Abstract
Background.
Nuclear size is a tightly regulated cellular feature. Mechanisms that regulate nuclear size and the functional significance of this regulation are largely unknown. Nuclear size and morphology are often altered in many diseases, such as cancer. Therefore, understanding the mechanisms that regulate nuclear size is crucial to provide insight into the role of nuclear size in disease.
Scope and Approach.
The goal of this review is to summarize the most recent studies about the mechanisms and functional significance of nuclear size control using the Xenopus model system. First, this review describes how Xenopus egg extracts, embryos, and embryo extracts are prepared and used in scientific research. Next, the review focuses on the mechanisms and functional effects of proper nuclear size control that have been learned using the Xenopus system.
Key Findings and Conclusions.
Xenopus is an excellent in vivo and in vitro experimental platform to study mechanisms of nuclear size control. Given its close evolutionary relationship with mammals and that most cellular processes and pathways are highly conserved between Xenopus and humans, the Xenopus system has been a valuable tool to advance biomedical research. Some of the mechanisms that regulate nuclear size include components of nuclear import such as importin α and NTF2, nuclear lamins, nucleoporins, proteins that regulate the morphology of the endoplasmic reticulum, and cytoskeletal elements.
Keywords: Nuclear size, Xenopus, cytoplasmic extract, embryo, midblastula transition, cancer
Kratak sadržaj
Uvod.
Veličina ćelijskog jedra je strogo regulisana odlika ćelije. Mehanizmi koji regulišu veličinu jedra ćelije, kao i funkcionalni značaj ove regulacije su malo poznati. Veličina i morfologija jedra su često poremećeni u mnogim bolestima, kao sto su karcinomi. Stoga, razumevanje mehanizama koji regulišu veličinu jedra je od krucijalnog značaja za razumevanje uloge veličine jedra u bolesti.
Cilj i pristup.
Cilj ovog rada je da se sumiraju najnovija istraživanja na temu mehanizama i funkcionalnog značaja regulacije veličine jedra koristeći Xenopus model sistem. Ovaj rad najpre opisuje pripremu i korišćenje Xenopus jajnog ekstrakta, embriona i embrionalnog ekstrakta u svrhe naučnog istraživanja. Rad se potom fokusira na opisivanje mehanizama i funkcionalnog značaja kontrole odgovarajuće veličine jedra koji su proučeni koristeći Xenopus sistem.
Ključni nalazi i zaključak.
Xenopus predstavlja odličnu in vivo i in vitro eksperimentalnu platformu za proučavanje mehanizama koji regulišu veličinu ćelijskog jedra. Obzirom na blisku evolutivnu vezu sa sisarima i to da je većina ćelijskih procesa i puteva visoko očuvana između Xenopus-a i ljudi, Xenopus predstavlja neprocenjivo oruđe za unapređenje bio-medicinskog istraživanja. Neki od mehanizama koji kontrolišu veličinu jedra uključuju komponente jedarnog transporta, kao što su importin α i NTF2, jedarni lamini, nukleoporini, proteini koji regulišu građu endoplazmatičnog retikuluma i elementi citoskeleta.
INTRODUCTION
Proper morphology of intracellular organelles is a precisely regulated cellular feature important for organellar function. The sizes of organelles, including the nucleus and mitotic spindle, generally positively scale with cell size to maintain a proper organelle-to-cytoplasmic volume ratio (Wuhr et al., 2008; Levy & Heald, 2012; Jevtic et al., 2015; Jevtic et al., 2016). Dysregulation of this ratio and organelle morphology affects normal cell, tissue, and organismal physiology and is linked to diseases. For example, defects in mitochondrial and lysosomal morphology and function are linked to neurodegenerative and encephalomyopathic diseases (Liesa et al., 2009; Hockey et al., 2015). Altered nuclear shape, size, and nuclear-to-cytoplasmic (N/C) volume ratio are associated with a variety of diseases that include Hutchinson-Gilford progeria syndrome, Emery-Dreifuss muscular dystrophy, limb-girdle muscular dystrophy, dilated cardiomyopathy, and different cancer types (Isermann & Lammerding, 2013; Jevtic et al., 2014; Jevtic & Levy, 2014). Nuclear size measurement has been long used by cytopathologists as an important morphological parameter to diagnose, stage and prognose many cancers (Zink et al., 2004; Jevtic & Levy, 2014). Changes in the N/C volume ratio have been implicated to regulate the onset of the midblastula transition (MBT) during early embryogenesis in many animal species (Kobayakawa & Kubota, 1981; Newport & Kirschner, 1982a, 1982b; Edgar et al., 1986; Kane & Kimmel, 1993; Jevtic & Levy, 2015).
Understanding mechanisms that regulate nuclear size is necessary to provide insight into the role of nuclear size in numerous diseases and physiological processes. In the past few years, a number of studies have begun to reveal mechanisms of nuclear size control and functional effects of this regulation. In this review, we focus our attention on the most exciting recent findings regarding nuclear size regulation using the Xenopus model system.
Xenopus model system
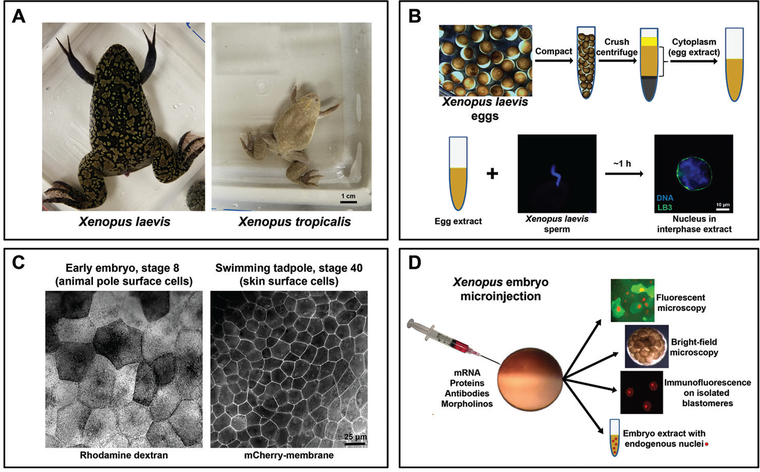
Two closely related Xenopus frog species are widely used as model organisms, the bigger pseudo-tetraploid X. laevis and smaller diploid X. tropicalis (Fig. 1A). The genomes of both Xenopus species have been sequenced and show high structural similarity with the human genome. There is a high percentage of homologous genes between Xenopus and human, and most cellular processes and pathways are highly conserved between the two (Hellsten et al., 2010; Session et al., 2016). This makes Xenopus a unique model system that is widely used to study molecular and cell biology and embryonic development and to model human diseases. Frog oocytes, eggs, and embryonic cells are large and easy to manipulate and microinject with different materials (Fig. 1C and1D). Moreover, cytoplasmic extracts can be prepared from both eggs and embryos providing a powerful in vitro cell-free system. Therefore, Xenopus frogs are excellent in vivo and in vitro experimental platforms to study mechanisms of nuclear size control.
Figure 1.
Xenopus is an important model system to study mechanisms and functions of nuclear size regulation. A. Comparison of adult frogs Xenopus laevis and Xenopus tropicalis. B. Eggs collected from the frog are compacted and fractionated by centrifugation, and cell-free cytoplasm is isolated. Addition of demembranated X. laevis sperm chromatin to egg extract triggers nuclear formation. LB3, lamin B3. C. Comparison of surface cells from a stage 8 embryo and stage 40 tadpole. The left image shows cells with fluorescent rhodamine-labeled dextran that serves as a tracer that was microinjected at the one-cell stage. The right image shows cells expressing mCherry fused to a membrane-targeting sequence expressed from mRNA microinjected at the one-cell stage. D. One-cell stage embryos can be microinjected with various materials to alter nuclear size, offering an excellent experimental approach to study the mechanisms and functional significance of nuclear size control. Live microinjected embryos expressing fluorescent proteins can be imaged by fluorescence microscopy to study changes in nuclear dynamics. Bright-field time lapse microscopy can be performed on whole microinjected embryos to quantify cell division timing. Single blastomeres can be isolated from embryos at different developmental stages for nuclear staining, imaging, and quantification. Different stage microinjected embryos can be used to generate embryo extracts containing endogenous nuclei, which can be further used for biochemical experiments, nuclear isolation, immunofluorescence studies, immunoblot analyses, etc.
Xenopus egg extracts, embryos and embryo extracts
Xenopus egg extract is a robust cell-free in vitro system consisting of undiluted cytoplasm. Xenopus female frogs are primed and induced to lay eggs with gonadotropin hormone injections. After a series of buffer washes, eggs are subjected to a centrifugation crushing step and the nearly undiluted cytoplasm is collected. This extract contains all of the proteins and membranes necessary to assemble organelles in vitro but lacks egg chromosomes (Fig. 1B) (Murray, 1991; Desai et al., 1999; Brown et al., 2007). Addition of demembranated Xenopus sperm chromatin triggers nuclear envelope (NE) assembly around each mass of sperm chromatin, ultimately giving rise to import-competent functional nuclei (Fig. 1B) (Chan & Forbes, 2006). Because it is an open biochemical system, recombinant proteins can be added and/or endogenous proteins can be immunodepleted from the egg extract. The extract can be supplemented with small chemical molecules to activate or inhibit different cellular processes that might regulate organelle size. Addition of fluorescently labeled proteins enables visualization of intracellular structures, performance of functional assays, and live time lapse microscopy to study organelle dynamics.
Xenopus eggs can be in vitro fertilized with crushed frog testes to generate hundreds of synchronized embryos. Xenopus early embryogenesis represents a powerful cellular scaling system, because cell divisions are rapid but without changes in the size of the embryo itself. The ~1.2 mm diameter single cell embryo undergoes twelve rapid, synchronous cell cycles (each 25–30 minutes long) to reach the first important developmental transition called the midblastula transition (MBT) or stage 8 containing more than 4000 ~100 μm diameter cells (Nieuwkoop & Faber, 1967; Jevtic & Levy, 2015). A few hours later, embryos proceed through gastrulation (stages 10.5–12) with further reductions in cell size, reaching ~ 20 μm diameter cells in the tadpole (our unpublished measurements) (Fig. 1C). As cell sizes decrease during early development, nuclei also scale smaller, providing an excellent system to study the mechanisms and functional significance of nuclear size regulation during early embryogenesis (Edens & Levy, 2014; Jevtic et al., 2015; Jevtic & Levy, 2015; Edens et al., 2017). Embryos can be microinjected with mRNAs to ectopically overexpress proteins of interest, antibodies or small chemical molecules to inhibit proteins, or morpholino oligonucleotides to deplete protein levels (Fig. 1D). Therefore, Xenopus embryos provide a robust in vivo system to study mechanisms of developmental organelle size regulation.
Similar to egg extract, embryo extract can be prepared from different developmental stages (Jevtic & Levy, 2015; Edens & Levy, 2016). Embryo extracts contain their endogenous nuclei, which is an advantage compared to egg extracts because nuclei can be easily studied in vitro in their native cytoplasm (Fig. 1D).
Nuclear structure
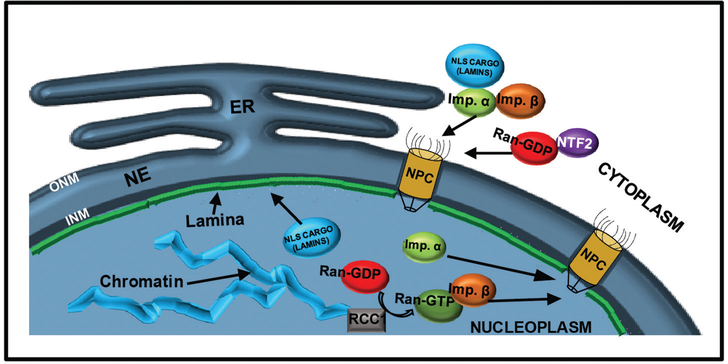
Many structural components of the nucleus are involved in nuclear morphology control. The nucleus is a large membrane-bound organelle that separates the genome from the cytoplasm. The NE consists of two phospholipid bilayers, the inner nuclear membrane (INM) and the outer nuclear membrane (ONM). The ONM is continuous with the endoplasmic reticulum (ER), and NE assembly and growth depend on intact ER membranes (Anderson & Hetzer, 2007). The INM is lined by the nuclear lamina, a meshwork of intermediate lamin filaments that provides mechanical support to the NE and connects it to the chromatin (Fig. 2). The nuclear lamina generally consists of three types of nuclear lamin proteins that include lamin A/C (LA), lamin B1 (LB1), and lamin B2 (LB2). Lamin B3 (LB3) is a frog oocyte specific lamin protein and it is the only lamin isoform present in Xenopus eggs and cleavage stage embryos. The INM meets the ONM at the nuclear pore complex (NPC) that mediates nucleocytoplasmic transport of molecules. Classical nuclear import is regulated by nuclear import receptors such as importin α, importin β, and nuclear transport factor 2 (NTF2). In the cytoplasm, importin α binds to nuclear localization signal (NLS) containing cargo proteins and importin β, which interacts with the NPC. Once this complex enters the nucleus, Ran-GTP binds to importin β causing the release of NLS cargo from importin α. NTF2 binds to Ran-GDP in the cytosol and shuttles it back to the nucleus where Ran-GDP is converted to Ran-GTP by its guanine nucleotide exchange factor, chromatin-bound RCC1 (Fig. 2) (Gruenbaum et al., 2003; Anderson & Hetzer, 2007, 2008; Dechat et al., 2008; Walters et al., 2012).
Figure 2.
Schematic representation of nuclear structure and transport. Nuclear envelope (NE); Outer nuclear membrane (ONM); Inner nuclear membrane (INM); Endoplasmic reticulum (ER); Nuclear pore complex (NPC); Nuclear localization signal (NLS); Importin (Imp.).
Mechanisms of nuclear size regulation
Interspecies nuclear scaling has been recapitulated studying nuclei assembled in egg extracts from the bigger pseudo-tetraploid X. laevis, and the smaller diploid X. tropicalis. Larger nuclei formed in X. laevis egg extract, correlating with a higher nuclear import rate, importin α concentration, and rate of LB3 import. Conversely, X. tropicalis egg extract, in which smaller nuclei formed, had a higher concentration of NTF2, lower concentration of importin α, and reduced bulk and LB3 import rates (Levy & Heald, 2010). Altering the levels of importin α and NTF2 was sufficient to explain observed differences in nuclear size and import of LB3, the only egg lamin known to be required for NE growth (Newport et al., 1990). To demonstrate that titratable cytoplasmic factors are major determinants of nuclear size as opposed to ploidy, X. tropicalis sperm, which has approximately half the DNA content of X. laevis sperm, was added to X. laevis egg extract. Generated nuclei were only slightly smaller than nuclei assembled using X. laevis sperm (Levy & Heald, 2010). Reductions in nuclear size during X. laevis early development also correlated with reduced cytoplasmic importin α levels and bulk import at the MBT. Overexpression of importin α and/or LB3 in early X. laevis embryos was sufficient to increase nuclear size at the MBT, showing that changes in the import rate and import of LB3 contribute to developmental nuclear scaling in MBT embryos (Levy & Heald, 2010; Jevtic & Levy, 2015).
Both the total lamin concentration and the expression of different lamin isoforms change during early X. laevis development (Stick & Hausen, 1985; Jevtic et al., 2015). To test how nuclear lamin isoforms and expression levels affect nuclear size in vitro, X. laevis egg and embryo extracts were supplemented with increasing concentrations of different single recombinant lamin proteins (LB3, LA, LB1, and LB2) or combinations of different lamin proteins. Similarly, one cell-stage embryos were microinjected with different concentrations of single recombinant lamins or lamin combinations. Both in vitro and in vivo, we found that nuclear size was sensitive to changes in total lamin levels irrespective of lamin type. Ectopic addition of low concentrations of any single lamin type increased nuclear size. Conversely, high concentrations of any single lamin isoform or combination of different lamins decreased nuclear size. Based on these results we propose that total lamin expression levels influence nuclear size, dependent on the developmental stage and cell type (Jevtic et al., 2015).
To further elucidate the mechanism of action of NTF2, we studied two different NTF2 point mutants, one that is defective for Ran binding and another with reduced affinity for the NPC. We found that the NTF2 Ran binding mutant failed to inhibit nuclear growth and import of large cargos, such as LB3, in X. laevis egg extract. The NTF2 mutant with reduced affinity for the NPC was also defective in limiting nuclear growth, suggesting that binding of NTF2 to the NPC also contributes to nuclear size regulation. Transmission electron microscopy experiments showed that overexpression of NTF2 decreases the apparent diameter of the NPC, while overexpression of the NTF2 Ran binding mutant largely failed to change NPC size. Furthermore, ectopic NTF2 expression in X. laevis early embryos also caused altered nuclear size. These data suggest that increased levels of NTF2 bound to Ran at the NPC reduce the effective diameter of the NPC pore to inhibit import of large cargos and nuclear growth (Vukovic et al., 2016).
As already mentioned, nuclear size reductions correlated with decreased bulk import and cytoplasmic importin α levels during the early cleavage stages of embryogenesis and up to the MBT. From the MBT through gastrulation, nuclear size decreased further and this reduction in size correlated with and was dependent on increased activity and intranuclear localization of conventional protein kinase C (cPKC) (Edens & Levy, 2014). Further study identified a novel cPKC phosphorylation site in LB3. Nuclear size was sensitive to phosphorylation at this site, and X. laevis gastrula stage embryos overexpressing a mutant version of LB3 that cannot be phosphorylated at this site exhibited larger nuclei. Conversely, overexpression of phosphomimetic mutant LB3 caused smaller nuclei. Furthermore, confocal fluorescence recovery after photobleaching experiments showed that cPKC mediated phosphorylation of LB3 increased lamina dynamics. This suggests that cPKC interphase phosphorylation of lamin proteins contributes to their decreased localization to the NE and reduced nuclear size (Edens et al., 2017).
Xenopus studies have also revealed how other structural elements of the nucleus, the ER, and the cytoskeleton can determine nuclear size. Nucleoporins (Nups) are important constituents of the NPC and have been shown to regulate nuclear expansion. Depletion of Nup188 from Xenopus egg extract caused an increase in nuclear size and import of INM proteins (Shaulov et al., 2011). Conversely, smaller nuclei were generated by supplementing Xenopus egg extract with a dominant-negative fragment of Nup POM121 (Shaulov et al., 2011).
The ER is an interconnected membrane network that is directly connected to the NE and consists of highly curved ER tubules and flat ER sheet membranes. Proteins in the reticulon family (Rtn) that contain hydrophobic transmembrane domains shape ER membranes into highly curved tubules (Voeltz et al., 2006). Given that the NE is continuous with the ER, alterations in ER morphology and ER sheet membrane amounts can affect NE reassembly and nuclear size. Levels of Rtns can impact nuclear size through a tug-of-war competition that exists between ER tubule membranes and the NE. Higher Rtn levels increased the proportion of ER tubular membranes, decreased ER sheet membranes, and resulted in smaller nuclear sizes (Anderson & Hetzer, 2008). Overexpression of Rtn4a or Rtn4b proteins in early X. laevis embryos altered nuclear size (Jevtic & Levy, 2015). Post-mitotic nuclear reformation is mediated by targeting of ER tubules to chromosomes and spreading of membrane around chromatin. Disrupting ER tubule formation in egg extract using Rtn4a neutralizing antibodies caused a failure in nuclear formation. Furthermore, small nuclei were formed when ER membranes were disturbed using shear mechanical stress, and NE growth resumed upon ER network reformation (Anderson & Hetzer, 2007).
Nuclear scaling has been recapitulated using microfluidic technology that involves encapsulation of Xenopus egg extract and preassembled nuclei into microfluidic chambers of defined dimensions. Nuclear growth positively scaled with chamber dimensions and the amount of available cytoplasm, and above a certain threshold volume of available cytoplasm, nuclear growth reached a plateau. This threshold volume corresponded to the size of the centrosomal array of microtubules emanating from the NE. It was proposed that the volume occupied by this microtubule aster regulates nuclear expansion by determining the rate of dynein-mediated transport of membranes to the nucleus (Hara & Merten, 2015).
Functional significance of nuclear size during the MBT
The MBT is the first transition stage during early embryonic development when abrupt zygotic transcription is activated and cell cycles become longer and asynchronous. This critical developmental transition is the trigger for cell differentiation and prepares the embryo for upcoming gastrulation. It has been proposed that the ratio of the total DNA amount to total cytoplasmic volume in the embryo (DNA-to-cytoplasm ratio) controls MBT timing (Kobayakawa & Kubota, 1981; Newport & Kirschner, 1982a, 1982b; Edgar et al., 1986). The model is that when a critical total amount of DNA reaches a threshold at this stage of development, maternally loaded MBT inhibitors become fully titrated by the DNA, leading to MBT onset.
Prior to MBT, both nuclear and cytoplasmic average volumes decrease, ~three-fold and ~70-fold, respectively. As a consequence, the N/C volume ratio increases rapidly during early X. laevis development, reaching maximum around MBT. To test the contribution of nuclear size to MBT timing regulation, we altered nuclear size in early X. laevis embryos by microinjecting mRNAs to ectopically alter the expression of nuclear transport factors, nuclear lamins, or tubule-shaping components of the ER, followed by monitoring for cellular and molecular hallmarks of the MBT. Increasing nuclear size increased the N/C volume ratio and led to premature onset of zygotic transcription and cell cycle lengthening. Conversely, decreasing nuclear size decreased the N/C volume ratio and delayed the onset of the MBT (Jevtic & Levy, 2015).
As a follow-up to these studies, we tested the relative contributions of DNA amount and the N/C volume ratio to MBT timing control, by simultaneously altering nuclear size and ploidy in X. laevis embryos. Compared to diploid embryos with control nuclear size, haploid control embryos exhibited a delay in the onset of zygotic transcription and cell cycle lengthening. Both haploid and diploid embryos with increased nuclear size expressed higher levels of zygotic transcripts and exhibited premature lengthening of cell cycles compared to their haploid and diploid control counterparts. Interestingly, haploids with increased nuclear size showed intermediate timing effects, while reducing N/C volume ratios in haploid embryos further delayed MBT onset. These data suggest that neither mechanism has a dominant effect in determining MBT timing, with both DNA amount and the N/C volume ratio contributing to the regulation of MBT timing (Jevtic & Levy, 2017).
How might nuclear volume regulate MBT timing? Recently, a few potential DNA-binding MBT inhibitors have been identified (Collart et al., 2013; Murphy & Michael, 2013; Amodeo et al., 2015; Collart et al., 2017), but none of these factors fully account for the extent of changes in zygotic gene expression and cell cycle duration associated with the MBT. Perhaps changes in total embryonic nuclear volume during development determine nuclear concentrations of maternally loaded MBT inhibitors. Another possibility is that changes in NE surface area may regulate import capacity and import of potential DNA-binding limiting components. It seems likely that the MBT is controlled by multifactorial intertwining mechanisms, with some zygotic genes being more sensitive to changes in DNA amount, while other zygotic genes preferentially respond to changes in the N/C volume ratio (Veenstra, 2002; Jevtić and Levy, 2017).
CONCLUSION
Because of its close evolutionary relationship with mammals, the Xenopus system has been a valuable tool to advance biomedical research. Its extensive use has contributed many important discoveries in the fields of molecular biology, cell biology, developmental biology, physiology, and neurobiology (Dominguez-Sola et al., 2007; Fuller et al., 2008; Raschle et al., 2008; Bajpai et al., 2010; Cruciat et al., 2010; Hellsten et al., 2010; Levy & Heald, 2010; Poulsen et al., 2010). Advances in existing and emerging technologies, such as microfluidics (Liu & Singh, 2013; Bermudez et al., 2017), microscopy (Zumbusch et al., 2013; Puah et al., 2017), and high-throughput imaging (Shachar et al., 2015), will offer many new approaches for studying nuclear size regulation in the Xenopus system.
Nuclear size changes during early embryogenesis, differentiation, and cell division to maintain proper N/C volume ratios, which must have important effects on cell physiology. Changes in nuclear size are likely to affect nuclear and sub-nuclear function by altering chromatin organization and gene expression (Schuster-Bockler & Lehner, 2012). Aberrations in nuclear size and the N/C volume ratio are often associated with different disease states, namely cancers (Jevtic & Levy, 2014). It is largely unknown what the link is between altered nuclear size and cancer and how deregulated nuclear size and N/C volume ratios contribute to carcinogenesis. Therefore, understanding the mechanisms and functional significance of nuclear size control will shed light on the role of the cancer nucleus in pathogenesis and diagnosis.
Acknowledgements
Work in the Levy laboratory is supported by the NIH (R01GM113028) and American Cancer Society (RSG-15–035-01-DDC).
Footnotes
Competing interests
The authors declare that they have no competing interests.
REFERENCES
- Amodeo AA, Jukam D, Straight AF, Skotheim JM 2015. Histone titration against the genome sets the DNA-to-cytoplasm threshold for the Xenopus midblastula transition. Proc Natl Acad Sci U S A, 112(10), E1086–1095. doi: 10.1073/pnas.1413990112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Hetzer MW 2007. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol, 9(10), 1160–1166. doi: 10.1038/ncb1636 [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Hetzer MW 2008. Reshaping of the endoplasmic reticulum limits the rate for nuclear envelope formation. J Cell Biol, 182(5), 911–924. doi: 10.1083/jcb.200805140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, Helms J, Chang CP, Zhao Y, Swigut T, Wysocka J 2010. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature, 463(7283), 958–962. doi: 10.1038/nature08733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez JG, Chen H, Einstein LC, Good MC 2017. Probing the biology of cell boundary conditions through confinement of Xenopus cell-free cytoplasmic extracts. Genesis, 55(1–2). doi: 10.1002/dvg.23013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KS, Blower MD, Maresca TJ, Grammer TC, Harland RM, Heald R 2007. Xenopus tropicalis egg extracts provide insight into scaling of the mitotic spindle. J Cell Biol, 176(6), 765–770. doi: 10.1083/jcb.200610043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Forbes DI 2006. In vitro study of nuclear assembly and nuclear import using Xenopus egg extracts. Methods Mol Biol, 322, 289–300. [DOI] [PubMed] [Google Scholar]
- Collart C, Allen GE, Bradshaw CR, Smith JC, Zegerman P 2013. Titration of four replication factors is essential for the Xenopus laevis midblastula transition. Science, 341(6148), 893–896. doi: 10.1126/science.1241530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart C, Smith JC, Zegerman P 2017. Chk1 Inhibition of the Replication Factor Drf1 Guarantees Cell-Cycle Elongation at the Xenopus laevis Mid-blastula Transition. Dev Cell, 42(1), 82–96 e83. doi: 10.1016/j.devcel.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C 2010. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science, 327(5964), 459–463. doi: 10.1126/science.1179802 [DOI] [PubMed] [Google Scholar]
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD 2008. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev, 22(7), 832–853. doi: 10.1101/gad.1652708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Murray A, Mitchison TJ, Walczak CE 1999. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol, 61, 385–412. [DOI] [PubMed] [Google Scholar]
- Dominguez-Sola D, Ying CY, Grandori C, Ruggiero L, Chen B, Li M, Galloway DA, Gu W, Gautier J, Dalla-Favera R 2007. Non-transcriptional control of DNA replication by c-Myc. Nature, 448(7152), 445–451. doi: 10.1038/nature05953 [DOI] [PubMed] [Google Scholar]
- Edens LJ, Dilsaver MR, Levy DL 2017. PKC-mediated phosphorylation of nuclear lamins at a single serine residue regulates interphase nuclear size in Xenopus and mammalian cells. Mol Biol Cell, 28(10), 1389–1399. doi: 10.1091/mbc.E16-11-0786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens LJ, Levy DL 2014. cPKC regulates interphase nuclear size during Xenopus development. J Cell Biol, 206(4), 473–483. doi: 10.1083/jcb.201406004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens LJ, Levy DL 2016. A Cell-Free Assay Using Xenopus laevis Embryo Extracts to Study Mechanisms of Nuclear Size Regulation. J Vis Exp(114). doi: 10.3791/54173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, Kiehle CP, Schubiger G 1986. Cell cycle control by the nucleo-cytoplasmic ratio in early Drosophila development. Cell, 44(2), 365–372. doi: 0092–8674(86)90771–3 [pii] [DOI] [PubMed] [Google Scholar]
- Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P, Brautigan DL, Stukenberg PT, Kapoor TM 2008. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature, 453(7198), 1132–1136. doi: 10.1038/nature06923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y, Goldman RD, Meyuhas R, Mills E, Margalit A, Fridkin A, Dayani Y, Prokocimer M, Enosh A 2003. The nuclear lamina and its functions in the nucleus. Int Rev Cytol, 226, 1–62. [DOI] [PubMed] [Google Scholar]
- Hara Y, Merten CA 2015. Dynein-Based Accumulation of Membranes Regulates Nuclear Expansion in Xenopus laevis Egg Extracts. Dev Cell, 33(5), 562–575. doi: 10.1016/j.devcel.2015.04.016 [DOI] [PubMed] [Google Scholar]
- Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, Ovcharenko I, Putnam NH, Shu S, Taher L, Blitz IL, Blumberg B, Dichmann DS, Dubchak I, Amaya E, Detter JC, Fletcher R, Gerhard DS, Goodstein D, Graves T, Grigoriev IV, Grimwood J, Kawashima T, Lindquist E, Lucas SM, Mead PE, Mitros T, Ogino H, Ohta Y, Poliakov AV, Pollet N, Robert J, Salamov A, Sater AK, Schmutz J, Terry A, Vize PD, Warren WC, Wells D, Wills A, Wilson RK, Zimmerman LB, Zorn AM, Grainger R, Grammer T, Khokha MK, Richardson PM, Rokhsar DS 2010. The genome of the Western clawed frog Xenopus tropicalis. Science, 328(5978), 633–636. doi: 10.1126/science.1183670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S 2015. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci, 128(2), 232–238. doi: 10.1242/jcs.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isermann P, Lammerding J 2013. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol, 23(24), R1113–1121. doi: 10.1016/j.cub.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P, Edens LJ, Li X, Nguyen T, Chen P, Levy DL 2015. Concentration-dependent Effects of Nuclear Lamins on Nuclear Size in Xenopus and Mammalian Cells. J Biol Chem, 290(46), 27557–27571. doi: 10.1074/jbc.M115.673798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P, Edens LJ, Vukovic LD, Levy DL 2014. Sizing and shaping the nucleus: mechanisms and significance. Curr Opin Cell Biol, 28, 16–27. doi: 10.1016/j.ceb.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P, Levy DL 2014. Mechanisms of nuclear size regulation in model systems and cancer. Adv Exp Med Biol, 773, 537–569. doi: 10.1007/978-1-4899-8032-8_25 [DOI] [PubMed] [Google Scholar]
- Jevtic P, Levy DL 2015. Nuclear size scaling during Xenopus early development contributes to midblastula transition timing. Curr Biol, 25(1), 45–52. doi: 10.1016/j.cub.2014.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P, Levy DL 2017. Both Nuclear Size and DNA Amount Contribute to Midblastula Transition Timing in Xenopus laevis. Sci Rep, 7(1), 7908. doi: 10.1038/s41598-017-08243-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P, Milunovic-Jevtic A, Dilsaver MR, Gatlin JC, Levy DL 2016. Use of Xenopus cell-free extracts to study size regulation of subcellular structures. Int J Dev Biol, 60(7–8-9), 277–288. doi: 10.1387/ijdb.160158dl [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane DA, Kimmel CB 1993. The zebrafish midblastula transition. Development, 119(2), 447–456. [DOI] [PubMed] [Google Scholar]
- Kobayakawa Y, Kubota HY 1981. Temporal pattern of cleavage and the onset of gastrulation in amphibian embryos developed from eggs with the reduced cytoplasm. J Embryol Exp Morphol, 62, 83–94. [PubMed] [Google Scholar]
- Levy DL, Heald R 2010. Nuclear size is regulated by importin alpha and Ntf2 in Xenopus. Cell, 143(2), 288–298. doi: 10.1016/j.cell.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DL, Heald R 2012. Mechanisms of intracellular scaling. Annu Rev Cell Dev Biol, 28, 113–135. doi: 10.1146/annurev-cellbio-092910-154158 [DOI] [PubMed] [Google Scholar]
- Liesa M, Palacin M, Zorzano A 2009. Mitochondrial dynamics in mammalian health and disease. Physiol Rev, 89(3), 799–845. doi: 10.1152/physrev.00030.2008 [DOI] [PubMed] [Google Scholar]
- Liu Y, Singh AK 2013. Microfluidic platforms for single-cell protein analysis. J Lab Autom, 18(6), 446–454. doi: 10.1177/2211068213494389 [DOI] [PubMed] [Google Scholar]
- Murphy CM, Michael WM 2013. Control of DNA replication by the nucleus/cytoplasm ratio in Xenopus. J Biol Chem, 288(41), 29382–29393. doi: 10.1074/jbc.M113.499012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AW 1991. Cell cycle extracts. Methods Cell Biol, 36, 581–605. doi: 10.1016/S0091-679X(08)60298-8 [PubMed] [Google Scholar]
- Newport J, Kirschner M 1982a. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell, 30(3), 675–686. [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M 1982b. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell, 30(3), 687–696. [DOI] [PubMed] [Google Scholar]
- Newport JW, Wilson KL, Dunphy WG 1990. A lamin-independent pathway for nuclear envelope assembly. J Cell Biol, 111(6 Pt 1), 2247–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J 1967. Normal Table of Xenopus laevis (Daudin) (2nd ed.). Amsterdam: North-Holland Publishing Company. [Google Scholar]
- Poulsen H, Khandelia H, Morth JP, Bublitz M, Mouritsen OG, Egebjerg J, Nissen P 2010. Neurological disease mutations compromise a C-terminal ion pathway in the Na(+)/K(+)-ATPase. Nature, 467(7311), 99–102. doi: 10.1038/nature09309 [DOI] [PubMed] [Google Scholar]
- Puah WC, Chinta R, Wasser M 2017. Quantitative microscopy uncovers ploidy changes during mitosis in live Drosophila embryos and their effect on nuclear size. Biol Open, 6(3), 390–401. doi: 10.1242/bio.022079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle M, Knipscheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger TE, Scharer OD, Walter JC 2008. Mechanism of replication-coupled DNA interstrand crosslink repair. Cell, 134(6), 969–980. doi: 10.1016/j.cell.2008.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster-Bockler B, Lehner B 2012. Chromatin organization is a major influence on regional mutation rates in human cancer cells. Nature, 488(7412), 504–507. doi: 10.1038/nature11273 [DOI] [PubMed] [Google Scholar]
- Session AM, Uno Y, Kwon T, Chapman JA, Toyoda A, Takahashi S, Fukui A, Hikosaka A, Suzuki A, Kondo M, van Heeringen SJ, Quigley I, Heinz S, Ogino H, Ochi H, Hellsten U, Lyons JB, Simakov O, Putnam N, Stites J, Kuroki Y, Tanaka T, Michiue T, Watanabe M, Bogdanovic O, Lister R, Georgiou G, Paranjpe SS, van Kruijsbergen I, Shu S, Carlson J, Kinoshita T, Ohta Y, Mawaribuchi S, Jenkins J, Grimwood J, Schmutz J, Mitros T, Mozaffari SV, Suzuki Y, Haramoto Y, Yamamoto TS, Takagi C, Heald R, Miller K, Haudenschild C, Kitzman J, Nakayama T, Izutsu Y, Robert J, Fortriede J, Burns K, Lotay V, Karimi K, Yasuoka Y, Dichmann DS, Flajnik MF, Houston DW, Shendure J, DuPasquier L, Vize PD, Zorn AM, Ito M, Marcotte EM, Wallingford JB, Ito Y, Asashima M, Ueno N, Matsuda Y, Veenstra GJ, Fujiyama A, Harland RM, Taira M, Rokhsar DS 2016. Genome evolution in the allotetraploid frog Xenopus laevis. Nature, 538(7625), 336–343. doi: 10.1038/nature19840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachar S, Voss TC, Pegoraro G, Sciascia N, Misteli T 2015. Identification of Gene Positioning Factors Using High-Throughput Imaging Mapping. Cell, 162(4), 911–923. doi: 10.1016/j.cell.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulov L, Gruber R, Cohen I, Harel A 2011. A dominant-negative form of POM121 binds chromatin and disrupts the two separate modes of nuclear pore assembly. J Cell Sci, 124(Pt 22), 3822–3834. doi: 10.1242/jcs.086660 [DOI] [PubMed] [Google Scholar]
- Stick R, Hausen P 1985. Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell, 41(1), 191–200. [DOI] [PubMed] [Google Scholar]
- Veenstra GJ 2002. Early Embryonic Gene Transcription in Xenopus. Advances in Developmental Biology and Biochemistry, 12, 85–105. [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA 2006. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell, 124(3), 573–586. doi: 10.1016/j.cell.2005.11.047 [DOI] [PubMed] [Google Scholar]
- Vukovic LD, Jevtic P, Zhang Z, Stohr BA, Levy DL 2016. Nuclear size is sensitive to NTF2 protein levels in a manner dependent on Ran binding. J Cell Sci, 129(6), 1115–1127. doi: 10.1242/jcs.181263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters AD, Bommakanti A, Cohen-Fix O 2012. Shaping the nucleus: Factors and forces. J Cell Biochem, 113(9), 2813–2821. doi: 10.1002/jcb.24178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhr M, Chen Y, Dumont S, Groen AC, Needleman DJ, Salic A, Mitchison TJ 2008. Evidence for an upper limit to mitotic spindle length. Curr Biol, 18(16), 1256–1261. doi: 10.1016/j.cub.2008.07.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink D, Fischer AH, Nickerson JA 2004. Nuclear structure in cancer cells. Nat Rev Cancer, 4(9), 677–687. doi: 10.1038/nrc1430 [DOI] [PubMed] [Google Scholar]
- Zumbusch A, Langbein W, Borri P 2013. Nonlinear vibrational microscopy applied to lipid biology. Prog Lipid Res, 52(4), 615–632. doi: 10.1016/j.plipres.2013.07.003 [DOI] [PubMed] [Google Scholar]