental delay. Med Health R I 2011;94: 131, 134–7. [PubMed] [Google Scholar]
- 31. Reiff M, Ross K, Mulchandani S, et al. . Physicians’ perspectives on the uncertainties and implications of chromosomal microarray testing of children and families. Clin Genet 2013;83:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Desale M, Worden LT, Cohen JS, Wilms Floet AM, Hoon AH Jr. Diagnostic evaluation in children with developmental delay: A cautionary tale for genetic testing. Clin Pediatr (Phila) 2012;51:1208–10. [DOI] [PubMed] [Google Scholar]
- 33. Dumont-Driscoll M. Genetics and the general pediatrician: Where do we belong in this exploding field of medicine?Curr Probl Pediatr Adolesc Health Care 2002;32:6–28. [DOI] [PubMed] [Google Scholar]
- 34. Houwink EJ, van Luijk SJ, Henneman L, van der Vleuten C, Jan Dinant G, Cornel MC. Genetic educational needs and the role of genetics in primary care: A focus group study with multiple perspectives. BMC Fam Pract 2011;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rinke ML, Driscoll A, Mikat-Stevens N, et al. . A quality improvement collaborative to improve pediatric primary care genetic services. Pediatrics 2016;137:e20143874. [DOI] [PubMed] [Google Scholar]