Abstract
Surface modification is of significant interest in biomaterials, biosensors, and device biocompatibility. Immobilization of bioactive or biomimetic molecules is a common method of disguising a foreign body as host tissue to decrease the foreign body response (FBR) and/or increase device–tissue integration. For example, in neural interfacing devices, immobilization of L1, a neuron-specific adhesion molecule, has been shown to increase neuron adhesion and reduce inflammatory gliosis on and around the implants. However, the activity of modified surfaces is limited by the relatively low concentration of the immobilized component, in part due to the low surface area of flat surfaces available for modification. In this work, we demonstrate a novel method for increasing the device surface area by attaching a layer of thiolated silica nanoparticles (TNPs). This coating method results in an almost two-fold increase in the immobilized L1 protein. L1 immobilized nanotextured surfaces showed a 100% increase in neurite outgrowth than smooth L1 immobilized surfaces without increasing the adhesion of astrocytes in vitro. The increased bioactivity observed in the cell assay was determined to be mainly due to the higher protein surface density, not the increase in surface roughness. In addition, we tested immobilization of a superoxide dismutase mimic (SODm) on smooth and roughened substrates. The SODm immobilized rough surfaces demonstrated an increase of 145% in superoxide scavenging activity compared to chemically matched smooth surfaces. These results not only show promise in improving biomimetic coating for neural implants, but may also improve surface immobilization efficacy in other fields such as catalysts, protein purification, sensors, and tissue engineering devices.
Introduction
Surface modification plays a major role in solid-state catalyst design, corrosion resistance, wear resistance, and biocompatibility. For implantable medical devices, bioactive and biomimetic coatings are of particular interest in biochemical sensing, promoting host tissue integration, and reducing foreign body responses (FBRs). For example, heparin coatings have been shown to decrease restenosis of vascular stents.1 Incorporation of biological molecules such as the brain-derived neurotropic factor (BDNF)2 increases tissue regeneration when applied to regenerative scaffolds for tissue engineering. Biosensors incorporate bio-recognition molecules such as aptamers,3 enzymes,4,5 and antibodies6 onto their surface. Immobilized enzymes may aid in CO2 removal for artificial lungs.7,8 In neural devices, immobilized proteins, anti-inflammatory peptides, and antioxidants may decrease the FBR and increase tissue integration in the peripheral9 and central nervous system.10,11
However, surface immobilization of functional molecules is limited by the availability of binding sites and surface area presented by the pristine substrate. 2D surface coatings such as immobilized catalysts or proteins are only capable of being bound at a relatively low density to the surface of the substrate. The activity of these coatings may be increased if the surface area was increased. A simple mathematical calculation reveals that by using the hexagonal sphere packing method, we can convert a smooth surface to a sphere-coated surface, which nearly doubles the available surface area, with hexagonal sphere packing increasing the area by a factor of 1.9, or up to 4.1 if the underside of the sphere can still be accessed. As such, nanoparticle immobilization onto the surface of a substrate offers a promising method for increasing surface area available for immobilization without the risk of damaging the substrate, which is possible when using destructive methods of texturization such as surface etching. Silica nanoparticles are commonly employed for drug delivery12 and have been well characterized as biocompatible.13,14 In addition, the surface properties can be easily modified via direct functionalization15 or by incorporating different monomers into the reaction solution,15,16 producing nanoparticles with active thiol16,17 or amine15 groups. Together, these properties make silica nanoparticles attractive candidates for surface modification when biocompatibility is a concern.
Implanted neural electrodes are one example where surface modification has shown benefits in promoting device–tissue integration. The potential of neural interfacing devices to restore the neurological function of patients is profound, but most neural electrodes suffer from chronic degradation in recording quality. Biologically, this long-term degradation is often attributed to the host inflammatory response to the implant, in which the native glial cells (notably astrocytes and microglia) play a critical role.18 In response, increasing the biocompatibility of these devices has become an extensive field of research. Nanopatterned surface ridges on silicon implants have been shown to reduce the activity of astrocytes in vitro,19 while surface topographic features can guide neurite outgrowth and alignment.20,21 Meanwhile, superoxide dismutase mimics such as Mn(iii)tetrakis(4-benzoic acid)porphyrin and amine functionalized meso-tetra(2-pyridyl)porphine (referred to as iSODm)22,23 have been covalently bound to the surface of neural implants to reduce harmful reactive oxygen species (ROS) released both acutely after insertion injury and chronically as a component of inflammatory tissue responses. Biomimetic coatings utilizing peptides24,25 and proteins10,26,27 exhibit pronounced effects in reducing the inflammatory response and/or encouraging neurite attachment as well as decreasing the activation of glia. Laminin-coated surfaces can modulate the inflammation around the implant, increasing the microglia activation acutely but decreasing the glial scar after 4 weeks.11 Neural electrodes modified with the neuron specific adhesion protein L1 have shown attenuated microglia activation immediately after implantation,27 as well as increased neuron attachment, neuron health, and reduced glial encapsulation over many weeks of implantation.10
Here we describe a novel surface modification technique utilizing thiol functionalized silica nanoparticles to increase the available surface area for protein and antioxidant catalyst binding (Scheme 1). We utilize neural electrodes as a model system, which may benefit from an increase in the density of bioactive molecules at the surface. Through nanoparticle surface roughening, we can increase the area of a flat neural probe, proportionally increasing the number of active sites for crosslinking and bioactive molecule attachment, thereby increasing the effectiveness of surface immobilization. We test three immobilizable molecules: iSODm, L1, and laminin. First, we show that increased antioxidant attachment (iSODm) on the textured surface is more effective in eliminating reactive oxygen species (ROS), in comparison to the smooth surface control. Second, we demonstrate that increased protein attachment (L1 and laminin) results in increased neurite outgrowth of primary neurons cultured on the nanotextured substrate compared to protein-bound smooth controls. Third, increasing surface area alone does not lead to increased cell attachment.
Scheme 1.
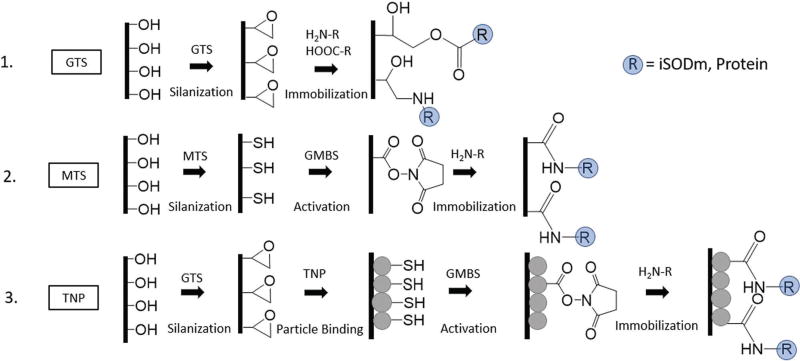
Scheme of surface modification. (1) GTS surfaces. Smooth silicon and glass substrates are first activated then coated by a layer of epoxide containing silane. Epoxide modified surfaces are linked to amine/carboxyl containing antioxidant or protein via nucleophilic ring opening (GTS surfaces). (2) MTS surfaces. Smooth silicon and glass substrates are activated then coated with a thiol containing silane. Thiol groups are linked to amine containing molecules by an N-malaimidobutyryl-oxysuccinimide ester (GMBS) crosslinker. (3) TNP surfaces. Thiol coated nanoparticles are linked to the GTS modified substrate by carbonate catalysed ring opening to produce a roughened surface with thiol groups which can link to amine containing molecules via GMBS.
Experimental
Materials and characterization
All chemicals were purchased from Sigma-Aldrich and used as received unless otherwise noted. Ultrapure di-water was used for all experiments (18.2 MΩ, Milli-Q). Glass coverslips (8 mm diameter) were purchased from Electron Microscopy Sciences. Silicon wafers were ordered from University Wafer. Pregnant and post-natal rats were ordered from Taconic. All animal procedures were performed in compliance with the United States Department of Agriculture and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Transmission electron microscopy (TEM) images were taken using a Joel 2100F TEM microscope. For TEM, nanoparticles were collected from the suspension via centrifugation and resuspended in 100% ethanol to form a dilute suspension. To produce reduced particles, 15 mg of tris(2-carboxyethyl)-phosphine (TCEP) was added to 2 mL of the suspension for 10 minutes prior to collection by centrifugation. 1 µL of the suspension was added dropwise over TEM grids (400 mesh carbon on copper, Ted Pella). Scanning electron microscopy (SEM) was performed using a JSM6335. SEM samples were made conductive by sputter coating a 3.5 nm thick layer of Au/Pd alloy (108Auto, Cressington). Atomic Force Microscopy (AFM) was performed by asylum MFP3D with silicon probes (HQ-300-Au, Oxford Instruments) on dehydrated samples on silicon substrates. Dynamic Light Scattering (DLS) was performed using a Malvern ZS90 Zetasizer. All spectrophotometric measurements were performed using a SpectraMax i3x, Molecular Devices. Water contact angle (WCA) measurements and ellipsometry were performed using an AST Products VCA Optima and J. A. Woollam α-SE, respectively. Ellipsometry data were fitted with a Cauchy model, using a silica refractive index of 1.46. Fluorescence microscopy images were taken using a Leica DMI4000b.
Nanoparticle fabrication
Thiol functionalized nanoparticles were prepared via a sol–gel process. 50 mL of 0.014 M NaOH in H2O was heated to 70 °C. While vigorously stirring, 500 µL of tetraethyl orthosilicate (TEOS) was quickly pipetted into the flask. After 5 minutes, 100 µL of mercaptopropyl trimethoxysilane (MTS) was added and the solution was allowed to react for 2 h to form a slightly cloudy nanoparticle suspension. Nanoparticles were collected by centrifugation.
Nanoparticle characterization
For Dynamic Light Scattering, 0.5 mg of nanoparticles was re-suspended in 50 mg of TCEP dissolved in 2 mL of water to break any disulphide bonds between particles and sonicated for 5 minutes. The particles were again collected, suspended in 2 mL of water, pipetted into a disposable polystyrene cuvette, and analysed. Note that TCEP was only used for nanoparticle size characterization by DLS and TEM.
Thiol concentration was measured using Ellman’s reagent. A calibration curve was established by dissolving MTS in pH 7.4 phosphate buffered saline (PBS), and reacting with equal volumes of Ellman’s reagent (2 mg mL−1) in PBS. 0.2 mg of TNPs was suspended in 1 mL of PBS and was allowed to react with 1 mL of Ellman’s reagent solution for 15 minutes. Particles were spun out of the solution prior to measuring absorbance. The colour change was measured by spectrophotometry at 420 nm.
Surface functionalization
Surface modification routes of experimental and control surfaces are illustrated in Scheme 1. Prior to functionalization, glass coverslips or silicon wafers were cleaned by sonicating in acetone for 3 × 15 minutes and isopropyl alcohol for 3 × 15 minutes. Substrates were then submerged in piranha solution (3 : 1 H2SO4 : 30% H2O2) for 30 minutes to remove any surface contamination. Cleaned substrates were dried under nitrogen and placed into a 48 well plate. 500 µL of 0.1 M NaOH was added to each well for 1 hour followed by 20 minutes of sonication. Substrates were washed thoroughly in water followed by ethanol and dried under nitrogen. 500 µL of 2.5% (3-glycidyloxypropyl) trimethoxysilane (GTS) in ethanol and 0.1 M acetic acid were added to each well and allowed to react for 1 hour. Wells were washed with ethanol and dried under a nitrogen stream. To some of these GTS functionalized samples, thiolated nanoparticles were added to create the textured surface. Nanoparticle suspensions were created by centrifuging 2 mL of the prepared TNP solutions and resuspending the TNPs in sodium carbonate (100 mg mL−1) aqueous solution. 300 µL of the nanoparticle suspension was added to each well containing GTS functionalized coverslips and incubated at 80 °C for 30 minutes to form TNP-coated coverslips. To other GTS functionalized samples, iSODm or proteins were added later directly to form GTS smooth surface controls.
Additionally, smooth MTS coated substrates were prepared as surface chemistry matched controls to examine the effect of surface texturization. NaOH treated substrates were added to 25 mL of anhydrous toluene which was then degassed under N2, and then kept under a nitrogen atmosphere. 625 µL of MTS was syringed into the flask, and allowed to react for 1 hour. Samples were washed with ample toluene and then dried under N2.
The L1 protein was isolated from P4 postnatal rat pup brains using affinity column chromatography as described in a previous study.28 Laminin was purchased from Invitrogen. 1 mg of sulfo-GMBS was dissolved in a minimum amount of dimethylformamide, and then the mixture was brought to a final concentration of 0.2 mg mL−1 in absolute ethanol. 200 µL of GMBS solution was added over MTS and TNP functionalised coverslips in dried wells for 1 hour followed by ethanol and PBS washing. Protein immobilization occurred upon reaction of protein amine groups with epoxides on the GTS surface, or oxy-succinimide esters present on GMBS that are coupled to MTS and TNP modified coverslips, respectively. 100 µL of 20 µg mL−1 protein solution was added over the functionalised coverslips (GTS, MTS + GMBS, and TNP + GMBS) and incubated for 1.5 hours at 37 °C followed by 30 minutes at 23 °C.
Superoxide dismutase mimics (SODm) based on a reactive metal centre coordinated to a porphyrin are well characterized for their ability to cause reduction in the presence of free radicals.29 To produce the immobilizable form of SODm (iSODm), meso-tetra(2-pyridyl)porphine (Frontier Scientific) was allowed to react with excess 3-bromo-propylamine to form amine side groups, followed by MgCl2 to create a reactive centre as described in a previous study.23 For immobilization, iSODm was dissolved in water (4 mg mL−1), and 350 µL was plated over GTS, MTS + GMBS, or TNP + GMBS modified coverslips for 1 hour at 23 °C.
Evaluation of antioxidant coatings
Cytochrome-c assay was performed to determine the catalytic superoxide scavenging properties of the immobilized iSODm using different surface modification methods. Specifically, superoxide (O2•−) was generated upon reaction of xanthine oxidase (0.1 mg mL−1) and xanthine (0.1 mg mL−1) in the presence of cytochrome-c (2 mg mL−1) in 1 × PBS. To prevent interference from H2O2, catalase (1 mg mL−1) was added to the reaction. The assay was conducted in a 48-well tissue culture polystyrene dish at room temperature, and each well received 100 µL of reagent solutions. After 30 minutes, the reaction product from the 48-well plate was transferred to a 96-well half area plate for absorbance measurement of the oxidized cytochrome c at 550 nm.
Surface protein concentration
Protein-coated samples were washed with 1% Tween-20 in PBS to remove any non-covalently bound protein and then transferred to a glass vial. 400 µL of 6 M HCl was added to the vials, which were then placed in an 80 °C incubator for 36 hours to digest the amide bonds. The solution containing the digested amino acids was analysed by spectrophotometry for absorbance at 280 nm.
Surface characterization
Five samples were prepared as described above, and each sample was measured at 2 separate points for WCA and ellipsometry. Stepwise reactions were monitored using WCA values. Prior to contact angle measurements, samples were washed with water and dried. The thickness and surface roughness of the samples were measured by ellipsometry. Prior to measurements, the samples were dried using compressed gas to remove any dust from the surface. Nanoparticle distribution was visualised by SEM. Nanoparticle adhesion was qualitatively examined by a scotch tape assay, described further in the ESI.†
Cell culture
In vitro neuron and astrocyte cultures were used to assess the biocompatibility and bioactivity of the surface. Neurons were collected from E18 rat foetuses. E18 pregnant Sprague-Dawley rats were euthanized under CO2 followed by decapitation. Rat pups were removed and washed with 70% ethanol solution, followed by removal of the foetal pup brain. Brains were homogenized by soaking in 0.25% trypsin/EDTA for 15 minutes and then mechanically dispersed by repeated trituration using a fire polished Pasteur pipette. 10 µL aliquots of cell suspension were added to 10 µL of trypan blue solution prior to counting using a haemocytometer. Only cells deemed viable were counted. Three coverslips per condition were prepared as described above and placed in a 48 well plate (TCPS, Falcon). Neurons were plated at a density of 25 000 cells per well in B27/GlutiMax/PenStrep supplemented Neurobasal media and grown for 36 hours. Cultures were repeated 3 times to ensure the validity of the results.
Astrocytes were isolated from E18 rat foetuses. E18 pup brains were collected as described above. 6 pup brains were homogenized by soaking in 0.05% trypsin/EDTA for 25 minutes followed by mechanical dispersion by trituration. Cells were resuspended in astrocyte culture media (10% FBS, 1% PenStrep in DMEM) and plated over a poly-lysine coated 75 cm2 cell culture flask (Corning) for 10 days, and the media was replaced every third day. Once the astrocyte layer was confluent, microglia and oligodendrocytes were removed by vigorously shaking the culture flask, followed by 3 successive washes with PBS. Purified astrocytes were split between two culture dishes. For culture, astrocytes were suspended in 0.05% trypsin/EDTA and collected by centrifugation. Three coverslips per condition were prepared as described above and placed in a 48 well plate (TCPS, Falcon). Astrocytes were plated at a density of 5000 cells per well in astrocyte culture media for 36 hours. Culturing was repeated 3 times.
Cell staining and imaging
After growth, cells were fixed with 4% PFA, followed by permeabilizing with 0.2% Triton-X. Primary antibodies Beta-III-Tubulin (Neurons) and Glial Fibrillary Acidic Protein (GFAP, Astrocytes) (Invitrogen) were diluted 1 : 1000 in 5% goat serum/PBS and introduced over cells for 2 hours at 37 °C. After washing in PBS, secondary antibodies were diluted 1 : 1000 in 5% goat serum/PBS and introduced for 45 minutes at room temperature. Nuclei were stained with Hoechst stain (Invitrogen) diluted 1 : 1000/PBS for 10 minutes at 37 °C. Each sample was imaged 3 times by fluorescence microscopy at 100× magnification, for a total of 9 samples per experimental group. Neuron culture images were analysed using a NeuriteTracer ImageJ plugin.30 Astrocyte cultures were analysed using a custom MatLab script. Astrocyte images were treated by a binary threshold method to determine area coverage. Nuclei were counted manually.
Statistics
Unless otherwise stated, statistical significance was determined by the analysis of variance (ANOVA) followed by Tukey’s post hoc test. The statistical significance of WCA values was determined by two-way Student’s t-test of unequal variance. Ellipsometry roughness was compared using a paired t-test. The threshold of statistical significance was set to p < 0.05.
Results and discussion
Nanoparticle fabrication
Surface modification using nanoparticles may greatly increase the surface area available for surface immobilization of proteins and other bioactive compounds. Ideally, the nanoparticles used for modification should be able to bind strongly to both the substrate and the desired immobilizable compound. For biologically interfacing devices, the particles must not produce toxic degradation products or present other biocompatibility issues. Thiol modified silica nanoparticles were chosen due to their ability to be easily crosslinked to amine containing compounds through a GMBS linker, to attack epoxides present on the substrate surface, and to bind to each other through di-sulphide bonds. Furthermore, silica nanoparticles are non-toxic, commonly being employed in drug delivery applications.12 The resulting surface modification of TNPs endows them with a biocompatible and complex 3-dimensional structure.
Thiol functionalized nanoparticles were synthesized by a sol–gel process and characterized by TEM and DLS (Fig. S1, ESI†). Determination of individual nanoparticle diameters by DLS was achieved in the presence of TCEP, a reducing agent that can react with disulphides and prevent the nanoparticles from aggregating. Synthesized TNPs have a peak hydrodynamic diameter of approximately 50 nm, with a median hydrodynamic diameter approaching 60 nm. A polydispersity index of 0.161 indicates a narrow particle size distribution. In the absence of TCEP, the thiol groups are free to form di-sulphide bonds, and the particles aggregate together into large clusters, as shown in the TEM images and DLS of the unreduced TNP samples (Fig. S1A and C, ESI†). Unreduced TNPs have a greater peak diameter of 164 nm as well as a larger range of diameter values. Ellman’s reagent was used to measure the surface thiol concentration of the TNP. A 0.2 mg sample of nanoparticles resulted in an equivalent colour change to 12.4 nmol of MTS, which was used in combination with the size and surface area data from DLS to calculate and determine the surface thiol concentration of one thiol per 2.8 nm2. Assuming uniformity, this high thiol density should result in more than enough binding sites for L1 and laminin.
Surface modification
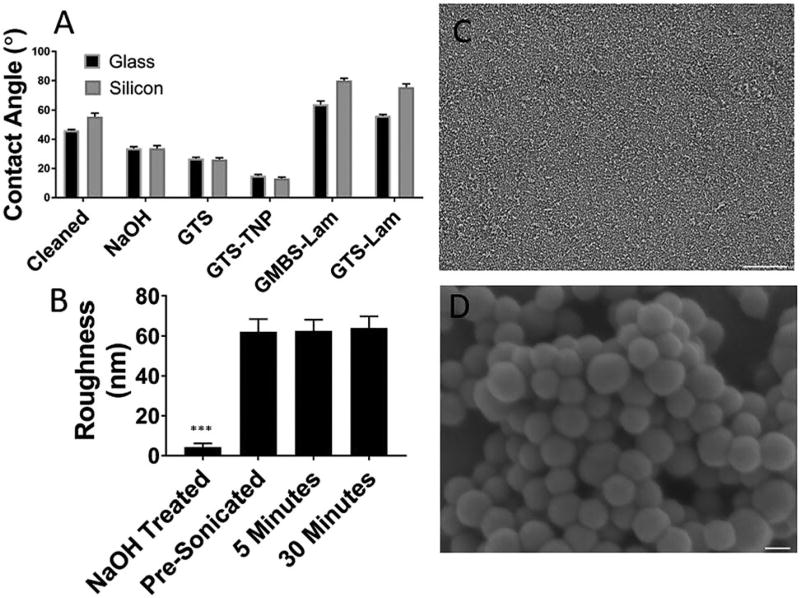
Surface modification was tracked stepwise by water contact angle (Fig. 1A) measurements. GTS was chosen as a base silane because of its ability to directly interact with proteins and the TNP. This should form an active base layer, where any portion of the surface not modified by the TNP can still be used for immobilization. As expected, both NaOH treatment and GTS functionalization caused a decrease in the water contact angle. Direct binding of hydrophobic laminin to the GTS-modified surface increased the water contact angle, indicating that proteins can bind to the epoxide-modified surface. Protein binding to the surface of the GTS-modified substrates is likely to occur due to combination of slower amine–epoxide interactions, and faster carboxylic acid–epoxide interactions.31
Fig. 1.
Surface modification and stability of nanoparticle coatings. (A) WCA demonstrating the stepwise reaction on glass and silicon substrates. All changes are significant (p < 0.05). (B) Surface roughness of the NaOH cleaned silicon substrate measured by ellipsometry in comparison to TNP coated surface both before and after sonication. (C) SEM of TNP coated substrates, scale bar 100 mm. (D) Magnified image of (C). Large clusters of nanoparticles are visible on the surface, scale bar = 50 nm. (***p < 0.001).
TNPs were bound to the GTS-modified surface through sodium carbonate catalysed epoxide ring opening by the thiols present on the TNP. Binding of TNPs to the surface of the GTS-modified glass further decreased the contact angle. This decrease may be attributed to changes in surface roughness and/or potentially further etching of the glass/silicon by the sodium carbonate catalyst. Finally, laminin immobilization on the surface of the GMBS linked TNP modified surface increased the water contact angle. All changes were significant (p < 0.05) compared to the prior measurement. Changes in surface roughness were tracked by ellipsometry (Fig. 1B). Substrates were limited to silicon with native oxide to minimize the initial roughness. A large increase in surface roughness was observed after the immobilization of TNPs on the surface, indicating an increase in surface features and the resulting surface area. The surface roughness of the TNP was also determined to be 58 nm by AFM (Fig. 3E) with a standard deviation of 6 nm, similar to the ellipsometry results. To investigate the stability of TNP coating, samples were subjected to 5 and 30 minutes of sonication. Examining the surfaces through ellipsometry (Fig. 1B) and SEM (Fig. S2, ESI†) revealed no substantial changes in surface roughness after sonication, displaying a strong adhesion between the TNP and the GTS modified surface. For chronic neural implant applications, adhesion of coatings is of great importance. Sonication has been used to test the adhesion of the neural electrode coating such as Pt black.32 The fact that the TNP coating is capable of withstanding sonication is a promising result. Surface adhesion of the TNP was further characterized by a scotch tape assay (Fig. S3, ESI†). Precleaned TNP coated silicon did no leave a detectable residue on the scotch tape, again showing the strong interaction between the TNP and the epoxide coating at forces larger than those experienced during implantation. However, to fully understand the chronic stability, future in vivo studies should be conducted to examine the mechanical and chemical stability of the immobilized nanoparticles during extended implantation periods.
Fig. 3.
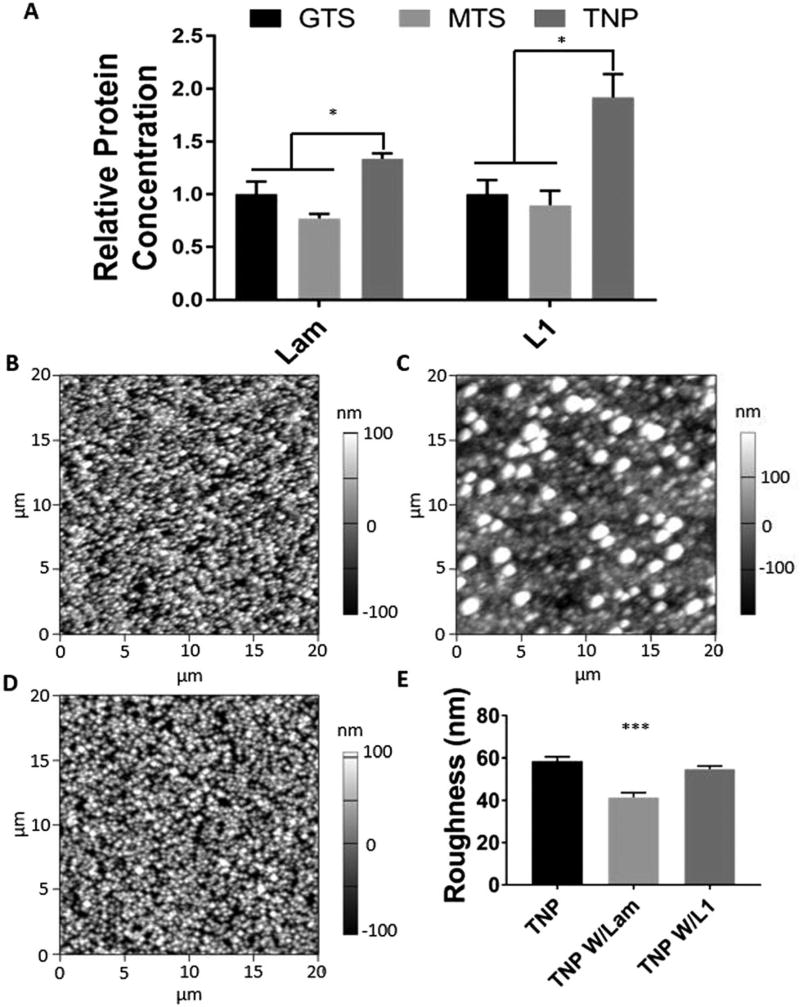
(A) Relative protein concentration bound to substrates as determined by UV spectroscopy of the digested solution measure at 280 nm. (B–D) AFM images of bare, laminin, and L1 modified TNP coated substrates, respectively. Left and bottom scaled in µm, height scaled in nm. (E) Surface roughness as determined by AFM. (*p < 0.05, ***p < 0.001).
Antioxidant immobilization
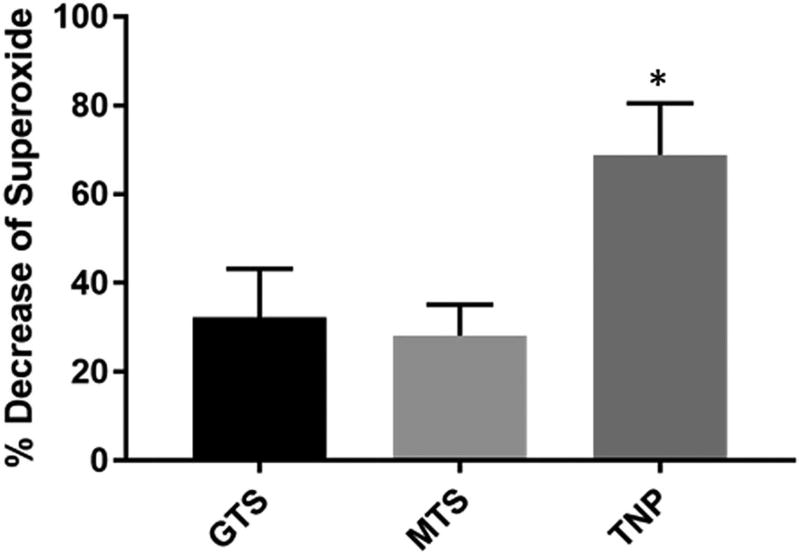
Previous reports have suggested that both free33,34 and immobilized22 antioxidants decrease inflammation and improve neuronal health at the site of implantation. Increasing the surface antioxidant concentration and antioxidant activity may allow for a greater effect of immobilized antioxidant coatings. To examine the effect of surface roughening on antioxidant attachment, the antioxidant iSODm was bound to the surface of GTS, MTS and TNP modified substrates. As expected, all iSODm modified substrates demonstrated superoxide scavenging capacity, with control bare glass samples serving as a 0% reference (Fig. 2). The superoxide scavenging activity of the iSODm bound to roughened TNP modified substrates is approximately 145% and 113% greater than that bound to MTS and GTS smooth substrates respectively (Fig. 2). This large increase in antioxidant activity is likely due to the respective increase in the amount of the bound iSODm, as the small size of the iSODm allows the antioxidant to take full advantage of the surface features presented by nanoparticle roughening. The observed increase in activity of the immobilized antioxidant on roughened surfaces will likely further decrease the reactive oxygen and nitrogen species released in response to an implanted neural device, thereby reducing the damage to both the host tissues and the device itself. Additionally, these results may likely translate to applications where catalyst immobilization is involved such as blood deoxygenation and chemical sensing.
Fig. 2.
Relative decrease in superoxide level determined by the oxidation of cytochrome-c, measured at 550 nm. Superoxide was produced by xanthine oxidase in the presence xanthine over GTS, MTS and TNP modified glass coverslips with immobilized antioxidant iSODm. Percent decrease of superoxide from control where cytochrome-c was oxidized over bare glass is shown (*p < 0.05).
Surface density of immobilized proteins
We hypothesized that nanoparticle surface modification increases the protein quantity bound to the surface of TNP-modified substrates compared to the smooth controls. Protein attachment was quantified by digesting the protein off the surfaces and the absorbance of the digested solution was analysed at 280 nm (Fig. 3A). The absorbance values are normalized to the control GTS surface without the protein. The digested solution from the nanoparticle coated surface exhibited significantly a higher absorbance compared to either smooth surfaces (GTS and MTS), indicating that the surface density of the immobilized protein was significantly higher on the TNP-coated substrates for both laminin and L1. Notably, there was a substantial difference in the percent increase in protein attachment of laminin and L1 to TNP roughened surfaces, with L1 concentrations increasing by 90% while laminin concentrations increased by 30%. This discrepancy may be due to the differences in the relative size of the proteins. While L1 is a protein with a mass of 220 kDa,35 laminin is a much larger molecule composed of 3 separate chains with a total molar mass of 810 kDa.36 Being the larger protein, laminin occupies a greater area and volume, likely decreasing its ability to take advantage of the smaller features of the nanoparticle-modified surface. Laminin also tends to form polymerized structures,37 further increasing its relative size. As such, the relationship between the size of the immobilized molecule and the textural features has a large impact on the efficacy of roughening prior to immobilization.
AFM was used to examine the changes in surface roughness after protein immobilization (Fig. 3B–E). Three TNP-modified samples, bare, with laminin, or with L1, were examined. The surface of the bare TNP sample and the laminin-coated sample exhibits stark differences in surface topography, with a quantifiable decrease in the surface roughness after laminin immobilization from 58 nm to 41 nm (Fig. 3E). The L1-coated surface did not appear to be significantly altered, and the decrease in surface roughness was not significant. These results support the previous finding that laminin is less capable of utilizing the increased surface area presented by nanoparticle immobilization, potentially due to the large scale polymerization of the protein over the surface.
Given these findings, it is reasonable to assume that decreasing the diameter of these particles would likely exaggerate these differences until particles become so small that neither protein is able to make the effective use of the textural features. In addition, there is a direct relationship between the size of the immobilized particle and the resulting footprint of the device upon implantation. For neural interfacing devices, small changes in the width of the device may have pronounced effects on the resulting inflammatory response, while other applications are likely more robust to the changes in device/particle size. While not directly investigated in this work, there may be value in optimizing the size of the nanoparticle based layer to best fit the application and the protein of choice.
Bioactivity of the immobilized proteins
Neuronal loss and glia scarring around a neural implant is one of the most common responses to neural interfacing devices. A protein coating which is capable of inhibiting the formation of this scar, either by promoting neural health or by minimizing astrocyte activation and spreading, has potential to increase chronic recording viability. Laminin and L1 are two proteins that have been shown to promote neurite outgrowth in vitro and have been used to reduce the inflammatory response to implantation in vivo.10,11 The biological effects of the surface roughening and protein immobilization were examined with cultures of primary neurons and astrocytes. Fig. 4 displays the outgrowth of neuron cultures on bare and protein modified substrates. Two smooth surfaces (GTS and MTS) and a nanoparticle-modified surface (TNP) were used to evaluate the bioactivity of laminin and L1 immobilized to roughened substrates. Both laminin and L1 bound to the GTS modified surfaces show an increase in the neurite outgrowth when compared to bare controls (Fig. 4J), indicating that the GTS modified surface is capable of coupling to proteins while retaining their bioactivity. GTS is used to modify the surface prior to TNP attachment (Scheme 1), and the ability of GTS modified substrates to increase neurite outgrowth with immobilized proteins allows any substrate area that is not covered by the TNP modification to produce a bioactive coating. This may fill in any holes resulting from incomplete nanoparticle coverage, as well as produce an active base layer where small molecules may be able to bind.
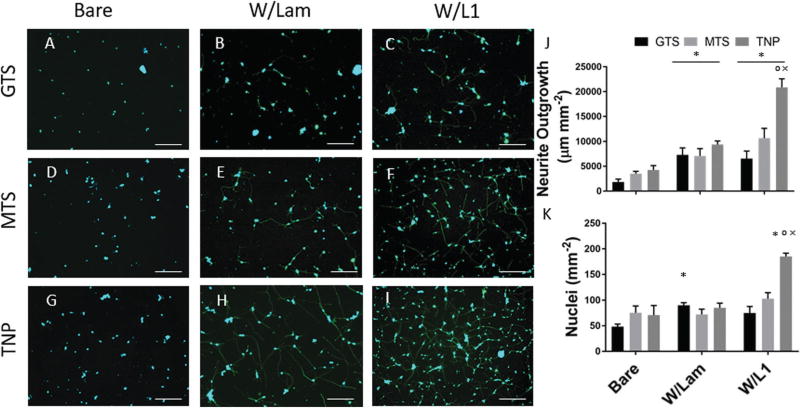
Fig. 4.
(A)–(I) Representative images of neurons grown on different substrates. Stained for Beta-III-Tubulin (green) and DAPI (blue), scale bar = 100 µm. (J) Total neurite outgrowth per mm2. (K) Nuclei counts per mm2. (*p < 0.05 compared to bare, °p < 0.05 compared to laminin, ×p < 0.05 compared to respective protein coated smooth surface).
MTS and TNP surfaces present the same reactive thiol group at the surface and utilize the same chemistry for immobilization on a smooth and nanoparticle roughened surface, respectively. After protein immobilization, smooth GTS and MTS surfaces with immobilized laminin and L1 both exhibited significant increases in neurite outgrowth when compared to their respective bare samples. Consistent with previous findings, L1 and laminin retained their bioactivity after immobilization.26 We expect that by increasing the concentration of proteins bound to the surface, there will be a resulting increase in neurite outgrowth, and nanoparticle modified substrates have shown to increase the amount of bound protein (Fig. 3A). Consequently, TNP-modified surfaces show a significant increase in neurite outgrowth compared to the smooth MTS modified substrates (Fig. 4J). Furthermore, TNP W/L1 surfaces showed the highest number of neuronal nuclei among all groups. Increases in the number of neurons attached may be attributed to (1) improved neuronal survival and/or (2) increased neurite extension resulting in a more stable attachment capable of surviving after the multiple washing steps involved in cell staining because of the higher surface density of L1 for the cells to interact with.
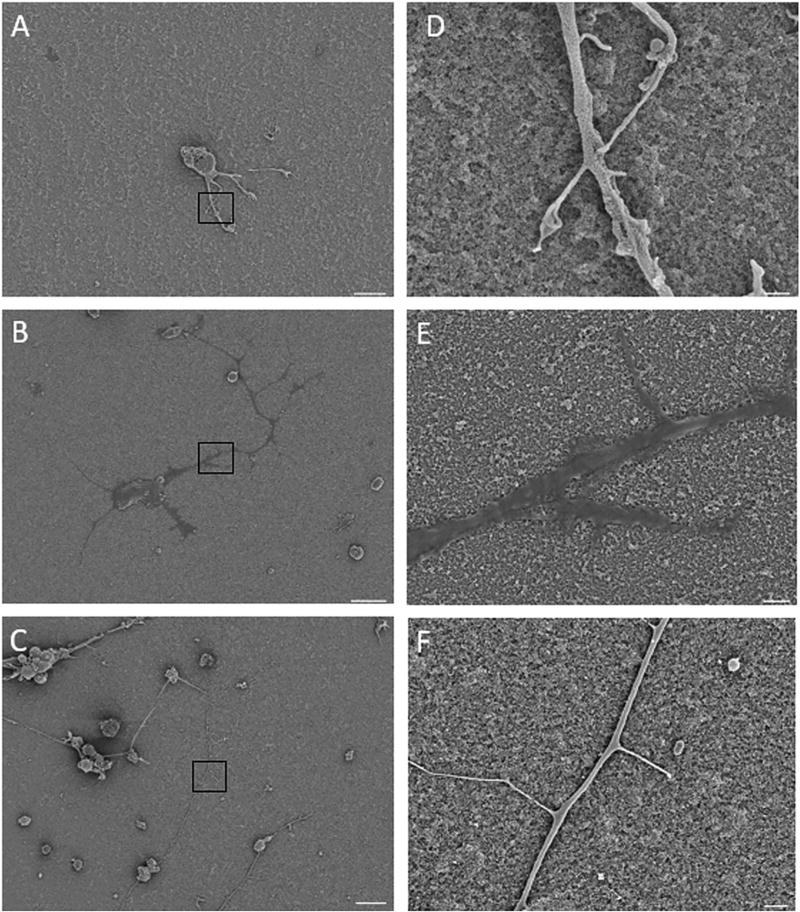
Examining the SEM images of the primary neuron cultures (Fig. 5A–F) reveals differences in surface topography and neuron morphology. Magnified images in Fig. 5D–F show a decrease in the roughness after protein immobilization, as shown by AFM (Fig. 3B–E). The roughness and standard deviation of bare, laminin and L1 coated TNP samples were determined to be 58 (5.9), 41 (6.4), and 54 (4.2) nm by AFM, and the values for laminin are significantly lower than those of other samples. As previously stated, this may be attributed to the smaller size of the L1 in comparison to laminin, as well as the nature of laminin to form large superstructures. The shape of the neurites is also noticeably different on laminin and L1 coated TNP substrates. Qualitatively, cells grown on laminin coated TNP substrates show flattened neurites, whereas cells grown on L1 coated TNP substrates showed a more 3-dimensional shape for the neurites. In addition, there appears to be a heightened degree of neurite sprouting from the larger processes of neurons grown on L1 adhered substrates.
Fig. 5.
SEM images of neurons grown of TNP roughened surfaces. (A–C) are bare, laminin, and L1 modified TNP coated substrates, respectively, scale bar = 10 µm. (D–F) and are magnified regions of A–C, respectively, scale bar = 1 µm.
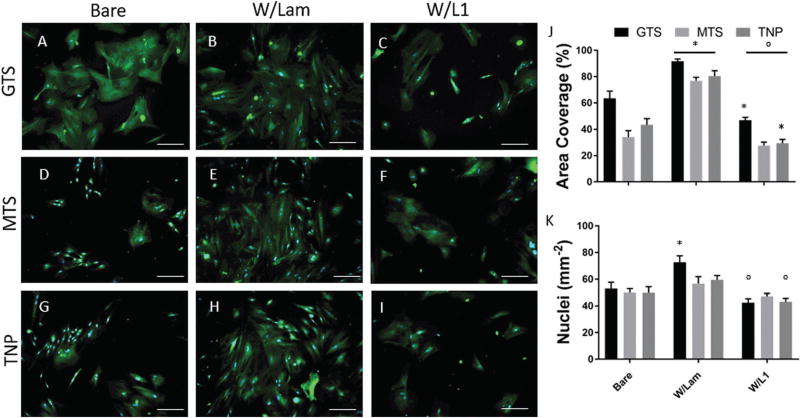
Astrocyte activation and adhesion may be indicative of in vivo astrocytic scar formation. Here we test the effect of two different protein coatings and roughening on the spreading of primary astrocyte cells. As in the primary neuron cultures, astrocytes were grown on bare or protein modified substrates, with two smooth (GTS and MTS) and 1 roughened (TNP) surface. Regardless of roughening, astrocyte outgrowth was significantly higher for groups coated with laminin compared to bare or L1 coated samples (Fig. 6J).
Fig. 6.
(A)–(I) Representative images of astrocytes grown on different substrates. Stained for GFAP (green) and DAPI (blue), scale bar = 100 µm. (J) Area covered. (K) Nuclei counts per mm2. (*p < 0.05 compared to bare, °p < 0.05 compared to laminin).
L1-modified groups show the opposite trend, as all L1 modified substrates show a significantly lower astrocyte spreading compared to laminin groups, and both GTS and TNP groups with L1 exhibit a lower astrocyte spreading than their respective bare samples. This is consistent with previous findings for L1 and laminin coated smooth surfaces.26,38 Laminin is a non-specific extracellular matrix protein that promotes the attachment of multiple cell types including astrocytes, while L1 selectively promotes neurons38 and inhibits astrocyte proliferation and astrocytic differentiation from neural stem cells.39 The behaviours of neurons and astrocytes on L1 vs. laminin immobilized TNP surfaces are consistent with their known biological properties, which is an important criterion for surface immobilization. Likewise, astrocyte nuclei counts were significantly lower in 2 groups where L1 was coupled to the surface compared to laminin, while they were higher in one of the laminin coated groups (Fig. 6K).
Compared to surface chemistry matched MTS, TNP surfaces did not produce a significant change in astrocyte spreading and adhesion, with or without bound L1. This suggests that the modified surface roughness does not increase glial attachment to the substrate. This result is different from previous reports which showed that silicon with nano-scale etched ridges reduced astrocyte proliferation and GFAP expression in vitro when compared to smooth substrates,19 but it is difficult to compare different methods of topographical surface modification directly without accounting for the geometry of the texture or changes in surface chemistry. We find that roughening prior to protein adhesion is most promising for L1-coated samples. Importantly, the ability of TNP W/L1 surfaces to further increase neuron outgrowth without increasing astrocyte activation is a promising property for in vivo implementation, where the beneficial survival signalling and neural attachment may increase without negatively impacting glial activation.
Conclusions
Thiolated nanoparticles were successfully immobilized onto glass and silicon substrates via silane chemistry. This coating was stable under mechanical perturbation. Binding a small molecule antioxidant to the TNP functionalized surface increased the antioxidant activity by approximately 145% compared to the surface chemistry matched smooth surfaces. Roughening the surface with TNP also increased the surface density of proteins. The L1 protein binding was increased by 90% and laminin by 30%; this discrepancy may be due to the size of the bound molecules relative to the size of the surface features. L1 bound TNP surfaces greatly increased the neurite outgrowth compared to L1 bound smooth surfaces, while TNP roughening was less effective upon increasing the neuronal response to laminin. Furthermore, L1 bound TNP surfaces inhibited astrocyte attachment and growth, an effect observed previously for L1 smooth surfaces. On the other hand, laminin increases astrocyte attachment and growth, due to its non-specific cell adhesion promoting nature. These results confirm that the surface immobilization of bioactive molecules on the TNP surface preserved their specific biological function. Taken together, roughening surfaces with TNP effectively increased the surface density and the effect of bound molecules of different sizes. These results are not limited to neural devices. Surface modifications implemented in chemical sensing, device/tissue integration, and catalytic coatings may also benefit from this strategy.
Supplementary Material
Acknowledgments
This project was financially supported by the National Institute of Health, NINDS Grants (R01NS062019 and R01NS089688). We thank Professor Lei Li in the Chemical Engineering Department at the University of Pittsburgh for the use of WCA and ellipsometry instruments.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8tb00408k
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Hårdhammar PA, van Beusekom HMM, Emanuelsson HU, Hofma SH, Albertsson PA, Verdouw PD, Boersma E, Serruys PW, van der Giessen WJ. Reduction in Thrombotic Events With Heparin-Coated Palmaz-Schatz Stents in Normal Porcine Coronary Arteries. Circulation. 1996;93(3):423–430. doi: 10.1161/01.cir.93.3.423. [DOI] [PubMed] [Google Scholar]
- 2.Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong J, Wu J, Liang W, Chen L, Zhao Y, Chen B, Dai J. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials. 2010;31(35):9212–9220. doi: 10.1016/j.biomaterials.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 3.Taylor IM, Du Z, Bigelow ET, Eles JR, Horner AR, Catt KA, Weber SG, Jamieson BG, Cui XT. Aptamer-functionalized neural recording electrodes for the direct measurement of cocaine in vivo. J. Mater. Chem. B. 2017;5(13):2445–2458. doi: 10.1039/C7TB00095B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foulds NC, Lowe CR. Enzyme entrapment in electrically conducting polymers. Immobilisation of glucose oxidase in polypyrrole and its application in amperometric glucose sensors. J. Chem. Soc., Faraday Trans. 1. 1986;82(4):1259–1264. [Google Scholar]
- 5.Kang X, Wang J, Wu H, Aksay IA, Liu J, Lin Y. Glucose Oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009;25(4):901–905. doi: 10.1016/j.bios.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Conroy PJ, Hearty S, Leonard P, O’Kennedy RJ. Antibody production, design and use for biosensor-based applications. Semin. Cell Dev. Biol. 2009;20(1):10–26. doi: 10.1016/j.semcdb.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Arazawa DT, Kimmel JD, Federspiel WJ. Kinetics of CO2 exchange with carbonic anhydrase immobilized on fiber membranes in artificial lungs. J. Mater. Sci.: Mater. Med. 2015;26(6):193. doi: 10.1007/s10856-015-5525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimmel JD, Arazawa DT, Ye SH, Shankarraman V, Wagner WR, Federspiel WJ. Carbonic anhydrase immobilized on hollow fiber membranes using glutaraldehyde activated chitosan for artificial lung applications. J. Mater. Sci.: Mater. Med. 2013;24(11):2611–2621. doi: 10.1007/s10856-013-5006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, Endo K, Shimizu Y. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)–collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000;868(2):315–328. doi: 10.1016/s0006-8993(00)02207-1. [DOI] [PubMed] [Google Scholar]
- 10.Azemi E, Lagenaur CF, Cui XT. The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface. Biomaterials. 2011;32(3):681–692. doi: 10.1016/j.biomaterials.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei H, George CM, Ravi VB. Nanoscale laminin coating modulates cortical scarring response around implanted silicon microelectrode arrays. J. Neural Eng. 2006;3(4):316. doi: 10.1088/1741-2560/3/4/009. [DOI] [PubMed] [Google Scholar]
- 12.Slowing II, Vivero-Escoto JL, Wu C-W, Lin VSY. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Delivery Rev. 2008;60(11):1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Lin C-Y, Li W-P, Huang S-P, Yeh C-S, Yang C-M. Hollow mesoporous silica nanosphere-supported FePt nanoparticles for potential theranostic applications. J. Mater. Chem. B. 2017;5:7598–7607. doi: 10.1039/c7tb01812f. [DOI] [PubMed] [Google Scholar]
- 14.Lee C-H, Cheng S-H, Huang IP, Souris JS, Yang C-S, Mou C-Y, Lo L-W. Intracellular pH-Responsive Mesoporous Silica Nanoparticles for the Controlled Release of Anticancer Chemotherapeutics. Angew. Chem. 2010;122(44):8390–8395. doi: 10.1002/anie.201002639. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Lee Y, Youn JK, Na HB, Yu T, Kim H, Lee S-M, Koo Y-M, Kwak JH, Park HG, Chang HN, Hwang M, Park J-G, Kim J, Hyeon T. Simple Synthesis of Functionalized Superparamagnetic Magnetite/Silica Core/Shell Nanoparticles and their Application as Magnetically Separable High-Performance Biocatalysts. Small. 2008;4(1):143–152. doi: 10.1002/smll.200700456. [DOI] [PubMed] [Google Scholar]
- 16.Lai C-Y, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VSY. A Mesoporous Silica Nanosphere-Based Carrier System with Chemically Removable CdS Nanoparticle Caps for Stimuli-Responsive Controlled Release of Neurotransmitters and Drug Molecules. J. Am. Chem. Soc. 2003;125(15):4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Wu P, He Z, He H, Rong W, Li J, Zhou D, Huang Y. Mesoporous silica nanoparticles with lactose-mediated targeting effect to deliver platinum(IV) prodrug for liver cancer therapy. J. Mater. Chem. B. 2017;5:7591–7597. doi: 10.1039/c7tb01704a. [DOI] [PubMed] [Google Scholar]
- 18.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods. 2005;148(1):1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Ereifej ES, Matthew HW, Newaz G, Mukhopadhyay A, Auner G, Salakhutdinov I, VandeVord PJ. Nanopatterning effects on astrocyte reactivity. J. Biomed. Mater. Res., Part A. 2013;101A(6):1743–1757. doi: 10.1002/jbm.a.34480. [DOI] [PubMed] [Google Scholar]
- 20.Miller C, Jeftinija S, Mallapragada S. Synergistic effects of physical and chemical guidance cues on neurite alignment and outgrowth on biodegradable polymer substrates. Tissue Eng. 2004;8(3):367–378. doi: 10.1089/107632702760184646. [DOI] [PubMed] [Google Scholar]
- 21.Xie J, MacEwan MR, Li X, Sakiyama-Elbert SE, Xia Y. Neurite Outgrowth on Nanofiber Scaffolds with Different Orders, Structures, and Surface Properties. ACS Nano. 2009;3(5):1151–1159. doi: 10.1021/nn900070z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potter-Baker KA, Nguyen JK, Kovach KM, Gitomer MM, Srail TW, Stewart WG, Skousen JL, Capadona JR. Development of superoxide dismutase mimetic surfaces to reduce accumulation of reactive oxygen species for neural interfacing applications. J. Mater. Chem. B. 2014;2(16):2248–2258. doi: 10.1039/C4TB00125G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder N, Cui XT. Doctoral dissertation. University of Pittsburgh, Department of Bioengineering; 2015. Novel methods for the prevention of neurodegeneration around neural implants. [Google Scholar]
- 24.Stauffer WR, Cui XT. Polypyrrole doped with 2 peptide sequences from laminin. Biomaterials. 2006;27(11):2405–2413. doi: 10.1016/j.biomaterials.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Cui X, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, Martin DC. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. 2001;56:261–272. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Azemi E, Stauffer WR, Gostock MS, Lagenaur CF, Cui XT. Surface immobilization of neural adhesion molecule L1 for improving the biocompatibility of chronic neural probes: In vitro characterization. Acta Biomater. 2008;4(5):1208–1217. doi: 10.1016/j.actbio.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 27.Eles JR, Vazquez AL, Snyder NR, Lagenaur C, Murphy MC, Kozai TDY, Cui XT. Neuroadhesive L1 coating attenuates acute microglial attachment to neural electrodes as revealed by live two-photon microscopy. Biomaterials. 2017;113:279–292. doi: 10.1016/j.biomaterials.2016.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proc. Natl. Acad. Sci. U. S. A. 1987;84(21):7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvemini D, Muscoli C, Riley DP, Cuzzocrea S. Superoxide Dismutase Mimetics. Pulm. Pharmacol. Ther. 2002;15(5):439–447. doi: 10.1006/pupt.2002.0374. [DOI] [PubMed] [Google Scholar]
- 30.Pool M, Thiemann J, Bar-Or A, Fournier AE. NeuriteTracer: A novel ImageJ plugin for automated quantification of neurite outgrowth. J. Neurosci. Methods. 2008;168(1):134–139. doi: 10.1016/j.jneumeth.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 31.Orr CA, Cernohous JJ, Guegan P, Hirao A, Jeon HK, Macosko CW. Homogeneous reactive coupling of terminally functional polymers. Polymer. 2001;42(19):8171–8178. [Google Scholar]
- 32.Raeyoung K, Yoonkey N. Electrochemical layer-by-layer approach to fabricate mechanically stable platinum black microelectrodes using a mussel-inspired polydopamine adhesive. J. Neural Eng. 2015;12(2):026010. doi: 10.1088/1741-2560/12/2/026010. [DOI] [PubMed] [Google Scholar]
- 33.Kelsey AP-B, Wade GS, William HT, Chun TW, William DM, Nicholas PZ, Jeffrey RC. Implications of chronic daily anti-oxidant administration on the inflammatory response to intracortical microelectrodes. J. Neural Eng. 2015;12(4):046002. doi: 10.1088/1741-2560/12/4/046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozai TDY, Jaquins-Gerstl AS, Vazquez AL, Michael AC, Cui XT. Dexamethasone retrodialysis attenuates microglial response to implanted probes in vivo. Biomaterials. 2016;87(Supplement C):157–169. doi: 10.1016/j.biomaterials.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Galileo DS. Soluble L1CAM promotes breast cancer cell adhesion and migration in vitro, but not invasion. Cancer Cell Int. 2010;10(1):34. doi: 10.1186/1475-2867-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin-a glycoprotein from basement membranes. J. Biol. Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- 37.Yurchenco PD, Tsi-library EC, Charonis AS, Furthermayr H. Laminin Polymerization in Vitro. J. Biol. Chem. 1985;260(12):7636–7644. [PubMed] [Google Scholar]
- 38.Webb K, Budko E, Neuberger TJ, Chen S, Schachner M, Tresco PA. Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and fibroblasts. Biomaterials. 2001;22(10):1017–1028. doi: 10.1016/s0142-9612(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 39.Dihne M, Bernreuther C, Sibbe M, Paulus W, Schachner M. A new role for the cell adhesion molecule L1 in neural precursor cell proliferation, differentiation, and transmitter-specific subtype generation. J. Neurosci. 2003;23(16):6638–6650. doi: 10.1523/JNEUROSCI.23-16-06638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.