Summary
Chronic hepatitis C virus (HCV) infection has been associated with both organ-specific and systemic autoimmune diseases, with cryoglobulinemia being the most frequent associated disease. Experimental, virologic, and clinical evidence have demon-strated a close association between HCV infection and some systemic autoimmune diseases, especially Sjögren’s syndrome, but also rheumatoid arthritis and lupus. A higher prevalence of hematological processes has also been described in patients with HCV infection, including cytopenias and lymphoproliferative disorders (B-cell lymphoma). In addition, patients with chronic HCV infection have a higher frequency of other extrahepatic manifestations including endocrine, metabolic and cardiovascular disorders that may worse the prognosis of patients, along with neuropsychiatric manifestations and general symptoms that have a significant influence on the quality of life of the patient. Direct-acting antiviral therapies (DAAs) that have recently begun to be used are providing the opportunity to effectively cure chronic HCV infection and reduce the burden of both hepatic and extrahepatic complications.
Keywords: prisons, hepacivirus, hepatitis C, antiviral agents, autoinmune diseases, lymphoma, Spain, diabetes mellitus
Resumen
La infección crónica por el virus de la hepatitis C (VHC) se ha asociado tanto a enfermedades autoinmunes específicas de órganos como a enfermedades autoinmunes sistémicas, siendo la más frecuente la crioglobulinemia. Las evidencias experimentales, virológicas y clínicas han demostrado una estrecha asociación entre la infección por el VHC con algunas enfermedades autoinmunes sistémicas, especialmente el síndrome de Sjögren, junto con la artritis reumatoide y el lupus. Se ha descrito una mayor prevalencia de procesos hematológicos en pacientes con infección por VHC, incluyendo citopenias y trastornos linfoproliferativos como el linfoma B. Además, los pacientes con infección crónica por el VHC presentan una mayor frecuencia de otras manifestaciones extrahepáticas que incluyen alteraciones endocrinas, metabólicas y cardiovasculares que pueden afectar seriamente el pronóstico de los pacientes, junto con manifestaciones neuropsiquiátricas y de afectación del estado general que influyen notablemente en la calidad de vida del paciente. Las terapias antivirales de acción directa (DAA) que han empezado a utilizarse recientemente están proporcionando la oportunidad de curar eficazmente la infección crónica por VHC y reducir la carga causada por las complicaciones hepáticas y extrahepáticas.
Palabras clave: prisiones, hepacivirus, hepatitis C, antivirales, enfermedades autoinmunes, linfoma, España, diabetes mellitus
1. Introduction
Hepatitis C virus (HCV) was first isolated from an infected chimpanzee in 1988. It is a single-stranded RNA virus enveloped in a lipid bilayer belonging to the Flaviviridae family and more specifically to one of its three genera: hepacivirus. Its genome includes over 9400 nucleotides and several regions. HCV is one of the virus with a greatest genome variability. Its genome heterogeneity is one of the main features of HCV, which can be classified as follows: genotypes, subtypes and quasispecies. The underlying reason of such variability is a lack of the corrective activity of virus-dependent RNA-polymerase leading to the frequent introduction of nucleotide substitutions in the virus genome. Within the same genotype, sequences are homologous in around 95%, while between genotypes they are so in only 65% of cases. Continuous mutation of HCV significantly threatens the immune memory since the number of memory cells originating from the first exposure capable of recognizing the new varieties are less and less with each new mutation. 1
Autoimmunity and viral infections are closely related, and viruses have been acknowledged as potential etiologic or triggering agents for this kind of diseases (AID). For over twenty years, several authors described the relationship between HCV and a heterogeneous array of extrahepatic manifestations such as pulmonary fibrosis, cutaneous vasculitis, glomerulonephritis, Mooren’s ulcer, porphyria cutanea tarda or lichen planus. Currently though, it has been accepted that for some of this entities, a direct link is difficult to establish. 2
Recently, a higher prevalence of endocrine-metabolic, cardiovascular and neuropsychiatric diseases has been described among HCV patients. Endocrine-metabolic alterations which are most frequently found are affection of the thyroid gland, gonadal dysfunction and insulin resistance/type 2 diabetes mellitus. Chronic infection by HCV has been described as an independent factor for cardiovascular events such as carotid atherosclerosis, myocardial infarction and heart failure, all of which are associated with poor outcomes. On the other hand, the presence of neurological and psychiatric symptoms has been directly and indirectly associated with HCV.
The relevance of chronic infection by HCV among the imprisoned population is clearly established by its prevalence: up to 20 times higher than in the general population. This epidemiological data together with the special features of this population (risk factors, age, HIV coinfection, etc.) implies a complex situation affecting the common distribution of genotypes or the reinfection rate and thus, the therapeutic approach. 3 - 5 In this context, it is crucial to know what extrahepatic manifestations can imply underlying infection by HCV. In this review, we will go through the main extrahepatic manifestations that can contribute to an early diagnosis of HCV in this population.
2. Etiopathogenesis of extrahepatic manifestations
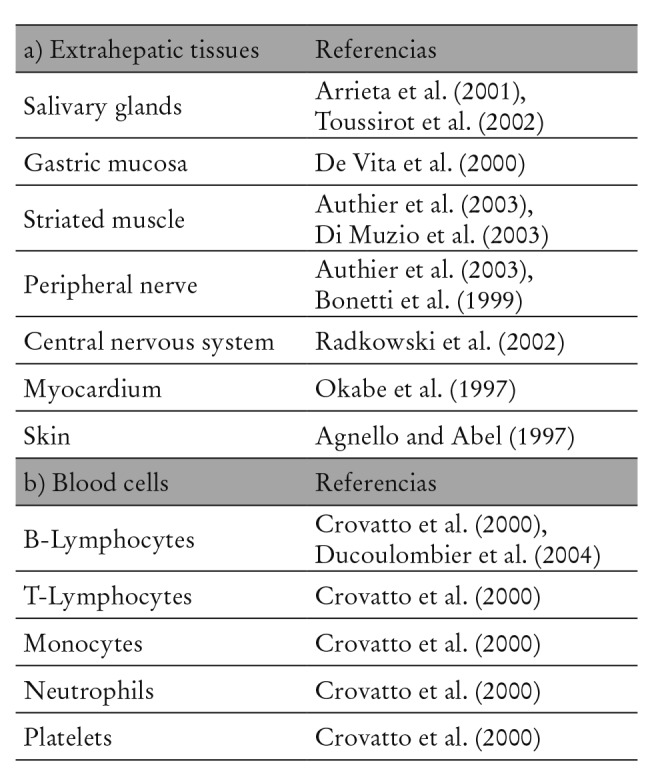
Obviously, the ultimate consequence of HCV infection (healing vs. chronification) depends upon the integrity of the host’s immune system and the virus’s features at the time of infection. In most cases, the virus overcomes the immune system and it “settles” within cells, where it remains isolated from the immune system (Table 1). It is possible that plasmatic lipoprotein binding and the existence of quasispecies are involved in this first escape from the immune system. The infection or chronic presentation of viral antigens to lymphocytes entails their monoclonal expansion therefore implying the presence of circulating cryoglobulins. The risk for developing cryoglobulinemia increases with the duration of HCV infection and thus, the presence of those cryoglobulins may finally produce vasculitis symptoms. Potentially, (infected) B lymphocyte proliferation could become uncontrollable, therefore inducing the development of lymphoma. Anyway, lymphoproliferation and its evolution from a benign form to a malign presentation is most probably a process with multiple stages where one or more infectious agents could play a determining role, as well as genetic and environmental factors. 6
Table 1: Extrahepatic localication of Hepatitis C Virus infection.
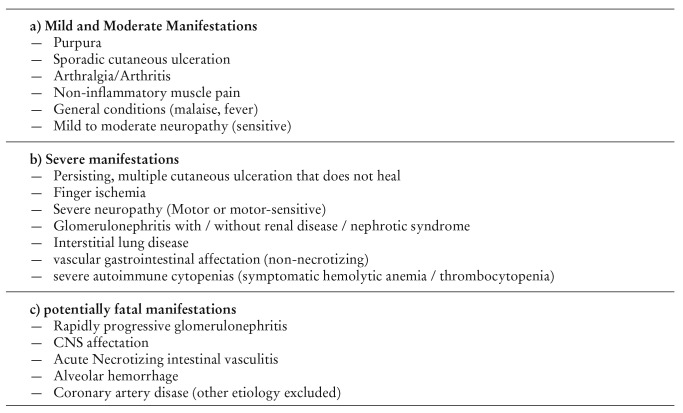
Patients with chronic HCV infection frequently present autoimmune phenomena with extremely variable clinical relevance: from subclinical alterations to very severe immunological alterations (Table 2). On the other hand, autoimmune manifestations associated to chronic HCV infection can eventually be the reason why HCV infection is diagnosed, due to the lack of specific clinical or biological manifestations of chronic HCV infection, with the existence of viremia in the absence of hepatic affectation.
Table 2. Organ-specific manifestations of HCV patients with extrahepatic manifestations according to severity.
3. Autoimmune manifestations
3.1. Systemic autoimmune diseases
The association between systemic autoimmune diseases hand chronic HCV infection has arisen great interest for the last few years. 7 Extrahepatic manifestations frequently found among patients with chronic HCV infection (both clinical and immunological) can lead to the fulfilling of current criteria for some system autoimmune diseases (Table 3).
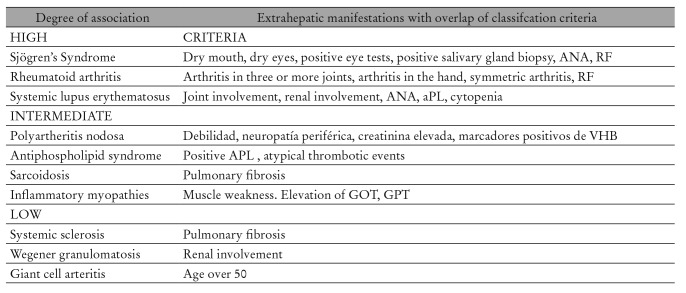
Table 3. Different degrees of association between HCV infection and systemic autoimmune diseases.
The clinical association of different SADs with chronic HCV infection can be analyzed from two different yet complementary points of view. First, a revision of literature concluded that around 500 patients with SADs coexisted with chronic HCV infection 5, Sjögren’s syndrome (SS), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and polyarteritis nodosa (PAN) being the most frequently found. 1 Next, the analysis of all series references of patients with SAD who underwent HCV testing showed a higher prevalence of HCV among patients with SS (17%), PAN (14.4%), SLE (9.6%) and RA (5.9%). 1 More recent studies have focused on the association between HCV chronic infection and circulating autoantibodies, organ-specific autoimmune diseases and lymphoproliferative disorders.
The largest study was published in 2009 by HISPAMEC International Study Group. 8 , 9 The HISPAMEC Registry is a multi-center international study group dedicated to data collection on systemic autoimmune diseases co-existing with chronic HCV infection. The study included 1020 patients with chronic HCV infection and co-existing SAD in Southern Europe (60%), North America (15%), Asia (14%), Northern Europe (9%), South America (1%) and Australia (1%). SAD most frequently described were SS (483 cases), RA (150 cases), SLE (129 cases), PAN (78 cases), antiphospholipid syndrome (APS) (59 cases), inflammatory myopathy (39 cases) and sarcoidosis (28 cases). Therefore, most frequently SAD associated with chronic HCV infection were SS (almost half of the cases), RA and SLE with almost two thirds of the cases reported in the Mediterranean area. In patients with coexisting SAD and HCV infection, the predominant immunological features were the presence of ANA, RF and cryoglobulins.
3.1.1. Vasculitis autoimmune manifestations
Cryoglobulinemia is the autoimmune manifestation most frequently described among HCV patients. Mixed cryoglobulins are identified in between 40 and 60% of patients with chronic HCV infection although cryoglobulinemic vasculitis in only observed in around 10% 10 . The main clinical manifestations include purpura, arthralgia, weakness, myalgia, polyneuropathy, glomerulonephritis and intestinal ischemia. Patients with cryoglobulinemia present a broad range of clinical features. 10 Although 50% of patients present a relatively benign evolution with good prognosis and survival, some patients can present a severe affectation of internal organs. 11 , 12 Two studies have assessed the clinical features of cryoglobulinemia associated with HCV in large series of patients. Sene et al. 13 retrospectively evaluated 123 patients with mixed cryoglobulinemia (MC) and concluded that cryoglobulinemic vasculitis was associated with older age, longer duration of HCV infection, MC type II and high levels of serum cryoglobulins. Ferri et al. 12 analyzed demographical, clinical and serological features and the survival of 231 patients with MC. One hundred and sixty eight patients were tested for HCV infection, which proved positive in 155 (92%). Malignant neoplasms were observed in 15% of patients mainly non-Hodgkin lymphoma (NHL) and hepato-cellular carcinoma and the main causes of death were associated with MC (64%), NHL (13%) and hepatic failure (13%).
The reason why some patients present severe forms of vasculitis (cryoglobulinemic) remains an open question. Ferri et al. 12 described that 35% of their patients with cryoglobulinemic patients had a moderate to severe clinical course and the prognosis was determined by both the cryoglobulinemic affectation and HCV-associated conditions such as hepatic failure. Cryoglobulinemia can be life threatening for HCV-patients in about 10-15% of cases. The presence of renal, intestinal, heart or CNS affectation entail a poor prognosis. Actually, recent data suggest that cryoglobulinemic glomerulonephritis significantly affects both prognosis and survival. 10 Around 30% of patients can develop moderate to severe forms of the disease and almost 15% can be potentially fatal. 10
Alike other virus-associated vasculitis (HBV, HIV), the activity of HCV-associated cryoglobulinemic vasculitis generally correlates with viremia 14 . Treatment should focus on potential causal agents. Therefore, antivirals are the cornerstone of therapies against this type of vasculitis. Currently, direct-acting antivirals (DAA) have provided higher rates of sustained virologic response (SVR) and lower burden of toxicity for HCV patients and more recently, the first significant series of patients with HCV-associated cryoglobulinemic vasculitis effectively treated with DAA have been published. 14 , 15
3.1.2 Non vasculitic autoimmune manifestations
a) Sjögren’s syndrome. Several experimental studies both virologic and clinical support a close relationship between HCV and SS. In 2002, the HCV-SS Study Group was formed, a multicenter international collaboration group which identified 137 patients with HCV and SS. 2 They determined that HCV-associated SS is indistinguishable from the primary form in most cases according to the most recent classification criteria and the term HCV-associated SS was proposed for all HCV patients fulfilling SS 2002 classification criteria. 2 HCV chronic infection should be considered an exclusion criterion for primary SS because the virus could be involved in the development of SS in a particular sub-group of patients, not due to the similarity of both forms. 2 Nevertheless, there is no conclusive evidence on the beneficial effect of antivirals for these patients.
b) Joint involvement and rheumatoid arthritis. Joint involvement in the form of arthralgia and arthritis is one of the extrahepatic manifestations most frequently reported. It is reasonable to therefore consider, that HCV patients with polyarthritis and positive RF suffer from some type of RA. HACV patients can present four of the 1998 ACR revised criteria: involvement of three or more joints, arthritis of the hand, symmetric arthritis and RF. Rosner and col. 16 revised thoroughly the prevalence and clinical features of HCV-associated arthritis and assessed the significant coincidence with RA. The most common presentation of HCV-associated arthritis is chronic inflammatory polyarthritis, which fulfills ACR classification criteria in over 50% of cases. The presence of morning stiffness, rheumatoid nodules and erosive arthritis (rarely reported in HCV infection) can be useful in diagnosing actual coexistence of RA and HCV. Patel et al. 17 identified 92 HCV + patients (5.1%) out of 1706 patients with RA. In comparison with AR patients negative for HCV, HCV positive patients were younger, predominantly Afro-American and smokers. Authors also concluded that RA patients with chronic HCV infection had higher scores for the activity of the disease (mainly associated with higher means reported by patients) and a higher probability of undergoing treatment with prednisone and anti-TNF alpha therapies and less likely to be treated with methotrexate than HCV negative patients. Recent studies have confirmed that anti-CCP antibodies can be useful in differentiating patients with HCV and a real RA from those with HCV-associated arthropathy. Ezzaz et al. 18 evaluated 30 patients with RA and 22 patients with HCV-associated polyarthritis and concluded that 83% of patients with RA presented anti-CCP antibodies while only 4.5% of HCV-associated polyarthopathy did. The determination of anti-CCP antibodies proved more specific for the diagnosis of RA than RF (95.4 vs. 18.2%) while sensitivity was similar (83.3 vs. 90%).
c) Lupus-related manifestations. Virus have been proposed as potential etiologic agents or triggering factors for SLE. HCV chronic infection can induce clinical and serological features (arthritis, nephropathy, cytopenia and low levels of ANA or anti-DNA) which, together, can fulfill 1982 ACR criteria for SLE. In this regard, some studies have suggested that HCV can fake SLE. Several authors have analyzed the prevalence of HCV infection in SLE patients and some case reports have been published on this topic. 19 Ramos-Casals et al. 20 analyzed a large series of SLE patients and concluded that HCV was present in 11% of an unselected SLE population. This prevalence is significantly higher than that found in the control group (1%) and in the general population of Catalonia (1.2%). This suggests a potential link between HCV infection and SLE. Similar results have been obtained by Ahmed et al. 21 in USA (10% in SLE patients in comparison with 1.3% among the general population).
d) Pulmonary involvement and sarcoidosis. The first association between sarcoidosis and HCV was described in 1993 strictly related with the initiation of interferon-alpha treatment. Ramos-Casals et al. 22 analyzed clinical features and results of 68 cases of sarcoidosis coexisting with chronic HCV infection, in 75% of which sarcoidosis was triggered by antiviral therapy. 22 Other patterns of association between sarcoidosis and HCV have been described: 1) The co-existence of both diseases in patients with HCV infection with no prior treatment and 2) the reactivation of preexisting sarcoidosis in HCV patients later treated with anti-HCV agents. The association with idiopathic pulmonary fibrosis and other forms of interstitial lung disease (ILD) have also been described.
e) Muscle involvement and inflammatory myopathy. The presence of muscle pain (myalgia) in patients with chronic HCV infection is very common, yet the diagnosis of inflammatory myopathy is rare. The association between these entities is mostly in isolated cases, with a total of 36 published reports out of which 21 were polymyositis. 23 On the other hand, only three studies have assessed the prevalence of HCV in series of patients with inflammatory myopathy and HCV infection was present in twelve out of 126 patients (9.5%). 23 Bohan and Peter criteria show a slight degree of overlap with HCV infection since HCV patients rarely present muscle weakness with elevated levels of muscle enzymes or electromyographic evidence of generalized myopathy. Therefore, currently, inflammatory myopathy is weakly associated with HCV infection. A recent case-control study 24 have analyzed the prevalence of HCV infection in 114 patients with inclusion body myositis and 44 patients with polymyositis aged similarly in the same period of time. A higher frequency of HCV infection was found among patients with inclusion body myositis than in those with polymyositis and the general Japanese population (28% vs. 4.5% vs. 3.4%) suggesting a plausible etiopathogenic link between HCV and inclusion body myositis.
3.2. Organ-specific autoimmune diseases
Autoimmune thyroiditis is the organ-specific autoimmune disease most frequently associated with chronic HCV infection. The role of HCV in the induction of thyroid autoimmunity remains unclear. Bini and Mehandru 25 described the development of thyroid disease (both evident and subclinical) in 11% of two hundred and twenty-five male patients with HCV infection and combined antiviral therapy although the thyroid disease responded to specific treatment and was reversible in most cases. Antonelli et al. 26 described a higher frequency of hypothyroidism (13%) and anti-thyroid antibodies (21%) in 630 patients with HCV infection and no treatment in comparison with normal controls and found similar results in a sub-group of patients with associated mixed cryoglobulinemia. However, other studies carried out in the same geographical area did not conclude this close association. Floreani et al. 27 tested six hundred and ninety-seven Italian patients for thyroid autoantibodies and anti-HCV antibodies. Out of 71 HCV positive patients, four (6%) were positive for at least one thyroid antibody, compared with seven (5%) of negative controls according to age and gender.
4. Hematologic disorders
The specific tropism of HCV for many types of extrahepatic cells (Table 1), especially for blood cells has been suggested by many studies, therefore establishing a clear bond between HCV and the development of autoimmune and proliferative disorders.
4.1. Cytopenia
Although HCV-associated cytopenia is not uncommon, in general terms it is considered a mild alteration with no clinical relevance, especially in patients with hypersplenism. Thrombocytopenia is the most common, with a chronic course and rarely entailing severe bleeding. Severe cytopenia is present in some HCV patients, regardless of antiviral therapy. Thrombocytopenia can be severe (under 30x 109/L) in HCV patients with no prior treatment and in some cases it is associated with concomitant autoimmune disorders, with cryoglobulinemia and with simultaneous HIV infection. Recently, two cases of autoimmune hemolytic anemia (AHA) with positive Coombs test in patients with no IFN treatment have been reported. Previously seventeen cases of AHA associated with HCV infection had been described, mostly associated with autoimmune disorders, among which cryoglobulinemia was the most common.
4.2. Lymphoma
Several studies have concluded a higher prevalence of lymphoproliferative disorders among HCV patients. 28 Matsuo et al. 29 and Iwata et al. 30 carried out a meta-analysis of twenty-three epidemiological studies on the association between HCV and NHL, including 4049 patients with NHL. Odds-ration (OR) for NHL in HCV patients was 5.70; 5.04 for B-cell NHL and 2.51 for T-cell NHL. 29 Dal Maso and Franceschi 31 carried out a similar meta-analysis to determine the strength and accuracy of the association between HCV and NHL, including only Z100 studies according to age and gender. Combined RR of all NHL in HCV patients was 2.5. Nieters et al. 32 studied the presence of HCV infection in samples form 1807 cases of NHL and 1788 controls and found HCV in 53 (2.9%) of lymphoma cases and in 41 (2.3%) of controls (OR 1.42). When limited to patients with positive HCV RNA, OR raised to 1.82. The most frequent case of lymphoma in HCV patients is B-cell lymphoma or NHL and marginal zone lymphoma the most common subtype. IN most cases, there is an excellent response to treatment after receiving antiviral therapy 7 .
5. Endocrine. Metabolic affectation
5.1. Diabetes mellitus
Several clinical studies have suggested a potential link between chronic hepatitis caused by HCV and the development of diabetes mellitus (DM). Antonelli et al. 33 described that the prevalence of type 2 DM is higher in HCV-MC patients. Diabetic patients with HCV infection and MC have also a more pronounced autoimmune reactivity than non-HCV diabetic patients. The development of hepatic fibrosis has been associated with insulin resistance in patients with chronic HCV infection 31 and DM can contribute to the presence and severity of hepatic encephalopathy regardless of the severity of the hepatic disorder. A recent meta-analysis describes that concomitant DM is associated with a higher risk of hepatocellular carcinoma, in association with body mass index (BMI) and steatosis 34 , while Hwang et al. 35 have described that chronic HCV infection increases the risk for developing end-stage renal disease in patients with DM.
5.2. Hepatic steatosis
Metabolic disorders in HCV patients can be associated to the development of hepatic steatosis, with a clinical relevance which has been recently emphasized. There are several factors associated with hepatic steatosis including obesity, use of alcohol, type 2 DM and hyperlipidemia. The infection by genotype 3 is particularly associated with fatty deposition in the liver. Leandro et al. 36 carried out a meta-analysis in 3068 patients with histologically confirmed chronic HCV infection. Steatosis was present in 1561 patients (51%) and fibrosis in 2688 (88%). Steatosis was independently associated with genotype 3, fibrosis, diabetes, hepatic inflammation, alcohol use, higher BMI and older age. Antiviral therapy and cure of the infection is associated with the disappearance of steatosis, especially in patients with genotype 3 infection, with no other risk factors. In patients with persisting steatosis, the control of metabolic factors such as over-weight and the modification of lifestyle seem important in the management of chronic HCV infection.
6. Atheromatosis and cardiovascular manifestarions
There is a higher risk for the development of cardiovascular disorders in patients with chronic HCV infection 7 . This infection is a proven independent risk factor for the development of cardiovascular manifestations such as carotid atherosclerosis, heart failure and stroke. It has all been attributed with a higher cardiovascular mortality throughout the course of chronic HCV infection and thus, with a relevant impact in the prognosis of the patient. Chronic HCV infection can be reasonably considered as potentially iatrogenic since it promotes insulin resistance and type 2 diabetes, and due to the replication of HCV within carotid plaques 7 .
7. Neurophyschiatric manifestations and general symptoms
Neuropsychiatric manifestations are included in the array of extrahepatic manifestations of HCV infection. Their etiopathogenesis is complex and can include several mechanisms such as direct neuro-invasion, immunologically mediated lesion due to antibodies against antigens in the nervous tissue or ischemic alterations secondary to vasculitis/atheromatosis. Up to 50% of patients with HCV infection can develop a variable combination of clinical or subclinical neuropsychiatric manifestations including peripheral polyneuropathy, CNS manifestations (encephalopathy, myelitis, vascular events) and neuro-psychological/psychiatric manifestations 7 .
Neuropsychiatric symptoms have been directly related with an important affectation of the quality of life of patients together with other symptoms such as sexual dysfunction and depression, chronic fatigue, chronic joint and muscle pain and fibromyalgia. The treatment of chronic HCV infection including interferon can further jeopardize patients’ quality of life since some of its adverse effects include fatigue, muscle pain, depression and cognitive impairment. New agents, with different adverse effects profiles and different regimens can allow patients to achieve SVR and preserve their quality of life.
8. Therapeutic implications
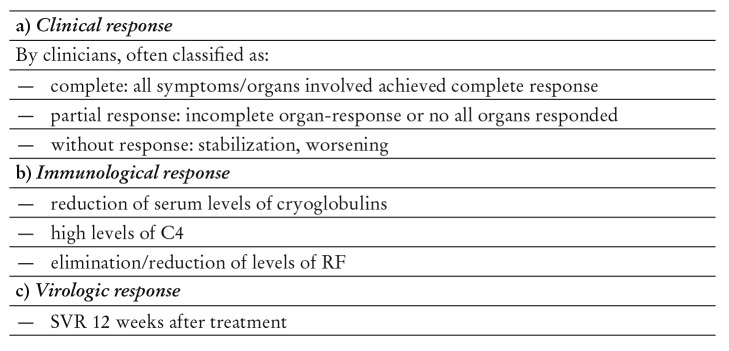
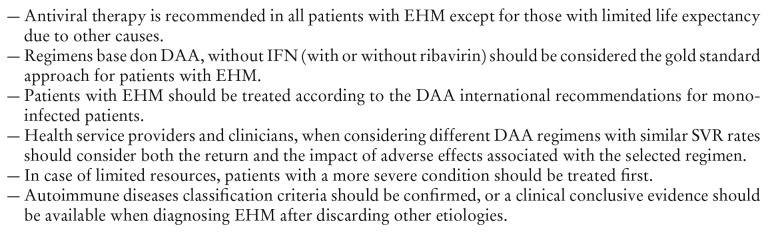
Currently there are no international recommendations on the treatment of HCV patients with extrahepatic manifestations (EHM). The first approaches are based on immunosuppressive therapies such as those used in non-HCV vasculitis. The introduction of the first combination of antiviral agents (interferon and ribavirin) clearly improved survival rates. Nevertheless, this approach had a poor virologic efficacy (eradication under 50% for genotype 1 HCV) and often included several months and high rates of intolerance. 37 Direct-acting antivirals (DAA) have been recently introduced as an effective alternative for HCV infection, with short duration of treatment, minimum adverse effects and an efficacy close to 100%. 38 These new agents are allowing an efficient cure for HCV infection with a reduced burden due to hepatic and extrahepatic complications, therefore providing the opportunity of a dramatic change in the results of these patients. The objective of a recently published multidisciplinary international consensus 39 is to provide the first set of evidence-based recommendations on a homogenous therapeutic approach for HCV patients with extrahepatic manifestations in the new DAA era (Tables 4 and 5).
Table 4. Evaluation of therapeutic response: results mainly used in published studies on patients with EHM.
Tabla 5. Recommendations for the treatment of extrahepatic manifestations 39.
9. Conclusions
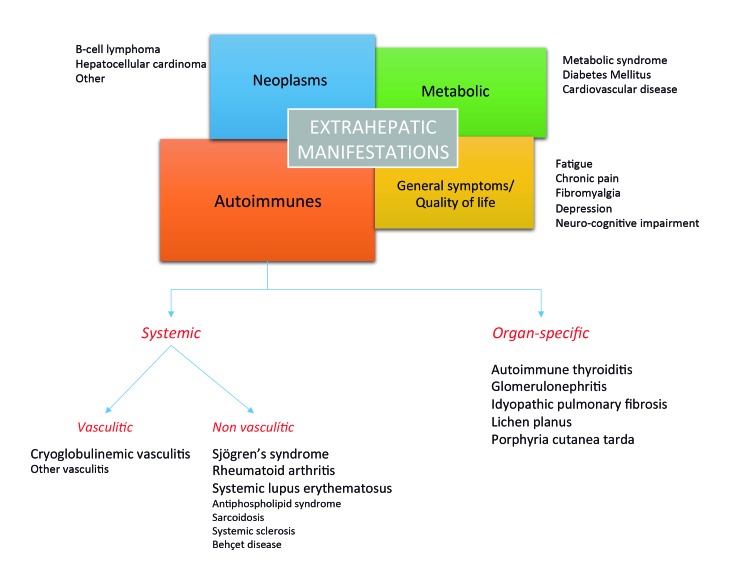
Obviously, HCV infection should be approached from a systemic point of view due to the strong association with different types of extrahepatic manifestations, of autoimmune, hematological or metabolic origin (Figure 1). Clinical extrahepatic manifestations can be observed in several localizations the most frequent being: joint involvement (arthralgia and/or arthritis), skin conditions (purpura), neurological disorders (polyneuropathy), renal manifestations (glomerulonephritis), pulmonary disorders (alveolitis), thyroid (thyroiditis), eye and mucosae affectation (dryness) and soft tissue manifestations (fibromyalgia). Currently, available data underline the importance of a new approach on the prevalence of so-considered “extrahepatic manifestations” due to large series of patients and the use of specific techniques. Therefore, we could consider the following “common” extrahepatic manifestations (with a prevalence ranging between 10 and 20%): arthralgia, mucous dryness and fibromyalgia and “relatively common” (5-10%) cutaneous vasculitis, polyneuropathy and glomerulonephritis (directly related with cryoglobulinemia). Finally, there are three manifestations classically considered as HCV-associated and whose relationship has not been established in the later publications: Thyroid manifestations (associated with interferon therapies and some age groups), pulmonary fibrosis and Mooren corneal ulcers. Finally, it is worth considering that the appearance of extrahepatic manifestations and positive immunological results can lead to the diagnosis of a specific system autoimmune disorder, according to the corresponding criteria. Research throughout the last thirty years on HCV points to two clear facts. The first is that cryoglobulinemia is associated with many of the autoimmune manifestations (both clinical and serological) in HCV patients and that cryoglobulinemic syndrome seems to be the great simulator of systemic autoimmune disease. The second is that there are certain autoimmune diseases such as SLE, DD or RA that are frequently found among patients with chronic HCV infection.
Figure 1. Classification of extrahepatic manifestations associated with chronic HCV infection.
References
- 1.Ramos-Casals M, Font J, Ingelmo M. Prevalence and clinical significance of hepatitis C virus infection in systemic autoimmune diseases. Med Clin (Barc) 2001 May 19;116(18):701–709. doi: 10.1016/s0025-7753(01)71958-6. [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Casals M, Font J. Extrahepatic manifestations in patients with chronic hepatitis C virus infection. Curr Opin Rheumatol. 2005 Jul;17(4):447–455. doi: 10.1097/01.bor.0000166386.62851.49. [DOI] [PubMed] [Google Scholar]
- 3.Juan Jd, de la Hoya PS, Marco A, Antón JJ, Faraco I, Yllobre C, et al. Multicenter study on the discontinuation and efficacy of chronic hepatitis C treatment in the Spanish penitentiary population (EPIBAND study) Eur J Gastroenterol Hepatol. 2014 Oct;26(10):1083–1089. doi: 10.1097/MEG.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 4.Marco A, Gallego C, Caylà JA. Incidence of hepatitis C infection among prisoners by routine laboratory values during a 20-year period. PLoS One. 2014 Feb 28;9(2):e90560. doi: 10.1371/journal.pone.0090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marco A, Esteban JI, Solé C, da Silva A, Ortiz J, Roget M, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013 Jul;59(1):45–51. doi: 10.1016/j.jhep.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Casals M, García-Carrasco M, Cervera R, Font J. Is hepatitis C virus a sialotropic virus? Am J Pathol. 2001 Oct;159(4):1593–1594. doi: 10.1016/S0002-9440(10)62543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferri C, Ramos-Casals M, Zignego AL, Arcaini L, Roccatello D, Antonelli A, et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun Rev. 2016 Dec;15(12):1145–1160. doi: 10.1016/j.autrev.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Casals M, Muñoz S, Medina F, Jara LJ, Rosas J, Calvo-Alen J, et al. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry) J Rheumatol. 2009 Jul;36(7):1442–1448. doi: 10.3899/jrheum.080874. [DOI] [PubMed] [Google Scholar]
- 9.Ramos-Casals M, Jara LJ, Medina F, Rosas J, Calvo-Alen J, Mañá J, et al. Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the HISPAMEC Registry): patterns of clinical and immunological expression in 180 cases. J Intern Med. 2005 Jun;257(6):549–557. doi: 10.1111/j.1365-2796.2005.01490.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012 Jan 28;379(9813):348–360. doi: 10.1016/S0140-6736(11)60242-0. [DOI] [PubMed] [Google Scholar]
- 11.Retamozo S, Díaz-Lagares C, Bosch X, Bové A, Brito-Zerón P, Gómez ME, et al. Life-Threatening Cryoglobulinemic Patients With Hepatitis C: Clinical Description and Outcome of 279 Patients. Medicine (Baltimore) 2013 Sep;92(5):273–284. doi: 10.1097/MD.0b013e3182a5cf71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin. Arthritis Rheum. 2004;33:355–374. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Sene D, Ghillani-Dalbin P, Thibault V, Guis L, Musset L, Duhaut P, et al. Longterm course of mixed cryoglobulinemia in patients infected with hepatitis C virus. J. Rheumatol. 2004;31:2199–2206. [PubMed] [Google Scholar]
- 14.Bonacci M, Lens S, Londoño MC, Mariño Z, Cid MC, Ramos-Casals M, et al. Virologic, Clinical, and Immune Response Outcomes of Patients With Hepatitis C Virus-Associated Cryoglobulinemia Treated With Direct-Acting Antivirals. Clin Gastroenterol Hepatol. 2017;15:575–83.e1. doi: 10.1016/j.cgh.2016.09.158. [DOI] [PubMed] [Google Scholar]
- 15.Saadoun D, Pol S, Ferfar Y, Alric L, Hezode C, Ahmed SNS, et al. Efficacy and Safety of Sofosbuvir Plus Daclatasvir for Treatment of HCV-Associated Cryoglobulinemia Vasculitis. Gastroenterology. 2017;153:49–52.e5. doi: 10.1053/j.gastro.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Rosner I, Rozenbaum M, Toubi E, Kessel A, Naschitz JE, Zuckerman E. The case for hepatitis C arthritis. Semin. Arthritis Rheum. 2004;33:375–387. doi: 10.1016/j.semarthrit.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Patel R, Mikuls TR, Richards JS, Kerr G, Cannon GW, Baker JF. Disease characteristics and treatment patterns in veterans with rheumatoid arthritis and concomitant hepatitis C infection. Arthritis Care Res (Hoboken) 2015;67:467–474. doi: 10.1002/acr.22463. [DOI] [PubMed] [Google Scholar]
- 18.Ezzat WM, Raslan HM, Aly AA, Emara NA, El Menyawi MM, Edrees A. Anti-cyclic citrullinated peptide antibodies as a discriminating marker between rheumatoid arthritis and chronic hepatitis C-related polyarthropathy. Rheumatol Int. 2011;31:65–69. doi: 10.1007/s00296-009-1225-8. [DOI] [PubMed] [Google Scholar]
- 19.Ramos-Casals M. Viruses and lupus the viral hypothesis. Lupus. 2008 Mar;17(3):163–165. doi: 10.1177/0961203307086268. [DOI] [PubMed] [Google Scholar]
- 20.Ramos-Casals M, Font J, García-Carrasco M, Cervera R, Jiménez S, Trejo O, et al. Hepatitis C virus infection mimicking systemic lupus erythematosus: study of hepatitis C virus infection in a series of 134 Spanish patients with systemic lupus erythematosus. Arthritis Rheum. 2000 Dec;43(12):2801–2806. doi: 10.1002/1529-0131(200012)43:12<2801::AID-ANR21>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed MM, Berney SM, Wolf RE, Hearth-Holmes M, Hayat S, Mubashir , et al. Prevalence of active hepatitis C virus infection in patients with systemic lupus erythematosus. Am. J. Med. Sci. 2006;331:252–256. doi: 10.1097/00000441-200605000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Ramos-Casals M, Mañá J, Nardi N, Brito-Zerón P, Xaubet A, Sánchez-Tapias JM, et al. Sarcoidosis in patients with chronic hepatitis C virus infection analysis of 68 cases. Medicine (Baltimore) 2005 Mar;84(2):69–80. doi: 10.1097/01.md.0000157577.69729.e6. [DOI] [PubMed] [Google Scholar]
- 23.Ramos-Casals M, Jara LJ, Medina F, Rosas J, Calvo-Alen J, Mañá J, et al. HISPAMEC Study Group Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the HISPAMEC Registry): patterns of clinical and immunological expression in 180 cases. J. Intern. Med. 2005;257:549–557. doi: 10.1111/j.1365-2796.2005.01490.x. [DOI] [PubMed] [Google Scholar]
- 24.Uruha A, Noguchi S, Hayashi YK, Tsuburaya RS, Yonekawa T, Nonaka I, et al. Hepatitis C virus infection in inclusion body myositis: A case-control study. Neurology. 2016;86:211–217. doi: 10.1212/WNL.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 25.Bini EJ, Mehandru S. Incidence of thyroid dysfunction during interferon alfa-2b and ribavirin therapy in men with chronic hepatitis C: a prospective cohort study. Arch. Intern. Med. 2004;164:2371–2376. doi: 10.1001/archinte.164.21.2371. [DOI] [PubMed] [Google Scholar]
- 26.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, et al. Thyroid disorders in chronic hepatitis C. Am. J. Med. 2004;117:10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Floreani A, Betterle C, Carderi I, Presotto F, Pedini B, Moscon A, et al. Arsita-one Research Group. Is hepatitis C virus a risk factor for thyroid autoimmunity? J. Viral Hepat. 2006;13:272–277. doi: 10.1111/j.1365-2893.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 28.Ramos-Casals M, la Civita L, de Vita S, Solans R, Luppi M, Medina F, et al. Characterization of B cell lymphoma in patients with Sjögren's syndrome and hepatitis C virus infection. Arthritis Rheum. 2007 Feb 15;57(1):161–170. doi: 10.1002/art.22476. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin's lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95:745–752. doi: 10.1111/j.1349-7006.2004.tb03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwata H, Matsuo K, Takeuchi K, Kishi Y, Murashige N, Kami M. High incidences of malignant lymphoma in patients infected with hepatitis B or hepatitis C virus. Haematologica. 2004;89:368–370. [PubMed] [Google Scholar]
- 31.Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol. Biomarkers Prev. 2006;15:2078–2085. doi: 10.1158/1055-9965.EPI-06-0308. [DOI] [PubMed] [Google Scholar]
- 32.Nieters A, Kallinowski B, Brennan P, Ott M, Maynadié M, Benavente Y, et al. Hepatitis C and risk of lymphoma: results of the European multicenter case-control study EPILYMPH. Gastroenterology. 2006;131:1879–1886. doi: 10.1053/j.gastro.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Antonelli A, Ferri C, Fallahi P, Sebastiani M, Nesti C, Barani L, et al. Type 2 diabetes in hepatitis C-related mixed cryoglobulinaemia patients. Rheumatol. (Oxford) 2004;43:238–240. doi: 10.1093/rheumatology/keh011. [DOI] [PubMed] [Google Scholar]
- 34.Dyal HK, Aguilar M, Bartos G, Holt EW, Bhuket T, Liu B, et al. Diabetes Mellitus Increases Risk of Hepatocellular Carcinoma in Chronic Hepatitis C Virus Patients: A Systematic Review. Dig Dis Sci. 2016;6:636–645. doi: 10.1007/s10620-015-3983-3. [DOI] [PubMed] [Google Scholar]
- 35.Hwang JC, Jiang MY, Lu YH, Weng SF. Impact of HCV Infection on Diabetes Patients for the Risk of End-Stage Renal Failure. Medicine (Baltimore) 2016;95:e2431. doi: 10.1097/MD.0000000000002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, et al. HCV Meta-Analysis (on) Individual Patients' Data Study Group Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–1642. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Retamozo S, Brito-Zerón P, Quartuccio L, De Vita S, Ramos-Casals M. Introducing treat-to-target strategies of autoimmune extrahepatic manifestations of chronic hepatitis C virus infection. Expert Rev Clin Pharmacol. 2017 Jul 27;:1–17. doi: 10.1080/17512433.2017.1357466. [DOI] [PubMed] [Google Scholar]
- 38.Zignego AL, Ramos-Casals M, Ferri C, Saadoun D, Arcaini L, Roccatello D, et al. International therapeutic guidelines for patients with HCV-related extrahepatic disorders. A multidisciplinary expert statement. Autoimmun Rev. 2017 May;16(5):523–541. doi: 10.1016/j.autrev.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Casals M, Zignego AL, Ferri C, Brito-Zerón P, Retamozo S, Casato M, et al. Evidence-based recommendations on the management of extrahepatic manifestations of chronic hepatitis C virus infection. J Hepatol. 2017 Jul;66(6):1282–1299. doi: 10.1016/j.jhep.2017.02.010. [DOI] [PubMed] [Google Scholar]