Abstract
Introduction
The AXIS trial established axitinib as a standard of care treatment for patients with metastatic renal cell carcinoma (mRCC) after failure of a prior tyrosine kinase inhibitor. Axitinib dosing begins at 5 mg twice daily, with escalation of doses to 7 and 10 mg after consecutive 2-week intervals if tolerated (as per the drug label). Given clinical concerns about drug-related toxicity, we have used a pragmatic strategy where dose escalations were made only after disease progression or where rapid responses were clinically required.
Methods
We performed a retrospective review of electronic health records and radiology of all patients with mRCC treated with axitinib for >2 weeks at Addenbrooke’s Hospital, Cambridge, UK, over a 37 -month period to determine the clinical and radiological effects of dose escalations made according to the above strategy.
Results
42 patients fitting these criteria were identified, 29 having ≥1 dose escalation event (DEE). 60 DEEs were identified (median of two per patient), and the objective radiological consequences of 53 DEEs could be evaluated. The disease control rate (partial response or stable disease) after the first DEE instituted for disease progression was similar to that after the second DEE (68.8% vs 70%). 56.6 % of all DEEs and 63.6 % of DEEs made as a result of disease progression resulted in disease control. The median OS from the commencement of axitinib for all dose-escalated patients was 19.9 months, and 16.5 months for the entire cohort. The mean dose (for all patients) at 90 days after starting axitinib was 5.92 mg.
Conclusion
These data suggest that dose escalation of axitinib after disease progression may be an effective dosing strategy for patients with mRCC, and this may be a preferred option in patients in whom there are particular concerns about drug-related toxicity, quality of life optimisation or healthcare-associated costs.
Keywords: renal cell carcinoma, axitinib, tyrosine kinase inhibitors, dose escalation, toxicity
Summary box.
What is already known about this subject?
Axitinib is an effective treatment for patients with metastatic renal cell carcinoma but is associated with significant drug-related toxicity.
The optimal dosing strategy for axitinib is not known, and the axitinib label dosing strategy is to commence treatment at 5 mg twice daily, escalating to 7 mg and then 10 mg doses at 2 weekly intervals if tolerated.
What does this study add?
Our retrospective review of patients undergoing dose escalations of axitinib for disease progression, or for a clinical need to achieve a rapid response, suggests that this may be an effective dosing strategy for axitinib in patients with metastatic renal cell carcinoma.
Our study shows favourable outcomes in a less fit patient population than that treated in the AXIS trial.
How might this impact on clinical practice?
Dose escalation of axitinib on demonstration of disease progression, rather than demonstration of drug tolerance, may result in good clinical outcomes with less drug-related toxicity and lower direct and indirect healthcare-associated costs.
It may be an option for patients in whom rapid disease control is not required, or where there are concerns about drug-related toxicity and quality-of-life optimisation.
Introduction
Renal cell carcinoma accounts for up to 5% of adult cancers, and its incidence is rising.1 Tyrosine kinase inhibitors (TKIs) are the longest established treatment options for patients with metastatic renal cell carcinoma.2 The efficacy of TKIs must be carefully balanced with their attendant toxicities in individual patients, often necessitating dose and schedule changes, and treatment breaks, in order to optimise both drug exposure and quality of life.3 Results from a randomised phase III trial (AXIS) established the efficacy of the multiple TKI axitinib in the second-line setting for the treatment of patients with metastatic renal cell carcinoma (mRCC) with a clear cell component, given superior progression-free survival (PFS) in the axitinib arm versus the sorafenib comparator arm (6.7 months vs 4.7 months; HR 0.665, 95% CI 0.544 to 0.812; p<0.0001).4 Median overall survival (OS) was 20.1 months with axitinib.5 The most common ≥grade 3 toxicities in the axitinib arm were hypertension (16% of patients), diarrhoea (11%), fatigue (11%), reduced appetite (5%), palmar-plantar erythrodysaesthesia (5%) and asthenia (5%). Patients in this trial were ECOG performance status 0 or 1 on entry, with a life expectancy of ≥12 weeks. The protocol for axitinib dosing in this study built on previous clinical evidence, with axitinib-assigned patients being commenced on 5 mg twice daily and, if tolerated, dose-escalated to 7 mg and then 10 mg doses twice daily after consecutive 2-week intervals. The AXIS study led to the approval of axitinib in Europe and elsewhere for the second-line treatment of mRCC after failure of a prior TKI or cytokine therapy, and the drug label uses the same dose escalation guidance as the AXIS trial. However, it remains unclear if this is the optimal dosing strategy, particularly given associated drug toxicity. For example, it remains uncertain if dose escalation after demonstration of tolerance, or after demonstration of clinical failure at that dosage level, is optimal in terms of the balance between therapeutic efficacy, quality-of-life considerations, and direct and indirect healthcare-associated costs, particularly in real-world populations where patients are less fit than in clinical trials.6 7
Our centre’s early clinical experience with axitinib suggested that dose escalation as per its label was associated with significant drug-related toxicity (particularly asthenia and anorexia), not necessarily predicted by the 2-week windows (suggested by the available guidance) to assess tolerance. Also, we observed unambiguous clinical responses to axitinib at the starting dose of 5 mg twice daily, where dose escalation (with its attendant risk of increasing drug-related toxicity) seemed clinically inappropriate. Over time we adopted a pragmatic strategy where doses of axitinib were only escalated after unambiguous radiological or clinical progression, or if there was a pressing clinical need to achieve a swift response (as judged by the treating physician, on grounds of high disease burden/symptomatic masses/high-risk location of metastases (eg, intracranial)), with dose reductions made only on the grounds of drug-related toxicity. We present here a retrospective analysis of the clinical outcomes from this strategy. We use a defined data cut-off before immune checkpoint inhibitors became standard of care treatment in order not to confound the outcomes of this dosing strategy.
Methods
We examined the medical records of all patients with metastatic renal cell carcinoma treated at Cambridge University Hospitals NHS Foundation Trust (Cambridge, UK) that were commenced on axitinib between the 1 June 2013 and 12 July 2016. Patients fulfilling these criteria were identified using reporting from our current electronic healthcare record system (EPIC) and its predecessor (EMR). Patients who received axitinib as part of a clinical trial were excluded, as were patients who failed to complete 2 weeks of axitinib dosing. The following data were obtained: (1) age at diagnosis; (2) patient sex; (3) histology subtype; (4) date of diagnosis of metastatic renal cell carcinoma (with both histopathological and radiological evidence); (5) prior treatment lines and their respective start and finish dates; (6) axitinib starting date and starting dose; (7) reasons for any changes in axitinib dose or discontinuation; (8) the results of any changes made in dosing (either clinical or radiological); (9) date of final progression on axitinib (if applicable) and (10) date of death (if applicable). From these, OS from starting axitinib was calculated as (10)-(6). The cut-off for all analyses was 12 October 2016 before the routine use of immune checkpoint inhibitors commenced in our centre in order to minimise survival data confounding by subsequent or prior immune checkpoint inhibitors. All radiological responses were determined by cross-sectional imaging (CT/MRI/both). Objective response to axitinib was determined by the results of the first radiological imaging after commencement of axitinib (regardless of axitinib dosing at that time). Responses to dose escalations were determined by the first radiological imaging after starting and continuing on the new dosage (although short dose intermissions were permitted). Kaplan-Meier curves were produced using Prism software (GraphPad), with statistical analyses of comparisons being performed using the log-rank (Mantel-Cox) test.
Results
Demographics and baseline patient characteristics
We retrospectively identified 42 patients with mRCC meeting the above criteria and treated with axitinib for at least 2 weeks. Epidemiological factors were similar to baseline mRCC populations reported in the AXIS trial,4 although 10 patients (23.8%) had non-clear cell histology (table 1).
Table 1.
Characteristics of the patient population reported in this study [n (%)]
| Gender | Male | 31 (73.8) |
| Female | 11 (26.2) | |
| Histology | Clear cell | 32 (76.2) |
| Papillary | 7 (16.7) | |
| Chromophobe | 1 (2.4) | |
| SDHB-associated | 1 (2.4) | |
| NOS | 1 (2.4) | |
| Prior treatment lines | TKI | 42 (100) |
| TKI only | 29 (69.0) | |
| TKI+ immunotherapy | 6 (14.3) | |
| TKI+ everolimus | 5 (11.9) | |
| TKI+ everolimus +immunotherapy | 3 (7.1) | |
| ECOG performance status (all patients) | 0 | 17 (40.5) |
| 1 | 12 (28.6) | |
| 2 | 12 (28.6) | |
| 3 | 0 | |
| 4 | 1 (2.4) | |
| ECOG performance status (dose-escalated patients) | 0 | 15 (51.7) |
| 1 | 9 (31.0) | |
| 2 | 5 (17.2) | |
| 3 | 0 | |
| 4 | 0 | |
| ECOG performance status (non-dose-escalated patients) | 0 | 2 (15.4) |
| 1 | 3 (23.1) | |
| 2 | 7 (53.8) | |
| 3 | 0 | |
| 4 | 1 (7.7) |
ECOG, Eastern Cooperative Oncology Group; NOS, histology not otherwise specified (non-clear cell)); SDHB, succinate dehydrogenase B; TKI, tyrosine kinase inhibitor other than axitinib.
All patients were previously treated with a TKI, with 29 patients (69.0%) having only received prior TKI therapy. Only two patients (6.9%) were started on a dose other than 5 mg (2 mg given significant prior TKI toxicities). Of these 42 patients, 29 (69.0%) were identified as having ≥1 dose escalation of axitinib. The Eastern Cooperative Oncology Group Performance Statuses (ECOG PS) of patients were significantly higher than those in the axitinib arm of the AXIS trial4 with 40.5% of patients in this study being ECOG PS 0 (cf. 54% in the AXIS trial), and 31% of patients being ECOG PS 2–4 (cf. <1% in the AXIS trial). 17.2% of patients having dose escalations of axitinib were ECOG PS 2–4 compared with 61.5% of patients who did not have dose escalations (table 1).
Dose escalation events
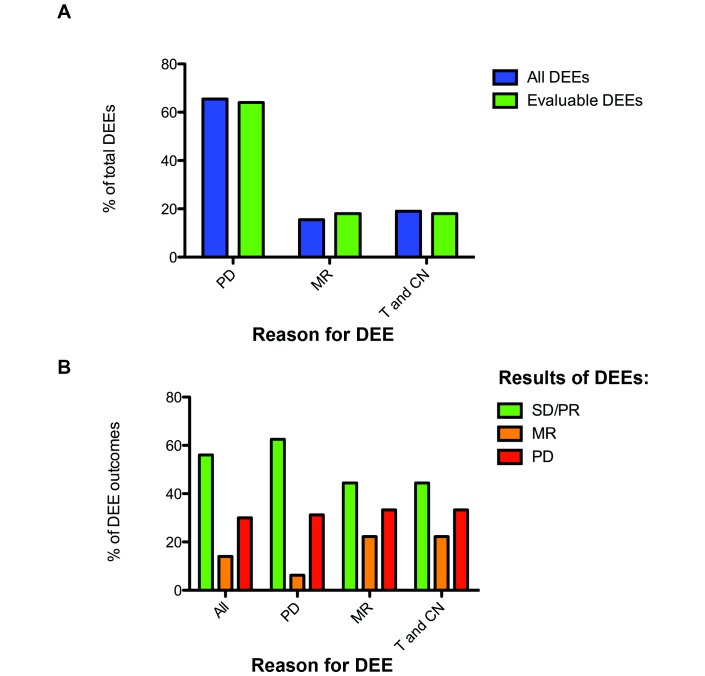
Sixty individual dose escalation events (DEEs) were identified in these 29 patients, with a median of 2 DEEs per patient. Twenty-eight patients had at least one DEE for progression/MR. The results of 53 of these individual DEEs were radiologically evaluable. The others could not be objectively evaluated owing to toxicity or patient death, or to no follow-up imaging having been performed (with follow-up imaging awaited at the time of analysis cut-off, or further DEEs having been made owing to tolerance and a need to achieve a swift response without interval imaging). Evaluable DEEs were made for definitive radiological disease progression (PD; 62.3%), a mixed radiological response (MR; 20.8%) or a pressing clinical need to achieve a swift response in a patient tolerating treatment (T and CN; 17.0%). This is summarised in figure 1A. Nine dose-escalated patients required a dose reduction for reasons of toxicity as their first dose modification from the starting dose (31.0%).
Figure 1.
(A) Bar chart showing the proportion of total and radiologically evaluable axitinib dose escalation events (DEEs) that were made for reasons for progressive disease (PD), a mixed radiological response (MR) or drug tolerance and a clinical need to dose escalate (T and CN).(B) Bar chart showing the radiological outcomes (results) for evaluable DEEs for all DEEs (ALL), and those that were made for reasons for PD, an MR or drug tolerance and a clinical need to dose escalate (T and CN). MR, mixed response; PD, progressive disease; PR, partial response; SD, stable disease.
Results of DEEs
Of these 53 evaluable DEEs, 30 (56.6%) resulted in disease control (stable disease (SD; n=23) or partial response (PR; n=7); 12 DEEs resulted in some degree of tumour shrinkage (TS)). Eight resulted in a mixed response (MR; 15.1%), while 15 (28.3%) resulted in disease progression (figure 1B). The disease control rate (DCR) when DEEs were made as a reaction to disease progression was 63.6% (n=33; n(PR)=5; n(SD)=16; n(TS)=8), higher than the DCR when DEEs were made as a reaction to a mixed radiological response (45.5%; n=11; n(PR)=2; n(SD)=3; n(TS)=4) or drug tolerance and a clinical need (44.4%; n=9; n(PR)=0).
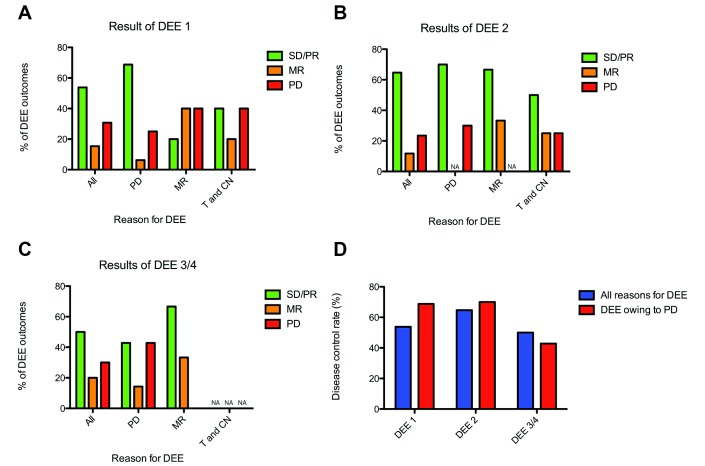
We next examined if disease control after a DEE was affected by having previous axitinib dose escalations. The DCR for a patient’s first DEE was 53.8% overall (n=26; PR in two patients; SD in 12 patients; four patients having TS), and 68.8% if the DEE was made as a result of disease progression (n=16; n(PR)=2; n(SD)=9; n(TS)=4) (figure 2A). The DCR for a patient’s second DEE was 64.7% overall (n=17; n(PR)=3; n(SD)=8; n(TS)=5), and 70% if the DEE was made as a result of disease progression (n=10; n(PR)=2; n(SD)=4; n(TS)=3) (figure 2B). The DCR for a patients third/fourth DEE was 50.0% overall (n=10; n(PR)=2; n(SD)=3; n(TS)=3), and 42.9% if the DEE was made as a result of disease progression (n=7; n(PR)=1; n(SD)=2; n(TS)=1) (figure 2C). Disease control rates by DEE sequence are summarised in figure 2D.
Figure 2.
(A–C) Bar chart showing the radiological outcomes (results) for evaluable dose escalation events (DEEs) for first (figure 2A), second (figure 2B) and subsequent (figure 2C) DEEs, subclassified by reason for DEE (progressive disease (PD), a mixed radiological response (MR) or drug tolerance and a clinical need to dose escalate (T and CN)). (D) Bar chart of disease control rates for first, second and subsequent DEEs, showing all DEEs and those made as a result of disease progression). PR, partial response; SD, stable disease.
We then examined if patients who achieved disease control (on the first imaging test performed) after starting axitinib were more or less likely to achieve a response to dose escalation. The overall DCR on axitinib at first interval imaging (regardless of any DEE(s)) for all evaluable patients was 62.5% (n=40; n(PR)=7; n(SD)=18) and 62.1% (n=29; n(PR)=5; n(SD)=13) for those who subsequently had DEE(s). Of the 12 evaluable patients who achieved initial disease control on axitinib and had a subsequent DEE, the DCR for the first DEE was 75%, and for those who did not (MR/progression after starting axitinib) the DCR was 35.7% (n=14).
We then examined if initial disease control after the first DEE predicted disease control after the second DEE. This was fully objectively evaluable for 15 patients (n=8 for patients with disease control after the first DEE; n=7 for patients with MR/progression after the first DEE). Of those patients initially having disease control after a first DEE, the DCR to the second DEE was 75%, while in those without initial disease control after a first DEE, the DCR to the second DEE was 57.1%.
The average length of time to the first DEE above the starting dose was 165.2 days (ranging from 21 to 785 days; median 84 days). For patients commenced on 5 mg of axitinib, the mean dose at 28 days in our dose-escalated patients was 4.93 mg (median=5 mg; n=27), lower than 5 mg as a result of some dose reductions being made owing to toxicity. The mean dose at 90 days in this cohort was 5.92 mg (median=7 mg; n=26).
Time to dose escalation and patient survival
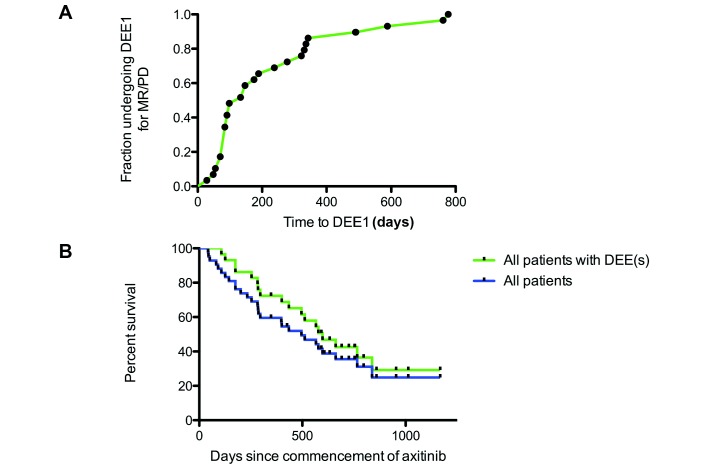
For those undergoing a first DEE for reasons of MR/PD, the time from commencement of axitinib to the time of dose escalation ranged from 28 to 777 days (median 133 days; figure 3A). The median OS for all 42 patients treated with axitinib was 496 days (16.5 months) from the commencement of treatment with axitinib (figure 3B). For the 29 patients who underwent DEE(s), their median OS was 598 days (19.9 months) from the commencement of treatment with axitinib (figure 3B), and 201 days (6.7 months) for patients who did not have DEE(s) (n=13; note that these patients had significantly higher ECOG PS). For cancers with a clear cell component (n=32), the median OS for all patients was 435 days, and 598 for those who underwent DEE(s) (n=22). The median time on axitinib (from first dose to last dose) for all patients was 329 days (462 days for those undergoing DEE(s) and 99 days for those who did not have DEE(s). For dose-escalated patients, the median PFS from starting axitinib was 189 days (range 28–785 days) and the median PFS after DEE1 was 182 days (range 28–648 days). The equivalent PFSs for cancers with a clear cell component were 231 and 182 days, respectively, and for non-clear cell cancers were 84 and 224 days, respectively. Previous studies have shown that an increased PFS on a prior TKI is associated with an increased OS after starting axitinib.5 We therefore examined if this held in our patients. For DEE evaluable patients who had higher than median time on their prior TKI, the median OS has not yet been reached at the time of cut-off (with a 62.9% 2-year survival), while this was 496 days (16.5 months) for those with less than median time on their prior TKI (p=0.0459; n=25). At the time of data cut-off, only six patients had received subsequent anticancer treatment (everolimus (n=3); nivolumab (n=2) and savolitinib (n=2)).
Figure 3.
(A) Kaplan-Meier curve showing the time from commencement of axitinib to the time of the first DEE for reasons of mixed response (MR)/progressive disease (PD). (B) Kaplan-Meier survival curves showing the overall survival of all patients included in our analysis since starting axitinib (n=42) and those who had DEEs (n=29). DEE, dose escalation event.
Discussion
We have presented retrospective data from a real-world cohort of patients with renal cell carcinoma treated with axitinib after failure of a prior TKI, using a dosing schedule of axitinib that starts at 5 mg twice daily, escalating to higher doses if there is evidence of disease progression or if the drug is tolerated and there was a pressing need to achieve rapid disease control. This strategy was adopted after our early clinical experience which suggested that our patient population tolerated higher doses of axitinib less well than the population treated within the AXIS trial, as well as unambiguous clinical responses being achieved at the starting dose of 5 mg twice daily.
For radiologically evaluable DEEs, the DCR on the first subsequent scan after commencing axitinib was 62.5%, lower than that reported for the AXIS trial.8 However, subsequent dose escalations for disease progression were able to achieve disease control in the majority of patients, and disease control with dose escalation of axitinib has been reported by others: in a study of 22 patients having dose escalations of TKIs after disease progression, the best response after dose escalation was PR in 6% of patients and SD in 72% of patients, with 78% of patients having some degree of TS—17 patients in this study had dose escalations of axitinib, although results were not broken down by TKI type.9 These results strongly suggest that if rapid gain of disease control is clinically indicated dose escalation should proceed as per the axitinib label. In other cases, where there are particular concerns about drug-related toxicity (eg, in patients who did not tolerate any previous TKI lines well, or in elderly patients or those with relevant comorbidities), it is an option to commence at the 5 mg twice daily dose level, and dose modify according to toxicity and clinical/objective progression (we suggest dose escalation in cases where even single lesions are progressing in the context of overall SD/PR/MR, and in cases of symptomatic metastases or where clinical consequences of local disease progression are likely to be severe and waiting for objective progression may lead to harm (eg, intracranial/spinal metastases)). Our survival data suggest that the outcomes of our dose escalation strategy may be comparable with those in the AXIS trial, in a significantly less fit patient population (as judged by ECOG PS), providing added reassurance about the longer term outcomes of more cautious dose escalation.
Concerns about TKI-related toxicities have led others to investigate alternative TKI dosing strategies, including the 2-week-on, 1-week-off strategy for sunitinib (as opposed to 4-week-on, 2-week-off), which has promising results in terms of toxicity and efficacy.10 Dose escalation on progression also appears feasible and efficacious with sunitinib.11 While ours is a small, retrospectively reviewed cohort, we believe that the outcomes for the patients presented here supports cautious, clinically guided dose escalation of axitinib, particularly in clear patients with whom there are specific concerns about axitinib-related drug toxicity (although prospective toxicity data were not collected in our study), quality of life or drug-related cost. Thirty-one per cent of patients undergoing DEEs in this study required dose reductions from their starting dose as the first dose modification event, underlining our concerns with axitinib tolerance in real-world patient populations. Although it is appreciated that not all patients will tolerate the label’s dose escalation schedule (to 10 mg twice daily in 28 days), compared with this dose escalation schedule our strategy may result in reduced toxicity and a significant cost saving. We also believe that our results may inform dosing strategies in trials of combination approaches with axitinib, and any subsequent integration of new combination treatments into clinical practice. A prospective trial comparing axitinib dosing strategies may confirm which strategy results in optimal efficacy and quality of life for patients.
Conclusions
Diligent management of TKI dosing and drug-related toxicity is required for the optimal clinical care of patients with renal cell carcinoma. While the AXIS trial confirmed the efficacy and safety of axitinib in the second-line setting, toxicity was common and it can be challenging to balance drug-related toxicity and efficacy in the real-world setting. Our results suggest that cautious dose escalation of axitinib is clinically feasible, with reassuring long-term results. Although we did not measure toxicity prospectively, cautious dose escalation is likely to result in lower toxicity, higher quality of life and reduced direct and indirect healthcare-associated costs. We suggest that where rapid disease control is clinically required axitinib dosing should proceed as per the drug label. In other settings, particularly in patients in whom there are particular concerns about drug-related toxicity, our data suggest that dose escalation as a result of disease progression (rather than drug tolerance) may be safely considered.
Footnotes
Contributors: GD and TE designed the study. Data collection and analysis were performed by GD and DL. GD wrote the first draft of the manuscript and all other authors contributed to its revision. All authors were involved in the clinical management of the patients in this study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AM is employed by AstraZeneca. KF has received honoraria for advisory board activities from Esai, Ipsen, Roche and Novartis, and speaker fees from Bristol Myers Squibb and Pfizer. TE is employed by AstraZeneca and has received honoraria for consultancy and advisory board activities from GSK, Pfizer, Roche, Novartis and Bristol Myers Squibb.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Sánchez-Gastaldo A, Kempf E, González Del Alba A, et al. Systemic treatment of renal cell cancer: a comprehensive review. Cancer Treat Rev 2017;60:77–89. 10.1016/j.ctrv.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 2. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27 v58–68. 10.1093/annonc/mdw328 [DOI] [PubMed] [Google Scholar]
- 3. Gangadaran SGD. Current management options in metastatic renal cell cancer. Oncol Rev 2017;11:339 10.4081/oncol.2017.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–9. 10.1016/S0140-6736(11)61613-9 [DOI] [PubMed] [Google Scholar]
- 5. Escudier B, Michaelson MD, Motzer RJ, et al. Axitinib versus sorafenib in advanced renal cell carcinoma: subanalyses by prior therapy from a randomised phase III trial. Br J Cancer 2014;110:2821–8. 10.1038/bjc.2014.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell AP, Harrison MR, George DJ. Clinical trial subjects compared to “real world” patients: Generalizability of renal cell carcinoma trials. J Clin Oncol 2014;32:6510. [Google Scholar]
- 7. Kennedy-Martin T, Curtis S, Faries D, et al. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 2015;16:495 10.1186/s13063-015-1023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013;14:1287–94. 10.1016/S1470-2045(13)70465-0 [DOI] [PubMed] [Google Scholar]
- 9. Ornstein MC, Wood L, Elson P, et al. Clinical effect of dose escalation after disease progression in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 2017;15:e275–280. 10.1016/j.clgc.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 10. Jonasch E, Slack RS, Geynisman DM, et al. Phase II study of two weeks on, one week off sunitinib scheduling in patients with metastatic renal cell carcinoma. J Clin Oncol 2018;36:1588–93. 10.1200/JCO.2017.77.1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raphael J, Thawer A, Bjarnason GA. Sunitinib dose-escalation after disease progression in metastatic renal cell carcinoma. Urol Oncol 2018;36:12.e1–6. 10.1016/j.urolonc.2017.09.004 [DOI] [PubMed] [Google Scholar]