Abstract
Background
We present a European multicenter study, comparing safety data and patient-reported outcomes (PRO) from patients undergoing synovial biopsy using ultrasound-guided needle biopsy (US-NB), ultrasound-guided portal and forceps (US-P&F) or arthroscopic-guided (AG) procedures.
Objectives
To describe safety and PRO data on joint indices of pain, stiffness and swelling before and after biopsy, procedural discomfort, joint status compared with before biopsy and willingness to undergo a second biopsy for each technique and compare the three techniques. To evaluate the impact on PRO and safety data of corticosteroid therapy as part of the biopsy procedure and sequential biopsy procedures.
Methods
Data were collected on the day of biopsy and 7–14 days postprocedure. Joint pain, swelling and stiffness indices were recorded as 0–100 mm Visual Analogue Scale; qualitative outcome variables on five-point Likert scales. Groups were compared with linear regression, adjusting for disease activity, corticosteroid therapy and prebiopsy PRO value and accounting for repeated measurements.
Results
A total of 524 synovial biopsy procedures were documented (402 US-NB, 65 US-P&F and 57 AGSB). There were eight adverse events (1.5%) with no difference between biopsy methods (p=0.55). All PROs were improved 2 weeks postprocedure, and there were no differences in postbiopsy change in PROs between biopsy methods. Corticosteroid administration, whether intramuscular (n=62) or intra-articular (n=38), did not result in more adverse events (p=0.81) and was associated with reduction in postbiopsy swelling (p<0.01). Sequential biopsy procedures (n=103 patients) did not result in more adverse events (p=0.61) or worsening in PRO data.
Conclusion
Overall, our results do not suggest a significant difference in safety or patient tolerability between US-NB, US-P&F and AGSB sampling. Further, corticosteroid therapy as part of the biopsy procedure and sequential biopsies is safe and well tolerated in patients.
Keywords: patient perspective, arthritis, rheumatoid arthritis, early rheumatoid arthritis, ultrasonography
Key messages.
What is already known about this subject?
Arthroscopic-guided synovial biopsy, ultrasound-guided needle biopsy and ultrasound-guided portal and forceps methods has been shown to be a safe and well-tolerated procedures, although without comparison of patient-reported outcomes and safety between the methods.
What does this study add?
This is the largest comparative study evaluating the tolerability and safety of ultrasound-guided and arthroscopic biopsy techniques and we found no significant difference between ultrasound-guided needle biopsy, ultrasound-guided portal and forceps or the arthroscopic-guided synovial biopsy methods.
Further, corticosteroid therapy as part of the biopsy procedure and sequential biopsies is safe and well tolerated in patients.
How might this impact on clinical practice?
From a safety and patient perspective, all three synovial biopsy methods can be used in both trials and clinical practice.
Introduction
Synovial tissue analysis could provide a step change towards personalising diagnosis, disease stratification and treatment selection of patients with inflammatory arthritis.1 2Arthroscopic-guided synovial biopsy (AGSB) is to date regarded as the ‘gold standard’ for synovial tissue acquisition due to extensive validation examining synovial biopsy performance and patient tolerability and safety.3 4 However, the technical skills and equipment required generally restrict the use of arthroscopy for research purposes to targeting large joints and within academic centres in rheumatology practice.2 4–6 By contrast, minimally invasive ultrasound-guided needle biopsy (US-NB) or portal and forceps (US-P&F) are becoming widely adopted.7–9 These approaches permit access to synovial tissue in a wider range of joints, are technically simple, relatively inexpensive to perform and have a generally good safety record.5 7 10 11 However, the wide scale adoption of US-guided techniques in clinical trials and in routine care requires further studies evaluating patient safety and tolerability of the techniques, particularly compared with the gold standard of arthroscopy.4
The aim of this multicenter retrospective cohort study of patients with inflammatory arthritis undergoing US-NB, US-P&F and AGSB was to evaluate whether there were significant differences in safety and tolerability between the techniques.
Patients and methods
Participating centers
The study protocol was presented at the European Synovitis Study Group European League Against Rheumatism (EULAR) meeting in June 2017. Centres with consecutive cohorts of patients with inflammatory arthritis undergoing US-NB or US-P&F and AGSB procedures with patient-reported outcome (PRO) data and safety data were invited to participate in the study. Academic rheumatology centres with suitable cohorts of patients included in the study were: Barts and the London School of Medicine and Dentistry (UK) (data from the multicenter studies Stratification of Biologic Therapies for Rheumatoid Arthritis by Pathobiology (STRAP) and Response—Resistance to Rituximab vs Tocilizumab in Rheumatoid Arthritis (R4RA) trials), Odense University Hospital (Denmark) (data from the SynRA study), Birmingham (UK) (data from the Birmingham Early Arthritis Cohort (BEACON)), Brussels (Belgium) and Lisbon Academic Medical Centre (data from mini-arthroscopy cohort, Portugal).
Biopsy procedures
US-guided needle biopsy
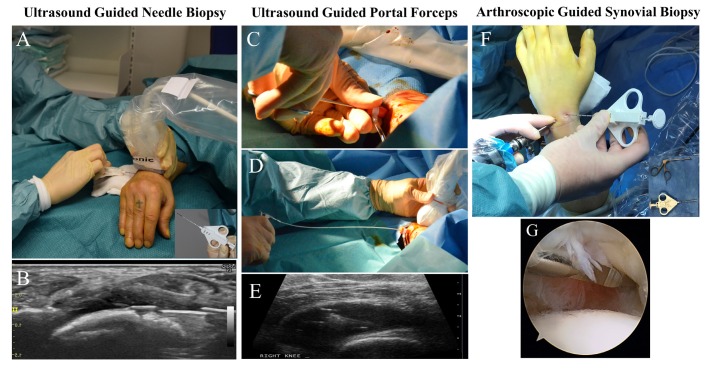
US-NB procedures (figure 1A) were performed in a clean procedure room or operating theatre, on both large joints and small joints, as previously described.7 Briefly, local anaesthetic was injected into the soft tissue up to the joint capsule and into the joint space and a Quick-Core biopsy needle (16/14-gauge; Cook Medical) was then guided by ultrasound and placed within the joint capsule (figure 1B) to retrieve synovium.
Figure 1.
(A) The ultrasound-guided needle biopsy (US-NB) procedure of wrist, in outpatient clinic using local anaesthesia. In the lower right corner example of biopsy needle. (B) Image of ultrasound guidance during US-NB procedure. (C) and (D) Portal and forceps technique: using rigid and flexible forceps through a disposable portal under local anaesthesia, (E) Synovial sampling within the knee joint under ultrasound control. (F) Arthroscopy of wrist, in operating theatre, patient under general anaesthesia. In lower corner examples of biopsy equipment. (G) Video guidance used to visualise synovium during arthroscopy.
US-guided portal and forceps
US-P&F procedures (figure 1C–E) were performed in a clean procedure room on large joints (knee, ankle and wrist).8 9 Briefly, local anaesthetic was injected into the soft tissue up to the joint capsule and into the joint space. A single 7 Fr disposable portal was placed using Seldinger technique under ultrasound guidance, within the joint capsule. Hereafter, synovial biopsies were obtained, through the portal, using rigid 2.2 mm (Hartmann Herzfeld, Medicon, Germany) and flexible 2.2 mm (Tontarra, Germany) forceps (figure 1C–E).
Arthroscopic-guided synovial biopsy
AGSB procedures (figure 1F) were performed in an operating theatre setting or a clean procedure room, on both small and large joints, as previously described.5 12 13 Arthroscopy was performed using local, regional or general anaesthesia.
Briefly, for the wrist joint, patients underwent general anaesthesia and up to 5 kg of traction across the wrist. Two standard arthroscopic portals were inserted into the radiocarpal joint, one for arthroscopic visualisation and the other for instrumentation. Synovial biopsies were obtained under direct visual inspection (figure 1G), using a Quick-Core biopsy needle (16/14-gauge; Cook Medical) (figure 1F) a standard wrist arthroscopy punch was also used (figure 1F, lower right corner insert). For the knee joint, standardised procedures for synovial tissue collection in clinical trials, under local anaesthesia, were followed as previously described.13 Arthroscopies were performed with (‘wet’) and without (‘dry’) flow of saline through the joint during the procedure.
Patients
Patient characteristics including age, gender, diagnosis, disease duration, joint biopsied, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and rheumatological medication (conventional synthetic (cs) disease-modifying antirheumatic drugs (csDMARD), biological DMARDs (bDMARDs), corticosteroid), disease activity score in 28 joints with CRP or ESR (DAS28CRP or DAS28ESR), use of intramuscular (IM) or intra-articular (IA) corticosteroids immediately following biopsy were collected.
PRO data
Prebiopsy (day of biopsy) and postbiopsy (7–14 days postprocedure) pain, stiffness and swelling (Visual Analogue Scale (VAS) from 0 to 100 mm) were collected for each biopsy procedure. Further, PROs if available, on the degree of discomfort during the procedure, prebiopsy and postbiopsy evaluation of worsening/improvement of joint symptoms and the willingness of the patient to undergo another biopsy were also collected using five-point Likert scales.
Safety data
All registered adverse events (no time restriction) between time of biopsy to November 2017 were collected, including, but not limited to, data on presyncope, syncope, tenosynovitis, joint infection, deep vein thrombosis, nerve or tendon damage or haemarthrosis.
Statistical analysis
Categorical variables on patient characteristics were presented as numbers and percentages, and continuous variables as means with SD. Between-group PRO data comparisons were performed using both unadjusted and adjusted linear regression, adjusting for biopsy method, disease activity, IM or IA corticosteroid use during biopsy procedure and the prebiopsy PRO value.14 Robust cluster estimation was used in the linear regressions to account for minor deviations from the model assumptions on normality and to account for repeated measurements of some patients. Differences in answers between biopsy methods in the questions measured on Likert scales were analysed by multinomial logistic regression using robust cluster estimation and adjusting for disease activity and IM or IA corticosteroid used during biopsy procedure. In the analysis of discomfort during procedure, AGSB procedures performed in general anaesthesia were excluded. Tests for interaction between biopsy technique and use of peri-biopsy IM or IA corticosteroid were conducted in relation to the PRO outcomes on discomfort and rebiopsy. Comparison of PRO data in the cohort of patients with repeated biopsies was performed using linear regression with robust cluster estimation, both unadjusted and adjusting for disease activity, corticosteroid use during biopsy procedure and in post-PRO values, the prebiopsy PRO data value. P values <0.05 were taken as statistically significant. Data were analysed on Stata V.15.
Results
Patients and procedures
Data from a total of 524 synovial biopsy procedures were included from 421 patients. Of these, 43% (228/524) of the procedures were performed as part of the multicentre R4RA (14 centres) and STRAP (nine centres) trials led by Barts and the London, while the remaining procedures were included from Brussels, n=122 (23%), Odense, n=98 (19%), Birmingham, n=56 (11%) and Lisbon, n=20 (4%) centres. A total of 318 patients (75.5%) underwent one synovial biopsy and 103 (24.5%) a repeat biopsy. Overall, 77% (402/524) of procedures were performed using US-NB, 12% (65/524) were performed using US-P&F and 11% (57/524) were performed using AGSB. For details on distribution of biopsy methods between centres, please see online supplementary table 1A and B. In the arthroscopy group, 77% (44/57) were performed using local anaesthesia and 23% (13/57) under general anaesthesia. In the US-NB group, biopsies were primarily from the wrist (69%) and, as the only method, also from small joints as metacarpophalangeal (MCP) (18%) and metatarsophalangeal (MTP) (1%) joints. In the US-P&F and arthroscopy groups, the primary joint biopsied was the knee (86% and 68%, respectively). Characteristics of patients at the time of biopsy are shown in table 1
Table 1.
Patient characteristics
| US-NB | US-P&F | AGSB | Total | P values | Missing (%) | |
| Biopsy procedures, n (%) | 402 (76.7) | 65 (12.4) | 57 (10.9) | 524 (100.0) | ||
| Age, mean (SD) | 55.93 (13.79) | 52.43 (12.07) | 52.30 (17.00) | 55.12 (14.00) | 0.05 | 1.9 |
| Gender, n (%) | ||||||
| Female, n (%) | 285 (72.5) | 35 (53.8) | 38 (70.4) | 358 (69.9) | 0.01 | 2.3 |
| Diagnosis, n (%) | ||||||
| Rheumatoid arthritis, n (%) | 360 (89.6) | 42 (64.6) | 27 (47.4) | 429 (81.9) | ||
| Undifferentiated arthritis, n (%) | 20 (5.0) | 8 (12.3) | 2 (3.5) | 30 (5.7) | ||
| Spondyloarthritis, n (%) | 7 (1.7) | 0 (0.0) | 2 (3.5) | 9 (1.7) | ||
| Psoriatic arthritis, n (%) | 7 (1.7) | 9 (13.8) | 2 (3.5) | 18 (3.4) | ||
| Degenerative, n (%) | 0 (0.0) | 0 (0.0) | 13 (22.8) | 13 (2.5) | ||
| Other, n (%) | 8 (2.0) | 6 (9.2) | 11 (19.3) | 25 (4.8) | <0.01 | 0 |
| Disease duration (years) | 8.21 (9.74) | 0.53 (1.35) | 9.09 (9.40) | 7.25 (9.42) | <0.01 | 8.5 |
| Time from diagnosis to biopsy | ||||||
| Under 1 month, n (%) | 53 (13.2) | 40 (61.5) | 1 (1.8) | 94 (17.9) | ||
| One to 12 months, n (%) | 42 (10.4) | 15 (23.1) | 11 (19.3) | 68 (13.0) | ||
| Over 1 year, n (%) | 307 (76.4) | 10 (15.4) | 45 (78.9) | 362 (69.1) | <0.01 | 0 |
| Joint biopsied | ||||||
| Wrist, n (%) | 277 (69.3) | 1 (1.5) | 18 (31.6) | 296 (56.7) | ||
| Knee, n (%) | 34 (8.5) | 56 (86.2) | 39 (68.4) | 129 (24.7) | ||
| MCP, n (%) | 74 (18.5) | 0 (0.0) | 0 (0.0) | 74 (14.2) | ||
| Ankle, n (%) | 2 (0.5) | 8 (12.3) | 0 (0.0) | 10 (1.9) | ||
| Elbow, n (%) | 8 (2.0) | 0 (0.0) | 0 (0.0) | 8 (1.5) | ||
| MTP, n (%) | 5 (1.3) | 0 (0.0) | 0 (0.0) | 5 (1.0) | <0.01 | 0.4 |
| Disease activity score (DAS28-CRP/ESR), n (%) | ||||||
| EULAR remission | 28 (10.4) | 4 (6.3) | 2 (5.7) | 34 (9.3) | ||
| EULAR low disease activity | 14 (5.2) | 7 (11.1) | 7 (20.0) | 28 (7.6) | ||
| EULAR moderate disease activity | 98 (36.4) | 31 (49.2) | 18 (51.4) | 147 (40.1) | ||
| EULAR high disease activity | 129 (48.0) | 21 (33.3) | 8 (22.9) | 158 (43.1) | <0.01 | 29 |
| Treatment | ||||||
| Naive | 131 (35.2) | 58 (89.2) | 25 (51.0) | 214 (44.0) | <0.01 | 7.2 |
| csDMARDs | 218 (58.6) | 7 (10.8) | 23 (46.9) | 248 (51.0) | <0.01 | 7.2 |
| Oral corticosteroid | 64 (15.9) | 2 (3.1) | 13 (22.8) | 79 (15.1) | <0.01 | 7.2 |
| bDMARDs | 53 (14.2) | 1 (1.5) | 1 (2.0) | 55 (11.3) | <0.01 | 7.2 |
| IM or IA corticosteroid at biopsy procedure | ||||||
| IM corticosteroid at biopsy, n (%) | 55 (13.7) | 3 (4.6) | 4 (7.0) | 62 (11.8) | ||
| IA corticosteroid at biopsy, n (%) | 26 (6.5) | 0 (0.0) | 12 (21.1) | 38 (7.3) | <0.01 | 0 |
AGSB, arthroscopic-guided synovial biopsy; CRP, C-reactive protein; DMARD, disease-modifying antirheumatic drugs; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; IA, intra-articular; IM, intramuscular; MCP, metacarpophalangeal; MTP, metatarsophalangeal; US-NB; ultrasound-guided needle biopsy; US-P&F, ultrasound-guided portal and forceps.
rmdopen-2018-000799supp001.docx (26.2KB, docx)
When patients were categorised according to biopsy technique, significant differences in gender, diagnosis, disease duration, time from diagnosis to biopsy, joint biopsied and EULAR disease activity criteria between groups were demonstrated, reflecting a diversity in the patients with inflammatory arthritis recruited at each centre. In addition, significant differences in rheumatological medication were found between biopsy groups: 44% (214/524) of patients were treatment naive with the greatest proportion in the US-P&F group (89.2% (58/65), p<0.01). In total, 51%, (248/524) of patients were being treated with one or more csDMARD. The prevalence of bDMARDs was highest in the US-NB group (14% (53/402), p<0.01), while use of steroid during biopsy procedure (IM or IA) was lowest in the US-P&F group (3% (3/63), p<0.01). Detailed information on csDMARD and bDMARD therapy is shown in online supplementary table 2.
Tolerability of biopsy procedures
In order to determine whether there were significant differences in tolerability of the procedures between the three biopsy techniques, we next evaluated differences in pain, stiffness and swelling before and after the biopsy procedure. Although we found significantly higher prebiopsy levels of pain and swelling within the arthroscopic group (p<0.01), we found no significant differences in postbiopsy pain, swelling and stiffness levels. Further, we found that the changes in pain, stiffness and swelling from baseline were not significantly different between biopsy techniques (online supplementary table 3).
For all patients biopsied, irrespective of method and for each method individually, we found that pain, swelling and stiffness all decreased significantly after the procedure (figure 2A). These results indicate that all biopsy techniques evaluated were generally well tolerated, often with significant reduction in pain, swelling and stiffness of joints following biopsy.
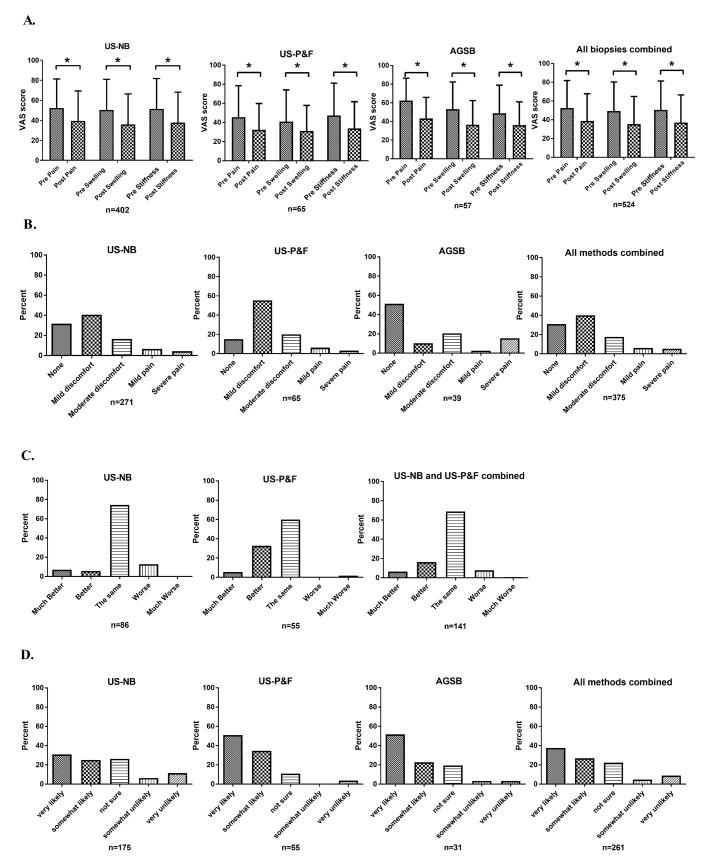
Figure 2.
(A) Graphs of patient reported data on Visual Analogue Scale for (a) ultrasound-guided needle biopsy (US-NB), (b) ultrasound-guided portal and forceps (US-P&F) and (c) arthroscopic-guided synovial biopsy (AGSB) and (d) all methods combined are presented. *P<0.05. (B) Answer to question on discomfort during procedure. The AGSB procedures using general anaesthesia not included. (C) Answer to question on how the joint feels during compared with before biopsy. Arthroscopy not applicable due to small amount of data. (D) Answer to question on whether the patient would participate in another biopsy.
Among patients undergoing procedures under local anaesthesia, we found that a high proportion of patients undergoing each biopsy technique experienced absent or only mild discomfort (US-NB 72% (196/271), US-P&F 71% (46/65), AGSB 62% (24/39)), we found no significant difference in outcomes in the adjusted analysis between methods (p=0.72) (figure 2B).
In the patients undergoing US-NB and US-P&F, we were also able to evaluate answers to the question ‘How does joint feel compared with before biopsy’ (figure 2C). In both the US-NB and US-P&F group, 87% (75/86) and 99% (54/55), respectively, of the patients answered that the joint felt the same or better. When comparing the answers in adjusted analysis, the US-P&F group scored better compared with the US-NB group (p<0.01).
Finally, we evaluated differences regarding willingness to undergo a repeat biopsy (figure 2D). In all groups, the majority of patients were somewhat or very likely to agree to another biopsy (US-NB 57% (98/175), US-P&F 85% (47/55), AGSB 64% (23/31)). Adjusted analysis demonstrated that patients undergoing both US-P&F and AGSB were more willing than those undergoing US-NB to undergo a repeat procedure (US-P&F vs US-NB: p<0.01 and AGSB vs US-NB: p<0.01), while there was no significant difference between US-P&F and AGSB (p=0.85).
Outcomes in patients undergoing repeated biopsy procedures
In the 103 patients who had undergone two biopsy procedures, we evaluated differences in VAS scores for preprocedure and postprocedure pain, stiffness and swelling and differences in willingness to undergo a repeat biopsy. Of the 103 patients, the majority (84.5%, (87/103)) had undergone US-NB and 11% (11/103) US-P&F and 5% (5/103) AGSB (table 2).
Table 2.
Repeated biopsy procedures
| Baseline | Repeat biopsy | P values | P values* | Missing (%) | |
| n | 103 | 103 | |||
| Months between biopsies† | 5.37 (5.50) | ||||
| Biopsy method | |||||
| US-NB, n (%) | 87 (84.5) | 88 (85.4) | |||
| US-P&F, n (%) | 11 (10.7) | 11 (10.7) | |||
| AGSB, n (%) | 5 (4.9) | 4 (3.9) | 0.94 | ||
| Diagnosis | |||||
| RA, n (%) | 100 (97.1) | 100 (97.1) | |||
| UA, n (%) | 1 (1.0) | 1 (1.0) | |||
| PsA, n (%) | 2 (1.9) | 2 (1.9) | 1.00 | ||
| Joint biopsied | |||||
| Elbow, n (%) | 2 (1.9) | 2 (1.9) | |||
| Wrist, n (%) | 70 (68.0) | 70 (68.0) | |||
| MCP, n (%) | 8 (7.8) | 8 (7.8) | |||
| Knee, n (%) | 20 (19.4) | 20 (19.4) | |||
| Ankle, n (%) | 2 (1.9) | 2 (1.9) | |||
| MTP, n (%) | 1 (1.0) | 1 (1.0) | 1.00 | 0.4 | |
| EULAR disease activity | |||||
| Remission, n (%) | 0 (0.0) | 28 (43.8) | |||
| Low activity, n (%) | 3 (3.4) | 4 (6.3) | |||
| Moderate activity, n (%) | 42 (47.7) | 21 (32.8) | |||
| High activity, n (%) | 43 (48.9) | 11 (17.2) | <0.01 | 29.9 | |
| Patient-reported outcome measures | |||||
| Pre-pain, mean (SD) | 54.96 (28.01) | 32.90 (26.01) | <0.01 | <0.01 | 2.5 |
| Post-pain, mean (SD) | 39.30 (29.29) | 27.05 (24.12) | <0.01 | 0.6 | 7.5 |
| Pre-swelling, mean (SD) | 50.46 (29.56) | 28.70 (25.32) | <0.01 | <0.01 | 5.3 |
| Post-swelling, mean (SD) | 34.94 (30.55) | 20.14 (22.79) | <0.01 | <0.01 | 9.5 |
| Pre-stiffness, mean (SD) | 57.16 (27.62) | 31.11 (24.27) | <0.01 | <0.01 | 5.3 |
| Post-stiffness, mean (SD) | 35.60 (29.55) | 22.54 (23.84) | <0.01 | 0.52 | 9.3 |
| Delta pain, mean (SD) | −15.23 (29.93) | −5.53 (21.13) | 0.01 | 0.62 | 7.6 |
| Delta swelling, mean (SD) | −15.04 (31.87) | −7.67 (26.41) | 0.06 | 0.70 | 9.9 |
| Delta stiffness, mean (SD) | −21.30 (32.70) | −7.90 (21.58) | <0.01 | 0.52 | 9.7 |
| Participate in another biopsy? | |||||
| Very unlikely, n (%) | 6 (10.5) | 5 (8.6) | |||
| Somewhat unlikely, n (%) | 2 (3.5) | 3 (5.2) | |||
| Not sure, n (%) | 8 (14.0) | 16 (27.6) | |||
| Somewhat likely, n (%) | 8 (14.0) | 8 (13.8) | |||
| Very likely, n (%) | 33 (57.9) | 26 (44.8) | 0.25 | 0.51 | 50.2 |
*P values adjusted for baseline or follow-up biopsy, disease activity, intra-articular or intramuscular corticosteroid injection during biopsy. P-values on post and delta values were also adjusted for prevalue.
†Mean for all in group.
EULAR, European League Against Rheumatism; GSB, arthroscopic-guided synovial biopsy; MCP, metacarpophalangeal; MTP, metatarsophalangeal; PsA, psoriatic arthritis; RA, rheumatoid arthritis; UA, undifferentiated arthritis; US-NB, ultrasound-guided needle biopsy; US-P&F, ultrasound-guided portal and forceps.
The majority of repeat biopsies were performed in patients with rheumatoid arthritis (97.1% (100/103)) who were biopsied from the wrist (68% (70/103)). Disease activity was significantly higher on first biopsy compared with second (96.6% moderate or high disease activity at biopsy 1 vs 44.5% at second biopsy, p<0.01), with 44% in remission at the second biopsy with 0% at the first biopsy (table 2).
Comparing PRO data between the first and second biopsy, adjusting for potential confounders, we demonstrated lower pre-pain (p<0.01), pre-stiffness (p<0.01) and pre-swelling and post-swelling (both p<0.01) in patients undergoing a second biopsy, while other PRO data were not significantly different. No statistically significant differences were found in delta PRO values between first and second biopsy (table 2).
When evaluating differences in whether patients were willing to undergo a repeat biopsy between first and second biopsy, we saw no differences in whether they were somewhat likely or very likely to undergo a repeat biopsy (first biopsy 72% (41/57), second biopsy 59% (34/58)). Notably, there was no significant decline in willingness between first and second biopsy (p=0.51). Further, there were no significant differences in adjusted analyses of VAS data (pain, stiffness, swelling or delta values) between biopsy techniques in patients undergoing repeated biopsies (data not shown).
The effect of peri-biopsy corticosteroid therapy on PRO data
In order to determine whether the administration of peri-biopsy corticosteroids influenced patient tolerability, we evaluated whether significant differences in pre-pain and post-pain, stiffness and swelling for each biopsy technique differed according to administration of IM or IA corticosteroid.
First, we evaluated all procedures (n=424) where treatment with peri-biopsy corticosteroid was not used and in adjusted analysis found significant decreases in pain, stiffness and swelling after biopsy in the US-NB (n=321), US-P&F (n=62) and AGSB procedures (n=16) (table 3).
Table 3.
Effect of peri-biopsy corticosteroid therapy
| Prebiopsy | Postbiopsy | P values* | P values† | |
| Without IM or IA steroid | ||||
| US-NB (n=321) | ||||
| Pain (mean (SD)) | 53.6 (28.9) | 41.4 (30.4) | <0.001 | <0.001 |
| Stiffness (mean (SD)) | 51.9 (30.4) | 39.4 (30.8) | <0.001 | <0.001 |
| Swelling (mean (SD)) | 50.9 (30.9) | 38.2 (30.1) | <0.001 | <0.001 |
| US-P&F (n=62) | ||||
| Pain (mean (SD)) | 43.7 (32.5) | 31.6 (27.2) | 0.003 | 0.005 |
| Stiffness (mean (SD)) | 45.5 (33.7) | 33.4 (27.6) | 0.003 | 0.006 |
| Swelling (mean (SD)) | 39.3 (32.7) | 30.7 (26.4) | 0.021 | 0.029 |
| AGSB (n=41) | ||||
| Pain (mean (SD)) | 62.3 (23.5) | 49.3 (22.4) | 0.017 | 0.001 |
| Stiffness (mean (SD)) | 45.4 (31.5) | 38 (26.6) | 0.296 | 0.001 |
| Swelling (mean (SD)) | 49.6 (30.9) | 41.7 (28.2) | 0.198 | <0.001 |
| With IM or IA steroid | ||||
| US-NB (n=81) | ||||
| Pain (mean (SD)) | 46.3 (29.4) | 31.8 (26.9) | 0.001 | <0.001 |
| Stiffness (mean (SD)) | 49.8 (30.1) | 31.2 (28.2) | <0.001 | 0.001 |
| Swelling (mean (SD)) | 47.8 (29.9) | 27.3 (27.7) | <0.001 | <0.001 |
| US-P&F (n=3) | ||||
| Pain (mean (SD)) | 84.7 (7.2) | 49.3 (33.5) | 0.173 | 0.279 |
| Stiffness (mean (SD)) | 83.7 (8.1) | 40.7 (42.4) | 0.192 | 0.304 |
| Swelling (mean (SD)) | 76 (21.3) | 42 (36) | 0.189 | 0.300 |
| AGSB (n=16) | ||||
| Pain (mean (SD)) | 62.9 (26) | 28.9 (16.1) | <0.001 | <0.001 |
| Stiffness (mean (SD)) | 57.5 (25.2) | 31.8 (20.4) | <0.001 | <0.001 |
| Swelling (mean (SD)) | 61.9 (23.0) | 24.2 (14.2) | <0.001 | <0.001 |
*P value by unadjusted cluster linear regression.
†Here adjusted for disease activity and pre-PRO value.
AGSB, arthroscopic-guided synovial biopsy; IA, intra-articular; IM, intramuscular; US-NB, ultrasound-guided needle biopsy; US-P&F, ultrasound-guided portal and forceps.
In the cohort of patients treated with peri-biopsy corticosteroids (n=100) (US-NB (n=81), US-P&F (n=3), AGSB (n=16)), we demonstrated a significant decrease in all three PRO parameters for US-NB and both pain and stiffness for arthroscopic biopsy (table 3). In the US-P&F group, there was insufficient data (only three procedures) to demonstrate a possible decrease in the three PRO parameters.
When all procedures were combined and adjusted for biopsy method, disease activity and pre-PRO value, the use of corticosteroid significantly reduced post-swelling VAS data (p<0.01), but not postprocedure pain (p=0.15) or stiffness (p=0.13).
We found no interaction between biopsy technique and use of peri-biopsy IM or IA corticosteroid in relation to the discomfort (p=0.33) or rebiopsy questions (p=0.95). Analysis was not possible for ‘How joints feel compared with before biopsy’ in the arthroscopy group due to low numbers.
Safety data
We next collected data on all adverse events per biopsy technique and evaluated whether significant differences in rates of complications existed between procedures. Safety data was available on all procedures. Biopsy procedures were performed in the time interval between September 2012 and November 2017, with follow-up data available for a mean of 16 months (US-NB 15 months, US-P&F 12 months, AGSB 23 months).
Overall, there were n=8 (1.5%) adverse events. Although all complications were reported within the US-guided procedure group, overall there was no difference in number of adverse events between the biopsy methods (p=0.55) (table 4). The most common complication reported was postprocedure sensory disturbance (n=4), all within US-NB procedures.
Table 4.
Safety data
| Safety | US-NB | US-P&F | AGSB | Total | P values |
| Biopsy procedures, n (%) | 402 (76.7) | 65 (12.4) | 57 (10.9) | 524 (100.0) | |
| Syncope or presyncope, n (%) | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (0.4) | |
| Tenosynovitis | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.2) | |
| Neurological disturbance*, n (%) | 4 (1.0) | 0 (0.0) | 0 (0.0) | 4 (1.0) | |
| Tendon damage, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Haemarthrosis, n (%) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.2) | |
| Deep vein thrombosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Joint infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Total adverse events | 8 | 0 | 0 | 8 | 0.55† |
*Sensory impairment following biopsy, no motor involvement.
†Test for difference in total number of adverse events in each group, by Fishers exact.
AGSB, arthroscopic-guided synovial biopsy; US-NB, ultrasound-guided needle biopsy; US-P&F, ultrasound-guided portal and forceps.
Further, in the 100 procedures in which IM (n=62) or IA (n=38) corticosteroid therapy was administered we also evaluated the effect on adverse events. We found no higher risk of adverse events in the group receiving corticosteroid (p=0.81). Neither was there a higher risk in patients receiving bDMARDs at the time of biopsy (n=55, p=0.63). In the patients undergoing two biopsies there was no higher risk of adverse events when undergoing the second biopsy procedure compared with first (p=0.61).
Discussion
We herein present a multicentre study across European academic rheumatology centres evaluating PRO and safety data from 524 synovial biopsy procedures where either US-NB, US-P&F or AGSB techniques where used. Our results demonstrate a number of important findings. First, all three synovial biopsy techniques were safe and well tolerated with no significant differences demonstrated between techniques in PROs or adverse events demonstrated. Second, patients are broadly willing to undergo repeat ultrasound-guided procedures, and this willingness does not decrease when repeats occur. Third, our results suggest that peri-biopsy administration of IM or IA corticosteroid is safe and can improve tolerability of the procedure. Finally, our results suggest no new safety or tolerability issues in a large cohort of patients undergoing sequential synovial biopsy using any of the three techniques.
To our knowledge, this is the largest comparative study evaluating the tolerability and safety of US-guided and arthroscopic biopsy techniques. Such an evaluation is important as the application of US-guided techniques to sample synovial tissue has become increasingly common, driven by a minimally invasive approach with no requirement for operating theatre time and studies supporting the quality of synovial tissue obtained using these procedures.4 7 15 However, the previously held perception that minimally invasive US-guided procedures are better tolerated than arthroscopy is not supported by our data, suggesting that patients are able to tolerate all procedures with minimal discomfort or postprocedure pain.4 It is important to note that for all three biopsy techniques, the majority of patients would agree to a subsequent biopsy. Furthermore, despite a significant fall in disease activity measures in patients prior to undergoing a second biopsy, with a consequent reduction in the inflamed synovial tissue mass, available to sample, there were no significant increases in PROs including postprocedure pain, swelling or stiffness.
Further adding to the methods tolerability, in patients not receiving IM or IA steroid treatment, there was an overall improvement in postbiopsy pain, swelling and stiffness (table 3). This effect could be driven by other factors such as start or change of treatment, patient reassurance, good experience with procedure and treatment staff and the effect of more attention given to patients in clinical studies. The focus of this study was that there was no worsening in PRO data.
When considering the integration of synovial biopsy into clinical trials recruiting patients with highly active disease, peri-biopsy administration of corticosteroids is important for subsequent swift and effective control of synovitis. However, it is uncertain whether this is associated with an increased risk of adverse events. Our data suggests that clinicians can safely administer corticosteroids following synovial sampling with any of the three techniques examined and that corticosteroids reduced patient reported postprocedure swelling. It is also important to note that our results do not suggest an increased risk of adverse events in patients treated with biologics undergoing synovial biopsy.
The results on safety presented here are in line with previous studies. AGSB has been shown to be a safe and well-tolerated procedure with a low rate of complications.3 5 16 Both US-NB and US-P&F methods have previously been found reliable, safe and tolerable in both small and large joints in smaller cohorts.7–9
The comparative examination in this study of 524 synovial biopsy procedures remains the largest reported cohort to date, and the inclusion of patients with both early and established disease and with a wide variation in disease activity suggests the results may be generalisable. The study has limitations including the heterogenous characteristics of the patient cohorts, possible difference in number of retrieved biopsies per procedure, the relatively small number of AGSB procedures available for comparison, the number of contributing biopsy centres and the retrospective nature of the study. Nor were we able to take into account the ‘operator’ effect on biopsy outcomes and the procedure allocation. The tolerability and the PRO measures could also have been influenced by the fact that the majority of these patients have been included in large interventional treatment studies.
Further, although no new safety signals were identified in this study, the relative infrequency of adverse events makes ongoing evaluation of larger cohorts important.
Future efforts should focus on PRO measures driven and developed by patients undergoing the procedure and applied to prospective patient cohorts.17 The continued study of PRO data and development of the current instrument is essential in order to meet the needs of the diverse patient group that will undergo biopsy as the method is implemented in further studies.
Acknowledgments
We are indebted to all the study subjects and personnel at the centers contributing data to this study.
Footnotes
SAJ and FH contributed equally.
Contributors: All authors have contributed substantially in the process of completing this study. AF was involved in the development of the Biopsy PRO domains. SAJ was involved in the conception of the study. SAJ, HLI, FH, CP and AF contributed to the designing of the study. SAJ, HL, LB, PD, ES, RT, MS, JW, SR AP, EC, NG, MB, CE, PT, IM, CP, FH and AF were responsible for the
aggregation of data. SAJ and PVL contributed to the statistics. All authors contributed to the interpretation of data, drafting and revising, final approval and agreed to be accountable.
Funding: SAJ is supported by grants from The Danish Rheumatism Association and Odense University Hospital PhD Fund and Fund for clinical research. AF was supported by Arthritis Research UK Fellowship (grant 18547) and the Arthritis Research UK Rheumatoid Arthritis Pathogenesis Centre of Excellence (grant 20298) and the European Community’s Collaborative project FP7-HEALTH-F2-2012-305549 ‘Euro-TEAM’. This is a summary of independent research funded by the National Institute for Health Research’s (NIHR) Birmingham Biomedical Research Centre programme, supported by the National Institute for Health Research/Wellcome Trust Clinical Research Facility at University Hospitals Birmingham NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The STRAP trial is jointly funded through project grants from AR-UK and MRC and the R4RA trial funded by NIHR.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: UK, London: R4RA (12/WA/0307) and STRAP (14/WA/1209). UK, Birmingham: West Midlands Black Country Research Ethics Committee, references 12/WM/0258 and 07/H1203/57. Denmark, Odense: the SynRA study is approved by the regional ethics review board (S-20140062) and the Danish data protection agency (2008-58-0035). Belgium, Brussels: Cliniques Universitaires Saint Luc, Comité d’éthique, references CEHF-FORM-023 / REV 001. Portugal, Lisbon: Biobank of Instituto de Medicina Molecular (ethics approval from 17/09-2008 and National Data Protection Committee approval number 74/35 from 2011).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data in this study is property of the respective centres involved. Sharing of the data can therefore be accepted if a specific request is received, and all centers hereafter accept.
References
- 1. Orr C, Vieira-Sousa E, Boyle DL, et al. . Synovial tissue research: a state-of-the-art review. Nat Rev Rheumatol 2017;13:463–75. 10.1038/nrrheum.2017.115 [DOI] [PubMed] [Google Scholar]
- 2. Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Curr Opin Rheumatol 2013;25:334–44. 10.1097/BOR.0b013e32835fd8eb [DOI] [PubMed] [Google Scholar]
- 3. Lazarou I, D'Agostino MA, Naredo E, et al. . Ultrasound-guided synovial biopsy: a systematic review according to the OMERACT filter and recommendations for minimal reporting standards in clinical studies. Rheumatology 2015;54:1867–75. 10.1093/rheumatology/kev128 [DOI] [PubMed] [Google Scholar]
- 4. Humby F, Kelly S, Bugatti S, et al. . Evaluation of minimally invasive, ultrasound-guided synovial biopsy techniques by the omeract filter--determining validation requirements. J Rheumatol 2016;43:208–13. 10.3899/jrheum.141199 [DOI] [PubMed] [Google Scholar]
- 5. Kane D, Veale DJ, FitzGerald O, et al. . Survey of arthroscopy performed by rheumatologists. Rheumatology 2002;41:210–5. 10.1093/rheumatology/41.2.210 [DOI] [PubMed] [Google Scholar]
- 6. Wechalekar MD, Smith MD. Arthroscopic guided synovial biopsy in rheumatology: current perspectives. Int J Rheum Dis 2017;20:141–4. 10.1111/1756-185X.13020 [DOI] [PubMed] [Google Scholar]
- 7. Kelly S, Humby F, Filer A, et al. . Ultrasound-guided synovial biopsy: a safe, well-tolerated and reliable technique for obtaining high-quality synovial tissue from both large and small joints in early arthritis patients. Ann Rheum Dis 2015;74:611–7. 10.1136/annrheumdis-2013-204603 [DOI] [PubMed] [Google Scholar]
- 8. Yeo L, Adlard N, Biehl M, et al. . Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis. Ann Rheum Dis 2016;75:763–71. 10.1136/annrheumdis-2014-206921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scirè CA, Epis O, Codullo V, et al. . Immunohistological assessment of the synovial tissue in small joints in rheumatoid arthritis: validation of a minimally invasive ultrasound-guided synovial biopsy procedure. Arthritis Res Ther 2007;9:R101 10.1186/ar2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vordenbäumen S, Joosten LA, Friemann J, et al. . Utility of synovial biopsy. Arthritis Res Ther 2009;11:256 10.1186/ar2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koski JM, Helle M. Ultrasound guided synovial biopsy using portal and forceps. Ann Rheum Dis 2005;64:926–9. 10.1136/ard.2004.027409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolf JM, Dukas A, Pensak M. Advances in wrist arthroscopy. J Am Acad Orthop Surg 2012;20:725–34. 10.5435/JAAOS-20-11-725 [DOI] [PubMed] [Google Scholar]
- 13. van de Sande MG, Gerlag DM, Lodde BM, et al. . Evaluating antirheumatic treatments using synovial biopsy: a recommendation for standardisation to be used in clinical trials. Ann Rheum Dis 2011;70:423–7. 10.1136/ard.2010.139550 [DOI] [PubMed] [Google Scholar]
- 14. Senn S. Baselines and covariate information. Statistical issues in drug development. John Wiley & Sons, 2008: 95–112. [Google Scholar]
- 15. Humby F, Kelly S, Hands R, et al. . Use of ultrasound-guided small joint biopsy to evaluate the histopathologic response to rheumatoid arthritis therapy: recommendations for application to clinical trials. Arthritis Rheumatol 2015;67:2601–10. 10.1002/art.39235 [DOI] [PubMed] [Google Scholar]
- 16. Gerlag D, Tak PP. Synovial biopsy. Best Pract Res Clin Rheumatol 2005;19:387–400. 10.1016/j.berh.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 17. Nelson EC, Eftimovska E, Lind C, et al. . Patient reported outcome measures in practice. BMJ 2015;350:g7818 10.1136/bmj.g7818 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2018-000799supp001.docx (26.2KB, docx)