Abstract
Mouse embryonic stem cells (ESCs) are pluripotent stem cells, which have the ability to differentiate into all three germ layers: mesoderm, endoderm, and ectoderm. Proper levels of phosphorylated extracellular signal-regulated kinase (pERK) are critical for maintaining pluripotency, as elevated pERK evoked by fibroblast growth factor (FGF) receptor activation results in differentiation of ESCs, while, conversely, reduction of pERK by a MEK inhibitor maintains a pluripotent ground state. However, mechanisms underlying proper control of pERK levels in mouse ESCs are not fully understood. Here, we find that Klf5, a Krüppel-like transcription factor family member, is a component of pERK regulation in mouse ESCs. We show that ERK signaling is overactivated in Klf5-KO ESCs and the overactivated ERK in Klf5-KO ESCs is suppressed by the introduction of Klf5, but not Klf2 or Klf4, indicating a unique role for Klf5 in ERK suppression. Moreover, Klf5 regulates Spred1, a negative regulator of the FGF-ERK pathway. Klf5 also facilitates reprogramming of EpiSCs into a naïve state in combination with a glycogen synthase kinase 3 inhibitor and LIF, and in place of a MEK inhibitor. Taken together, these results show for the first time that Klf5 has a unique role suppressing ERK activity in mouse ESCs.
Introduction
Pluripotent stem cells (PSCs) can be established as embryonic stem cells (ESCs) in culture from the epiblast of a blastocyst [1,2]. PSCs can also be generated as induced pluripotent stem cells (iPSCs) through the induction of pluripotency from somatic cells by ectopic expression of defined factors such as Oct3/4, Sox2, Klf4, and c-Myc [3]. Pluripotency of mouse ESCs is regulated by extracellular stimuli such as leukemia inhibitory factor (LIF) [4], as well as nuclear factors such as Oct3/4, Sox2, and Nanog [5–9]. Pluripotency is also achieved by the combinatorial inhibition of extracellular signal-regulated kinase (ERK) signaling and glycogen synthase kinase 3β (GSK3β), called the ground state [10]. Conversely, extracellular stimuli elicited by fibroblast growth factor (FGF) activates the ERK pathway in mouse ESCs, thereby destabilizing the pluripotent state and promoting cellular differentiation [10–12].
Mouse epiblast stem cells (EpiSCs) are PSCs derived from post-implantation epiblast at E5.5, the egg cylinder stage [13,14]. Although EpiSCs retain the ability to differentiate into all three germ layers, EpiSCs hardly contribute to fetal tissues when injected into a blastocyst. Mouse EpiSCs and human ESCs share many properties such as gene expression patterns, epigenetic modifications, proliferative activities, and cytokine responsiveness [15]. Both EpiSCs and human ESCs depend on basic FGF and activin signaling for self-renewal, indicating that responsiveness of the FGF-ERK pathway is substantially different between mouse ESCs and human ESCs. PSCs capable of contributing to a chimera are defined to be in a naïve state, while PSCs that depend on FGF signaling and are incapable of contributing to chimeras are in a primed state [15]. A substantial number of studies have demonstrated that naïve PSCs differentiate into primed PSCs [16,17], while primed PSCs can be converted into the naïve state by defined factors such as Nanog and Klf2 [18–20].
Krüppel-like transcription factor family members (Klfs) such as Klf2, Klf4, and Klf5 have important functions in both maintenance of mouse ESC pluripotency and the cellular reprogramming process [16,21–26]. Previous studies clearly demonstrated an association between expression of Klfs and naïve pluripotency, and the self-renewal capacity of mouse ESCs was severely reduced when Klf2, Klf4, and Klf5 were knocked down [21] or knocked out (KO) [26]. While Klf2, Klf4, and Klf5 have redundant functions in the maintenance of pluripotency, our previous report indicated that Klf5-KO mouse ESCs exhibit a spontaneous differentiation phenotype [22]. Our recent study demonstrated overactivation of the ERK pathway in Klf5-KO preimplantation embryos and promotion of primitive endoderm specification of inner cell mass cells at the expense of epiblast in Klf5-KO embryos [27]. However, it is unknown whether Klf5 regulates FGF-ERK pathway in mouse ESCs.
Here, we show overactivation of ERK in Klf5-KO ESCs. Importantly, such overactivation is suppressed by the introduction of Klf5, but not Klf2 or Klf4, indicating a unique role of Klf5 in ERK suppression. Klf5 regulates Spred1, a negative regulator of the FGF-ERK pathway. Klf5 facilitates reprogramming of EpiSCs into a naïve state in combination with a GSK3 inhibitor and LIF, and in place of MEK inhibition. Taken together, our results demonstrate for the first time that Klf5 has a unique role in suppressing ERK activity in mouse ESCs.
Results
Loss of Klf5 results in an increased level of pERK in mouse ESCs
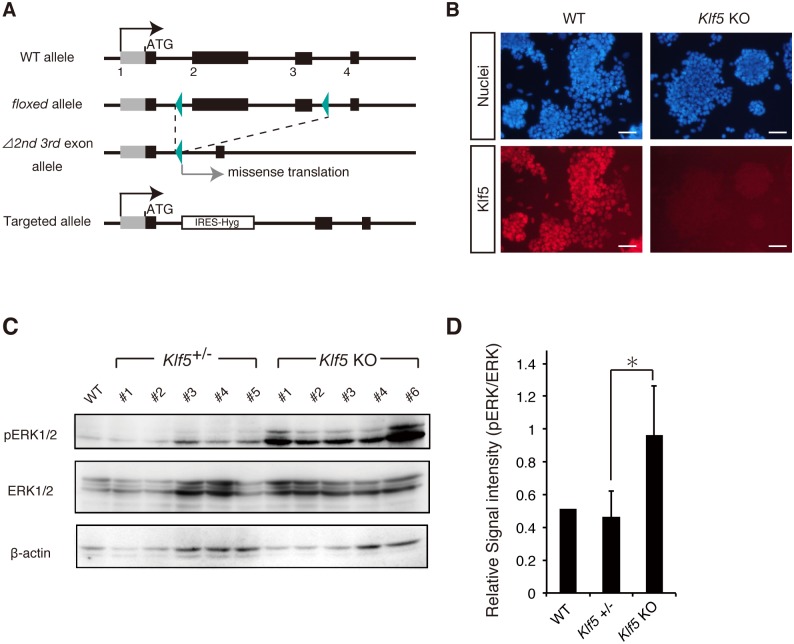
Proper levels of pERK are critical for maintaining pluripotency, yet how pERK levels are properly controlled in mouse ESCs is not fully understood. As our recent study showed that the ERK pathway is repressed by the transcription factor Klf5 in preimplantation mouse embryos [27], we examined whether Klf5 regulates ERK signaling in mouse ESCs. To evaluate pERK levels in only Oct3/4-positive PSCs, new Klf5-KO ESC lines were generated from Oct3/4-CFP::Rex1-GFP (OCRG9) ESCs in which a puromycin resistance gene was introduced into the Oct3/4 locus (Fig 1A and 1B), thereby enabling rapid selection of Oct3/4-positive PSCs [28]. After culture in the presence of puromycin, we performed western blotting analysis and found that pERK levels were significantly elevated in Klf5-KO ESC lines compared with WT and Klf5 heterozygous ESCs (Fig 1C and 1D).
Fig 1. Klf5 suppresses pERK in mouse ESCs.
(A) Schematic representation of Klf5 targeting vector to create Klf5-KO ESCs. (B) Expression of Klf5 protein in WT and Klf5-KO ESCs. Scale bar: 100 μm. (C) Western blot analysis of WT, Klf5 heterozygous, and Klf5-KO ESCs. Western blot analysis was performed with antibodies against pERK, ERK, and β-actin. (D) Quantified signal intensity for pERK relative to ERK. Asterisk indicates statistical significance: *P < 0.01; Mann-Whitney U test.
Klf5, but not Klf2 or Klf4, rescues elevated pERK levels in Klf5-KO ESCs
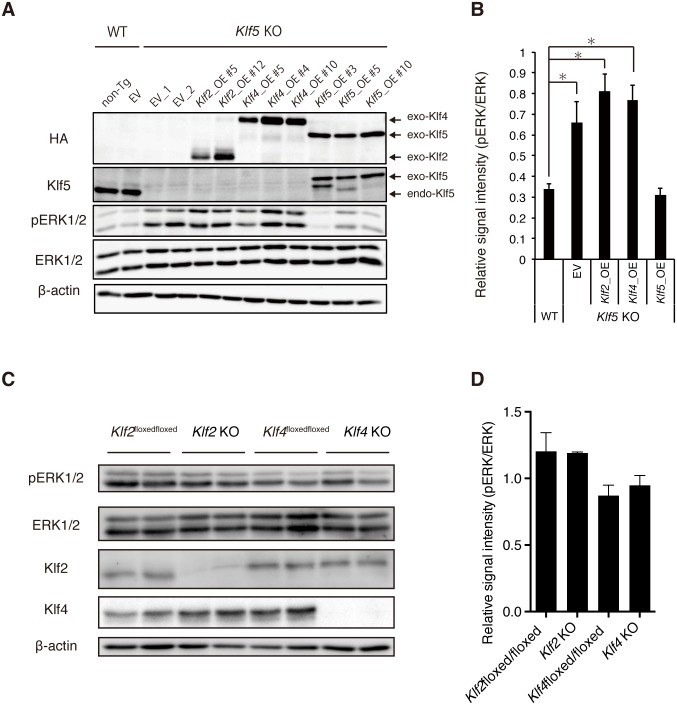
Previous reports showed that Klf family members such as Klf2, Klf4, and Klf5 have redundant functions to maintain the pluripotency of mouse ESCs [21,22,26]. To investigate whether Klf2, Klf4, and Klf5 have similar functions on pERK regulation in mouse ESCs, epitope-tagged versions of Klf2, Klf4, or Klf5 were introduced into Klf5-KO ESCs and respective Klf-Tg ESC lines were established (Fig 2A). Expression levels of epitope-tagged Klf were similar to endogenous Klf5 expression in WT ESCs (Fig 2A). Elevated pERK levels in Klf5-KO ESCs were reversed to normal levels in WT ESCs by the introduction of Klf5, but not Klf2 or Klf4 (Fig 2A and 2B), indicating a unique Klf5 function on suppression of pERK. To more directly investigate whether Klf2 and Klf4 are involved in pERK regulation, we examined pERK levels in Klf2- or Klf4-KO ESCs by western blot analysis; no significant changes were observed (Fig 2C and 2D). Collectively, these results demonstrate a unique role for Klf5 in pERK suppression in mouse ESCs.
Fig 2. Rescue of elevated pERK levels in Klf5-KO ESCs by Klf5, but not Klf2 or Klf4.
(A) Level of pERK in Klf5-KO ESCs overexpressing Klf2, Klf4, or Klf5. Western blot analysis of WT non-transgenic (non-Tg), empty vector (EV) control, Klf5-KO EV control, Klf2-overexpressing (Klf2_OE), Klf4_OE, and Klf5_OE ESCs were performed with antibodies against HA, Klf5, pERK, ERK, and β-actin. Exo; exogenous, endo; endogenous. (B) Quantified signal intensity for pERK relative to ERK. (C) Level of pERK in Klf2-or Klf4-KO ESCs. Western blot analysis of WT, Klf2-KO ESCs, and Klf4-KO ESCs. (D) Quantified signal intensity of pERK relative to ERK. Asterisk indicates statistical significance: *P < 0.01; Mann-Whitney U test.
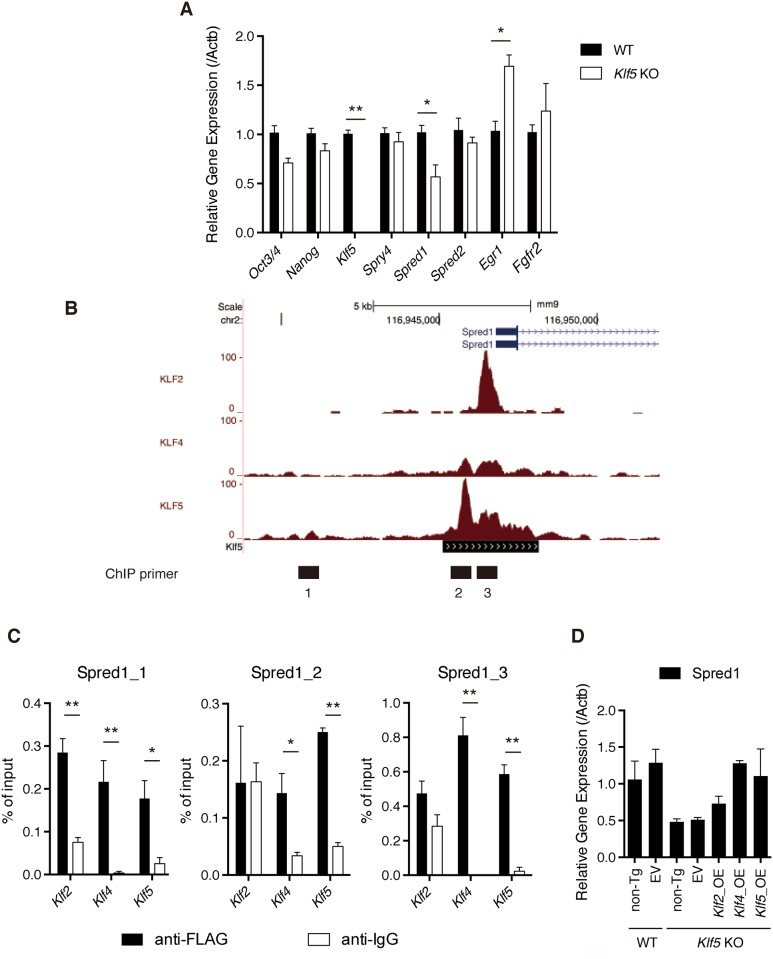
Given that pERK was elevated in Klf5-KO ESCs, we assessed whether expression of genes involved in the FGF-FGFR-ERK pathway were altered in Klf5-KO ESCs. RT-qPCR analysis indicated that Egr1 is induced in Klf5-KO ESCs, consistent with the notion that Egr1 is a direct target of ERK [29] and Spred1 [30], a suppressor for ERK that was also significantly reduced in Klf5-KO ESCs (Fig 3A). To investigate whether Klf5 binds to Spred1 directly, we surveyed genomic binding sites of Klf5 by examining ChIP-seq data and found that Spred1 was occupied by Klf5 (Fig 3B). To confirm this finding, we used ESC lines overexpressing epitope-tagged Klf5, and found that Klf5 bound preferentially to Spred1 loci (Fig 3C). These results indicated that Klf5 regulates Spred1 to maintain proper pERK levels in mouse ESCs. Notably, Spred1 was also occupied by Klf2 and Klf4 (Fig 3B and 3C), and the introduction of Klf2 or Klf4 significantly rescued Spred1 expression (Fig 3D). These results indicated that Klf2 and Klf4 also have ability to activate transcription of Spred1. However, given that the elevated pERK level in Klf5-KO ESCs was reversed by Klf5, but not Klf2 or Klf4, we speculate that there may be other FGF-FGFR-ERK pathway-related molecules regulated by Klf5, but not Klf2 or Klf4.
Fig 3. Klf5 regulates Spred1 in mouse ESCs.
(A) RT-qPCR analysis of genes involved in the FGF-FGFR-ERK pathway. (B) Binding peaks of Klf2, Klf4, and Klf5 to Spred1 locus. Numbers below binding peaks indicate regions for ChIP primers. (C) Manual ChIP assay of Klf2, Klf4, and Klf5 binding to Spred1 in mouse ESCs. (D) Spred1 mRNA expression in Klf5-KO ESCs overexpressing Klf2, Klf4, or Klf5. Asterisks indicate statistical significance: *P < 0.01, P < 0.001** (Mann-Whitney U test).
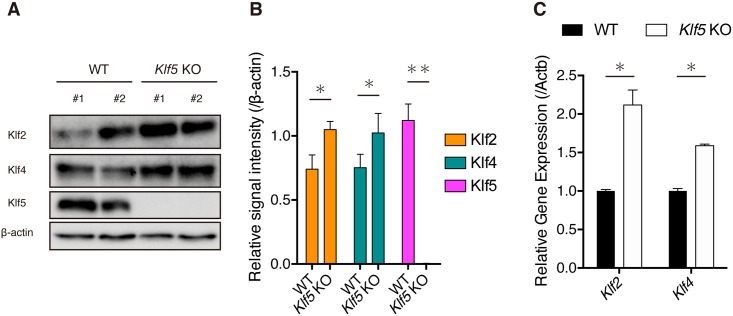
Previous studies demonstrated that both Klf2 and Klf4 proteins are phosphorylated by ERK1/2 before proteolysis by the proteasome-degradation pathway [25,31]. As pERK levels were elevated in Klf5-KO ESCs, we examined whether levels of Klf2 and Klf4 proteins were reduced (Fig 4A). Unexpectedly, levels of both Klf2 and Klf4 protein were significantly increased in Klf5-KO ESCs (Fig 4A and 4B). Thus, we examined mRNA levels and found that both Klf2 and Klf4 mRNA were significantly increased in Klf5-KO ESCs (Fig 4C). We speculate that compensatory upregulation of Klf2 and Klf4 mRNA may lead to an increase in both proteins, although degradation of Klf2 and Klf4 proteins may be promoted by elevated pERK.
Fig 4. Levels of Klf2 and Klf4 in Klf5-KO ESCs.
(A) Western blot analysis of WT and Klf5-KO ESCs. Western blot analysis was performed with antibodies against Klf2, Klf4, and β-actin. (B) Quantified signal intensity of Klf2 or Klf4 relative to β-actin. (C) RT-qPCR analysis of Klf2 and Klf4 in WT and Klf5-KO ESCs.
Klf5 facilitates reprogramming in combination with GSK3β inhibition
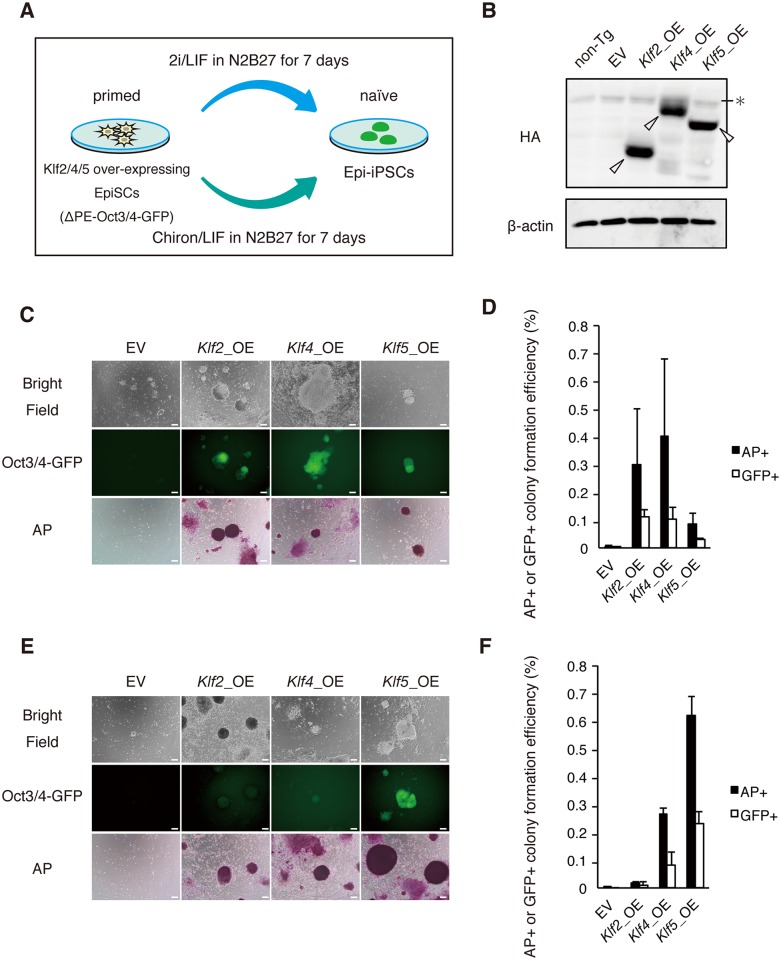
Mouse EpiSCs can be reprogrammed into Epi-iPSCs by induced expression of naïve transcription factors, such as Nanog and Esrrb, in the presence of 2i [16,20,32]. This system is widely used to evaluate the reprogramming ability of a putative reprogramming factor (Fig 5A). Previous studies reported that Klf2 and Klf4 have strong reprogramming activity from fibroblasts into iPSCs, whereas Klf5 has rather weak activity [16,20,24]. Similarly, Klf2 and Klf4 efficiently (0.1%–0.2%) facilitated the reprogramming of EpiSCs towards Epi-iPSCs, in contrast to Klf5 (0%) [20]. We reassessed the effect of Klf2, Klf4, and Klf5 on reprogramming using EpiSCs overexpressing epitope-tagged versions of these Klf proteins, and confirmed that all three proteins were expressed at similar levels (Fig 5B). First, we examined the efficiency of reprogramming in a typical reprogramming medium [N2B27 medium containing a MEK inhibitor and GSK3ß inhibitor (2i)] by alkaline phosphatase (AP) assay and green fluorescent protein (GFP) fluorescence driven by ΔPE-Oct3/4 transgene activity, which is only present in naïve PSCs. It was observed that Klf2 and Klf4 had strong reprogramming activities (0.3%–0.5%) (Fig 5C and 5D). Klf5 also had moderate activity (0.1%) (Fig 5C and 5D), consistent with our previous work [24]. Given that Klf5 represses the FGF-ERK pathway in pre-implantation embryos [27] and suppresses pERK in mouse ESCs (current study), we presumed that Klf5 might facilitate the reprogramming process in place of a MEK inhibitor. Thus, we cultured EpiSCs in the absence of a MEK inhibitor, and found that Klf5 generated a substantial number of Oct3/4-positive colonies with greater efficiency than Klf2 or Klf4 (Fig 5E and 5F).
Fig 5. Klf5 facilitates reprogramming towards naïve pluripotency in combination with Chiron.
(A) Experimental design to evaluate the reprogramming activity of Klf proteins. EpiSCs (3 × 104) were replated onto fibronectin-coated dishes and cultured in N2B27 medium containing 2i/LIF or Chiron/LIF. After 7 days in 2i/LIF or Chiron/LIF, iPSC colonies were picked for an AP assay. (B) Expression levels of epitope-tagged Klf2, Klf4, and Klf5 in EpiSCs. Open arrowheads indicate epitope-tagged Klf proteins. * indicates non-specific band. (C) Reprogramming ability of Klf2, Klf4, and Klf5 in the presence of 2i and LIF. Scale bar: 100 μm. (D) Comparison of reprogramming efficiency among Klf2, Klf4, and Klf5 in the presence of 2i by AP assay and counting of GFP-positive colonies. (E) Reprogramming ability of Klf2, Klf4, and Klf5 in the presence of Chiron and LIF. Scale bar: 100 μm. (F) Comparison of reprogramming efficiency among Klf2, Klf4, and Klf5 in the presence of Chiron by AP assay and counting of GFP-positive colonies.
Notably, the reprogramming efficiency elicited by Klf5 without MEK inhibition was higher than with MEK inhibition (Fig 5D and 5F). Recently, Yagi et al. and Choi et al. reported that prolonged culture in the presence of 1 μM MEK inhibitor causes loss of DNA methylation in mouse pluripotent stem cells, which erases of genomic imprinting and alters developmental potential into embryonic lineages [33,34]. Therefore, we speculated that treatment with 1 μM MEK inhibitor in combination with Klf5 may cause an additive effect for downstream genes, thereby reducing the reprogramming efficiency; although, the exact mechanism is currently unclear.
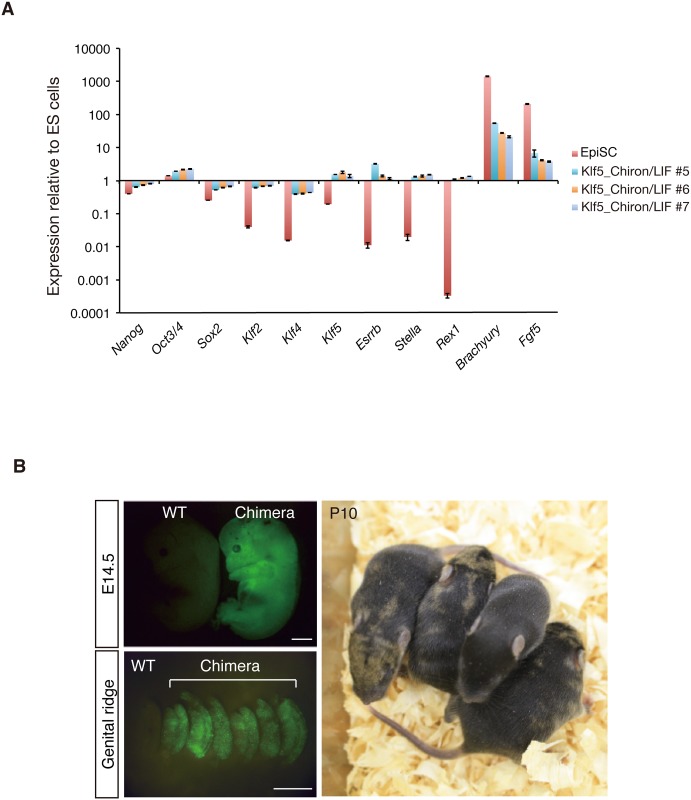
As Oct3/4-positive colonies exhibited very similar gene expression patterns to ESCs (Fig 6A), we performed blastocyst injections to examine the contribution of Oct3/4-positive cells to post-implantation embryos (Fig 6A and 6B). Injected embryos showed strong contributions to embryonic and postnatal chimeras (Fig 6B). These results suggest that Klf5 facilitates reprogramming in collaboration with GSK3β in the absence of a MEK inhibitor by regulating the FGF-ERK pathway.
Fig 6. Chimera-forming ability of EpiSCs reprogrammed by Klf5 with Chiron/LIF.
Reprogrammed Epi-iPSCs show the hallmarks of naïve pluripotency. (A) RT-qPCR analysis of expression of markers for naive pluripotency and differentiation. After the Klf5 expression cassette was removed by transient Cre expression, RT-qPCR analysis was performed. Levels of expression relative to those in ESCs are shown. (B) Chimera generated from Epi-iPSCs induced by Klf5 in combination with Chiron. Upper panels show a control WT embryo and embryo injected with GFP-labeled Epi-iPSCs on the left and right, respectively. Lower panel shows a postnatal chimera at P10 injected with GFP-labeled Epi-iPSCs. Note that brown coat color indicates the contribution of 129 Epi-iPSCs. Plotted results are mean and SEM of three independent experiments. Scale bar: 100 μm.
Discussion
Although proper pERK levels are known to be required for maintenance of pluripotency, how pERK levels are properly controlled in mouse ESCs is not well understood. Thus far, extrinsic BMP4 attenuates ERK activity by inducing the ERK-specific phosphatase DUSP9 to promote self-renewal [35,36]. The MYC/MAX complex also suppresses ERK activity by regulating Dusp2 and Dusp7 to maintain the naïve state of PSCs, as a lack of MAX causes defective self-renewal and differentiation accompanied by ERK activation [37,38]. Our recent study showed that the transcription factor Klf5 represses the ERK pathway via suppression of Fgf4 in preimplantation mouse embryos [27]. Although we previously showed that Fgf4 is not altered in Klf5-KO ESCs [27], current our study clearly indicates that Klf5 suppresses pERK in mouse ESCs via Spred1, a negative regulator for ERK signaling.
Previous reports have indicated that Klf2, Klf4, and Klf5 have redundant functions in the self-renewal of mouse ESCs and in the induction of pluripotency [23]. However, it appears that Klf4 and Klf5 have differential roles on cellular proliferation [22]. Yeo et al. reported that Klf2 is directly phosphorylated by Erk2, and phospho-Klf2 undergoes proteasome-dependent degradation [25]. Therefore, inhibition of MEK can prevent Klf2 phosphorylation and stabilize Klf2 protein, thereby activating genes related to naïve pluripotency, which explains in part how MEK inhibition promotes the formation of naïve PSCs. Similarly, ERK phosphorylates Klf4 as part of the proteasome pathway [31]. In this regard, it is interesting to note that pERK was elevated in Klf5-KO ESCs and increased pERK could be reversed by overexpression of Klf5, but not Klf2 or Klf4. Thus, these data clearly demonstrate a unique role for Klf5 in the suppression of pERK in ESCs.
Taken together, our results clearly demonstrate that Klf5 is a critical genetic component that suppresses MEK activity in naïve PSCs.
Materials and methods
Pluripotent stem cells
Mouse Klf5 hyg/floxed ESC lines were created by introducing a Klf5 hygromycin-resistant gene knock-in vector [22] and floxed Klf5 vector [27] into OCRG9 ESCs (Oct3/4-CFP::Rex1-GFP-irespuroR, a generous gift from Dr. Niwa). Subsequently, pCAG-NLS-Cre (a generous gift from Dr. Andras Nagy, Samuel Lunenfeld Research Institute) was transiently introduced to delete the second and third exons of Klf5, thus generating Klf5-KO (hyg/Δ) ESC lines. ESCs were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) in the presence of puromycin to enrich Oct3/4-positive PSCs. Klf2-floxed ESCs and Klf4-floxed ESCs were described previously [39].
OZ7 mouse EpiSCs, a generous gift from A. Smith [16], were maintained in N2B27 medium (Stem Cells Sciences) supplemented with 12 ng/μL basic fibroblast growth factor (bFGF) and 20 ng/μL activin A (R&D Systems). To assay the reprogramming of EpiSCs, an effector plasmid expressing Klf2, Klf4, or Klf5 was transfected into OZ7 cells using Lipofectamine 2000 (Invitrogen) and cells were cultured on fibronectin-coated dishes. Subsequently, EpiSCs (3 × 104) were replated onto fibronectin-coated dishes and cultured in N2B27 medium containing 2i (1 μM PD0325901 and 3 μM CHIR99021)/LIF, or 3 μM Chiron/LIF. After 7 days in 2i/LIF or Chiron/LIF, iPSC colonies were picked or an AP assay was performed using an AP staining kit (Sigma-Aldrich).
Immunohistochemistry and AP assay
PSCs were fixed in 4% paraformaldehyde for 10 min, permeabilized in 0.5% Triton X-100 for 10 min, and incubated in a blocking reagent [phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin and 0.01% Tween 20] for 1 hr. PSCs were incubated at 4°C overnight with primary antibodies (S1 Table). After three washes with PBS containing 0.2% Tween 20, secondary antibodies were incubated at room temperature for 1 hr. Nuclei were stained with Hoechst 33342 (10 μg/mL, Molecular Probes). AP assays to identify PSCs were performed with a leukocyte AP staining kit (Sigma-Aldrich).
Blastocyst injection
The Klf5 expression unit was removed from Epi-iPSC lines reprogrammed from 129 EpiSCs, which were cultured in DMEM + 10% FBS and transfected with pPB-UbC-GFP plasmid and a transposase, as described in Jeon et al. [24]. GFP-positive Epi-iPSCs were harvested and injected into C57BL/6 mouse blastocysts (SLC Inc., Shizuoka, Japan), and chimeras were dissected out at E9.5 and E13.5. Some chimeras were analyzed for their coat color at postnatal day 10. The sacrifice was carried out by cervical dislocation. The experimental procedures were approved by the ethics committee for Animal Experimentation of Shiga University of Medical Sciences (Approval number: 2016-11-8) (Committee members are Akira Andoh, Jun Udagawa, Masatsugu Ema, Kazumasa Ogasawara, Shinichiro Nakamura, Kazuhiko Nozaki, Seiji Hitoshi, Kihachiro Horiike, Yoshihito Muroji, Shigehiro Morikawa). Masatsugu Ema was not involved in the judgement.
Quantitative PCR analysis
For RT-PCR analysis, first-strand cDNA was synthesized from total RNA using a QuantiTect Reverse Transcription kit (Qiagen). Real-time PCR was performed with SYBR Premix Ex Taq II (TaKaRa) and analyzed on a Thermal Cycler Dice Real Time System (TP850; TaKaRa). Amounts of target RNA were estimated using an appropriate standard curve and normalized by dividing values by the estimated amount of β-actin. Sequences of primers used for quantitative PCR are listed in S2 Table.
Western blot analysis
ESCs were lysed and western blotting was performed as described previously [22]. Membranes were immunoblotted with rat anti-mouse Klf5 antibody (KM1784; Kyowa Kirin), rabbit anti-pERK antibody (Cell Signaling Technology), rabbit anti-ERK antibody (Cell Signaling Technology), rabbit anti-Klf2 antibody (Millipore), rabbit anti-Klf4 antibody (Abcam), or anti-β-actin antibody (MBL), followed by an appropriate secondary antibody [horseradish peroxidase-conjugated rabbit anti-mouse IgG (Invitrogen) or horseradish peroxidase-conjugated goat anti-rabbit IgG (Zymax)]. Immunoreactive proteins were detected using enhanced chemiluminescence (Chemilumi One; Nakalai) and an ImageQuant LAS 4000 imager (GE Healthcare). Signal intensity of western blotting was quantified using ImageJ. Signals were normalized to the intensity of ERK or β-actin.
Chromatin immunoprecipitation (ChIP)-sequencing data analysis
Data analysis was performed as described previously [27]. ChIP-seq data were downloaded from the EMBLE-EBI site [Klf2: ERR440998, Klf4: SRR952210, Klf5: SRR952211, Aksoy et al., 2014]. ChIP-seq reads were located to the mouse reference genome (mm9) using Burrows-Wheeler alignment software. Uniquely mapped reads were used for peak calling by CCAT3 version 3.0. Peak regions were filtered for false discovery rate values < 0.05. RefSeq genes that had a Klf5 binding site within 20 kb were identified by determining the overlap between ChIP-seq peak regions and RefSeq genes extended by 20 kb in both directions. To visualize ChIP-seq tag counts in the UCSC Genome Browser, mapped reads were extended and converted into the bedGrapgh format using the genomecov function of BEDTools.
Chromatin immunoprecipitation assays
ChIP assay was performed as described previously [27]. Sequences of primers used for ChIP-qPCR are listed in S3 Table.
Statistical analysis
Statistical analyses were performed using the Mann-Whitney U test. Data are expressed as mean and standard error (SEM). Differences were considered significant at P < 0.05.
Supporting information
Uncropped western blot images shown in Figures are presented.
(PDF)
Uncropped western blot images shown in Figures are presented.
(PDF)
Antibodies used in this study are presented.
(PDF)
Primer sequences for qRT-PCR analysis are presented.
(PDF)
Primer sequences for ChIP-qPCR analysis are presented.
(PDF)
Acknowledgments
We thank Dr. T. Tsukiyama for helpful discussion and reagents. This work was supported in part by a grant from PRESTO, Japan Science and Technology Agency (M. E.). We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by a grant number 2011 from Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency (M.E.). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292: 154–156. 10.1038/292154a0 [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci. 1981;78: 7634–7638. 10.1073/pnas.78.12.7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126: 663–76. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 4.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336: 688–90. 10.1038/336688a0 [DOI] [PubMed] [Google Scholar]
- 5.Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460: 118–122. 10.1038/nature08113 [DOI] [PubMed] [Google Scholar]
- 6.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113: 643–55. 10.1016/S0092-8674(03)00392-1 [DOI] [PubMed] [Google Scholar]
- 7.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113: 631–42. [DOI] [PubMed] [Google Scholar]
- 8.Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9: 625–35. 10.1038/ncb1589 [DOI] [PubMed] [Google Scholar]
- 9.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo dependes on the POU transcription factor Oct4. Cell. 1998;95: 379–391. 10.1016/S0092-8674(00)81769-9 [DOI] [PubMed] [Google Scholar]
- 10.Ying Q-L, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453: 519–23. 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134: 2895–902. 10.1242/dev.02880 [DOI] [PubMed] [Google Scholar]
- 12.Hamilton WB, Kaji K, Kunath T. ERK2 suppresses self-renewal capacity of embryonic stem cells, but is not required for multi-lineage commitment. PLoS One. 2013;8: e60907 10.1371/journal.pone.0060907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448: 191–5. 10.1038/nature05950 [DOI] [PubMed] [Google Scholar]
- 14.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448: 196–9. 10.1038/nature05972 [DOI] [PubMed] [Google Scholar]
- 15.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. Cell Press; 2009;4: 487–92. 10.1016/j.stem.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 16.Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136: 1063–9. 10.1242/dev.030957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13: 1070–5. 10.1038/ncb2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell. 2014;158: 1254–1269. 10.1016/j.cell.2014.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138: 722–37. 10.1016/j.cell.2009.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, et al. Oct4 and LIF/Stat3 Additively Induce Krüppel Factors to Sustain Embryonic Stem Cell Self-Renewal Cell Stem Cell. Elsevier Ltd; 2009;5: 597–609. 10.1016/j.stem.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Chan Y-S, Loh Y-H, Cai J, Tong G-Q, Lim C-A, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10: 353–60. 10.1038/ncb1698 [DOI] [PubMed] [Google Scholar]
- 22.Ema M, Mori D, Niwa H, Hasegawa Y, Yamanaka Y, Hitoshi S, et al. Krüppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell. 2008;3: 555–67. 10.1016/j.stem.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26: 101–6. 10.1038/nbt1374 [DOI] [PubMed] [Google Scholar]
- 24.Jeon H, Waku T, Azami T, Khoa LTP, Yanagisawa J, Takahashi S, et al. Comprehensive Identification of Krüppel-Like Factor Family Members Contributing to the Self-Renewal of Mouse Embryonic Stem Cells and Cellular Reprogramming. PLoS One. 2016;11: e0150715 10.1371/journal.pone.0150715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeo JC, Jiang J, Tan ZY, Yim GR, Ng JH, Göke J, et al. Klf2 is an essential factor that sustains ground state pluripotency. Cell Stem Cell. 2014;14: 864–872. 10.1016/j.stem.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 26.Yamane M, Ohtsuka S, Matsuura K, Nakamura A, Niwa H. Overlapping function of klf family targets multiple transcription factors to maintain naïve pluripotency of ES cells. Development. 2018;145: dev162404. 10.1242/dev.162404 [DOI] [PubMed] [Google Scholar]
- 27.Azami T, Waku T, Matsumoto K, Jeon H, Muratani M, Kawashima A, et al. Klf5 maintains the balance of primitive endoderm versus epiblast specification during mouse embryonic development by suppression of Fgf4. Development. 2017;144: 3706–3718. 10.1242/dev.150755 [DOI] [PubMed] [Google Scholar]
- 28.Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135: 909–18. 10.1242/dev.017400 [DOI] [PubMed] [Google Scholar]
- 29.Yu X, Shen N, Zhang M-L, Pan F-Y, Wang C, Jia W-P, et al. Egr-1 decreases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice. EMBO J. 2011;30: 3754–65. 10.1038/emboj.2011.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, Miyoshi K, et al. Spred is a sprouty-related suppressor of Ras signalling. Nature. 2001;412: 647–651. 10.1038/35088082 [DOI] [PubMed] [Google Scholar]
- 31.Kim MO, Kim S-H, Cho Y-Y, Nadas J, Jeong C-H, Yao K, et al. ERK1 and ERK2 regulate embryonic stem cell self-renewal through phosphorylation of Klf4. Nat Struct Mol Biol. 2012;19: 283–90. 10.1038/nsmb.2217 [DOI] [PubMed] [Google Scholar]
- 32.Festuccia N, Osorno R, Halbritter F, Karwacki-Neisius V, Navarro P, Colby D, et al. Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell Stem Cell. 2012;11: 477–90. 10.1016/j.stem.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagi M, Kishigami S, Tanaka A, Semi K, Mizutani E, Wakayama S, et al. Derivation of ground-state female ES cells maintaining gamete-derived DNA methylation. Nature. 2017;548: 224–227. 10.1038/nature23286 [DOI] [PubMed] [Google Scholar]
- 34.Choi J, Huebner AJ, Clement K, Walsh RM, Savol A, Lin K, et al. Prolonged Mek1/2 suppression impairs the developmental potential of embryonic stem cells. Nature. 2017;548: 219–223. 10.1038/nature23274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi X, Li T-G, Hao J, Hu J, Wang J, Simmons H, et al. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2004;101: 6027–32. 10.1073/pnas.0401367101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Fei T, Zhang J, Zhu G, Wang L, Lu D, et al. BMP4 Signaling Acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell. 2012;10: 171–82. 10.1016/j.stem.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 37.Hishida T, Nozaki Y, Nakachi Y, Mizuno Y, Okazaki Y, Ema M, et al. Indefinite self-renewal of ESCs through Myc/Max transcriptional complex-independent mechanisms. Cell Stem Cell. 2011;9: 37–49. 10.1016/j.stem.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 38.Chappell J, Sun Y, Singh A, Dalton S. MYC/MAX control ERK signaling and pluripotency by regulation of dual-specificity phosphatases 2 and 7. Genes Dev. 2013;27: 725–733. 10.1101/gad.211300.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamane M, Ohtsuka S, Matsuura K, Nakamura A, Niwa H. Overlapping functions of Krüppel-like factor family members: targeting multiple transcription factors to maintain the naïve pluripotency of mouse embryonic stem cells. Development. 2018;145: dev162404. 10.1242/dev.162404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Uncropped western blot images shown in Figures are presented.
(PDF)
Uncropped western blot images shown in Figures are presented.
(PDF)
Antibodies used in this study are presented.
(PDF)
Primer sequences for qRT-PCR analysis are presented.
(PDF)
Primer sequences for ChIP-qPCR analysis are presented.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.