Abstract
Background:
Outcomes of catheter ablation of ventricular tachycardia (VT) in patients with non-ischemic cardiomyopathy (NICM) could be related to etiology of NICM.
Objective:
To characterize VT ablation outcomes across NICM etiologies and adjust these outcomes by patient related comorbidities that could explain differences in arrhythmia recurrence rates.
Methods:
Data from 2075 patients with structural heart disease referred for catheter ablation of VT from 12 international centers was retrospectively analyzed. Patient characteristics and outcomes were noted for the 6 most common NICM etiologies. Multivariable Cox proportional hazard model was used to adjust for potential confounders.
Results:
Of 780 NICM patients (57±14 years, 18% female, LVEF 37±13%) underlying prevalences were dilated idiopathic cardiomyopathy (DCM) 66%, arrhythmogenic right ventricular cardiomyopathy (ARVC) 13%, valvular cardiomyopathy 6%, myocarditis 6%, hypertrophic cardiomyopathy 4%, and sarcoidosis 3%. One-year freedom from VT was 69%; and freedom from VT, heart transplantation, and death was 62%. On unadjusted competing risk analysis, VT ablation in ARVC demonstrated superior VT-free survival (82%) vs. DCM (p≤0.01). Valvular cardiomyopathy had the poorest unadjusted VT-free survival at 47% (p<0.01). After adjusting for comorbidities, including age, heart failure severity, ejection fraction, prior ablation and anti-arrhythmic use, myocarditis, ARVC, and DCM demonstrated similar outcomes, while hypertrophic cardiomyopathy, valvular cardiomyopathy and sarcoidosis had the highest risk of VT recurrence.
Conclusions:
Catheter ablation of VT in NICM is effective. Etiology of NICM is a significant predictor of outcomes, with ARVC, myocarditis, and DCM having similar but superior outcomes to hypertrophic cardiomyopathy, valvular cardiomyopathy and sarcoidosis, after adjusting for potential covariates.
Keywords: Ventricular tachycardia, ablation, non-ischemic, sarcoidosis, myocarditis, arrhythmogenic right ventricular cardiomyopathy, valvular
CONDENSED ABSTRACT
In this large multi-center retrospective study, competing risk analysis and Cox-proportional hazard models were used to report VT recurrence rates and freedom from VT, death, and transplantation of 780 NICM patients by etiology after VT ablation. Patients with myocarditis, DCM, and ARVC had similar and superior outcomes, while patients with HCM, sarcoidosis, and valvular cardiomyopathy had relatively poorer outcomes, after adjusting for important confounders including age, heart failure class, and LV ejection fraction. These findings impact physician-patient discussions of risks, benefits, and expectations in patients with specific etiologies of NICM and VT, who are being considered for catheter ablation procedures.
INTRODUCTION
Catheter ablation of ventricular tachycardia in patients with non-ischemic cardiomyopathy (NICM) has been reported to have less favorable outcomes and higher VT recurrence rates as compared to patients with ischemic cardiomyopathy.(1–3) However, non-ischemic cardiomyopathy can result from a wide-spectrum of disease processes and the differences in outcomes may be driven by the etiology of NICM. Single center studies have suggested differential outcomes for patients with various types of NICM, with arrhythmogenic right ventricular cardiomyopathy (ARVC) often reported to have the most favorable and sarcoidosis reported to have relatively poorer outcomes.(4,5) However, single center studies are limited by small numbers of patients. Further, the differences in outcomes observed of various etiologies could also be driven by patient comorbidities. The aim of this study was to use a large international registry of VT ablation patients to evaluate outcomes of NICM by etiology and to adjust these outcomes for significant comorbidities that may be driving the underlying arrhythmia success and recurrence rates. This data is not only important for prognosis and discussion of benefits and outcomes with patients, but can also be used to better understand substrates that underlie etiologies with less successful outcomes.
Methods
Data Collection
Data was collected by the International Ventricular Tachycardia Ablation Center Collaborative (IVTCC) from 12 international referral centers, as has been previously described,6 and included 2218 patients with structural heart disease who underwent catheter ablation for ventricular tachycardia. Patients with known etiology of NICM who underwent VT ablation between 2002 and 2014 included. Data from a total of 780 patients with NICM and an identified etiology in the database was retrospectively analyzed. Patients with mixed cardiomyopathy (ischemic and non-ischemic cardiomyopathy were excluded from the analysis). Patients with idiopathic dilated cardiomyopathy were used as reference. The six most common etiologies were used for analysis and included idiopathic dilated cardiomyopathy with EF <50%, arrhythmogenic ventricular cardiomyopathy, myocarditis, cardiac sarcoidosis, hypertrophic cardiomyopathy (HCM), and valvular cardiomyopathy. Valvular cardiomyopathy was defined as severe regurgitation or stenosis of any valve or history of valvular replacement due to severe valvular regurgitation or stenosis. All centers were contacted and confirmed etiologies of cardiomyopathy based on their institutional database and specific review of patient data records. The Institutional Review Boards of each participating center approved collection of the data.
Electrophysiology study and ablation
The primary strategy for VT ablation across centers was substrate-based modification of scar guided by electroanatomic mapping. Electroanatomic mapping was performed using CARTO (Biosense Webster, Diamond Bar, CA) or NAVX (St. Jude Medical, Minneapolis, MN) with standard low voltage settings (<1.5mV).(6) Procedures were performed under conscious sedation or general anesthesia. VT was induced with programmed stimulation using up to two sites, two drive drains, and up to three extra-stimuli as previously described.(7) The clinical VT followed by non-clinical VTs were targeted for ablation. If VT was hemodynamically tolerated, activation and entrainment mapping was performed.(8) Pace-mapping was used to targed potential VT exit sites, where matches with the targeted VT were observed.(9) Regions of late activation or local conduction delay as evidence by split, fractionated or isolated late potentials were tagged and targeted for ablation.(10,11) Elimination of sustained monomorphic VT inducibility served as the common desired procedural endpoint and programmed stimulation was performed after ablation unless hemodynamic instability or procedural duration was prohibitive.(12)
Percutaneous epicardial mapping and ablation was performed at the discretion of the operator. If percutaneous epicardial access was limited by prior cardiac surgery and/or adhesions, the epicardium was accessed surgically. Radiofrequency catheter ablation of VT was performed using non-irrigated catheters (Navi-Star, Biosense-Webster, Diamond Bar, CA), open-irrigated catheters (ThermoCool, ThermoCool SF, or Navistar RMT 3.5mm, Biosense-Webster, Diamond Bar, CA) or closed-loop irrigated catheters (Chilli, Boston Scientific, Marlborough, MA). Electroanatomic mapping was performed using CARTO (Biosense Webster, Diamond Bar, CA) or NAVX (St. Jude Medical, St. Paul, MN).
Follow-up
In patients who underwent multiple ablations, data from the most recent ablation was considered for follow-up and the date of ablation was used as the beginning of follow up. Follow-up for VT recurrence included office visits and device interrogations if a device was present. Endpoints included VT recurrence and composite outcome of VT recurrence, death, or orthotopic heart transplant (OHT). VT recurrence was defined as documented sustained VT (≥ 30 seconds) or any appropriate ICD therapy, including anti-tachycardia pacing.
Statistical analysis
Continuous data is reported as mean ± standard deviation and compared across etiology groups using a one way analysis of variance model. Categorical variables are reported as percentages or frequencies. Comparison of categorical variables across etiology groups was performed using Fisher’s exact test. Age across etiologies was compared using a one-way analysis of variance model. Ordinal NYHA class values across groups were compared using the non-parametric Kruskal-Wallis test, since NYHA classes did not follow a normal distribution.
Event free survival curves were computed using the Kaplan-Meier method and compared across the 6 etiologies using the Cox proportional hazard model. An attempt was made to control for the following confounders (primary: age, gender, NYHA class, VT storm, secondary: chronic kidney disease (CKD), diabetes mellitus, left ventricular ejection fraction (LVEF), VT ablation center, having previously failed two or more anti- arrhythmic agents, prior VT ablation, atrial fibrillation, hypertension, need for epicardial ablation, and interactions of LVEF with HCM and ARVC, by adding them to the Cox model. The backward stepwise method with a p ≤0.05 variable retention criterion was used to determine what subset of 15 potential confounders should be retained in the model, controlling for NICM etiology. A model using only the four primary potential confounders and NICM etiologies was also evaluated. Model accuracy was quantified using the Harrell C statistic.
Competing risks of VT recurrence and death/transplant incidence were similarly computed using the method of Gray(13) and compared across the 6 NICM etiologies using the corresponding Fine and Gray competing risk model, controlling for non-VT related death/heart transplantation. A similar backward stepwise method under this model was used to determine which of the 12 potential confounders to retain and for evaluating a model with only etiology and the four primary potential confounders included.
The Hazard ratio (HR) and its corresponding 95% confidence intervals are reported for the Cox or Fine-Gray models. Reported p values were adjusted for multiple comparisons using the Tukey test when appropriate. Analysis was performed using R software.
RESULTS
Patient and Procedural Characteristics
Between 2002 and 2014, 780 patients (mean age 57±14 years old, 18% female, LVEF 37±13%) with the six most common reported NICM etiologies in the database were identified. Underlying NICM etiologies were as follows: DCM in 518 (66%), ARVC 100 (13%), valvular 50 (6%), myocarditis 50 (6%), HCM 35 (4%), and sarcoidosis 27 (3%). Patient characteristics by etiologies are shown in Table 1. Prior ablation had been performed in 325 patients (45%). An implantable cardioverter defibrillator was present in 588 patients (80%) of patients at time of ablation.
Table 1.
Patient Characteristics by Etiology
| All | DICM | ARVC | Valvular | Myocarditis | HCM | Sarcoidosis | P Value | |
|---|---|---|---|---|---|---|---|---|
| N | 780 | 518 | 100 | 50 | 50 | 35 | 27 | |
| Age, mean (SD), y | 57 (14) | 60 (13) | 46 (16) | 65 (11) |
50 (15) ) |
59 (12) |
50 (11) |
<0.01 |
| Female (%) | 18% | 18% | 22% | 16% | 14% | 11% | 30% | 0.40 |
| LVEF , mean (SD) | 37 (13) |
33 (13) |
53 (11) |
31 (13) |
43 (16) |
41 (17) | 39 (15) |
<0.01 |
|
NYHA class I II |
40% | 31% | 73% | 30% | 62% | 44% | 48% | |
| 34% | 35% | 24% | 40% | 22% | 35% | 48% | <0.01 | |
| III | 21% | 27% | 2% | 26% | 14% | 15% | 4% | |
| IV | 1% | 7% | 1% | 4% | 2% | 6% | 0% | |
| HTN (% ) | 39% | 44% | 17% | 40% | 24% | 37% | 30% | <0.01 |
| DMII (%) | 14% | 16% | 2% | 16% | 6% | 20% | 15% | <0.01 |
| CKD (%) | 23% | 27% | 4% | 30% | 10% | 29% | 11% | <0.01 |
| VT storm (%) | 34% | 37% | 24% | 46% | 20% | 29% | 22% | 0.01 |
| >2 AAD (%) | 13% | 14% | 9% | 16% | 6% | 17% | 7.4% | 0.38 |
| Prior VT ablation (%) | 45% | 42% | 63% | 38% | 46% | 50% | 48% | ≥0.01 |
LVEF = left ventricular ejection fraction, NYHA = New York Heart Association, HTN = hypertension, DMII diabetes mellitus type II, CKD = chronic kidney disease, AAD = anti- arrhythmic medications.
Epicardial mapping was performed in 49% and epicardial ablation was performed in 38%. Acute procedural success, defined as VT no longer being inducible, was achieved in 436 (65%) of patients. Procedural characteristics by etiology are shown in Table 2. Overall complications rates of ablation were not statistically different amongst different etiologies, table 2. Type of complication by etiology is reported in supplemental tables 1 and 2.
Table 2.
Procedural Characteristics by Etiology
| All | DICM | ARVC | Valvular | Myocarditis | HCM | Sarcoid | P value | |
|---|---|---|---|---|---|---|---|---|
| No. of VTs induced | ||||||||
| 0 (%) | 74 (16) | 4 (4) | 6 (12) | 11 (23) | 5 (15) | 5 (20) | ||
| 1 (%) | 146 (31) | 35 (39) | 15 (30) | 19 (40) | 10 (29) | 8 (32) | 0.10 | |
| 2 (%) | 109 (23) | 24 (27) | 12 (24) | 10 (21) | 8 (24) | 3 (12) | ||
| ≥3 (%) | 136 (29) | 27 (30) | 17 (34) | 7 (15) | 11 (32) | 9 (36) | ||
| Percent inducible, n (%) | 606 (85) | 391 (84) | 86 (96) | 44 (88) | 36 (77) | 29 (85) | 20 (80) | 0.04 |
| Fastest TCL , mean±SD | 340±82 | 340±80 | 325±77 | 351±97 | 350±104 | 350±96 | 335±64 | 0.64 |
| Slowest TCL , mean±SD | 400±110 | 405±111 | 363±87 | 432±117 | 396±107 | 409±121 | 386±109 | 0.060 |
| At least 1 | 266/463 | 173/290 | 39/73 | 21/38 | 11/24 | 13/22 | 9/16 | 0.78 |
| unmappable VT (%) | (57) | (60) | (53) | (42) | (45) | (56) | (56) | |
| Epicardial ablation (%) | 278/708 (39) |
176/470 (37) |
43/86 (50) |
8/49 (16) | 31/46 (67) | 15/35 (43) | 5/22 (23) | <0.01 |
| Acute outcome | ||||||||
| Non-inducible (%) | 436 | 280 (63) | 75 (80) | 20 (44) | 31 (76) | 17 (63) | 13 (57) | |
| Partial/failure (%) | 202 | 138 (31) | 15 (16) | 23 (51) | 9 (22) | 9 (33) | 8 (35) | 0.010 |
| Not tested (%) | 35 | 25 (6) | 4 (4) | 2 (4) | 1 (2) | 1 (4) | 2 (9) | |
| Procedure time (min) | 295±128 | 290±131 | 297±131 | 305±126 | 299±111 | 329±112 | 318±103 | 0.66 |
| Procedural complication (%) | 46 (7) | 29 (7) | 3 (3) | 4 (9) | 4 (9) | 4 (11) | 2 (9) | 0.49 |
TCL = tachycardia cycle length.
Patient Outcomes
At one year post-ablation, 69% of the 780 patients with NICM were free from VT recurrence and 62% were free from the composite outcome of VT recurrence, death, and OHT, Figure 1. Median follow-up was 12.8 months (IQR 4–26 months). At the end of follow up, VT recurred in 318 patients (41%), death occurred in 137 patients (18%), and 47 patients (6%) underwent cardiac transplantation. Incidence of death and transplantation is shown in supplemental figure 1. In 78 patients (10%), death or cardiac transplantation occurred without VT recurrence. Kaplan Meier curves for VT recurrence are shown in Figure 2. In Gray/Kaplan Meier analysis, sarcoidosis and valvular cardiomyopathy had the highest VT recurrence rates (50% and 43%, respectively) at one year while myocarditis and ARVC had the best un-adjusted outcomes, with a VT recurrence rate of 23% and 17% at one year, respectively (p< 0.01 compared to DCM), Figure 2. VT recurrence rates at one year for DCM was 32% while for HCM was 38%. Unadjusted Kaplan Meier analysis also demonstrated higher VT/transplant free survival for patients with ARVC and myocarditis (82% and 75%, respectively) was superior at one year at one year to valvular cardiomyopathy (47%), and sarcoidosis (50%), Figure 3. Freedom from VT recurrence, death, or OHT was 59% for DCM and 56% for HCM.
Figure 1.
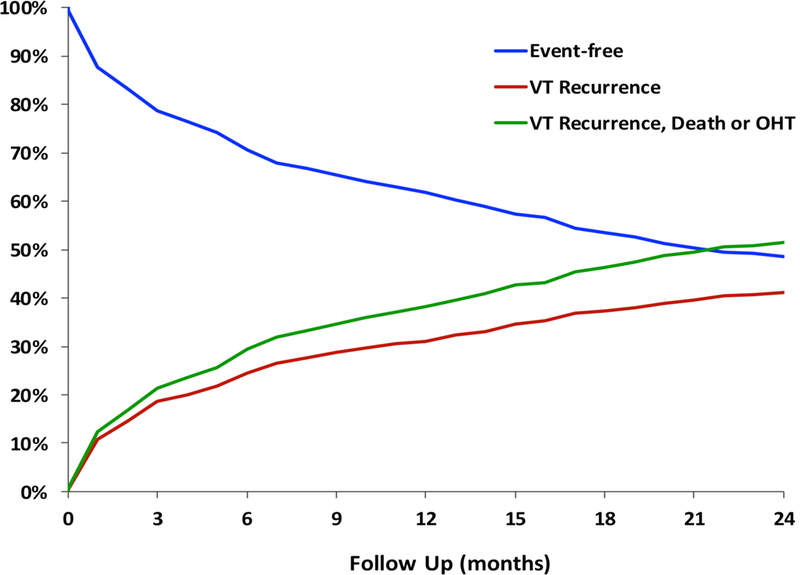
Competing risk analysis for the overall NICM population. Freedom from VT at follow up was 32% while the freedom from any event, including VT, death, and cardiac transplantation was 60% in the overall population of NICM patients.
Figure 2.
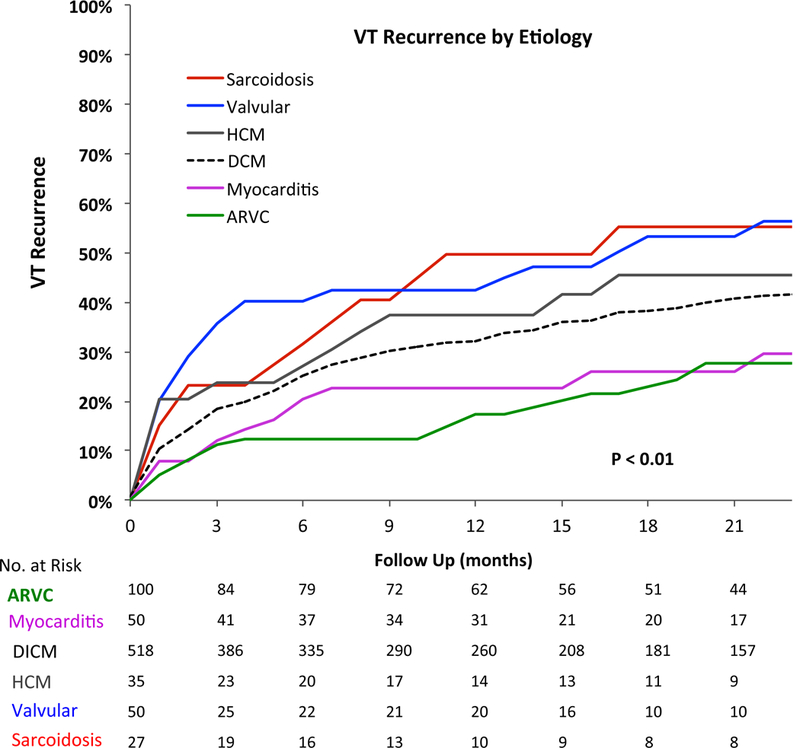
Unadjusted VT recurrence rates by etiology. Sarcoidosis and valvular cardiomyopathy have the highest rates of VT recurrence at one year.
Figure 3.
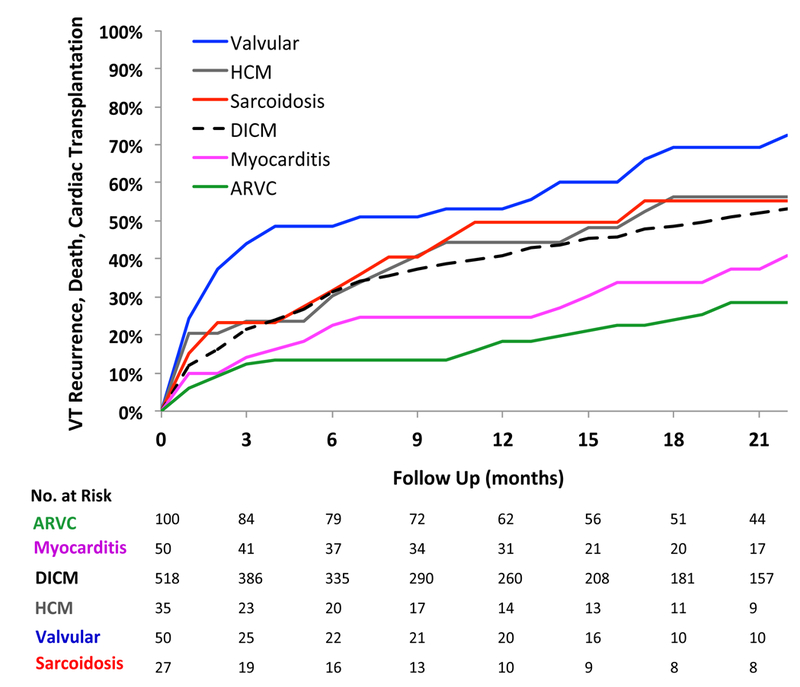
Unadjusted incidence of VT, death, and cardiac transplantation. Incidence of VT recurrence, death, and cardiac transplantation at on year was higher for Sarcoidosis, valvular cardiomyopathy, and hypertrophic cardiomyopathy patients and lower for those with ARVC and myocarditis
On multivariable analysis, significant variables for VT recurrence included age, LVEF, use of two or more anti-arrhythmic medications, prior VT ablation, and number induced VT’s in addition to etiology of cardiomyopathy. After adjusting for these variables and ablation center, VT storm, CKD, and epicardial ablation, which were significant on univariable analysis, were not significant for VT recurrence or the combined endpoint on multivariable analysis. After adjusting for age, LVEF, center, use of two or more anti-arrhythmic medications, prior VT ablation, and number of induced VTs, ARVC and myocarditis were associated with a similar risk of VT recurrence as DCM (HR 1.07 [95% CI 0.65–1.8] and HR 1.05 [95% CI 0.54–2.02] respectively), figure 4 (Central Illustration) and figure 5. On the other hand, sarcoidosis had the highest risk of VT recurrence (HR 1.97 [95% CI 1.09–3.55) followed by HCM (HR1.88 [95% CI 1.08–3.26] and valvular cardiomyopathy (HR 1.7 [CI 1.04–2.77]). Similarly, in multivariable analysis adjusting for the above-mentioned variables, ARVC and myocarditis had a similar risk for the combined endpoint of VT recurrence, transplantation, and death (HR 1.0 [95% CI 0.61–1.64] and HR 1.07 [0.6–1.93], respectively), figure 4 (Central Illustration) and figure 6, compared to DCM. In contrast, after adjusting for confounders including age, NYHA Class, LVEF, ablation center, prior VT ablation, number of anti-arrhythmic medication and number of induced VTs, valvular (HR 1.81 [95% CI 1.07–3.07]), HCM (HR 1.81 [95% CI 1.07–3.07]) had the highest hazard rations for the combined endpoint, of VT, death, and cardiac transplantation, figure 6. Other factors independently associated with less favorable combined outcomes included age, lower LVEF, NYHA class, use of two or more anti-arrhythmic medications, prior VT ablation, and number of induced VTs, figures 5 and 6. NYHA Class had a hazard ratio of 1.32 (p<0.01) for the combined endpoint but was not significant for VT recurrence alone, after adjusting for LVEF.
Figure 4 -.
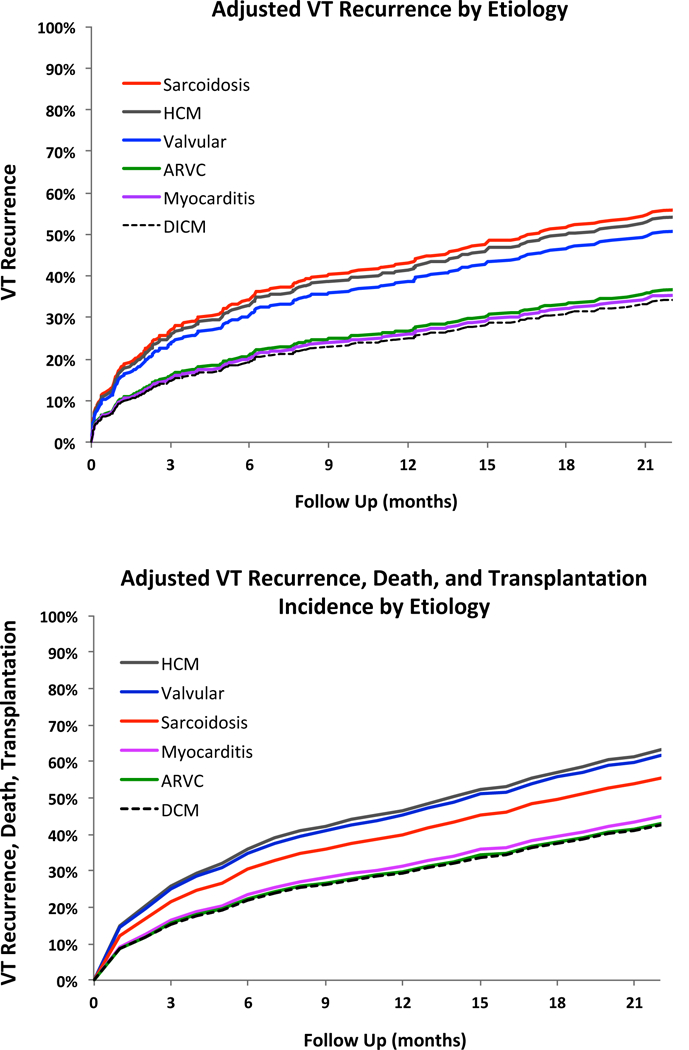
Central Illustration- Adjusted VT recurrence and adjusted VT recurrence, death, and transplantation by NICM etiology. (A) Adjusted VT recurrence rates by etiology and (B) adjusted combined endpoint of VT recurrence, death, and transplantation by etiology are shown. Valvular cardiomyopathy, hypertrophic cardiomyopathy, and sarcoidosis have the least favorable outcomes compared to DCM. There is no difference in the adjusted outcomes of ARVC and myocarditis as compared to DCM.
Figure 5.
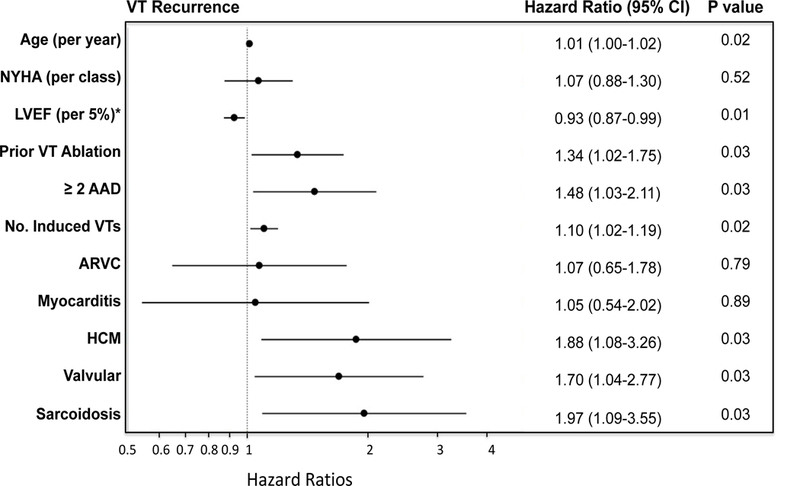
Characteristics associated with VT recurrence. Multivariable Cox proportional hazard ratio plot for VT recurrence demonstrates that sarcodosis, HCM and valvular cardiomyopathy are independently associated with higher risk of VT recurrence after adjusting for other comorbidities. Age, multiple anti-arrhythmic use, prior VT ablation and greater number of induced VTs were also associated with increased risk, while a higher LVEF was associated with a lower risk of recurrence. NYHA = New York Heart Association Functional Class, Abl = ablation, DCM was used as reference for etiologies. *adjusted for LVEF interactions with etiology of cardiomyopathy such as ARVC, variable represents the hazard ration for every 5% increase in LVEF.
Figure 6.
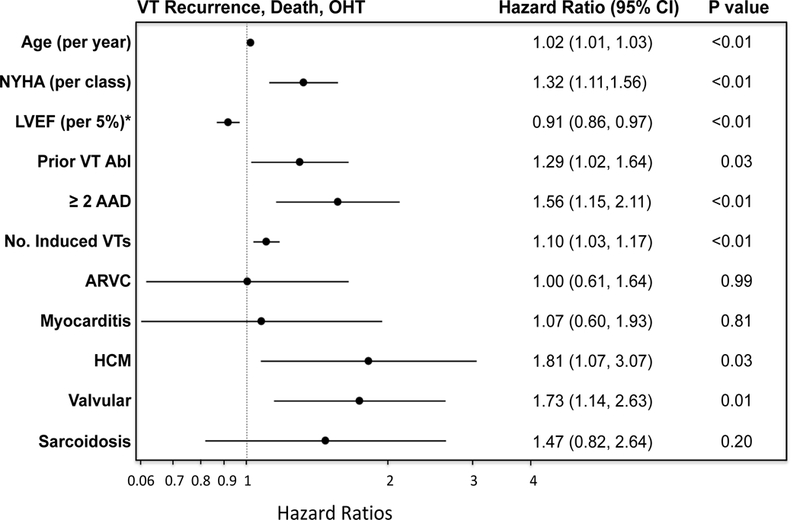
Characteristics associated with VT-free transplant-free survival. Multivariable Cox proportional hazard ratio plot for VT recurrence, death, and cardiac transplantation demonstrates that HCM and valvular cardiomyopathy are independently associated with poorer outcomes after adjusting for other comorbidities. Sarcoidosis had a high hazard ratio but did not reach statistical significance. Age, multiple anti-arrhythmic use, NYHA class, prior VT ablation, greater number of induced VTs were also associated with a poorer combined endpoint, while higher LVEF conferred a VT free survival benefit. ARVC and myocarditis had similar risk for the combined endpoint as DCM. NYHA = New York Heart Association Functional Class, Abl = ablation, DCM was used as reference for etiologies. *adjusted for LVEF interactions with etiology of cardiomyopathy such as ARVC, variable represents the hazard ration for every 5% increase in LVEF.
DISCUSSION
Major findings
In this large multi-center study, outcomes of various NICM etiologies were evaluated. This is the largest study to report VT ablation outcomes in patients with valvular heart disease, myocarditis, and hypertrophic cardiomyopathy. We show that success rates for VT ablation are driven by certain etiologies in NICM, while in other etiologies, additional patient characteristics contribute to the observed outcomes. Having a sufficient number of patient allowed us to be able to adjust for comorbidities, and we were able to show that idiopathic dilated cardiomyopathy had a similar risk of VT recurrence and VT recurrence, transplantation, and death compared to ARVC and myocarditis. Sarcoidosis, HCM and valvular cardiomyopathy had the highest risk for VT recurrence, while valvular cardiomyopathy and HCM had the highest risk for the combined endpoint of cardiac transplantation and death. Furthermore, despite having normal left ventricular ejection fractions, the adjusted outcomes in patients with HCM are relatively poor as compared to DCM.
VT ablation in NICM
VT ablation decreases VT recurrence in patients presenting with ICD shocks and VT. (14–16) It has been further reported that lack of VT recurrence after ablation is associated with improved mortality regardless of NYHA Class in those with moderate to severe systolic dysfunction.(7) In this multi-center study of NICM patients, 69% were free of VT at one-year of follow up, while freedom from VT, death, and cardiac transplantation was 62% at one year.These results are similar to those previously reported in single center studies with one year VT free survival rates ranging from 60 to 77%.(3,5,17,18)
NICM patients with VT are reported to have less favorable outcomes after catheter ablation as compared to ICM patients. The HELP-VT study, which prospectively compared dilated cardiomyopathy to ICM outcomes, reported a poorer VT-free survival at 1 year for dilated cardiomyopathy of 40.5% versus 57% for ICM patients at a follow up was 20–27 months.(l) Tung et al. reported that 77% of ischemic cardiomyopathy patients in the IVTCC registry were free of VT recurrence at one year, which was also higher than the VT free survival rate observed in the NICM population at one year.(7) It’s possible that the less favorable outcomes in the setting of NICM are partially driven by the heterogeneity in etiology.(5,17,19) In a single center study, Tokuda and colleagues(5) reported that patients with ARVC had better outcomes after catheter ablation of VT as compared to DCM patients, while patients with sarcoidosis appeared to have the poorest VT free survival rates. Santangeli and colleagues(20) reported favorable success rates for VT ablation in ARVC, with 71% VT free survival at 4.7 year follow up. Della Bella and colleagues(17) reported a 39% recurrence rate for DCM patients after catheter ablation as compared to 31% for patients with ARVC and 25% for HCM patients, though in this series, only 5 patients had HCM and only 13 had ARVC.(17) On the other hand, in another single center study of 166 patients which included 20 ARVC patients, Maury and colleagues observed similar VT recurrence rates for patients with DCM as compared to ARVC.(4) Of note, these studies did not adjust for potential confounders that could explain the differences in outcomes of various etiologies observed. Important factors known to influence outcomes include age, NYHA Class, as well as VT storm.(1,3–5)
In this large multi-center study of NICM patients, sarcoidosis and valvular cardiomyopathy and HCM had the least favorable outcomes for VT recurrence while valvular cardiomyopathy and HCM had the poorest VT, cardiac transplantation, and death, even after adjusting for potential confounders including age, NYHA Class, and history of VT storm. HCM was also associated with high risk of recurrence, transplantation, and death as compared to DCM. However, we found that after adjusting for comorbidities, catheter ablation after DCM had similar outcomes to ARVC and myocarditis. It’s interesting to note that after adjusting for other variables, including etiology of cardiomyopathy, prior VT ablation, LVEF, and number of induced VTs and anti-arrhythmic medications, VT storm was no longer significant in the multivariable analysis for outcomes, while NYHA class remained an independent predictor of the combined endpoint.
Influence of the substrate
In this study, sarcoidosis, valvular cardiomyopathy, and HCM were associated with a higher risk of VT recurrence. The relatively higher risk for VT recurrence in these etiologies may be driven by scar location. Although in this retrospective registry, the location of scar, as observed on imaging and electroanatomic data, was not specifically collected, it may explain the poorer outcomes seen with certain etiologies. Sarcoidosis and HCM patients often harbor septal scars, which can be difficult to access from either the endocardium or the epicardium.(21,22) Furthermore, it’s possible that scar and disease progression plays an important role in sarcoidosis, if untreated or refractory to immunosuppressive therapy. In this more challenging population, other adjunctive therapies such as arterial or venous alcohol ablation, surgical cryoablation, bipolar ablation, or coiling if the coronary anatomy is suitable, have been used to address septal and midmyocardial scars.(23–27) Valvular cardiomyopathy, in addition to presenting with potentially limited access due to mechanical valves or prior surgical pericardial adhesions, may present with basal scars, which given both the thickness of the ventricle and overlying epicardial fat in this region,(28,29) can pose a challenge to ablation. In contrast, both myocarditis and ARVC tend to have an epicardial substrate with better targets for ablation, including late potentials and fractionated electrograms(20,30–34), potentially leading to improved outcomes.
Finally, the number of patients with hypertrophic cardiomyopathy, valvular cardiomyopathy, and sarcoidosis was smaller than those with DICM and ARVC. Although the location of scar may have led to the poorer outcomes noted with hypertrophic cardiomyopathy, valvular cardiomyopathy, and sarcoidosis, it’s also possible that less experience with these substrates as compared to ARVC or DICM partially contributed to the outcomes observed.
Limitations
The present study is retrospective and represents outcomes reported at specialized VT ablation centers. The results, therefore, may not be applicable to all institutions. Etiology of cardiomyopathy was based on that reported in the database and identified by each center based on imaging data (magnetic resonance imaging and cardiac positron emission tomography), viral serology, and patient history. It’s possible that work up of the etiology of cardiomyopathy may have been variable at different institutions or that there might have been overlap of etiologies that limit the conclusions of this study. In particular, sarcoidosis can be under-diagnosed or misclassified.(35) However, any misclassification related to this would have biased the outcomes towards null. Therefore, our estimate of the differences in outcomes of sarcoidosis compared to DCM may represent a conservative estimate. Given retrospective collection of variables, effect of certain procedural variables, including high-density multi-electrode mapping catheters, could not be analyzed. Not all confounders could be evaluated in multivariable analysis, and therefore, only those thought to have a significant effect on outcomes were included in a step-wise fashion.
CONCLUSIONS
Catheter ablation of VT in NICM is effective, but certain etiologies significantly impact outcomes. Patients with ARVC and myocarditis had better outcomes after catheter ablation as compared to DCM patients, however, after adjusting for other confounders, these differences appear to be driven by comorbidities including age and NYHA Class in DCM patients. Patients with sarcoidosis, valvular cardiomyopathy, and HCM were at highest risk of VT recurrence, after catheter ablation, even after adjusting for potential comorbidities.
Supplementary Material
Perspectives
Competency in Medical Knowledge
Catheter ablation of ventricular tachycardia in non-ischemic cardiomyopathy is effective, but characterization of outcomes can be confounded by an underlying heterogeneous disease process. In this study, etiology of non-ischemic cardiomyopathy was a significant predictor of VT recurrence, death, and cardiac transplantation. Patients with arrhythmogenic right ventricular cardiomyopathy, myocarditis, and idiopathic dilated cardiomyopathy had similar, but superior outcomes after catheter ablation of VT, compared to those with hypertrophic and valvular cardiomyopathy and sarcoidosis.
Translational Outlook
Better characterization of the substrate in non-ischemic cardiomyopathies, such as valvular cardiomyopathy and sarcoidosis, could help inform both medical and procedural therapy and improve outcomes. Translational studies and specific models of non-ischemic cardiomyopathy targeted at specific etiologies are needed to better understand pathophysiological changes that cause ventricular arrhythmias and affect outcomes after catheter ablation in this population.
Acknowledgments
Funding Sources: The study was an unfunded, investigator-initiated collaborative study. M. Vaseghi is supported by NIH DP2 DP2HL132356–01, and K. Shivkumar is supported by NIH OT2OD023848.
ABBREVIATIONS
- AAD
anti-arrhythmic drug
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- CKD
chronic kidney disease
- DCM
dilated idiopathic cardiomyopathy
- HCM
hypertrophic cardiomyopathy
- HR
hazard ratio
- LVEF
left ventricular ejection fraction
- NICM
non-ischemic cardiomyopathy
- NYHA
New York Heart Association Class
- VT
ventricular tachycardia
Footnotes
Disclosures: M. Vaseghi: None. T.Y. Hu: None. R. Tung: None. P. Vergara: None. D.S. Frankel: None. L. Di Biase: Consultant for Stereotaxis, Boston Scientific, Abbott, Biosense Webster Inc. Compensation for services from Medtronic, Atricure, Pfizer, EpiEP and Biotronik Inc. U.B. Tedrow: Compensation for Services; Medtronic, Inc., St. Jude Medical, Boston Scientific Corp. Research Grants; Biosense Webster, Inc., St. Jude Medical. J. Gornbein: none. R. Yu: None. N. Mathuria: None. S. Nakahara: None. W.S. Tzou: None. W.H.Sauer: None. D.J. Burkhardt: Compensation for Services; Biosense Webster, Inc. V.N. Tholakanahalli: Research Grants; SJM. Intellectual Property Rights; St. Jude Medical. T. Dickfeld: Compensation for Services; Biosense Webster, Inc., SIEMENS, St. Jude Medical, Abbott Laboratories, Impulse Dynamics USA. Research Grants; GE Healthcare, Biosense Webster, Inc. J. Weiss: Compensation for Services; Stereotaxis, Inc. T. Bunch: Compensation for Services; Boston Scientific Corp. M. Reddy: None. D.J. Callans: Compensation for Services; Biosense Webster, Inc., Medtronic, Inc., St. Jude Medical. D.R. Lakkireddy: None. A. Natale: None. F.E. Marchlinski: None. W.G. Stevenson: Intellectual Property Rights; Brigham and Women’s Hospital. St. Jude Medical. P. Della Bella: Compensation for Services; St. Jude Medical, Biotronik, Biosense Webster, Inc.; K. Shivkumar: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dinov B, Fiedler L, Schonbauer R et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014;129:728–36. [DOI] [PubMed] [Google Scholar]
- 2.Proietti R, Essebag V, Beardsall J et al. Substrate-guided ablation of haemodynamically tolerated and untolerated ventricular tachycardia in patients with structural heart disease: effect of cardiomyopathy type and acute success on long-term outcome. Europace 2015;17:461–7. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Romero J, Mehta NK et al. Long-term outcomes after catheter ablation of ventricular tachycardia in patients with and without structural heart disease. Heart Rhythm 2016;13:1957–63. [DOI] [PubMed] [Google Scholar]
- 4.Maury P, Baratto F, Zeppenfeld K et al. Radio-frequency ablation as primary management of well-tolerated sustained monomorphic ventricular tachycardia in patients with structural heart disease and left ventricular ejection fraction over 30%. Eur Heart J 2014;35:1479–85. [DOI] [PubMed] [Google Scholar]
- 5.Tokuda M, Tedrow UB, Kojodjojo P et al. Catheter ablation of ventricular tachycardia in nonischemic heart disease. Circ Arrhythm Electrophysiol 2012;5:992–1000. [DOI] [PubMed] [Google Scholar]
- 6.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 2000;101:1288–96. [DOI] [PubMed] [Google Scholar]
- 7.Tung R, Vaseghi M, Frankel DS et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm 2015;12:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson WG, Friedman PL, Sager PT et al. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol 1997;29:1180–9. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson WG, Sager PT, Natterson PD, Saxon LA, Middlekauff HR, Wiener I. Relation of pace mapping QRS configuration and conduction delay to ventricular tachycardia reentry circuits in human infarct scars. J Am Coll Cardiol 1995;26:481–8. [DOI] [PubMed] [Google Scholar]
- 10.Arenal A, Glez-Torrecilla E, Ortiz M et al. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol 2003;41:81–92. [DOI] [PubMed] [Google Scholar]
- 11.Jais P, Maury P, Khairy P et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation 2012;125:2184–96. [DOI] [PubMed] [Google Scholar]
- 12.Aliot EM, Stevenson WG, Almendral-Garrote JM et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm 2009;6:886–933. [DOI] [PubMed] [Google Scholar]
- 13.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistcs 1988;16:1141–1154. [Google Scholar]
- 14.Carbucicchio C, Santamaria M, Trevisi N et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long term outcomes in a prospective single-center study. Circulation 2008;117:462–9. [DOI] [PubMed] [Google Scholar]
- 15.Sapp JL, Wells GA, Parkash R et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med 2016;375:111–21. [DOI] [PubMed] [Google Scholar]
- 16.Reddy VY, Reynolds MR, Neuzil P et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med 2007;357:2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della Bella P, Brugada J, Zeppenfeld K et al. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol 2011;4:653–9. [DOI] [PubMed] [Google Scholar]
- 18.Muser D, Santangeli P, Castro SA et al. Long-Term Outcome After Catheter Ablation of Ventricular Tachycardia in Patients With Nonischemic Dilated Cardiomyopathy. Circ Arrhythm Electrophysiol 2016;9. [DOI] [PubMed] [Google Scholar]
- 19.Goya M, Fukunaga M, Hiroshima K et al. Long-term outcomes of catheter ablation of ventricular tachycardia in patients with structural heart disease. J Arrhythm 2015;31:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santangeli P, Zado ES, Supple GE et al. Long-Term Outcome With Catheter Ablation of Ventricular Tachycardia in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy. Circ Arrhythm Electrophysiol 2015;8:1413–21. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Barbhaiya C, Nagashima K et al. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol 2015;8:87–93. [DOI] [PubMed] [Google Scholar]
- 22.Santangeli P, Di Biase L, Lakkireddy D et al. Radiofrequency catheter ablation of ventricular arrhythmias in patients with hypertrophic cardiomyopathy: safety and feasibility. Heart Rhythm 2010;7:1036–42. [DOI] [PubMed] [Google Scholar]
- 23.Dukkipati SR, d’Avila A, Soejima K et al. Long-term outcomes of combined epicardial and endocardial ablation of monomorphic ventricular tachycardia related to hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:185–94. [DOI] [PubMed] [Google Scholar]
- 24.Inada K, Seiler J, Roberts-Thomson KC et al. Substrate characterization and catheter ablation for monomorphic ventricular tachycardia in patients with apical hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2011;22:41–8. [DOI] [PubMed] [Google Scholar]
- 25.Tholakanahalli VN, Bertog S, Roukoz H, Shivkumar K. Catheter ablation of ventricular tachycardia using intracoronary wire mapping and coil embolization: description of a new technique. Heart Rhythm 2013;10:292–6. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen DT, Tzou WS, Brunnquell M et al. Clinical and biophysical evaluation of variable bipolar configurations during radiofrequency ablation for treatment of ventricular arrhythmias. Heart Rhythm 2016;13:2161–2171. [DOI] [PubMed] [Google Scholar]
- 27.Kreidieh B, Rodriguez-Manero M, Schurmann P, Ibarra-Cortez SH, Dave AS, Valderrabano M. Retrograde Coronary Venous Ethanol Infusion for Ablation of Refractory Ventricular Tachycardia. Circ Arrhythm Electrophysiol 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piers SR, Tao Q, Huls van Taxis CF van, Schalij MJ, van der Geest RJ, K Zeppenfeld Contrast-enhanced MRI-derived scar patterns and associated ventricular tachycardias in nonischemic cardiomyopathy: implications for the ablation strategy. Circ Arrhythm Electrophysiol 2013;6:875–83. [DOI] [PubMed] [Google Scholar]
- 29.van Huls van Taxis CF, Wijnmaalen AP, Piers SR, Geest RJ van der, Schalij MJ, Zeppenfeld K. Real-time integration of MDCT-derived coronary anatomy and epicardial fat: impact on epicardial electroanatomic mapping and ablation for ventricular arrhythmias. JACC Cardiovasc Imaging 2013;6:42–52. [DOI] [PubMed] [Google Scholar]
- 30.Maccabelli G, Tsiachris D, Silberbauer J et al. Imaging and epicardial substrate ablation of ventricular tachycardia in patients late after myocarditis. Europace 2014;16:1363–72. [DOI] [PubMed] [Google Scholar]
- 31.Berte B, Sacher F, Cochet H et al. Postmyocarditis ventricular tachycardia in patients with epicardial-only scar: a specific entity requiring a specific approach. J Cardiovasc Electrophysiol 2015;26:42–50. [DOI] [PubMed] [Google Scholar]
- 32.Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2009;120:366–75. [DOI] [PubMed] [Google Scholar]
- 33.Bai R, Di Biase L, Shivkumar K et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol 2011;4:478–85. [DOI] [PubMed] [Google Scholar]
- 34.Philips B, Madhavan S, James C et al. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol 2012;5:499–505. [DOI] [PubMed] [Google Scholar]
- 35.Vasaiwala SC, Finn C, Delpriore J et al. Prospective study of cardiac sarcoid mimicking arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol 2009;20:473–6.19017339 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


