Abstract
Background:
Culture-negative periprosthetic joint infection (PJI) is a challenging condition to treat. The most appropriate management of culture-negative PJI is not known, and there is immense variability in the treatment outcome of this condition. The purpose of this study was to elucidate the characteristics, outcomes, and risk factors for failure of treatment of culture-negative PJI.
Methods:
A retrospective review of 219 patients (138 hips and 81 knees) who had undergone surgery for the treatment of culture-negative PJI was performed utilizing a prospectively collected institutional PJI database. PJIs for which the results of culture were unavailable were excluded. An electronic query and manual review of the medical records were completed to obtain patient demographics, treatment, microbiology data, comorbidities, and other surgical characteristics. Treatment failure was assessed using the Delphi consensus criteria.
Results:
The prevalence of suspected culture-negative PJI was 22.0% (219 of 996), and the prevalence of culture-negative PJI as defined by the Musculoskeletal Infection Society (MSIS) was 6.4% (44 of 688). Overall, the rate of treatment success was 69.2% (110 of 159) in patients with >1 year of follow-up. Of the 49 culture-negative PJIs for which treatment failed, 26 (53.1%) subsequently had positive cultures; of those 26, 10 (38.5%) were positive for methicillin-sensitive Staphylococcus aureus. The rate of treatment success was greater (p = 0.019) for patients who had 2-stage exchange than for those who underwent irrigation and debridement.
Conclusions:
The present study demonstrates that culture-negative PJI is a relatively frequent finding with unacceptable rates of treatment failure. Every effort should be made to isolate the infecting organism prior to surgical intervention, including extending the incubation period for cultures, withholding antibiotics prior to obtaining culture specimens, and possibly using newly introduced molecular techniques.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence
Periprosthetic joint infection (PJI) remains one of the most devastating complications of total joint arthroplasty1-3. In particular, culture-negative PJI is a very perplexing condition to manage for the surgeon, patient, and infectious-disease team. In recent years, the prevalence of culture-negative PJI has been on the rise; traditional modalities for isolation of an infecting organism have failed in as many as 45% of patients in some series4. This increase may be attributed to a variety of reasons, including infection with low-virulence organisms that require a longer incubation period, premature treatment with antibiotics, and failure to use an enriched culture medium5,6. It has been demonstrated that withholding therapeutic antibiotics until specimens for culture have been obtained can help in isolating an organism6,7.
The existing literature suggests that outcomes after culture-negative PJI are similar to those after PJI with an identifiable infecting organism or that negative cultures are even a positive prognostic factor5,8,9. Furthermore, the most appropriate management of culture-negative PJI is not known, largely because of the immense variability in treatments from both an antimicrobial and a surgical standpoint5,8,10. The purpose of this study was to elucidate the characteristics and outcomes of culture-negative PJI and to investigate the risk factors for treatment failure.
Materials and Methods
A retrospective review of 219 patients (138 hips and 81 knees) who had undergone surgery for the treatment of culture-negative PJI between 2000 and 2014 was performed. These culture-negative PJIs were identified utilizing a prospectively collected institutional PJI database of 996 PJIs. A culture-negative infection was defined as one for which cultures of joint aspirate and/or intraoperative tissue samples did not isolate an organism. Patients were excluded from the study if the results of culture of material from the site of the PJI were unavailable; if they had 1 positive culture, a megaprosthesis, or a subsequent PJI in the same joint; or if they had been followed for <1 year.
An electronic query and manual review of the electronic medical record were performed to obtain patient demographics, treatment, microbiology data, comorbidities, and other surgical characteristics. The modified criteria of the Musculoskeletal Infection Society (MSIS) were utilized to further stratify the cohort on the basis of the presence of a sinus tract, white blood-cell count and differential, culture results, serological markers, and leukocyte esterase results11. Cutoffs for elevated serological markers were based on the thresholds established at the International Consensus Meeting on PJI12.
All primary total knee arthroplasties that were originally performed at our institution included antibiotic-impregnated cement, and all total hip arthroplasties at our institution were performed without cement. The primary total joint arthroplasty was performed at our institution in 60.1% of the cases in the PJI database. Intraoperative topical antibiotics or antibiotic beads were not routinely used. During irrigation and debridement, polyethylene exchanges were routinely performed concurrently. If a pathogen was isolated on solid media in the microbiology laboratory, regardless of the amount of growth, the cultures were considered positive and the PJI was excluded from the study. It is our generalized institutional protocol that multiple (3 to 5) tissue and fluid samples are obtained during revision surgery and are sent for aerobic and anaerobic, fungal, and acid-fast bacilli culture using both solid media and broth. The average number of samples for the patients in this study was 3.6 (range, 2 to 8). Tissue samples are obtained from 3 standardized surgical sites: the synovium, femoral medullary canal, and tibial medullary canal (for knees) or the capsule, femoral medullary canal, and acetabulum (for hips). Additional samples for culture are taken, on a case-by-case basis, from areas that appear to be high-yield. Both solid media and broth are used. A matrix-assisted laser desorption-ionization time-of-flight mass spectrometer (GE Healthcare) has been utilized in recent years to confirm the identity of pathogens isolated from culture.
Treatment success was assessed with use of the Delphi consensus criteria, which are based on (1) eradication of infection, characterized by a healed wound without fistula, drainage, pain, or recurrence of infection caused by the same strain of organism; (2) no subsequent surgical intervention for infection after reimplantation surgery; and (3) no occurrence of PJI-related mortality13.
All statistical analyses were performed with use of R 3.1 (R Foundation for Statistical Computing) using the RMS (regression modeling strategies) package for the logistic regression. An alpha level of 0.05 was used to determine significance. Kaplan-Meier survivorship curves were generated for 1, 2, and 5-year follow-up and for the different treatments, with treatment failure as the end point. Differences in survivorship were assessed using the log-rank test.
Results
The prevalence of suspected culture-negative PJI was 22.0% (219 of 996 joints) and that of MSIS-defined culture-negative PJI was 6.4% (44 of 688 joints). Overall, the rate of treatment success was 69.2% (110 of 159 joints) in patients who had been followed for >1 year. The rate of infection-free survival was 81.1% (95% confidence internal [CI]: 75.0% to 87.2%) at 1 year, 78.1% (95% CI: 71.6% to 84.6%) at 2 years, and 65.3% (95% CI: 57.3 % to 73.3%) at 5 years (Fig. 1). There was a high rate of complications in these patients, including 6 amputations and 3 PJI-related deaths.
Fig. 1.
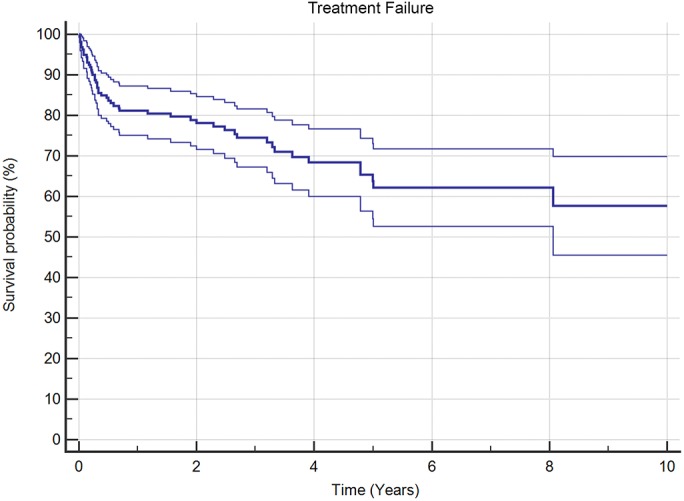
Kaplan-Meier survivorship curve (with 95% CI), with survival defined as treatment success according to the Delphi consensus criteria, for patients who had culture-negative PJI.
Treatment with 2-stage exchange resulted in improved survivorship (p = 0.019) compared with irrigation and debridement (Fig. 2). When stratified by procedures, the rate of treatment success was 71.2% (84 of 118 patients) for 2-stage exchange, 55.6% (15 of 27 patients) for irrigation and debridement, and 78.6% (11 of 14 patients) for 1-stage exchange. Furthermore, the rate of treatment success at last follow-up was 74.2% (69 of 93 patients) for patients treated with monotherapy and 64.3% (9 of 14 patients) for those treated with combination antibiotic therapy. There was no difference in survivorship between these antibiotic treatment groups (p = 0.248) (Fig. 3). The antibiotic treatment was unknown in 52 patients.
Fig. 2.

Kaplan-Meier survivorship curves (with 95% CIs), with survival defined as treatment success according to the Delphi consensus criteria, for patients managed with irrigation and debridement and those managed with 2-stage exchange.
Fig. 3.
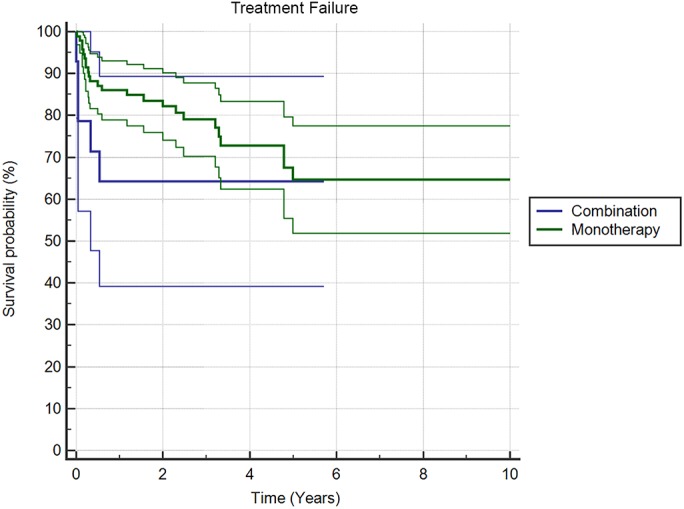
Kaplan-Meier survivorship curves (with 95% CIs), with survival defined as treatment success according to the Delphi consensus criteria, for patients managed with combination antibiotic treatment and those managed with monotherapy.
Of the 49 culture-negative PJIs that were not managed successfully, 26 (53.1%) subsequently had positive cultures, and for 10 (38.5%) of these patients the cultures showed methicillin-sensitive Staphylococcus aureus. The rate of negative cultures over the 15-year span of this study ranged from 11.9% to 33.3% and decreased by an average of only 0.35% per year (Fig. 4). The rate of treatment success over this time period was variable but increased by an average of 1.4% per year (Fig. 5).
Fig. 4.
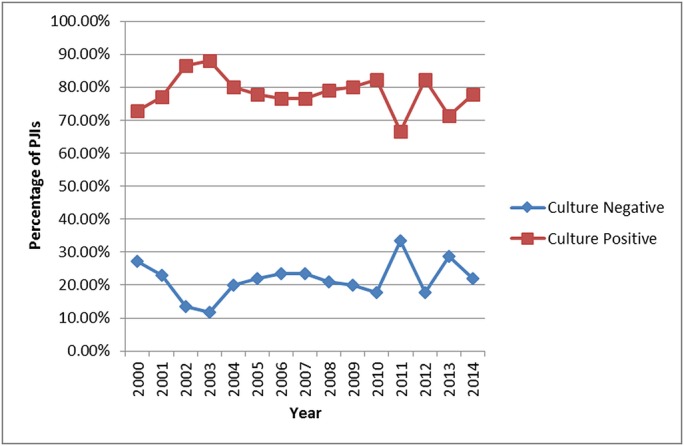
Line graph illustrating the 15-year rates of culture-negative and culture-positive PJI.
Fig. 5.

Line graph illustrating the rate of treatment success by year for patients with culture-negative PJI.
For the culture-negative PJIs in this study, multivariate analysis revealed that risk factors for treatment failure were knee joint involvement (adjusted odds ratio [OR], 4.60; p = 0.002) and surgical management with irrigation and debridement (adjusted OR, 3.10; p = 0.031). There was no difference in risk for patients treated with monotherapy or combination antibiotic therapy (OR, 0.612; p = 0.560).
Discussion
In the present study, culture-negative PJI was associated with poor outcomes and a high rate of salvage procedures. Furthermore, we found that irrigation and debridement had a low rate of eradication of infection, with a success rate of only 55.6%.
Given the poor outcomes associated with culture-negative PJI, it is important to identify the infecting organism. However, the sensitivity of routine cultures for identifying the infecting organisms in PJI is low, ranging from 39% to 70% in reported studies7,12,14,15. Several factors are associated with failure to isolate a microorganism and decreased yield on culture. First, premature administration of antibiotics may compromise culture yield; thus, antibiotic treatment should be withheld until organisms are grown on culture5,6. However, multiple studies have demonstrated that perioperative administration of prophylactic antibiotics has no influence on culture yield12,16-21. Tetreault et al. reported that intraoperative cultures yielded the same organisms as preoperative cultures in 82% (28 of 34) and 81% (25 of 31) of patients randomized to receive antibiotics before the skin incision or after specimens were obtained for culture, respectively20. A randomized study by Bedenčič et al. revealed no difference in the organisms grown from samples obtained before and after antimicrobial prophylaxis (OR, 0.99; p = 0.99)19. Despite this evidence, the recommendation of the International Consensus Meeting on PJI is that mandatory withholding of antibiotics is not justified but that “in cases in which PJI is diagnosed or suspected and a pathogen has yet to be identified, the use of prophylactic antibiotics is dependent upon clinical judgment.”12 Culturing techniques may also influence culture yield, particularly for less-virulent organisms such as Propionibacterium acnes or coagulase-negative Staphylococcus7. The use of blood culture bottles and flasks instead of conventional agar and broth cultures is 1 strategy that has been shown to improve the yield of positive cultures of both synovial fluid and tissue specimens in cases of PJI14,16. In addition, extending the incubation period and obtaining a sufficient number of samples may also increase the sensitivity of culture7,12. Current recommendations state that 3 to 5 distinct intraoperative tissue samples should be obtained and sent for aerobic and anaerobic cultures in suspected cases of periprosthetic joint infection12. Furthermore, it has been proposed that as many as 10 periprosthetic samples should be collected when infection with a low-virulence organism is suspected14.
As has been mentioned, increasing the incubation period may also increase the culture yield, particularly for low-virulence organisms. For instance, P. acnes has a prolonged incubation period (median, 6 days) before it can be identified on routine culture18. During this period, clinical suspicion of infection in addition to aspiration results should be considered and appropriate treatment for PJI should be administered until the results of culture are known.
While the preferred method of treatment for PJI when routine cultures are negative has not been determined, several studies have investigated treatment outcomes following culture-negative PJI5,9,22-24. Choi et al. found that infection control was actually greater for a group of 40 PJIs that had negative cultures of specimens from at least 3 separate areas than it was for 135 PJIs that had positive cultures (p = 0.006)22. Additionally, Li et al. reported a reinfection rate of 7.34% for patients with culture-negative PJI, compared with 11.1% for patients with culture-positive PJI (p = 0.94)9. Berbari et al. reported a treatment failure-free survival rate of 94% in a series of 60 PJIs for which cultures were negative after being incubated for 7 days5. One possible explanation for the high success rates for those culture-negative PJIs is that the infections may have been caused by less-virulent organisms, which are easier to treat than those caused by more virulent organisms such as methicillin-resistant S. aureus (MRSA)1. In contrast, the present study and several others have demonstrated equivalent and even worse outcomes for culture-negative PJIs compared with culture-positive PJIs. Huang et al., in a study in which 90% of patients had cultures of specimens from at least 2 locations, reported that the failure rate was 73% for both culture-negative PJIs and culture-positive PJIs (p = 1.0)23. Furthermore, in a multivariate analysis, Mortazavi et al. found that culture-negative PJI was a predictor of failure for 2-stage exchange arthroplasty of the knee (OR: 4.5; 95% CI, 1.3 to 15.7)25. While these culture-negative PJIs may be caused by less-virulent organisms, the inability to target a specific organism may explain these less-than-optimal results. However, throughout the literature and as found in our study, survivorship was better after 2-stage exchange arthroplasty than it was after irrigation and debridement. For instance, Berbari et al. reported a 5-year survivorship, defined as treatment success, of 94% (95% CI, 85% to 100%) for 2-stage exchange compared with 71% (95% CI, 44% to 100%) for irrigation and debridement5. In contrast, in our study, the overall survivorship at 5 years was dismal (65.3%). These low rates of eradication highlight the importance of minimizing the rate of PJI by employing medical optimization, perioperative strategies, and careful patient selection. The improved treatment outcomes in other studies may potentially be attributable to the fact that some joints believed to have had culture-negative PJI may not actually have been infected.
New technologies, such as improved culturing techniques or next-generation sequencing, are needed to help identify the infecting organism in order to tailor antibiotic treatment. Recent evidence suggests that next-generation sequencing may provide increased sensitivity in isolating organisms (at a rate of up to 89% for culture-negative PJI) that cannot be identified using conventional culture26-29. Such sequencing allows the identification of organisms within a sample by high-throughput parallel sequencing of all the microbial DNA present, followed by comparison of the generated sequence reads against a bioinformatic database of all known microorganisms. While previously cost-prohibitive, the price of this diagnostic technique has dramatically decreased in recent years, making it accessible for clinical use26,27. The technique may be particularly useful when there is strong clinical suspicion of infection but cultures or other diagnostic tests are negative12,27. In addition, several studies have demonstrated that sonication can improve the likelihood of identifying an organism through the removal of biofilm from the implant30-33. While sonication is a time-intensive procedure that requires specialized equipment and may not be available to many, the International Consensus Meeting on PJI recommended that it be used in cases of suspected or proven PJI for which preoperative cultures of aspirate do not yield positive culture and antibiotics were administered previously12,30-32.
The present study had a number of limitations. First, the study was retrospective and there was limited information regarding premature antimicrobial therapy for these patients because it was poorly documented in the medical record. In addition, patients who had had a 1-stage exchange were not included in the study because culture-negative PJI was considered a contraindication and only 8 patients at our institution were treated with 1-stage exchange arthroplasty. Additionally, we included patients in whom surgery was performed for PJI even though MSIS criteria may not have been met as it was very difficult to fulfill minor criteria when not a single positive culture was present. The rationale for inclusion of those patients was that serological markers and other aspiration results are lower in patients with low-virulence organisms such as coagulase-negative Staphylococcus or P. acnes, and many of these culture-negative PJIs are thus likely to arise from such organisms. Furthermore, only a 1-year minimum follow-up was used in our study. The sampling techniques, including the number of cultures and incubation periods, were variable among the surgeons and dependent on each surgeon’s suspicion of infection. Antibiotic information was unavailable for many patients because the orthopaedic and infectious-disease medical records were distinct and because many patients were followed by physicians unaffiliated with our institution. There was variability in the sampling technique between surgeons despite a generalized institutional protocol. Additionally, because many different surgeons treated these patients, different perioperative treatment strategies were used. Antibiotic therapy and treatments were very heterogeneous and could not be fully explored.
In summary, the present study demonstrates that culture-negative PJI is a relatively frequent finding and has an unacceptable rate of treatment failure. Because of the poor outcomes associated with culture-negative PJI, every effort should be made to isolate the infecting organism prior to surgical intervention. Methods such as extending the incubation period for culture samples, withholding antibiotics until samples have been sent for culture, and using molecular techniques can help in isolating the infecting organism.
Footnotes
Investigation performed at the Rothman Institute at Thomas Jefferson University, Philadelphia, Pennsylvania
Disclosure: No external funding was received for this study. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work and “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work (http://links.lww.com/JBJSOA/A52).
References
- 1.Gomez MM, Tan TL, Manrique J, Deirmengian GK, Parvizi J. The fate of spacers in the treatment of periprosthetic joint infection. J Bone Joint Surg Am. 2015. September 16;97(18):1495-502. [DOI] [PubMed] [Google Scholar]
- 2.Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013. December 18;95(24):2177-84. [DOI] [PubMed] [Google Scholar]
- 3.Shahi A, Tan TL, Chen AF, Maltenfort MG, Parvizi J. In-hospital mortality in patients with periprosthetic joint infection. J Arthroplasty. 2017. March;32(3):948-952.e1. Epub 2016 Sep 30. [DOI] [PubMed] [Google Scholar]
- 4.Bejon P, Berendt A, Atkins BL, Green N, Parry H, Masters S, McLardy-Smith P, Gundle R, Byren I. Two-stage revision for prosthetic joint infection: predictors of outcome and the role of reimplantation microbiology. J Antimicrob Chemother. 2010. March;65(3):569-75. Epub 2010 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007. November 01;45(9):1113-9. Epub 2007 Sep 26. [DOI] [PubMed] [Google Scholar]
- 6.Malekzadeh D, Osmon DR, Lahr BD, Hanssen AD, Berbari EF. Prior use of antimicrobial therapy is a risk factor for culture-negative prosthetic joint infection. Clin Orthop Relat Res. 2010. August;468(8):2039-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parvizi J, Erkocak OF, Della Valle CJ. Culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2014. March 05;96(5):430-6. [DOI] [PubMed] [Google Scholar]
- 8.Huang R, Buckley PS, Scott B, Parvizi J, Purtill JJ. Administration of aspirin as a prophylaxis agent against venous thromboembolism results in lower incidence of periprosthetic joint infection. J Arthroplasty. 2015. September;30(9)(Suppl):39-41. Epub 2015 Jul 7. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Ni M, Li X, Zhang Q, Li X, Chen J. Two-stage revisions for culture-negative infected total knee arthroplasties: A five-year outcome in comparison with one-stage and two-stage revisions for culture-positive cases. J Orthop Sci. 2017. March;22(2):306-12. Epub 2016 Dec 18. [DOI] [PubMed] [Google Scholar]
- 10.Restrepo C, Schmitt S, Backstein D, Alexander BT, Babic M, Brause BD, Esterhai JL, Good RP, Jørgensen PH, Lee P, Marculescu C, Mella C, Perka C, Pour AE, Rubash HE, Saito T, Suarez R, Townsend R, Tözün IR, Van den Bekerom MP. Antibiotic treatment and timing of reimplantation. J Arthroplasty. 2014. February;29(2)(Suppl):104-7. Epub 2013 Oct 1. [DOI] [PubMed] [Google Scholar]
- 11.Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014. July;29(7):1331. Epub 2014 Mar 21. [DOI] [PubMed] [Google Scholar]
- 12.Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD, Gambir A, Huang R, Kissin Y, Kobayashi H, Kobayashi N, Krenn V, Drago L, Marston SB, Meermans G, Perez J, Ploegmakers JJ, Rosenberg A, Simpendorfer C, Thomas P, Tohtz S, Villafuerte JA, Wahl P, Wagenaar FC, Witzo E. Diagnosis of periprosthetic joint infection. J Arthroplasty. 2014. February;29(2)(Suppl):77-83. Epub 2013 Dec 15. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res. 2013. July;471(7):2374-82. Epub 2013 Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen LH, Lange J, Xu Y, Schønheyder HC. Optimizing culture methods for diagnosis of prosthetic joint infections: a summary of modifications and improvements reported since 1995. J Med Microbiol. 2012. March;61(Pt 3):309-16. Epub 2012 Jan 5. [DOI] [PubMed] [Google Scholar]
- 15.Peel TN, Spelman T, Dylla BL, Hughes JG, Greenwood-Quaintance KE, Cheng AC, Mandrekar JN, Patel R. Optimal periprosthetic tissue specimen number for diagnosis of prosthetic joint infection. J Clin Microbiol. 2016. December 28;55(1):234-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peel TN, Dylla BL, Hughes JG, Lynch DT, Greenwood-Quaintance KE, Cheng AC, Mandrekar JN, Patel R. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. MBio. 2016. January 05;7(1):e01776-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008. October;128(10):1039-46. Epub 2007 Sep 15. [DOI] [PubMed] [Google Scholar]
- 18.Abdulmassih R, Makadia J, Como J, Paulson M, Min Z, Bhanot N. Propionibacterium acnes: time-to-positivity in standard bacterial culture from different anatomical sites. J Clin Med Res. 2016. December;8(12):916-8. Epub 2016 Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedenčič K, Kavčič M, Faganeli N, Mihalič R, Mavčič B, Dolenc J, Bajc Z, Trebše R. Does preoperative antimicrobial prophylaxis influence the diagnostic potential of periprosthetic tissues in hip or knee infections? Clin Orthop Relat Res. 2016. January;474(1):258-64. Epub 2015 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: Should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014. January;472(1):52-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wouthuyzen-Bakker M, Tornero E, Claret G, Bosch J, Martinez-Pastor JC, Combalia A, Soriano A. Withholding preoperative antibiotic prophylaxis in knee prosthesis revision: a retrospective analysis on culture results and risk of infection. J Arthroplasty. 2017. September;32(9):2829-33. Epub 2017 Apr 6. [DOI] [PubMed] [Google Scholar]
- 22.Choi HR, Kwon YM, Freiberg AA, Nelson SB, Malchau H. Periprosthetic joint infection with negative culture results: clinical characteristics and treatment outcome. J Arthroplasty. 2013. June;28(6):899-903. Epub 2013 Mar 20. [DOI] [PubMed] [Google Scholar]
- 23.Huang R, Hu CC, Adeli B, Mortazavi J, Parvizi J. Culture-negative periprosthetic joint infection does not preclude infection control. Clin Orthop Relat Res. 2012. October;470(10):2717-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan TL, Kheir MM, Tan DD, Parvizi J. Polymicrobial periprosthetic joint infections: outcome of treatment and identification of risk factors. J Bone Joint Surg Am. 2016. December 21;98(24):2082-8. [DOI] [PubMed] [Google Scholar]
- 25.Mortazavi SMJ, Vegari D, Ho A, Zmistowski B, Parvizi J. Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Relat Res. 2011. November;469(11):3049-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarabichi M, Shohat N, Goswami K, Alvand A, Silibovsky R, Belden K, Parvizi J. Diagnosis of periprosthetic joint infection: the potential of next generation sequencing. J Bone Joint Surg Am. 2018. January 17;100(2):147-54. [DOI] [PubMed] [Google Scholar]
- 27.Tarabichi M, Alvand A, Shohat N, Goswami K, Parvizi J. Diagnosis of Streptococcus canis periprosthetic joint infection: the utility of next-generation sequencing. Arthroplast Today. 2018. March;4(1):20-3. Epub 2017 Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012. December 19;94(24):2247-54. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Chang WH, Chen SC, Hsieh PH, Shih HN, Ueng SW, Lee GB. Molecular diagnosis of periprosthetic joint infection by quantitative RT-PCR of bacterial 16S ribosomal RNA. ScientificWorldJournal. 2013. December 17;2013:950548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothenberg AC, Wilson AE, Hayes JP, O’Malley MJ, Klatt BA. Sonication of arthroplasty implants improves accuracy of periprosthetic joint infection cultures. Clin Orthop Relat Res. 2017. July;475(7):1827-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007. August 16;357(7):654-63. [DOI] [PubMed] [Google Scholar]
- 32.Scorzolini L, Lichtner M, Iannetta M, Mengoni F, Russo G, Panni AS, Vasso M, Bove M, Villani C, Mastroianni CM, Vullo V. Sonication technique improves microbiological diagnosis in patients treated with antibiotics before surgery for prosthetic joint infections. New Microbiol. 2014. July;37(3):321-8. Epub 2014 Jul 1. [PubMed] [Google Scholar]
- 33.Portillo ME, Salvadó M, Sorli L, Alier A, Martínez S, Trampuz A, Gómez J, Puig L, Horcajada JP. Multiplex PCR of sonication fluid accurately differentiates between prosthetic joint infection and aseptic failure. J Infect. 2012. December;65(6):541-8. Epub 2012 Sep 4. [DOI] [PubMed] [Google Scholar]


