Supplemental Digital Content is available in the text
Keywords: acute pancreatitis in pregnancy (APIP), gamma-glutamyl transpeptidase (GGT), high-density lipoprotein (HDL), logistic regression analyses, neutrophil/lymphocyte (N/L) ratio
Abstract
Acute pancreatitis in pregnancy (APIP) is a rare but dangerous complication. APIP has common symptoms with acute abdomen. Assessment of an acute abdomen is more complicated during pregnancy because the gravid uterus could mask most of symptomatic signs. It has been a challenge to diagnose APIP by physical examination or diagnostic imaging. Case studies on APIP are also limited for analysis on the risk factors associated with the disease. This retrospective study evaluated a series of risk factors from a relatively substantial number of APIP cases to determine early predictors or prognosis markers for APIP.
Fifty-nine APIP patients together with 179 random normal pregnant women in Shengjing Affiliated Hospital of China Medical University were included for this retrospective study. Medical parameters of blood test in biochemistry and hematology were compared between 2 groups using t test. Multivariate logistic regression analysis was performed to investigate the relationship between various factors and APIP using Statistical Applied Software (SAS student version).
Compared with normal pregnant women, APIP patients have elevated values in alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen, creatinine, C-reactive protein, direct bilirubin, fibrin degradation products, gamma-glutamyl transpeptidase (GGT), glucose, lipase, pH and decreased values in albumin, fibrinogen, high-density lipoprotein (HDL), hemoglobin, low-density lipoprotein cholesterol (LDL-D), and total proteins from their blood tests. In addition, APIP patients have decreased numbers in red cells but increased numbers in white blood cells and increased ratio of neutrophil/lymphocyte (N/L). Among these factors, N/LR, GGT, lipase, and HDL are significantly associated with APIP. This study suggests that the combination of those factors serve as a panel of indicators for early-onset prognosis of APIP.
GGT, lipase, HDL, and N/LR can serve as a panel of factors to predict APIP. More case studies are important to further evaluate the predicting power of this panel factors in APIP.
1. Introduction
Acute pancreatitis in pregnancy (APIP) is a rare but dangerous disease.[1] APIP usually occurs during the third trimester or the early postpartum period. Its incidence varies approximately from 1 per 1000 to 12,000 pregnancies depending on medical history. The common cause of the disease is related with gallstones, hyperlipidemia,[2] or alcohol consumption.[3,4] Although the incidence of APIP has increased for undetermined conditions during the past 2 decades,[5] the reported case studies are still limited.
APIP causes a high incidence of maternal morbidity and neonatal death after premature birth. It has a rapid onset and progression.[6] The prompt diagnosis and treatment is critical for APIP patients. APIP is one of the diseases associated with the acute abdomen. Examination of an acute abdomen could be complicated during pregnancy because the enlarged uterus may mask diagnostic indicators. Thus, misdiagnosis for APIP patients is a serious issue for both maternal and fetal safety.[7] It remains a challenge to diagnose APIP by physical examination or diagnostic imaging.[8,9]
In China, APIP is also a challenging complication in terms of diagnosis and treatment. Clinical characteristics of APIP, including symptoms, mortality, and necessity of gestation termination were summarized based on 121 cases from the First Affiliated Hospital of Nanchang University.[10] Another study from 36 cases of APIP reported that hypertriglyceridemia was associated with poor outcomes of the disease.[11] The most recent study identified a correlation between fetal distress and fetal loss with APIP severity.[12] These studies reiterate the importance of precise diagnosis and treatment to decrease maternal and fetal mortality for APIP.
This retrospective study aimed to identify multiple risk factors associated with APIP based on 59 clinical cases of APIP from Shengjing Affiliated Hospital of China Medical University. This study compared medical parameters of blood test in biochemistry and hematology between normal pregnant women and APIP patients using t test. This study further conducted multivariate logistic regression analysis to investigate the relationship between numerous factors and APIP using Statistical Applied Software. Taken together, the present study applied the powerful statistical tool to the available case studies and identified a panel of indicators for early onset prognosis of APIP. These efforts could facilitate the development of prompt diagnosis and suitable treatment for APIP in China.
2. Methods
Patients with APIP admitted to emergency center of Shengjing Affiliated Hospital of China Medical University from 2013 to 2015 were studied retrospectively. A total of 59 APIP patients were monitored during the study period and 179 age-matched pregnant women were randomly selected as control. All patients in this study had data of blood test assessments in the record (Supplement Table 1). Patients who had missing blood test assessments in the record were excluded. As it was hard to obtain the laboratory assessment before patients were admitted to the hospital, case samples were only examined on the first days of hospitalization and follow-up samples were further measured to monitor the changes during the hospitalization. Pancreatitis was diagnosed by elevated pancreatic enzymes, correlated with clinical assessment and examinations. Serum levels of proteins or enzymes were measured using commercial medical assay kits.
Missing value was inputted by Markov chain Monte Carlo (MCMC) methods.[13] Analyses were 2-sided and a P value of <.05 was considered significant. Descriptive and univariate analyses were used to test each individual laboratory result and multivariable statistical analysis was used to identify predictors associated with APIP. Statistic LASSO selection was used to select significant risk factors.[14] Logistic regression analyses were performed to predict the relationship between different factors and APIP incidence using Statistical Applied Software (SAS student version).[15,16] A receiver operating characteristic (ROC) curve was constructed in order to assess the performance of the model.[17] The predicted model was then applied to the test samples to do cross-validation. The study was approved by the Committee on Ethics of Shengjing Affiliated Hospital of China Medical University. The informed consent was given by the patients who were enrolled in this study.
3. Results
3.1. Indicators associated with APIP
Pancreatitis was diagnosed by elevated circulating pancreatic enzymes.[3] Levels of enzymes or proteins were compared among all cases based on their blood tests (total 59 APIP patients and 179 normal pregnant women). According to the statistical analyses, values from APIP patients are significantly elevated in alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen, creatinine, C-reactive protein, direct bilirubin, D-dimer, fibrin degradation products, gamma-glutamyl transpeptidase (GGT), glucose, lipase, pH. Values from APIP patients are significantly dropped in albumin, fibrinogen, high-density lipoprotein (HDL), hemoglobin, low-density lipoprotein cholesterol (LDL-C), and total proteins (Table 1). In addition, values in hematology are also compared among all cases from their blood tests. Interestingly, APIP patients have significantly decreased numbers in red blood cells but increased neutrophil/lymphocyte (N/L) ratio and white blood cells (Table 2). Among those factors, some of them such as lipase, ALT, AST, and bilirubin have been considered as prognostic markers of AP by practicing physicians.[18] Serum C-reactive protein was known as a predictor for risk of vascular and gastrointestinal complications.[19,20] D-dimer was also considered as a marker of severity in patients with AP.[21]
Table 1.
Factors are significantly associated with APIP from blood test in biochemistry.

Table 2.
Factors are significantly associated with APIP from blood test in hematology.
3.2. Risk factors predicting APIP
To determine the predictive role of above variables listed in Tables 1 and 2, we performed LASSO (least absolute shrinkage and selection operator) selection and regularization to enhance the prediction accuracy of our statistical model. On the basis of the results from the LASSO selection, N/LR ratio, GGT, lipase, and HDL are significant predictors of APIP with odds ratio of 1.285, 1.075, 1.003, and 0.081, respectively. This can be interpreted as such: under any circumstances, if other factors keep same, for each 1-unit increase of N/LR, the likelihood of having APIP increases by relative 28.5%. Similarly, for each 1-unit increase of GGT, the likelihood of having APIP increases by relative 7.5%; for each 1-unit increase of lipase, the likelihood of having APIP increases by relative 0.3%; for each 1-unit decrease of HDL, the likelihood of having APIP increases by relative 91% (1/0.081).
In order to determine the predicted response of these factors while one of them is held constant, we constructed effect plots using the EFFECTPLOT statement through SAS.[15,16] Effect plots display the predicted response of those factors as a function of one of them is set as a constant. The plot in Fig. 1A shows that under a condition that N/LR is set constant as 5.396, if lipase is more than 500, GGT is less than 53.5, and HDL is less than 1.44, the predicted probability of APIP is more than 91%. Likewise, the plot in Fig. 1B shows that under a condition that lipase is set constant as 251.3, if GGT is more than 103, N/LR is more than 5.6, and HDL is less than 2.0, the predicted probability of APIP is more than 91%; the plot in Fig. 1C shows that under a condition that GGT is set constant as 19.47, if lipase is more than 473.1, N/LR is more than 5.6, and HDL is less than 0.5, the predicted probability of APIP is more than 91%; The plot in Fig. 1D shows that under a condition that HDL is set constant as 1.639, if GGT is more than 53.5, lipase is more than 500, and N/LR is more than 5.6, the predicted probability of APIP is more than 91%. This series of effect plots display curves of probability versus a continuous variable of predicted risk factors (HDL, Lipase, GTT, and N/LR) to create indicator values for risk factors (HDL, Lipase, GTT, and N/LR) and predict APIP.
Figure 1.
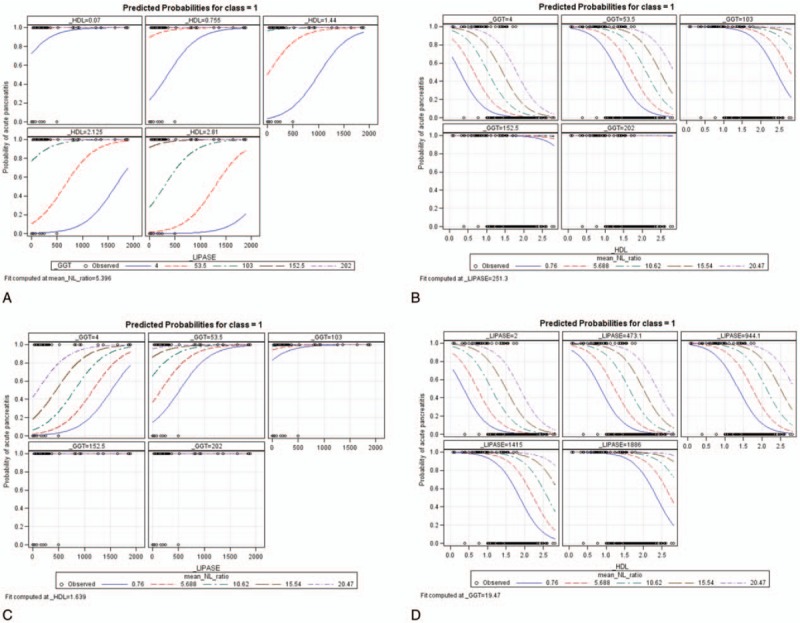
Effect plots of predicted probabilities of APIP upon continuous changes of 3 other variables if N/LR is fixed at 5.396 (A); if lipase levels are fixed at 251.3 (B); if GGT levels are fixed at 19.47 (C); if HDL was fixed at 1.639 (D).
The ROC curve was further computed to illustrate the performance of our model (Fig. 2). According to the ROC curve for the above 4-factor model, the value of AUC (area under the curve 0.914) is more than 90%, indicating that a combination of HDL, lipase, GTT, and N/LR can serve as a panel of predictors of APIP. The ROC curve for these individual factors, HDL, lipase, GTT, and N/LR has an AUC of 0.87, 0.51, 0.83, and 0.69, respectively. The comparison of those ROC curves indicates that HDL, lipase, GTT, and N/LR can be a panel of factors to predict APIP, which were superior to any individual factors.
Figure 2.
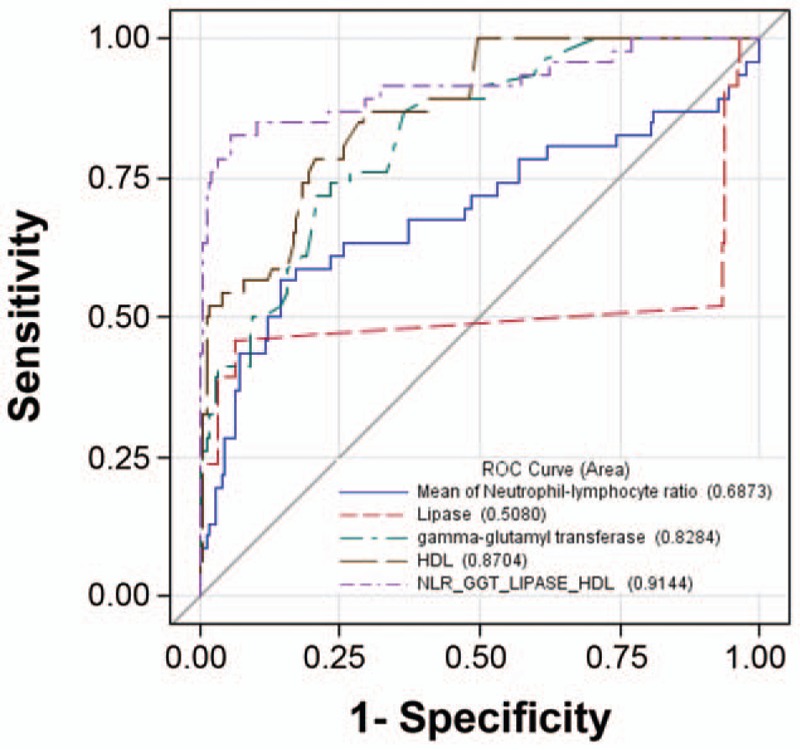
ROC curves of N/LR, lipase, GGT, HDL versus combining them as a panel to predict APIP.
4. Discussion
This retrospective study analyzed a relatively substantial number of APIP cases using statistical tools. A group of factors were found to be significantly associated APIP, including ALT, AST, blood urea nitrogen, creatinine, C-reactive protein, bilirubin, GGT, albumin, fibrinogen, HDL, and N/LR. Some of these factors were also reported in other AP or APIP cases. For example, hypoalbuminemia has been consistently observed in 130 patients with AP.[22] Levels of serum HDL cholesterol and LDL cholesterol are significantly lower in patients with severe AP.[23] Although our analysis could be far from exhaustive, it provides a relatively comprehensive array of biochemical diagnostic parameters to test APIP or AP from blood samples. In addition, our statistical model predicts that among those factors, N/LR ratio, GGT, lipase, and HDL as a panel could serve a predictive role for APIP.
The clinical diagnosis of APIP is based on 3 features: elevated serum amylase and lipase levels; severe abdominal pain; typical imaging features by computed tomography (CT) scan or magnetic resonance imaging (MRI).[24] Serum amylase levels were not included in our comparative studies because its levels were not available in normal pregnant women. That should not affect our analysis because it was reported before that amylase levels do not correlate with overall prognosis of AP.[1,25] Our model indicates that lipase is indeed one of factors in the predictive panel, which is aligned with the standard of the clinical diagnosis for APIP. However, lipase levels from blood test are not powerful enough to predict APIP. Addition of N/LR, GGT, and HDL to the panel including lipase significantly increases the power to predicate APIP. A recent study indicates that serum calcium level was negatively correlated with the severity of APIP. Unfortunately, serum calcium level was not considered as a parameter in our blood tests. Although our model is derived from APIP, given the similarity in the clinical diagnosis between AP and APIP, this panel of predicting factors could be also applied to AP. GGT and N/LR have already been considered as a predictor of adverse outcomes of AP and APIP.[25–28]
So far, there is no single blood test to predict APIP or AP in clinical practice.[29] On the basis of our model, this panel of factors from blood test could predict APIP with relatively high confidence. CT and MRI are essential for the clinical diagnosis of APIP, but the cost limits the accessibility of MRI in rural areas, especially in China.[30] Thus, a panel of parameters from blood test is more cost-effective for early diagnosis and classification of AP or APIP for clinical practice. This panel of factors are computed from 59 case studies to predict APIP. Due to the limited number of cases, it is important to further evaluate the predicting power of this panel factors in APIP with more case studies.
5. Conclusion
Taken together, we applied statistical modeling to 59 APIP case studies and found that GGT, lipase, HDL, and N/LR can serve as a panel of factors to predict APIP or AP. The predictive role of these factors in APIP or AP diagnosis needs to be further evaluated by practicing physicians in more clinical cases.
Author contributions
Conceptualization: Min Zhao.
Data curation: Yu Wang, Min Zhao, Shijie Cai.
Formal analysis: Shijie Cai.
Funding acquisition: Lichun Zhang.
Investigation: Jun Han.
Methodology: Haitao Shen.
Project administration: Haitao Shen.
Validation: Min Zhao, Shijie Cai.
Writing – original draft: Lichun Zhang, Shijie Cai.
Writing – review & editing: Lichun Zhang.
Supplementary Material
Footnotes
Abbreviations: ALT = alanine aminotransferase, APIP = acute pancreatitis in pregnancy, AST = aspartate aminotransferase, GGT = gamma-glutamyl transpeptidase, HDL = high-density lipoprotein, LDL-D = low-density lipoprotein cholesterol, MCMC = Markov chain Monte Carlo, N/L ration = neutrophil/lymphocyte ratio, ROC = receiver operating characteristic, SAS = Statistical Applied Software.
Funding/support: The work was supported internally by Shengjing Affiliated Hospital of China Medical University.
All authors have no conflict of interest or relationship to industry to disclose. Supplemental Digital Content is available for this article.
References
- [1].Abdullah B, Kathiresan Pillai T, Cheen LH, Ryan RJ. Severe acute pancreatitis in pregnancy. Case Rep Obstet Gynecol 2015;2015:239068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kayatas SE, Eser M, Cam C, et al. Acute pancreatitis associated with hypertriglyceridemia: a life-threatening complication. Arch Gynecol Obstet 2010;281:427–9. [DOI] [PubMed] [Google Scholar]
- [3].Windsor JA, Petrov MS. Acute pancreatitis reclassified. Gut 2013;62:4–5. [DOI] [PubMed] [Google Scholar]
- [4].Bouyou J, Gaujoux S, Marcellin L, et al. Abdominal emergencies during pregnancy. J Visc Surg 2015;152(6 suppl):S105–15. [DOI] [PubMed] [Google Scholar]
- [5].Jin J, Yu YH, Zhong M, et al. Analyzing and identifying risk factors for acute pancreatitis with different etiologies in pregnancy. J Matern Fetal Neonatal Med 2015;28:267–71. [DOI] [PubMed] [Google Scholar]
- [6].Tang SJ, Rodriguez-Frias E, Singh S, et al. Acute pancreatitis during pregnancy. Clin Gastroenterol Hepatol 2010;8:85–90. [DOI] [PubMed] [Google Scholar]
- [7].Vijayaraghavan G, Kurup D, Singh A. Imaging of acute abdomen and pelvis: common acute pathologies. Semin Roentgenol 2009;44:221–7. [DOI] [PubMed] [Google Scholar]
- [8].Ducarme G, Maire F, Chatel P, et al. Acute pancreatitis during pregnancy: a review. J Perinatol 2014;34:87–94. [DOI] [PubMed] [Google Scholar]
- [9].Lee CC, Chao AS, Chang YL, et al. Acute pancreatitis secondary to primary hyperparathyroidism in a postpartum patient: a case report and literature review. Taiwan J Obstet Gynecol 2014;53:252–5. [DOI] [PubMed] [Google Scholar]
- [10].Luo L, Zen H, Xu H, et al. Clinical characteristics of acute pancreatitis in pregnancy: experience based on 121 cases. Arch Gynecol Obstet 2018;297:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu Q, Wang S, Zhang Z. A 23-year, single-center, retrospective analysis of 36 cases of acute pancreatitis in pregnancy. Int J Gynaecol Obstet 2015;130:123–6. [DOI] [PubMed] [Google Scholar]
- [12].Tang M, Xu JM, Song SS, et al. What may cause fetus loss from acute pancreatitis in pregnancy: analysis of 54 cases. Medicine (Baltimore) 2018;97:e9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gurrin LC, Scurrah KJ, Hazelton ML. Tutorial in biostatistics: spline smoothing with linear mixed models. Stat Med 2005;24:3361–81. [DOI] [PubMed] [Google Scholar]
- [14].Leng C, Ma S. Path consistent model selection in additive risk model via Lasso. Stat Med 2007;26:3753–70. [DOI] [PubMed] [Google Scholar]
- [15].Jiang Y, He Y, Zhang H. Variable selection with prior information for generalized linear models via the prior LASSO method. J Am Stat Assoc 2016;111:355–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Penman AD, Johnson WD. A SAS program for calculating cumulative incidence of events (with confidence limits) and number at risk at specified time intervals with partially censored data. Comput Methods Programs Biomed 2008;89:50–5. [DOI] [PubMed] [Google Scholar]
- [17].Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract 2006;12:132–9. [DOI] [PubMed] [Google Scholar]
- [18].Kiriyama S, Gabata T, Takada T, et al. New diagnostic criteria of acute pancreatitis. J Hepatobiliary Pancreat Sci 2010;17:24–36. [DOI] [PubMed] [Google Scholar]
- [19].Hjalmarsson C, Stenflo J, Borgstrom A. Activated protein C-protein C inhibitor complex, activation peptide of carboxypeptidase B and C-reactive protein as predictors of severe acute pancreatitis. Pancreatology 2009;9:700–7. [DOI] [PubMed] [Google Scholar]
- [20].Digalakis MK, Katsoulis IE, Biliri K, et al. Serum profiles of C-reactive protein, interleukin-8, and tumor necrosis factor-alpha in patients with acute pancreatitis. HPB Surg 2009;2009:878490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ke L, Ni HB, Tong ZH, et al. D-dimer as a marker of severity in patients with severe acute pancreatitis. J Hepatobiliary Pancreat Sci 2012;19:259–65. [DOI] [PubMed] [Google Scholar]
- [22].Li HL, Jiang YH, Wei Y, et al. Clinical analysis of acute hyperlipidemic pancreatitis during pregnancy and postpartum period. Beijing Da Xue Xue Bao Yi Xue Ban 2014;46:125–9. [PubMed] [Google Scholar]
- [23].Khan J, Nordback I, Sand J. Serum lipid levels are associated with the severity of acute pancreatitis. Digestion 2013;87:223–8. [DOI] [PubMed] [Google Scholar]
- [24].Paramanathan A, Walsh SZ, Zhou J, et al. Laparoscopic cholecystectomy in pregnancy: an Australian retrospective cohort study. Int J Surg 2015;18:220–3. [DOI] [PubMed] [Google Scholar]
- [25].Talukdar R, Nageshwar Reddy D. Predictors of adverse outcomes in acute pancreatitis: new horizons. Indian J Gastroenterol 2013;32:143–51. [DOI] [PubMed] [Google Scholar]
- [26].Ilhan M, Ilhan G, Gok AF, et al. Evaluation of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and red blood cell distribution width-platelet ratio as early predictor of acute pancreatitis in pregnancy. J Matern Fetal Neonatal Med 2016;29:1476–80. [DOI] [PubMed] [Google Scholar]
- [27].Pienkowska J, Gwozdziewicz K, Skrobisz-Balandowska K, et al. Perfusion-CT: can we predict acute pancreatitis outcome within the first 24 hours from the onset of symptoms? PLoS One 2016;11:e0146965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Azab B, Jaglall N, Atallah JP, et al. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology 2011;11:445–52. [DOI] [PubMed] [Google Scholar]
- [29].Wu BU, Conwell DL. Update in acute pancreatitis. Curr Gastroenterol Rep 2010;12:83–90. [DOI] [PubMed] [Google Scholar]
- [30].Sang C, Wang S, Zhang Z, et al. Characteristics and outcome of severe preeclampsia/eclampsia concurrent with or complicated by acute pancreatitis: a report of five cases and literature review. J Matern Fetal Neonatal Med 2017;1–8. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


