Abstract
Stroke is one of leading causes of mortality and morbidity in the world with limited availability of therapeutic intervention. Exercise has been shown to improve stroke functional outcome in different preclinical and clinical setup. Exercise preconditioning induced neuroprotection in preclinical stroke models is believed to be mediated through its ability to restore brain vasculature and blood brain barrier integrity, promote neurogenesis, and help fight against neuroinflammation and excitotoxicity. In this short review, we will summarize the molecular mechanisms of exercise preconditioning described in preclinical stroke studies. We will also discuss the neuroprotective effects of pre-ischemic exercise.
Keywords: Stroke, exercise, preconditioning, cognition, physical activity
Introduction
Stroke is the fifth leading cause of death and most common cause of adult disability in the USA (Kochanek, Murphy et al. 2014). About 795,000 people in the United States suffer from stroke each year among which 140,000 Americans die of stroke (Mozaffarian, Benjamin et al. 2016). Unfortunately, due to difficulty in differentiating etiology and a limited therapeutic time window, the only FDA approved treatment for ischemic stroke, tissue-plasminogen activator, is available to 3–5% of all stroke patients (Fonarow, Smith et al. 2011, Roger, Go et al. 2012). Brain injury following stroke occurs by a complex interplay of multiple pathways (Doyle, Simon et al. 2008, Sierra, Coca et al. 2011). Despite extensive research and effort in both the preclinical and clinical setup, the development of effective neuroprotectants has largely been unsuccessful (Moskowitz, Lo et al. 2010, Traystman 2010). As a result, there is an immense need for therapies that could prevent or ameliorate stroke-induced brain injury.
The use of the brain’s endogenous mechanisms to protect itself against stroke injury through preconditioning will complement pharmacological treatments. Preconditioning is a procedure in which brief episodes of a noxious stimulus below the threshold of damage are applied to the target organ, which in return induces a robust protection against subsequent damaging injuries. Preconditioning in animal models has been used to induce resistance against the injury of ischemic stroke, trauma and different neurodegenerative diseases using a diverse range of stimuli, such as ischemia (Zhou, Li et al. 2004, Speetzen, Endres et al. 2013), hypoxia (Fan, Hu et al. 2011), hyperbaric oxygen (Cheng, Ostrowski et al. 2011), hypothermia (Nishio, Yunoki et al. 2000), hyperthermia (Xu, Aibiki et al. 2002), exposure to neurotoxins, and different pharmacological agents (Pinto, Simao et al. 2014, Cai, Yang et al. 2017). Preconditioning can have beneficial effects on different organs of the body. However, it has gained the highest clinical interest in brain injury due to the severity of stroke disability and limited regenerative properties of neurons.
Exercise, which can be considered a mild stressor (Arumugam, Gleichmann et al. 2006, Morton, Kayani et al. 2009) and thus follow the prototypical preconditioning stimulus, has beneficial effects on brain health and cognitive function (Cotman, Berchtold et al. 2007, Mattson 2012, Voss, Vivar et al. 2013). Exercise induced neuroprotection includes multiple targets, such as the blood brain barrier (BBB) and neurovascular unit maintenance (Ding, Li et al. 2006), cerebral inflammation reduction (Ding, Young et al. 2005, Barrientos, Frank et al. 2011), neurogenesis (Brandt, Maass et al. 2010), and neuronal apoptosis inhibition (Liebelt, Papapetrou et al. 2010) (Figure 2). This neuroprotective potential has generated a large interest in exercise as preconditioning in stroke treatment. In this review, we will discuss exercise as a preconditioning stimulus in stroke, review the proposed molecular mechanisms and discuss its clinical potential.
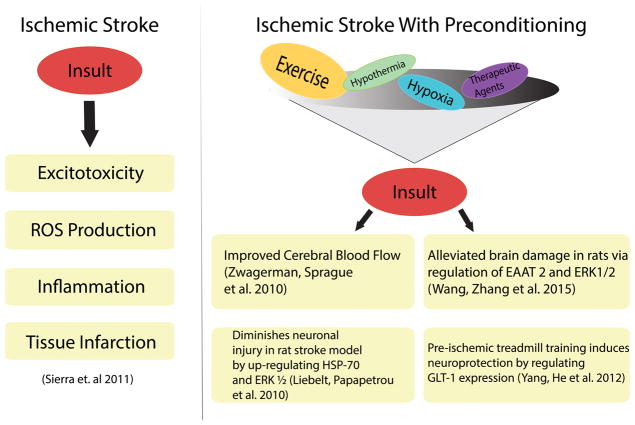
Figure 2. The beneficial effects of preconditioning on stroke progression.
The progression of classical ischemic stroke involves excitotoxicity, ROS production, inflammation, and tissue infarction. Preconditioning, specifically with exercise, helps reduce the typical deleterious effects of stroke. This includes improved blood flow (to the region of infarct) as well as upregulation of certain cellular pathways involved in glutamate transport to minimize the effects of excitotoxicity.
ROS, reactive oxygen species; EAAT 2, excitatory amino acid transporter; ERK1/2, extracellular signal-regulated kinases; HSP-70, heat shock protein 70; GLT-1, glutamate transporter 1.
Exercise induced neuroprotection in ischemic stroke
Exercise preconditioning in animal models
Previously reported studies have shown the beneficial effects of exercise on stroke-induced brain injuries in animal models, nicely reviewed in Zhang et al. (Zhang, Wu et al. 2011). The ischemic middle cerebral artery occlusion model in rats and mice was primarily used for these studies. The paradigms for exercise prior to stroke included either forced treadmill running (He, Wang et al. 2014) or voluntary free-wheel running (Kalogeraki, Pielecka-Fortuna et al. 2016) both of which showed a protective effect. At least 2 or 3 weeks of preischemic exercise is necessary to obtain a neuroprotective effect (Jia, Hu et al. 2009, Liebelt, Papapetrou et al. 2010). Exercise preconditioning derived neuroprotection has been shown to affect various systems including neurogenesis and angiogenesis stimulation, remodeling cerebral vasculature, modulating excitatory signaling, decreasing inflammatory markers and maintaining blood brain barrier integrity (Figure 1).
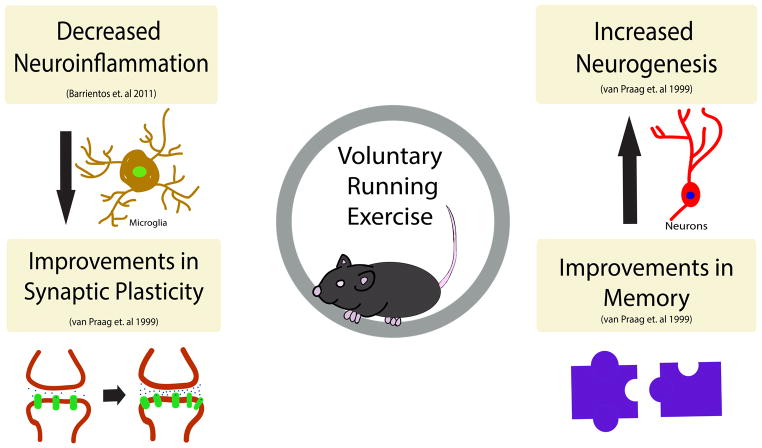
Figure 1. The beneficial effects of exercise on the brain.
Exercise has been shown to have many beneficial effects on the brain, including (but not limited to), increased neurogenesis in the dentate gyrus of the hippocampus as well as improvements in spatial learning and memory. Further, running activity decreases neuroinflammation in the brain and subsequently has been shown to decreased the overall number of microglia in the brain. Synaptic plasticity, the strengthening of synapses over time, has also been shown to take place following an exercise regimen.
Exercise preconditioning and neurogenesis
Stroke induces neurogenesis in the subventricular zone and in the subgranular zone in adult rodents (Ming and Song 2005, Xiong, Mahmood et al. 2010). Recent reports have also suggested the presence of stroke-induced neurogenesis in the human brain based on the presence of cells that express markers associated with newborn neurons in the ischemic penumbra (Jin, Wang et al. 2006). These neuronal progenitors can migrate to the site of injury, possibly aiding the repair. However, in the hippocampus it had been shown that the newborn neurons generated post-stroke show an abnormal morphology or fail to integrate into the circuit (Niv, Keiner et al. 2012, Woitke, Ceanga et al. 2017). Previous animal studies showed that exercise can induce hippocampal neurogenesis (van Praag, Kempermann et al. 1999, Brown, Cooper-Kuhn et al. 2003, Bednarczyk, Aumont et al. 2009) and improves contextual, spatial and temporal function by reorganizing the newly formed neuron circuitry (Vivar, Peterson et al. 2016, Sah, Peterson et al. 2017). Interestingly, running exercise post-MCAO in mice increased hippocampal neurogenesis and the use of spatial strategies. Despite this, it did not improve latency to find the hidden platform in the Morris water maze compared to non-runners with MCAO, nor did it change the morphology of newborn neurons (Woitke, Ceanga et al. 2017). However, blocking the proliferation of the progenitor cells reduces ischemic tolerance in the brain (Maysami, Lan et al. 2008). In summary, we suggest that exercise-induced neurogenesis might be a possible mechanism that helps restore neuronal circuitry and thus improve functional outcome in post stroke recovery.
Exercise preconditioning helps to maintain BBB integrity
Disruption of blood brain barrier (BBB) is an important pathological hallmark in ischemic stroke, negatively affecting the cerebral microenvironment. The BBB is a dynamic structure that results from the interplay of effective tight junctions, trans-endothelial transport systems, enzymes, and the regulation of leukocyte permeation (Abbott and Friedman 2012). Exercise preconditioning has been shown to improve different structural and functional components of the BBB. Treadmill pretraining reduced cerebral edema (Shamsaei, Erfani et al. 2017) by downregulating the aquaporin-4 transporter in a rat ischemic stroke model (He, Wang et al. 2014). 30-minute exercise preconditioning for three weeks has been shown to improve basal lamina by strengthening collagen IV (Davis, Mahale et al. 2007). Exercise preconditioning has also been shown to improve BBB function by upregulating TIMP-1 and inhibiting MMP-9 overexpression (Guo, Lin et al. 2008, Naderi, Alimohammadi et al. 2017). Preischemic exercise restores BBB function through the extracellular signal regulated kinases (ERK) pathway (Guo, Cox et al. 2008).
Exercise preconditioning effect on cerebral vasculature
Vascular remodeling is important to improve stroke outcome. Exercise in general has been shown to promote cerebral small vessel formation, increase capillary density, induce angiogenesis, and enhance both cerebrovascular integrity and neogenetic microvessel markers. In rat ischemic models, exercise preconditioning has shown improved cerebral blood flow (CBF) during reperfusion (Zwagerman, Sprague et al. 2010)and an additional study proposed that this was through regulation of endothelin-1 (ET-1) expression (Zhang, Zhang et al. 2014). Expression of Netrin-1 and its receptors deleted in colon cancer (DCC) and uncoordinated gene 5B (Unc5B), known mediators of neural and vascular activities, are also regulated in the exercise preconditioning in vascular activity (Liu, Huang et al. 2011). Vigorous exercise training, i.e. treadmill running 5 days/week for 8–19 weeks, improved the NOS-dependent vascular reactivity of cerebral arterioles and reduced infarct volume following MCAO (Arrick, Yang et al. 2014). Vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF) both play a vital role in cerebral vasculature angiogenesis which, in turn, is essential for vascular remodeling following ischemic stroke. Physical exercise has been shown to increase levels of both VEGF and IGF1 (Carro, Nuñez et al. 2000, Cotman, Berchtold et al. 2007, Tang, Xia et al. 2010) in the brain. Exercise preconditioning helps the brain resist ischemic stress by increasing glucose uptake and metabolism, resulting in increased ATP production following stroke; this promotes neuronal survival and cerebral tissue viability (Dornbos, Zwagerman et al. 2013). Based on the above studies, we can say that exercise preconditioning improves CBF, vascular activity and helps in vascularization via angiogenesis following induced ischemic stroke in animal models.
Exercise preconditioning targeting neuronal death
The ischemic core consists of necrotic tissue whereas the penumbral region surrounding the core shows signs of apoptosis. Proper intervention into the apoptotic program in the penumbra can provide neuroprotection. Heat shock protein (HSP-70) and ERK-mediated signaling pathways have been shown to be involved in ischemia-induced apoptosis (Zhang, Wu et al. 2011). Exercise preconditioning in rat stroke models diminishes neuronal injury by upregulating HSP-70 and ERK 1/2 (Liebelt, Papapetrou et al. 2010). Similarly, small heat shock protein (HSP-20), which protects neurons and glia against ischemia-induced apoptosis, and Matrix Metallopeptidase 9 (MMP-9) increased significantly upon exercise preconditioning and was associated with improved stroke outcomes in rats (Chaudhry, Rogers et al. 2010, Lin, Chang et al. 2015). Exercise preconditioning elevates midkine (MK) levels, a neurotropic factors that possesses anti-apoptotic and angiogenic activity (Muramatsu 2010), and thus provides a neuroprotective and regenerative role in cerebral ischemia (Otsuka, Sakakima et al. 2016). Exercise also reduces expression of the apoptosis associated gene bcl-X and neuronal death protein in the rat hippocampus (Tong, Shen et al. 2001). The hippocampus, a part of the brain important for learning and memory, is vulnerable to ischemic insults (Albasser, Amin et al. 2012). Previous studies suggested transient forebrain and global ischemia causes neuronal injury and loss in the CA1 region of hippocampus (Olsson, Wieloch et al. 2003, Ouyang, Voloboueva et al. 2007). Exercise preconditioning rescued ischemia-induced hippocampal CA1 neuronal degeneration in rat common carotid artery stroke model and thus prevented memory deficit (Shamsaei, Khaksari et al. 2015). Exercise prior to ischemic insult prevented hippocampal neuronal loss in CA1 and CA3 regions through BAX/BCL-2 ratio reduction and caspase-3 activation (Aboutaleb, Shamsaei et al. 2015, Aboutaleb, Shamsaei et al. 2016).
Exercise preconditioning in excitatory system
Excitotoxicity, due to excessive excitatory neurotransmitter glutamate release, is considered the primary reason of neuronal death in stroke (Lai, Zhang et al. 2014). Pre-ischemic exercise has been shown to decrease glutamate release (Zhang, Wu et al. 2010) and inhibit the expression of glutamate receptors, metabotropic glutamate receptor 5 (mGluR5) and N-methyl-D-aspartate receptor subunit type 2B (NR2B), following MCAO (Zhang, Jia et al. 2010, Zhang, Bai et al. 2012). Another study in rats showed that exercise preconditioning increases glutamate transporter-1 (GLT-1), also known as EAAT2, and thus exerts it neuroprotective potential by re-uptaking excessive glutamate after stroke possibly through extracellular signal-regulated kinase 1/2 (ERK1/2). Rats that underwent exercise preconditioning using treadmill running prior to MCAO had increased activity of superoxide dismutase (SOD) and decreased concentration of MDA, a marker of oxidative damage, suggesting exercise precondition decreases brain oxidative damage following ischemia reperfusion injury (Feng, Zhang et al. 2014). Another study on rats with three-week treadmill running demonstrated that preischemic exercise preconditioning alleviates neuronal oxidative damage by suppressing 4-hydroxy-2-nonenal-modified proteins, 8-hydroxy-2′-deoxyguanosine, and the levels of superoxide dismutase (SOD) following ischemic stroke (Hamakawa, Ishida et al. 2013).
Exercise preconditioning and inflammation
Different preclinical data have suggested that inflammation contributes to brain injury during ischemic stroke models (Wang, Tang et al. 2007, Barrientos, Frank et al. 2011). Various inflammatory mediators released by ischemic brain cells exacerbate the deleterious effects of ischemic brain injury (Mizuma and Yenari 2017). Several studies have shown that the ischemic reperfusion induced inflammatory response occurs through TLR4 mediated pathways, reviewed in Yang et al. (Yang, Xiang et al. 2010). Exercise has been shown to provide neuroprotection by regulating TLR4/NF-κB signaling pathway and thereby inhibiting central and peripheral inflammatory cascades during cerebral ischemic/reperfusion injury (Zhu, Ye et al. 2016). In summary, exercise preconditioning ameliorates the inflammatory response in ischemic stroke.
Exercise preconditioning in clinical studies
The beneficial effect of post stroke exercise has been well document in various preclinical (Zhang, Deng et al. 2013, Pan, Jiang et al. 2017, Stradecki-Cohan, Youbi et al. 2017) and clinical studies and meta-reviews (Belfiore, Miele et al. 2017, Oberlin, Waiwood et al. 2017, Robertson, Marzolini et al. 2017). In a few clinical studies, pre-stroke physical activity has been shown to be beneficial. However, there are fewer clinical studies that have assessed the effect of pre-stroke physical activity as a determinant for outcome. In a clinical study with 362 patients, lower severity and a better short-term stroke outcome were observed for patients who remained weakly to moderately physically active prior to cerebral ischemic stroke occurrence (Deplanque, Masse et al. 2006, Deplanque, Masse et al. 2012). In another clinical trial with 265 patients, which represents a subset of patients with first-ever stroke, enrolled into the ExStroke Pilot Trial, providing further evidence that physical activity prior to stroke was associated with a less severe stroke and better long-term outcome (Krarup, Truelsen et al. 2008). Self-reported higher physical activity prior to ischemic stroke was associated with functional advantages in a three-month follow-up as part of the Ischemic Stroke Genetics Study (Stroud, Mazwi et al. 2009). Higher-level pre-ischemic physical activity has also been shown to be correlated with smaller final infarct volume (Ricciardi, Lopez-Cancio et al. 2014). Maessen et al. report that lifelong exercise training is associated with increased tolerance against endothelial I/R, which could be cardio- and neuroprotective (Maessen, van Mil et al. 2017).
In humans, exercise increases cerebral blood volume in the hippocampal dentate gyrus and improves cognitive function in adult humans (Pereira, Huddleston et al. 2007). Physical activity, especially aerobic exercise, is also related to increased gray matter volume, increased hippocampal volume, and decreased cognitive impairment in aging (Colcombe, Erickson et al. 2006, Erickson, Raji et al. 2010, Erickson, Voss et al. 2011). Overall, better functional outcome and severity was observed in ischemic stroke with pre-ischemic physical activity in human study, but the molecular mechanism behind exercise preconditioning in stroke has not been explored in detail. The neurotrophin brain-derived neurotrophic factor (BDNF) has been reported to be elevated during exercise both in preclinical (Neeper, Gómez-Pinilla et al. 1995, Kobilo, Liu et al. 2011) and clinical studies (Rasmussen, Brassard et al. 2009, Erickson, Voss et al. 2011) and it is believed to be a mediator of neurogenesis (Pencea, Bingaman et al. 2001), hence it can be one of the molecular mediators for exercise preconditioning in ischemic stroke outcome. Another study reports that serum level of vascular endothelial growth factor (VEGF), an important neurotrophin, is increased significantly after ischemic stroke in patients with pre-ischemic physical activity (Lopez-Cancio, Ricciardi et al. 2017). These clinical studies strongly suggest that pre-stroke physical activity has been shown to be beneficial for the severity and the outcome following stroke. However, most studies could not exclude the beneficial effects of the higher fitness were due to improved overall health pre-stroke. Longitudinal studies with matched cohorts will be required to dissect these effects.
Conclusion
In conclusion, exercise preconditioning has been proven to be beneficial in both preclinical and clinical studies. Elucidating the mechanisms underlying exercise preconditioning-induced neuroprotection in stroke will provide us with the opportunity to explore new treatment strategies for stroke patients.
Acknowledgments
C.D.W. was supported by the NIH (NS087096), a NeuroBehavior Laboratory Pilot Project Research Award from the Harvard NeuroDiscovery Center (HNDC) and the Hassenfeld Cardiovascular Scholar Award. The authors declare no competing financial interests.
References
- Abbott NJ, Friedman A. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia. 2012;53(Suppl 6):1–6. doi: 10.1111/j.1528-1167.2012.03696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboutaleb N, Shamsaei N, Khaksari M, Erfani S, Rajabi H, Nikbakht F. Pre-ischemic exercise reduces apoptosis in hippocampal CA3 cells after cerebral ischemia by modulation of the Bax/Bcl-2 proteins ratio and prevention of caspase-3 activation. J Physiol Sci. 2015;65(5):435–443. doi: 10.1007/s12576-015-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboutaleb N, Shamsaei N, Rajabi H, Khaksari M, Erfani S, Nikbakht F, Motamedi P, Shahbazi A. Protection of Hippocampal CA1 Neurons Against Ischemia/Reperfusion Injury by Exercise Preconditioning via Modulation of Bax/Bcl-2 Ratio and Prevention of Caspase-3 Activation. Basic Clin Neurosci. 2016;7(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Albasser MM, Amin E, Lin TC, Iordanova MD, Aggleton JP. Evidence that the rat hippocampus has contrasting roles in object recognition memory and object recency memory. Behav Neurosci. 2012;126(5):659–669. doi: 10.1037/a0029754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrick DM, Yang S, Li C, Cananzi S, Mayhan WG. Vigorous exercise training improves reactivity of cerebral arterioles and reduces brain injury following transient focal ischemia. Microcirculation. 2014;21(6):516–523. doi: 10.1111/micc.12127. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Gleichmann M, Tang SC, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res Rev. 2006;5(2):165–178. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31(32):11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk MR, Aumont A, Decary S, Bergeron R, Fernandes KJ. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus. 2009;19(10):913–927. doi: 10.1002/hipo.20621. [DOI] [PubMed] [Google Scholar]
- Belfiore P, Miele A, Galle F, Liguori G. Adapted physical activity and stroke: a systematic review. J Sports Med Phys Fitness. 2017 doi: 10.23736/S0022-4707.17.07749-0. [DOI] [PubMed] [Google Scholar]
- Brandt MD, Maass A, Kempermann G, Storch A. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. Eur J Neurosci. 2010;32(8):1256–1264. doi: 10.1111/j.1460-9568.2010.07410.x. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17(10):2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Cai M, Yang Q, Li G, Sun S, Chen Y, Tian L, Dong H. Activation of cannabinoid receptor 1 is involved in protection against mitochondrial dysfunction and cerebral ischaemic tolerance induced by isoflurane preconditioning. Br J Anaesth. 2017;119(6):1213–1223. doi: 10.1093/bja/aex267. [DOI] [PubMed] [Google Scholar]
- Carro E, Nuñez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20(8):2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry K, Rogers R, Guo M, Lai Q, Goel G, Liebelt B, Ji X, Curry A, Carranza A, Jimenez DF, Ding Y. Matrix metalloproteinase-9 (MMP-9) expression and extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation in exercise-reduced neuronal apoptosis after stroke. Neurosci Lett. 2010;474(2):109–114. doi: 10.1016/j.neulet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42(2):484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Davis W, Mahale S, Carranza A, Cox B, Hayes K, Jimenez D, Ding Y. Exercise preconditioning ameliorates blood-brain barrier dysfunction in stroke by enhancing basal lamina. Neurol Res. 2007;29(4):382–387. doi: 10.1179/016164107X204701. [DOI] [PubMed] [Google Scholar]
- Deplanque D, Masse I, Lefebvre C, Libersa C, Leys D, Bordet R. Prior TIA, lipid-lowering drug use, and physical activity decrease ischemic stroke severity. Neurology. 2006;67(8):1403–1410. doi: 10.1212/01.wnl.0000240057.71766.71. [DOI] [PubMed] [Google Scholar]
- Deplanque D, Masse I, Libersa C, Leys D, Bordet R. Previous leisure-time physical activity dose dependently decreases ischemic stroke severity. Stroke Res Treat. 2012;2012:614925. doi: 10.1155/2012/614925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YH, Li J, Yao WX, Rafols JA, Clark JC, Ding Y. Exercise preconditioning upregulates cerebral integrins and enhances cerebrovascular integrity in ischemic rats. Acta Neuropathol. 2006;112(1):74–84. doi: 10.1007/s00401-006-0076-6. [DOI] [PubMed] [Google Scholar]
- Ding YH, Young CN, Luan X, Li J, Rafols JA, Clark JC, McAllister JP, 2nd, Ding Y. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol. 2005;109(3):237–246. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- Dornbos D, 3rd, Zwagerman N, Guo M, Ding JY, Peng C, Esmail F, Sikharam C, Geng X, Guthikonda M, Ding Y. Preischemic exercise reduces brain damage by ameliorating metabolic disorder in ischemia/reperfusion injury. J Neurosci Res. 2013;91(6):818–827. doi: 10.1002/jnr.23203. [DOI] [PubMed] [Google Scholar]
- Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55(3):310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75(16):1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Hu WW, Dai HB, Zhang JX, Zhang LY, He P, Shen Y, Ohtsu H, Wei EQ, Chen Z. Activation of the central histaminergic system is involved in hypoxia-induced stroke tolerance in adult mice. J Cereb Blood Flow Metab. 2011;31(1):305–314. doi: 10.1038/jcbfm.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng RUI, Zhang MIN, Wang X, Li WB, Ren SQ, Zhang F. Pre-ischemic exercise alleviates oxidative damage following ischemic stroke in rats. Experimental and Therapeutic Medicine. 2014;8(4):1325–1329. doi: 10.3892/etm.2014.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- Guo M, Cox B, Mahale S, Davis W, Carranza A, Hayes K, Sprague S, Jimenez D, Ding Y. Pre-ischemic exercise reduces matrix metalloproteinase-9 expression and ameliorates blood-brain barrier dysfunction in stroke. Neuroscience. 2008;151(2):340–351. doi: 10.1016/j.neuroscience.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Guo M, Lin V, Davis W, Huang T, Carranza A, Sprague S, Reyes R, Jimenez D, Ding Y. Preischemic induction of TNF-alpha by physical exercise reduces blood-brain barrier dysfunction in stroke. J Cereb Blood Flow Metab. 2008;28(8):1422–1430. doi: 10.1038/jcbfm.2008.29. [DOI] [PubMed] [Google Scholar]
- Hamakawa M, Ishida A, Tamakoshi K, Shimada H, Nakashima H, Noguchi T, Toyokuni S, Ishida K. Repeated short-term daily exercise ameliorates oxidative cerebral damage and the resultant motor dysfunction after transient ischemia in rats. J Clin Biochem Nutr. 2013;53(1):8–14. doi: 10.3164/jcbn.12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Wang X, Wu Y, Jia J, Hu Y, Yang X, Li J, Fan M, Zhang L, Guo J, Leung MC. Treadmill pre-training ameliorates brain edema in ischemic stroke via down-regulation of aquaporin-4: an MRI study in rats. PLoS One. 2014;9(1):e84602. doi: 10.1371/journal.pone.0084602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Hu YS, Wu Y, Liu G, Yu HX, Zheng QP, Zhu DN, Xia CM, Cao ZJ. Pre-ischemic treadmill training affects glutamate and gamma aminobutyric acid levels in the striatal dialysate of a rat model of cerebral ischemia. Life Sci. 2009;84(15–16):505–511. doi: 10.1016/j.lfs.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103(35):13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeraki E, Pielecka-Fortuna J, Huppe JM, Lowel S. Physical Exercise Preserves Adult Visual Plasticity in Mice and Restores it after a Stroke in the Somatosensory Cortex. Front Aging Neurosci. 2016;8:212. doi: 10.3389/fnagi.2016.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18(9):605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek KD, Murphy SL, Xu J, Arias E. Mortality in the United States, 2013. NCHS Data Brief. 2014;(178):1–8. [PubMed] [Google Scholar]
- Krarup LH, Truelsen T, Gluud C, Andersen G, Zeng X, Korv J, Oskedra A, Boysen G. Prestroke physical activity is associated with severity and long-term outcome from first-ever stroke. Neurology. 2008;71(17):1313–1318. doi: 10.1212/01.wnl.0000327667.48013.9f. [DOI] [PubMed] [Google Scholar]
- Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Liebelt B, Papapetrou P, Ali A, Guo M, Ji X, Peng C, Rogers R, Curry A, Jimenez D, Ding Y. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166(4):1091–1100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- Lin CM, Chang CK, Chang CP, Hsu YC, Lin MT, Lin JW. Protecting against ischaemic stroke in rats by heat shock protein 20-mediated exercise. Eur J Clin Invest. 2015;45(12):1297–1305. doi: 10.1111/eci.12551. [DOI] [PubMed] [Google Scholar]
- Liu N, Huang H, Lin F, Chen A, Zhang Y, Chen R, Du H. Effects of treadmill exercise on the expression of netrin-1 and its receptors in rat brain after cerebral ischemia. Neuroscience. 2011;194:349–358. doi: 10.1016/j.neuroscience.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Lopez-Cancio E, Ricciardi AC, Sobrino T, Cortes J, de la Ossa NP, Millan M, Hernandez-Perez M, Gomis M, Dorado L, Munoz-Narbona L, Campos F, Arenillas JF, Davalos A. Reported Prestroke Physical Activity Is Associated with Vascular Endothelial Growth Factor Expression and Good Outcomes after Stroke. J Stroke Cerebrovasc Dis. 2017;26(2):425–430. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Maessen MFH, van Mil A, Straathof Y, Riksen NP, Rongen G, Hopman MTE, Eijsvogels TMH, Thijssen DHJ. Impact of lifelong exercise training on endothelial ischemia-reperfusion and ischemic preconditioning in humans. Am J Physiol Regul Integr Comp Physiol. 2017;312(5):R828–r834. doi: 10.1152/ajpregu.00466.2016. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16(6):706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maysami S, Lan JQ, Minami M, Simon RP. Proliferating progenitor cells: a required cellular element for induction of ischemic tolerance in the brain. J Cereb Blood Flow Metab. 2008;28(6):1104–1113. doi: 10.1038/jcbfm.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mizuma A, Yenari MA. Anti-Inflammatory Targets for the Treatment of Reperfusion Injury in Stroke. Front Neurol. 2017;8:467. doi: 10.3389/fneur.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JP, Kayani AC, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009;39(8):643–662. doi: 10.2165/00007256-200939080-00003. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(4):410–425. doi: 10.2183/pjab.86.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi S, Alimohammadi R, Hakimizadeh E, Roohbakhsh A, Shamsizadeh A, Allahtavakoli M. The effect of exercise preconditioning on stroke outcome in ovariectomized mice with permanent middle cerebral artery occlusion. Can J Physiol Pharmacol. 2017 doi: 10.1139/cjpp-2017-0157. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nishio S, Yunoki M, Chen ZF, Anzivino MJ, Lee KS. Ischemic tolerance in the rat neocortex following hypothermic preconditioning. J Neurosurg. 2000;93(5):845–851. doi: 10.3171/jns.2000.93.5.0845. [DOI] [PubMed] [Google Scholar]
- Niv F, Keiner Krishna S, Witte OW, Lie DC, Redecker C. Aberrant neurogenesis after stroke: a retroviral cell labeling study. Stroke. 2012;43(9):2468–2475. doi: 10.1161/STROKEAHA.112.660977. [DOI] [PubMed] [Google Scholar]
- Oberlin LE, Waiwood AM, Cumming TB, Marsland AL, Bernhardt J, Erickson KI. Effects of Physical Activity on Poststroke Cognitive Function: A Meta-Analysis of Randomized Controlled Trials. Stroke. 2017;48(11):3093–3100. doi: 10.1161/STROKEAHA.117.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T, Wieloch T, Smith ML. Brain damage in a mouse model of global cerebral ischemia. Effect of NMDA receptor blockade. Brain Res. 2003;982(2):260–269. doi: 10.1016/s0006-8993(03)03014-2. [DOI] [PubMed] [Google Scholar]
- Otsuka S, Sakakima H, Sumizono M, Takada S, Terashi T, Yoshida Y. The neuroprotective effects of preconditioning exercise on brain damage and neurotrophic factors after focal brain ischemia in rats. Behav Brain Res. 2016;303:9–18. doi: 10.1016/j.bbr.2016.01.049. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27(16):4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Jiang T, Zhang L, Zheng H, Luo J, Hu X. Physical Exercise Promotes Novel Object Recognition Memory in Spontaneously Hypertensive Rats after Ischemic Stroke by Promoting Neural Plasticity in the Entorhinal Cortex. Front Behav Neurosci. 2017;11:185. doi: 10.3389/fnbeh.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21(17):6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto MC, Simao F, da Costa FL, Rosa DV, de Paiva MJ, Resende RR, Romano-Silva MA, Gomez MV, Gomez RS. Sarcosine preconditioning induces ischemic tolerance against global cerebral ischemia. Neuroscience. 2014;271:160–169. doi: 10.1016/j.neuroscience.2014.04.054. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Ricciardi AC, Lopez-Cancio E, Perez de la Ossa N, Sobrino T, Hernandez-Perez M, Gomis M, Munuera J, Munoz L, Dorado L, Millan M, Davalos A, Arenillas JF. Prestroke physical activity is associated with good functional outcome and arterial recanalization after stroke due to a large vessel occlusion. Cerebrovasc Dis. 2014;37(4):304–311. doi: 10.1159/000360809. [DOI] [PubMed] [Google Scholar]
- Robertson AD, Marzolini S, Middleton LE, Basile VS, Oh PI, MacIntosh BJ. Exercise Training Increases Parietal Lobe Cerebral Blood Flow in Chronic Stroke: An Observational Study. Front Aging Neurosci. 2017;9:318. doi: 10.3389/fnagi.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah N, Peterson BD, Lubejko ST, Vivar C, van Praag H. Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Sci Rep. 2017;7(1):10903. doi: 10.1038/s41598-017-11268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsaei N, Erfani S, Fereidoni M, Shahbazi A. Neuroprotective Effects of Exercise on Brain Edema and Neurological Movement Disorders Following the Cerebral Ischemia and Reperfusion in Rats. Basic Clin Neurosci. 2017;8(1):77–84. doi: 10.15412/J.BCN.03080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsaei N, Khaksari M, Erfani S, Rajabi H, Aboutaleb N. Exercise preconditioning exhibits neuroprotective effects on hippocampal CA1 neuronal damage after cerebral ischemia. Neural Regen Res. 2015;10(8):1245–1250. doi: 10.4103/1673-5374.162756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra C, Coca A, Schiffrin EL. Vascular mechanisms in the pathogenesis of stroke. Curr Hypertens Rep. 2011;13(3):200–207. doi: 10.1007/s11906-011-0195-x. [DOI] [PubMed] [Google Scholar]
- Speetzen LJ, Endres M, Kunz A. Bilateral common carotid artery occlusion as an adequate preconditioning stimulus to induce early ischemic tolerance to focal cerebral ischemia. J Vis Exp. 2013;(75):e4387. doi: 10.3791/4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradecki-Cohan HM, Youbi M, Cohan CH, Saul I, Garvin AA, Perez E, Dave KR, Wright CB, Sacco RL, Perez-Pinzon MA. Physical Exercise Improves Cognitive Outcomes in 2 Models of Transient Cerebral Ischemia. Stroke. 2017;48(8):2306–2309. doi: 10.1161/STROKEAHA.117.017296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud N, Mazwi TM, Case LD, Brown RD, Jr, Brott TG, Worrall BB, Meschia JF. Prestroke physical activity and early functional status after stroke. J Neurol Neurosurg Psychiatry. 2009;80(9):1019–1022. doi: 10.1136/jnnp.2008.170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Xia FC, Wagner PD, Breen EC. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir Physiol Neurobiol. 2010;170(1):16–22. doi: 10.1016/j.resp.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8(6):1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Traystman RJ. Neuroprotection: introduction. Stroke. 2010;41(10 Suppl):S63. doi: 10.1161/STROKEAHA.110.598557. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vivar C, Peterson BD, van Praag H. Running rewires the neuronal network of adult-born dentate granule cells. Neuroimage. 2016;131:29–41. doi: 10.1016/j.neuroimage.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17(10):525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1–2):53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitke F, Ceanga M, Rudolph M, Niv F, Witte OW, Redecker C, Kunze A, Keiner S. Adult hippocampal neurogenesis poststroke: More new granule cells but aberrant morphology and impaired spatial memory. PLoS One. 2017;12(9):e0183463. doi: 10.1371/journal.pone.0183463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11(3):298–308. [PMC free article] [PubMed] [Google Scholar]
- Xu H, Aibiki M, Nagoya J. Neuroprotective effects of hyperthermic preconditioning on infarcted volume after middle cerebral artery occlusion in rats: role of adenosine receptors. Crit Care Med. 2002;30(5):1126–1130. doi: 10.1097/00003246-200205000-00028. [DOI] [PubMed] [Google Scholar]
- Yang QW, Xiang J, Zhou Y, Zhong Q, Li JC. Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia. Front Biosci (Schol Ed) 2010;2:1081–1091. doi: 10.2741/s119. [DOI] [PubMed] [Google Scholar]
- Zhang A, Bai Y, Hu Y, Zhang F, Wu Y, Wang Y, Zheng P, He Q. The effects of exercise intensity on p-NR2B expression in cerebral ischemic rats. Can J Neurol Sci. 2012;39(5):613–618. doi: 10.1017/s0317167100015341. [DOI] [PubMed] [Google Scholar]
- Zhang F, Jia J, Wu Y, Hu Y, Wang Y. The effect of treadmill training pre-exercise on glutamate receptor expression in rats after cerebral ischemia. Int J Mol Sci. 2010;11(7):2658–2669. doi: 10.3390/ijms11072658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wu Y, Jia J. Exercise preconditioning and brain ischemic tolerance. Neuroscience. 2011;177:170–176. doi: 10.1016/j.neuroscience.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wu Y, Jia J, Hu YS. Pre-ischemic treadmill training induces tolerance to brain ischemia: involvement of glutamate and ERK1/2. Molecules. 2010;15(8):5246–5257. doi: 10.3390/molecules15085246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang L, Yang X, Wan Y, Jia J. The effects of exercise preconditioning on cerebral blood flow change and endothelin-1 expression after cerebral ischemia in rats. J Stroke Cerebrovasc Dis. 2014;23(6):1696–1702. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Zhang QW, Deng XX, Sun X, Xu JX, Sun FY. Exercise promotes axon regeneration of newborn striatonigral and corticonigral projection neurons in rats after ischemic stroke. PLoS One. 2013;8(11):e80139. doi: 10.1371/journal.pone.0080139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou AM, Li WB, Li QJ, Liu HQ, Feng RF, Zhao HG. A short cerebral ischemic preconditioning up-regulates adenosine receptors in the hippocampal CA1 region of rats. Neurosci Res. 2004;48(4):397–404. doi: 10.1016/j.neures.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Zhu L, Ye T, Tang Q, Wang Y, Wu X, Li H, Jiang Y. Exercise Preconditioning Regulates the Toll-Like Receptor 4/Nuclear Factor-kappaB Signaling Pathway and Reduces Cerebral Ischemia/Reperfusion Inflammatory Injury: A Study in Rats. J Stroke Cerebrovasc Dis. 2016;25(11):2770–2779. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.033. [DOI] [PubMed] [Google Scholar]
- Zwagerman N, Sprague S, Davis MD, Daniels B, Goel G, Ding Y. Pre-ischemic exercise preserves cerebral blood flow during reperfusion in stroke. Neurol Res. 2010;32(5):523–529. doi: 10.1179/016164109X12581096796431. [DOI] [PubMed] [Google Scholar]