Abstract
Cyclin-dependent kinase 12 (CDK12) belongs to the cyclin-dependent kinase (CDK) family of serine/threonine protein kinases that regulate transcriptional and post-transcriptional processes, thereby modulating multiple cellular functions. Early studies characterized CDK12 as a transcriptional CDK that complexes with cyclin K to mediate gene transcription by phosphorylating RNA polymerase II. CDK12 has been demonstrated to specifically upregulate the expression of genes involved in response to DNA damage, stress, and heat shock. More recent studies have implicated CDK12 in regulating mRNA splicing, 3’ end processing, pre-replication complex assembly, and genomic stability during embryonic development. Genomic alterations in CDK12 have been detected in esophageal, stomach, breast, endometrial, uterine, ovarian, bladder, colorectal, and pancreatic cancers, ranging from 5–15% of sequenced cases. An increasing number of studies point to CDK12 inhibition as an effective strategy to inhibit tumor growth, and synthetic lethal interactions have been described with MYC, EWS/FLI, and PARP/CHK1 inhibition. Herein, we discuss the present literature on CDK12 in cell function and human cancer, highlighting important roles for CDK12 as a clinical biomarker for treatment response and potential as an effective therapeutic target.
Keywords: cancer genetics, cell biology, C-MYC oncogene, breast cancer, ovarian cancer
INTRODUCTION
CDK12 (cyclin-dependent kinase 12; CRKRS, CRKR, or CRK7) was first identified from cDNA screens for cell cycle regulators related to cdc2 kinases[1]. In contrast to cyclin-dependent kinases (CDKs) that promote transition between different phases of the cell cycle, CDK12 is a transcriptional CDK with specific roles in regulating transcription of genes involved in cellular responses to DNA damage and stress[2–4]. Emerging studies continue to dissect the role of CDK12 in cell function and cancer and have illuminated its potential clinical use as a biomarker and therapeutic target.
STRUCTURE & EXPRESSION
On chromosome 17q12, the CDK12 gene encodes a 1490 amino acid protein with a molecular weight of 164 kDa[5]. The closely related CDK13 (located on 7p14) shares 43% sequence homology and a largely conserved kinase domain (Figure 1). The central kinase domain (KD) mediates phosphorylation of RNA Pol II [6], consisting of ~300 amino acids that shares 42% identity to human cdc2 and featuring characteristic analogous threonine and tyrosine residues required for cdc2 inactivation[5].
Figure 1.
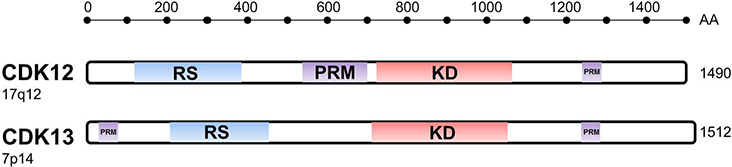
Schematic structures of the CDK12 and CDK13 genes and chromosomal location of the respective genes. RS = arginine/serine rich motifs; PRM = proline rich motifs; KD = kinase domain.
Arginine/serine rich (RS) motifs are critical components of proteins involved in pre-mRNA processing and can function as a nuclear localization signal[7]. CDK12 contains 21 RS motifs within the first 400 amino acids[5]. Proline rich motifs (PRM) are found between the RS domain and central kinase domain, as well as at the C-terminal region (Figure 1). These regions contain consensus binding sites for SRC Homology 3 (SH3) and WW domains, indicating potential protein interaction partners from a wide range of signaling pathways[8 9]. A unique C-terminal helix outside the canonical kinase fold of CDK12 facilitates its interaction with cyclin K[10 11]. The flexibility of this C-terminal extension was found to be critical for the kinase and ATP-binding activity of CDK12, and has directed the development of novel inhibitors to CDK12/13[12 13].
CDK12 is ubiquitously expressed, as demonstrated by human tissue northern blots in a panel of RNAs from different human tissues[5]. RNA sequencing analysis and immunohistochemical staining of 95 human individuals representing 27 tissue types also detected CDK12 in all tested tissues[14]. Compared to other tissues, higher CDK12 expression was generally detected in male and female reproductive tissues, endocrine tissues, bone marrow, spleen, and lymph nodes. Staining for CDK12 expression was also mainly localized to the nucleus, as suggested by its RS motifs and cellular functions[14].
FUNCTIONS
CDK12 functions as a complex with cyclin K, with its most well characterized roles in the regulation of gene transcription[3]. The strong functional link between CDK12 and cyclin K is reflected in the fact that knockdown of either protein results in similar phenotypes and affected genes, leading to genomic instability[3].
Transcription, mRNA processing, and the DNA damage response.
The CDK12/cyclin K complex phosphorylates RNA Pol II at Ser2 (Ser2p-RNA Pol II), which is thought to be a critical step in transition from transcriptional initiation to elongation[3 6 15 16] (Figure 2A). In vitro, CDK12/cyclin K was also shown to phosphorylate Ser5 of RNA Pol II, suggesting potential regulation of transcription initiation[11]. In cells depleted of CDK12, expression microarrays demonstrated that only 2.67% of tested microarray genes were altered[3]. Moreover, of the genes altered, the majority were downregulation of genes with large numbers of exons. Gene classification revealed an enrichment of genes involved in DNA replication, recombination, and repair centered on the BRCA1 module, and cells with knockdown of CDK12 had significantly lower levels of BRCA1, ATR, FANCI, and FANCD2[3]. CDK12 or cyclin K knockdown sensitized cells to DNA-damaging agents[3], suggesting that CDK12/cyclin K is a master regulator of proteins specifically involved in DNA damage repair (DDR) and response to DNA damage. CDK12 was also reportedly required for function of CncC, the Drosophila homolog of the stress-activated Nrf2 transcription factor, and expression of oxidative stress response genes, but not that of general housekeeping genes or cell viability[4]. Collectively, these studies indicate that CDK12 regulates specific subsets of genes involved in cellular responses to DNA damage, stress, and heat shock[3 4 6].
Figure 2.
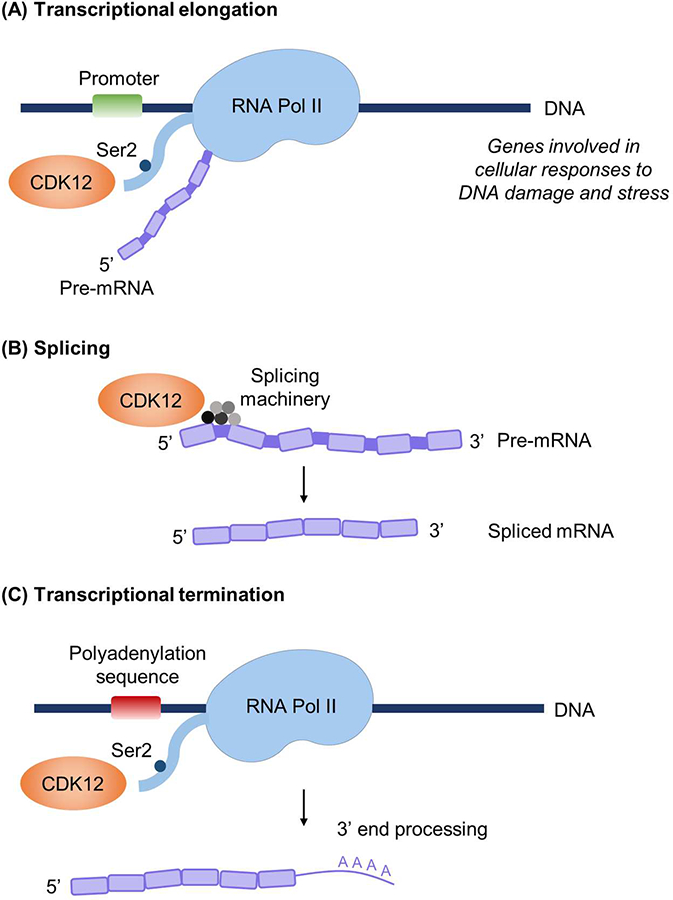
Known functions of CDK12. (A) CDK12 phosphorylates RNA polymerase II (RNA Pol II) at Ser2, which promotes transcriptional elongation. (B) CDK12 interacts with RNA-processing factors to regulate splicing. (C) CDK12-mediated phosphorylation of RNA Pol II couples transcription and mRNA 3’ end processing. CDK12 reportedly regulates the expression of a distinct subset of genes, including those involved in the DNA damage response, cellular stress, and heat shock.
The characteristic RS motifs of CDK12 strongly indicate functions in pre-mRNA processing[5]. Splicing factors are thought to be stored in subnuclear structures known as nuclear speckles[17], and CDK12 localizes to nuclear speckles and spliceosome components[5]. Indeed, mass spectrometry of CDK12-associated proteins identified a strong enrichment for RNA-processing factors[18] and an enrichment of genes involved in RNA splicing machinery[19] (Figure 2B). These studies also showed that CDK12 regulates the expression and alternative last exon (ALE) splicing of genes with long transcripts and large numbers of exons[19]. CDK12 can also indirectly regulate RNA processing by regulating phospho-epitopes on the C-terminal domain of RNA Pol II[18].
Ser2p-RNA Pol II couples transcription and mRNA 3’ end processing by interacting with polyadenylation and termination machinery at the 3’ ends of mRNA[20]. Davidson et al demonstrated that CDK12-mediated phosphorylation of Ser2p-RNA Pol II recruits the cleavage and polyadenylation factor CstF77 to ensure efficient 3’ end formation[21] (Figure 2C). CDK12 was demonstrated to be required for optimal pre-mRNA processing of the MYC gene, with gene depletion reducing levels of polyadenylated MYC RNA[21].
DNA Replication.
A recent study demonstrated that the CDK12/cyclin K complex is required for mammalian cell proliferation[22]. Specifically, CDK12/cyclin K mediates phosphorylation of cyclin E1 at Ser366, which blocks interaction with its binding partner, CDK2, during prereplicative complex assembly in early G1 phase[22]. Although cyclin E/CDK2 normally accumulates at the G1/S transition to promote S phase entry, aberrant cyclin E1/CDK2 activity in early G1 has been shown to inhibit pre-replicative complex formation[23]. Regulation of efficient pre-replication complex assembly by CDK12/cyclin K suggests novel roles in mediating crosstalk between DNA replication and gene transcription[22].
Development.
CDK12 expression is critical in mouse embryonic development. In vivo, Cdk12 activity was found to be critical from stage E3.5, and Cdk12 deficiency leads to arrest and embryonic lethality by stage E6.5[24]. Cdk12−/− embryos grown in vitro display spontaneous DNA damage and reduced expression of DNA damage response genes, including Atr, Brca1, Fanci, and Fancd2[24]. CDK12/13 and cyclin K are also required for self-renewal in embryonic stem cells, with knockdown of these proteins leading to differentiation[25]. Roles in proper development of neural cells have been described, with mice expressing conditional deletion of Cdk12 in neural progenitor cells (NPCs) dying shortly after birth and exhibiting microcephaly. NPCs of these mutant mice also displayed lower expression of DDR genes, increased double-strand breaks, and increased apoptosis[26]. These findings recapitulate critical roles for CDK12 in maintaining genomic stability and expression of DDR genes in development.
CDK12 and CDK13 are known to share similar biological processes, with both regulating RNA splicing and alternative splicing by virtue of their RS motifs[27–29], maintaining self-renewal in mouse embryonic stem cells[25], and regulating axonal elongation[30]. Despite their similarity in sequence and interaction with cyclin K, CDK12 and CDK13 do not have identical functions, and are likely to have evolved separately from a common ancestor gene[30]. Recent studies indicate that CDK13 regulates distinct subsets of genes and biological processes from CDK12, including snRNA and snoRNA gene expression[31], and extracellular/growth signaling pathways[32].
CDK12 MUTATIONS IN HUMAN TUMORS
Analysis of the CDK12 gene across The Cancer Genome Atlas (TCGA) revealed mutations, amplifications, or deep deletions in 30/32 tumor types (Figure 3). Tumor types with the highest percentage of cases with aberrant CDK12 (ranging from 5–15% of sequenced cases) include esophageal, breast, endometrial, uterine, and bladder carcinomas. Stomach, colorectal, pancreatic ductal, and ovarian serous adenocarcinomas also feature significant levels of aberrations.
Figure 3.
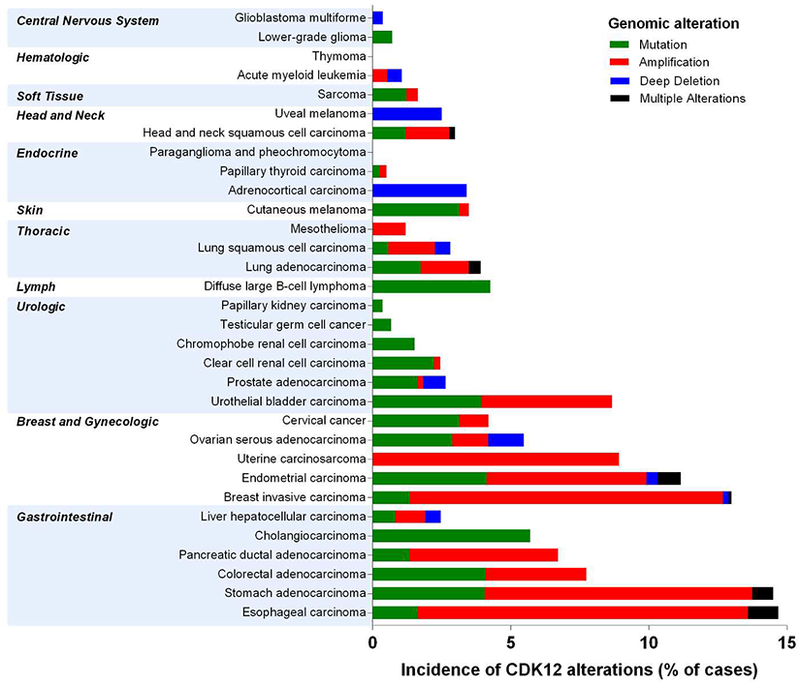
Genomic alterations of the CDK12 gene across The Cancer Genome Atlas (TCGA). Data downloaded from TCGA Provisional data sets on cBioportal (http://www.cbioportal.org/) in May 2018.
The functional roles of CDK12 gene mutations, amplifications, deletions, or variations in its expression remain incompletely understood, with both tumor-suppressive and tumorigenic roles proposed for CDK12. As far as its tumor-suppressive roles, two studies[33 34] have reported that the majority of CDK12 mutations in high-grade serous ovarian carcinoma (HGSOC) are homozygous point mutations in the kinase domain that abrogate the catalytic activity of CDK12. Loss-of-function of CDK12 results in decreased homologous recombination and enhanced sensitivity to DNA cross-linking agents and poly (ADP-ribose) polymerase (PARP) inhibitors.
Inactivation of the CDK12 gene has been associated with a unique genomic instability pattern characterized by up to 800 tandem duplications (TD; up to 10 Mb in size) per tumor that were quasi-randomly distributed along the genome[35]. Denoted the CDK12 TD-plus phenotype, these TDs affected over 10% of the genome and were observed in 4% of serous ovarian carcinomas and up to 2% of prostate adenocarcinomas[35].
On chromosome 17, the CDK12 gene is located approximately 200kb proximal to the HER2 (ERBB2) oncogene and is frequently co-amplified in breast tumors[36 37]. In cohorts of primary breast cancer, high CDK12 expression correlated with HER2 status, suggesting oncogenic roles for CDK12 in this context[38]. Tumorigenic roles for CDK12 were recently proposed through ALE splicing of DNAJB6, a HSP40 family chaperone, which promoted cell invasion and migration in HER2-amplified breast tumor cells[19]. Other studies reported that 13% of HER2-positive breast cancers showed out-of-frame rearrangements of CDK12 resultant from the amplification breakpoint in the HER2 amplicon converging on CDK12[39]. This led to decreases in CDK12 expression, loss-of-function, and sensitivity to PARP inhibitors[39]. In HER2-amplified MKN7 gastric cancer cells, gene fusions involving CDK12 and HER2 were reported[40]. These fusion transcripts were predicted to result in truncation of CDK12 protein, but were not in-frame to HER2. CDK12 mutations have also been reported in non-small cell lung cancer[41], lung adenocarcinoma[42], follicular lymphoma[43], esophagogastric tumors[44], and advanced carcinoma of unknown primary[45].
It is clear from these studies that the functional implications of CDK12 mutations are case- and context-dependent. Continued elucidation of the specific roles of CDK12 will be important for its use as a biomarker to inform patient stratification for therapeutic intervention[38] (Figure 4).
Figure 4.
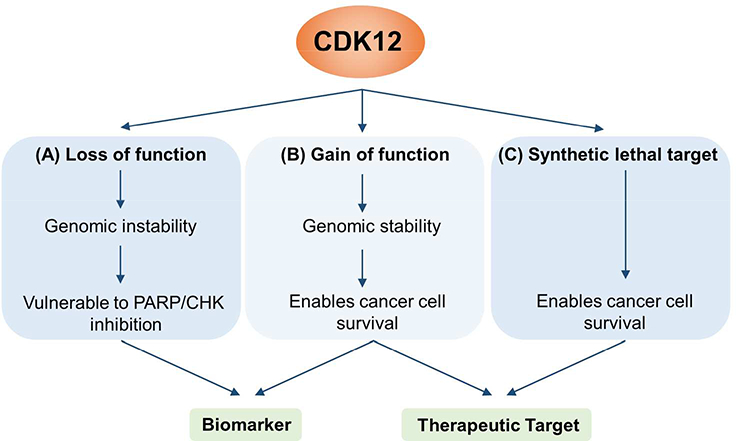
Potential for clinical use of CDK12 as a biomarker and/or therapeutic target. (A) CDK12 mutations that confer loss of function have been reported to promote genomic instability, rendering cancer cells more susceptible to PARP/CHK inhibitors. (B) On the other hand, CDK12 mutations that cause gain of function (e.g. amplification) could theoretically potentiate cancer cell survival by promoting expression of DNA damage repair genes. Though there are currently few reports of this, such a situation would enable use of CDK12 as a biomarker of drug response/clinical outcome, or as a drug target. (C) In cases where CDK12 is not necessarily mutated, CDK12 can enable tumor progression driven by genes such as MYC and EWS/FLI. These synthetic lethal interactions also provide an opportunity for therapeutic targeting.
CDK12 AS A THERAPEUTIC TARGET: SYNTHETIC LETHAL PARTNERS IN THE CONTEXT OF CANCER
In addition to its potential role as a clinical biomarker, recent studies have highlighted CDK12 as a therapeutic target for cancer (Figure 4). Inhibition of transcriptional CDKs could be an effective strategy to overcome resistance to targeted therapies, including erlotinib and crizotinib[46]. Numerous other studies have identified specific genetic or cellular contexts that confer enhanced sensitivity to CDK12 inhibition, including MYC dependency, PARP inhibition, and EWS/FLI rearrangement.
MYC.
MYC is a global transcription factor and central driver of many human cancers, which has proven to be difficult to inhibit directly[47 48]. To discover synthetic lethal genes with the MYC oncogene in an isogenic setting, we performed a siRNA screen using human fibroblasts with overexpression of cMYC[49]. In this study, we first reported CDK12 as synthetic lethal with cMYC. These findings were corroborated in an independent study demonstrating that CDK inhibition triggered massive downregulation of cMYC expression and its related genes[50].
Amongst the top genes identified as synthetic lethal with MYC were genes that regulate RNA polymerase II and cell cycle checkpoint control, including GTF2H4, POLR2E, RAD21, and WEE1[51]. Additionally, deregulated MYC is known to induce replicative stress by accelerating the rate of DNA replication, pointing to replication-coupled DNA damage repair as a targetable weakness in MYC-driven tumors[52 53]. The overlap between this MYC signature and the known cellular functions of CDK12, as well as the requirement of CDK12 for optimal processing of cMYC[21], collectively indicate CDK12 could be an effective therapeutic target for MYC-dependent cancers.
PARP and CHK1 inhibitors.
A genome-wide PARP1/2 inhibitor screen identified CDK12 as a sensitizer to olaparib and found CDK12 mutations were a clinically relevant biomarker of PARP1/2 inhibitor sensitivity in HGSOC[54]. Moreover, most HGSOC cases displaying CDK12 mutations were mutually exclusive with BRCA1/2 mutations, suggesting cells can utilize one of multiple strategies to achieve similar phenotypes. Supporting this, primary and acquired resistance to PARP inhibitors could be overcome by CDK12 inhibition in BRCA wild-type and mutated models of triple negative breast cancer[55]. This has led to clinical trials exploring this combination in advanced solid tumors (NCT01434316). Recently, Paculova et al proposed that CDK12- or BRCA1-deficient cells are reliant on the downstream S phase checkpoint kinase CHK1 for survival[56]. Loss of CDK12 or BRCA1 was found to potentiate the anti-tumor activity of CHK1 inhibitors irrespective of p53 status[56].
EWS/FLI.
Ewing sarcoma tumors are characterized by chromosomal rearrangements resulting in the fusion protein EWS/FLI, a potent transcriptional activator and transforming gene in this disease[57]. A recent study reported that inhibition of CDK12 was specifically responsible for synthetic lethality in Ewing sarcoma cells with EWS/FLI rearrangement[58]. Treatment of these cells with the specific CDK12/13 inhibitor, THZ531, preferentially repressed expression of DNA damage repair genes and was synergistic with PARP inhibitors. Interestingly, CDK12 is rarely mutated in Ewing sarcoma tumors (TCGA), suggesting that mutations in CDK12 are not necessary to confer its role as an effective therapeutic target.
Tumors driven by oncogenes such as MYC and EWS/FLI are highly dependent on transcriptional programs that converge on RNA Pol II [59 60] and the need for DDR gene expression to facilitate rapid replication [61]. Thus, impairing the function of CDK12 as both a transcriptional co-activator and specific regulator of DNA damage related proteins could explain the synthetic lethal interactions described above, representing a promising therapeutic strategy for these cancer types.
TAKE HOME MESSAGES.
CDK12 complexes with cyclin K to regulate transcriptional elongation, mRNA processing, proliferation, and development. It regulates specific subsets of genes involved in cellular responses to stress, heat shock, and DNA damage.
Genomic alterations in CDK12 are frequently observed in human cancers. In HGSOC, HER2-positive breast cancer, and lung adenocarcinoma, loss-of-function mutations have been reported, which decrease homologous recombination and enhance sensitivity to chemotherapy and PARP inhibitors. In contrast, CDK12 gene amplification could contribute to cancer fitness by constitutive engagement of DNA repair pathways.
Synthetic lethal interactions have been reported for CDK12 with MYC, EWS/FLI, and PARP inhibitors, and has led to growing interest as a therapeutic target and biomarker for response in cancer treatment.
ACKNOWLEDGMENTS & FUNDING
Results shown are in part based on data generated by the TCGA Research Network: http://cancergenome.nih.gov/. We apologize to the authors of articles that could not be cited due to space restrictions. G.L. acknowledges funding from the Ovarian Cancer Research Fund Alliance. C.K. is funded by grants from the NIH/NCI (U01 CA217883, U54 CA132381). The authors thank members of the Kemp laboratory for helpful discussions.
Footnotes
COMPETING INTERESTS
C.G. and C.K. are co-founders of and have ownership interests in SEngine Precision Medicine.
REFERENCES
- 1.Marques F, Moreau JL, Peaucellier G, et al. A new subfamily of high molecular mass CDC2-related kinases with PITAI/VRE motifs. Biochem Biophys Res Commun 2000;279(3):832–7. [DOI] [PubMed] [Google Scholar]
- 2.Malumbres M Cyclin-dependent kinases. Genome Biol 2014;15(6):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blazek D, Kohoutek J, Bartholomeeusen K, et al. The Cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev 2011;25(20):2158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Chatterjee N, Spirohn K, Boutros M, Bohmann D. Cdk12 Is A Gene-Selective RNA Polymerase II Kinase That Regulates a Subset of the Transcriptome, Including Nrf2 Target Genes. Sci Rep 2016;6:21455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko TK, Kelly E, Pines J. CrkRS: a novel conserved Cdc2-related protein kinase that colocalises with SC35 speckles. J Cell Sci 2001;114(Pt 14):2591–603. [DOI] [PubMed] [Google Scholar]
- 6.Bartkowiak B, Liu P, Phatnani HP, et al. CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev 2010;24(20):2303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepard PJ, Hertel KJ. The SR protein family. Genome Biol 2009;10(10):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci 2001;114(Pt 7):1253–63. [DOI] [PubMed] [Google Scholar]
- 9.Bedford MT, Chan DC, Leder P. FBP WW domains and the Abl SH3 domain bind to a specific class of proline-rich ligands. EMBO J 1997;16(9):2376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon-Clarke SE, Elkins JM, Cheng SW, Morin GB, Bullock AN. Structures of the CDK12/CycK complex with AMP-PNP reveal a flexible C-terminal kinase extension important for ATP binding. Sci Rep 2015;5:17122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosken CA, Farnung L, Hintermair C, et al. The structure and substrate specificity of human Cdk12/Cyclin K. Nat Commun 2014;5:3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T, Kwiatkowski N, Olson CM, et al. Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors. Nat Chem Biol 2016;12(10):876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johannes JW, Denz CR, Su N, et al. Structure-Based Design of Selective Noncovalent CDK12 Inhibitors. ChemMedChem 2018;13(3):231–35. [DOI] [PubMed] [Google Scholar]
- 14.Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 2014;13(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng SW, Kuzyk MA, Moradian A, et al. Interaction of cyclin-dependent kinase 12/CrkRS with cyclin K1 is required for the phosphorylation of the C-terminal domain of RNA polymerase II. Mol Cell Biol 2012;32(22):4691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buratowski S Progression through the RNA polymerase II CTD cycle. Mol Cell 2009;36(4):541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galganski L, Urbanek MO, Krzyzosiak WJ. Nuclear speckles: molecular organization, biological function and role in disease. Nucleic Acids Res 2017;45(18):10350–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartkowiak B, Greenleaf AL. Expression, purification, and identification of associated proteins of the full-length hCDK12/CyclinK complex. J Biol Chem 2015;290(3):1786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tien JF, Mazloomian A, Cheng SG, et al. CDK12 regulates alternative last exon mRNA splicing and promotes breast cancer cell invasion. Nucleic Acids Res 2017;45(11):6698–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn SH, Kim M, Buratowski S. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3’ end processing. Mol Cell 2004;13(1):67–76. [DOI] [PubMed] [Google Scholar]
- 21.Davidson L, Muniz L, West S. 3’ end formation of pre-mRNA and phosphorylation of Ser2 on the RNA polymerase II CTD are reciprocally coupled in human cells. Genes Dev 2014;28(4):342–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei T, Zhang P, Zhang X, et al. Cyclin K regulates prereplicative complex assembly to promote mammalian cell proliferation. Nat Commun 2018;9(1):1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekholm-Reed S, Mendez J, Tedesco D, Zetterberg A, Stillman B, Reed SI. Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J Cell Biol 2004;165(6):789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juan HC, Lin Y, Chen HR, Fann MJ. Cdk12 is essential for embryonic development and the maintenance of genomic stability. Cell Death Differ 2016;23(6):1038–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai Q, Lei T, Zhao C, et al. Cyclin K-containing kinase complexes maintain self-renewal in murine embryonic stem cells. J Biol Chem 2012;287(30):25344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen HR, Juan HC, Wong YH, Tsai JW, Fann MJ. Cdk12 Regulates Neurogenesis and Late-Arising Neuronal Migration in the Developing Cerebral Cortex. Cereb Cortex 2017;27(3):2289–302. [DOI] [PubMed] [Google Scholar]
- 27.Chen HH, Wang YC, Fann MJ. Identification and characterization of the CDK12/cyclin L1 complex involved in alternative splicing regulation. Mol Cell Biol 2006;26(7):2736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen HH, Wong YH, Geneviere AM, Fann MJ. CDK13/CDC2L5 interacts with L-type cyclins and regulates alternative splicing. Biochem Biophys Res Commun 2007;354(3):735–40. [DOI] [PubMed] [Google Scholar]
- 29.Even Y, Durieux S, Escande ML, et al. CDC2L5, a Cdk-like kinase with RS domain, interacts with the ASF/SF2-associated protein p32 and affects splicing in vivo. J Cell Biochem 2006;99(3):890–904. [DOI] [PubMed] [Google Scholar]
- 30.Chen HR, Lin GT, Huang CK, Fann MJ. Cdk12 and Cdk13 regulate axonal elongation through a common signaling pathway that modulates Cdk5 expression. Exp Neurol 2014;261:10–21. [DOI] [PubMed] [Google Scholar]
- 31.Liang K, Gao X, Gilmore JM, et al. Characterization of human cyclin-dependent kinase 12 (CDK12) and CDK13 complexes in C-terminal domain phosphorylation, gene transcription, and RNA processing. Mol Cell Biol 2015;35(6):928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greifenberg AK, Honig D, Pilarova K, et al. Structural and Functional Analysis of the Cdk13/Cyclin K Complex. Cell Rep 2016;14(2):320–31. [DOI] [PubMed] [Google Scholar]
- 33.Joshi PM, Sutor SL, Huntoon CJ, Karnitz LM. Ovarian cancer-associated mutations disable catalytic activity of CDK12, a kinase that promotes homologous recombination repair and resistance to cisplatin and poly(ADP-ribose) polymerase inhibitors. J Biol Chem 2014;289(13):9247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekumi KM, Paculova H, Lenasi T, et al. Ovarian carcinoma CDK12 mutations misregulate expression of DNA repair genes via deficient formation and function of the Cdk12/CycK complex. Nucleic Acids Res 2015;43(5):2575–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popova T, Manie E, Boeva V, et al. Ovarian Cancers Harboring Inactivating Mutations in CDK12 Display a Distinct Genomic Instability Pattern Characterized by Large Tandem Duplications. Cancer Res 2016;76(7):1882–91. [DOI] [PubMed] [Google Scholar]
- 36.Luoh SW. Amplification and expression of genes from the 17q11 approximately q12 amplicon in breast cancer cells. Cancer Genet Cytogenet 2002;136(1):43–7. [DOI] [PubMed] [Google Scholar]
- 37.Kauraniemi P, Kallioniemi A. Activation of multiple cancer-associated genes at the ERBB2 amplicon in breast cancer. Endocr Relat Cancer 2006;13(1):39–49. [DOI] [PubMed] [Google Scholar]
- 38.Naidoo K, Wai PT, Maguire SL, et al. Evaluation of CDK12 Protein Expression as a Potential Novel Biomarker for DNA Damage Response-Targeted Therapies in Breast Cancer. Mol Cancer Ther 2018;17(1):306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Natrajan R, Wilkerson PM, Marchio C, et al. Characterization of the genomic features and expressed fusion genes in micropapillary carcinomas of the breast. J Pathol 2014;232(5):553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zang ZJ, Ong CK, Cutcutache I, et al. Genetic and structural variation in the gastric cancer kinome revealed through targeted deep sequencing. Cancer Res 2011;71(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K, Zhang M, Zhu J, Hong W. Screening of gene mutations associated with bone metastasis in nonsmall cell lung cancer. J Cancer Res Ther 2016;12(Supplement):C186–C90. [DOI] [PubMed] [Google Scholar]
- 42.Biswas R, Gao S, Cultraro CM, et al. Genomic profiling of multiple sequentially acquired tumor metastatic sites from an “exceptional responder” lung adenocarcinoma patient reveals extensive genomic heterogeneity and novel somatic variants driving treatment response. Cold Spring Harb Mol Case Stud 2016;2(6):a001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geyer JT, Subramaniyam S, Jiang Y, et al. Lymphoblastic transformation of follicular lymphoma: a clinicopathologic and molecular analysis of 7 patients. Hum Pathol 2015;46(2):260–71. [DOI] [PubMed] [Google Scholar]
- 44.Riches JC, Schultz N, Ku GY, et al. Genomic profiling of esophagogastric (EG) tumors in clinical practice. J Clin Oncol 2015;33(3_suppl):57–57. [Google Scholar]
- 45.Subbiah IM, Tsimberidou A, Subbiah V, Janku F, Roy-Chowdhuri S, Hong DS. Next generation sequencing of carcinoma of unknown primary reveals novel combinatorial strategies in a heterogeneous mutational landscape. Oncoscience 2017;4(5–6):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rusan M, Li K, Li Y, et al. Suppression of Adaptive Responses to Targeted Cancer Therapy by Transcriptional Repression. Cancer Discov 2018;8(1):59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaub FX, Dhankani V, Berger AC, et al. Pan-cancer Alterations of the MYC Oncogene and Its Proximal Network across the Cancer Genome Atlas. Cell Syst 2018;6(3):282–300 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dang CV, Reddy EP, Shokat KM, Soucek L. Drugging the ‘undruggable’ cancer targets. Nat Rev Cancer 2017;17(8):502–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toyoshima M, Howie HL, Imakura M, et al. Functional genomics identifies therapeutic targets for MYC-driven cancer. Proc Natl Acad Sci U S A 2012;109(24):9545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delehouze C, Godl K, Loaec N, et al. CDK/CK1 inhibitors roscovitine and CR8 downregulate amplified MYCN in neuroblastoma cells. Oncogene 2014;33(50):5675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cermelli S, Jang IS, Bernard B, Grandori C. Synthetic lethal screens as a means to understand and treat MYC-driven cancers. Cold Spring Harb Perspect Med 2014;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dominguez-Sola D, Ying CY, Grandori C, et al. Non-transcriptional control of DNA replication by c-Myc. Nature 2007;448(7152):445–51. [DOI] [PubMed] [Google Scholar]
- 53.Robinson K, Asawachaicharn N, Galloway DA, Grandori C. c-Myc accelerates S-phase and requires WRN to avoid replication stress. PLoS One 2009;4(6):e5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bajrami I, Frankum JR, Konde A, et al. Genome-wide profiling of genetic synthetic lethality identifies CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity. Cancer Res 2014;74(1):287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson SF, Cruz C, Greifenberg AK, et al. CDK12 Inhibition Reverses De Novo and Acquired PARP Inhibitor Resistance in BRCA Wild-Type and Mutated Models of Triple-Negative Breast Cancer. Cell Rep 2016;17(9):2367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paculova H, Kramara J, Simeckova S, et al. BRCA1 or CDK12 loss sensitizes cells to CHK1 inhibitors. Tumour Biol 2017;39(10):1010428317727479. [DOI] [PubMed] [Google Scholar]
- 57.May WA, Lessnick SL, Braun BS, et al. The Ewing’s sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1. Mol Cell Biol 1993;13(12):7393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iniguez AB, Stolte B, Wang EJ, et al. EWS/FLI Confers Tumor Cell Synthetic Lethality to CDK12 Inhibition in Ewing Sarcoma. Cancer Cell 2018;33(2):202–16 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang L, Chansky HA, Hickstein DD. EWS.Fli-1 fusion protein interacts with hyperphosphorylated RNA polymerase II and interferes with serine-arginine protein-mediated RNA splicing. J Biol Chem 2000;275(48):37612–8. [DOI] [PubMed] [Google Scholar]
- 60.Bradner JE, Hnisz D, Young RA. Transcriptional Addiction in Cancer. Cell 2017;168(4):629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Connor MJ. Targeting the DNA Damage Response in Cancer. Mol Cell 2015;60(4):547–60. [DOI] [PubMed] [Google Scholar]


