Abstract
Preclinical studies report that the effective dose for morphine is approximately 2-fold higher in females than males. Following systemic administration, morphine is metabolized via Phase II glucuronidation in the liver and brain into two active metabolites: morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G), each possessing distinct pharmacological profiles. M6G binds to μ opioid receptors and acts as a potent analgesic. In contrast, M3G binds to toll-like receptor 4 (TLR4), initiating a neuroinflammatory response that directly opposes the analgesic effects of morphine and M6G. M3G serum concentrations are 2-fold higher in females than males, however, sex-specific effects of morphine metabolites on analgesia and glial activation in vivo remain unknown. The present studies test the hypothesis that increased M3G, and subsequent TLR4-mediated activation of glia, is a primary mechanism driving the attenuated response to morphine in females. We demonstrate that intra-PAG M6G results in a greater analgesic response in females than morphine alone. M6G analgesia was reversed with co-administration of (−)-naloxone, but not (+)-naloxone, suggesting that this effect is μ opioid receptor mediated. In contrast, intra-PAG administration of M3G significantly attenuated the analgesic effects of systemic morphine in males only, increasing the 50% effective dose of morphine two-fold (5.0 vs 10.3 mg/kg) and eliminating the previously observed sex difference. An increase in IL-1β, IL-6 and TNF was observed in females following intra-PAG morphine or M6G. In males, only IL-1β levels increased following morphine. Changes in cytokine levels following M3G were limited to TNF in females. Together, these data implicate sex differences in morphine metabolism, specifically M3G, as a contributing factor in the attenuated response to morphine observed in females.
Keywords: glucuronide, M6G, M3G, sex differences, analgesia, periaqueductal gray
1. Introduction
Opioids such as morphine are widely used for the treatment of severe pain, with 3–5% of adults in the US receiving long-term opioid therapy [1]. Preclinical studies using both acute and chronic pain assays report that morphine is less effective in females than in males [2–5]. Indeed, greater antinociception is observed in male rats for almost every opioid tested [6–8].
The midbrain periaqueductal gray (PAG) is a key neural locus for opioid action [9, 10]. Direct PAG administration of morphine induces long-lasting analgesia, while intra-PAG administration of the opioid antagonist (−)-naloxone or lesions of PAG μ opioid receptor (MOR) completely abolish the antinociceptive effects of systemic morphine [3]. Sex differences are also evident following intra-PAG administration of morphine, with the half-maximal antinociceptive dose (ED50) in males ranging from 1.2–1.6 μg/μl, while in females ED50 values range from 16 to >50 μg/μl [3, 11, 12]. The PAG contains a high density of MOR-containing neurons [13–15], and we have previously reported the PAG MOR levels are significantly reduced in females [3]. Indeed, during proestrus, MOR levels are 40% lower in females compared to males; interestingly, this corresponds to the time period when intra-PAG morphine produces minimal changes in somatosensory thresholds. The finding that sex differences in MOR expression and signaling contribute to the dimorphic effects of morphine are also supported by Bernal et. al. [16] who showed that reducing PAG MOR availability had a significantly greater impact on opioid antinociception in females than in males.
In addition to neuronal mechanisms, recent data suggest that the innate immune receptor, toll-like receptor 4 (TLR4), contributes to the sexually dimorphic effects of morphine [17]. Many opioids, including morphine, bind to myeloid differentiation factor 2 (MD-2), a co-receptor of TLR4, located on glial cells [18, 19]. Although the classical μ opioid receptor (MOR) binds only the (−)-stereoisomer of opioids, TLR4 binds opioids in a non-stereoselective manner, such that both the (−) and (+) isomers of opioid ligands modulate glial signaling [19]. TLR4 activation initiates a neuroimmune response that is characterized by the release of proinflammatory compounds including cytokines (tumor necrosis factor alpha [TNF], interleukins [IL-1β, IL-6, IL-10]), chemokines (CXCL3) and prostaglandin E2 (PGE2) [19–22]. These inflammatory factors increase neuronal excitability, resulting in hyperalgesia [23–26] and paradoxically, reducing the analgesic efficacy of morphine [18, 25, 27, 28]. Our previous research demonstrates that inhibition of TLR4 in the ventrolateral PAG (vlPAG) with the TLR4-specific antagonist (+)-naloxone potentiates analgesia in females and abolishes the sex difference in morphine response [17].
Recent studies suggest that morphine’s primary metabolites contribute significantly to its immunomodulatory effects [29, 30]. Following administration, approximately 90% of morphine is metabolized in the liver, peripheral macrophages and brain microglia to form two active glucuronide metabolites: morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G), each with distinct pharmacological properties [31–33]. M6G binds to MOR with high affinity, and is a potent analgesic [34, 35]. M3G, on the other hand, does not bind to MOR and does not produce analgesia [36]. Rather, M3G binds with high affinity to TLR4 [29, 37] to induce allodynia and hyperalgesia and actively oppose the analgesic effects of morphine and M6G, likely via upregulation of pro-inflammatory IL-1 [29, 38–42]. Importantly, unlike the vast majority of opioids, M6G does not bind to TLR4 and is not associated with a pro-inflammatory response [19, 43].
Several studies in rats have observed sex differences in morphine metabolism, such that approximately two-times greater concentrations of M3G are observed in the plasma of females compared with males [44, 45]. Though M3G concentrations are higher in females, there is no direct evidence that the increased M3G:M6G ratio contributes to the reduced efficacy of morphine observed in females. The present studies test the hypothesis that M3G activates TLR4 and opposes morphine analgesia to a greater degree in females than males. Most importantly, the present studies investigate whether MOR activation with M6G, in the absence of morphine/M3G-induced glial activation, results in equipotent analgesia in both males and females.
2. Materials and Methods
2.1. Subjects
Age matched (60–90 day old; 250–400g) intact male and normally cycling female Sprague Dawley rats (Charles River) were used. Animals were pair-housed with the same sex in ventilated cages on the same vivarium rack and maintained on a 12:12 h light/dark cycle (lights on at 08:00). Access to food and water was available ad libitum throughout the experiments except during behavioral testing. All studies were approved by the Institutional Animal Care and Use Committee at Georgia State University, and performed in compliance with Ethical Issues of the International Association for the Study of Pain and National Institutes of Health. All efforts were made to reduce the number of animals used in these experiments and to minimize pain and suffering.
2.1.1. Vaginal cytology
Vaginal lavages were performed daily beginning 7 days prior to testing to confirm that all female rats were cycling normally and to record cycle stage at the time of testing [10].
2.2. Procedures
2.2.1. Intra-vlPAG cannulae implantation and injections
Animals were anesthetized to a deep surgical plane with 5% isoflurane (maintained at 2–5% throughout surgery; Henry Schein Animal Health) and bilateral guide cannulae (22 gauge; Plastics One) aimed at the vlPAG (anterior—posterior: 1.7 mm; mediolateral: ±0.6 mm; dorsoventral: −5.0 mm from lambda) were implanted stereotaxically as previously described [46, 47]. Animals were allowed to recover 11–14 days post-cannula implantation before behavioral testing. Animals with blocked cannula were retained as no injection controls. No significant differences were observed between non-injected animals and animals receiving intra-PAG saline so these groups are pooled. Animals with bilateral cannulae located outside of the vlPAG (e.g., in the aqueduct or deep mesencephalic nucleus) were considered “cannula misses” and were not included in the analyses.
2.2.2. Behavioral testing
Thermal nociception was assessed using the paw thermal stimulator [17, 46, 48–50]. Briefly, for this test, the rat is placed in a clear Plexiglas box resting on an elevated glass plate maintained at 30°C. A radiant beam of light is positioned under the hindpaw and the time for the rat to remove the paw from the thermal stimulus is electronically recorded as the paw withdrawal latency (PWL) in seconds (s). A maximal PWL of 20 s was used to prevent tissue damage due to repeated application of the noxious thermal stimulus. Animals were acclimated to the testing apparatus 30–60 minutes per day for three consecutive days prior to the start of the experiment and on the day of testing. All behavioral testing took place between 10:00 and 15:00 (lights on at 08:00). Temperature of the thermal stimulus was recorded before and after each trial to maintain consistent recordings between groups and did not exceed a range of 60–64°C throughout the course of the experiments. All testing was conducted blind with respect to group assignment. Data were normalized to the percent maximum possible effect (%MPE) using the following formula: %MPE = (Paw withdrawal latency / 20s) * 100.
2.2.3. qPCR.
At the end of each experiment, brains were removed, flash frozen, and sectioned at 300 μm with a Leica CM3050S cryostat and mounted on to sterile slides. One-millimeter bilateral micropunches were taken from 6 levels of the vlPAG (Bregma −6.72, −7.04, −7.64, −8.0, −8.30, −8.80) and RNA was extracted with TRIzol (Life Technologies; 15596026) using standard procedures, followed by the addition of Glycoblue (Life Technologies; AM5916) for visualization. Concentrations of RNA (ng/μl) were calculated using a NanoDrop ND-1000 Spectrophotometer (Version 3.8, Thermo Fisher; DE). Following RNA extraction, RNA was diluted to a standard concentration and converted to cDNA using an AMV First-Strand Synthesis Kit (Invitrogen). PCR was performed using FastStart Essential DNA Green MasterMix (Roche) and analyzed using a Roche LightCycler 96 and accompanying software (Version 1.1.0.1320, 2011 Roche Diagnostics; Switzerland). Primer sequences are provided in Table 1.
Table 1. Primer sequences for qPCR of inflammatory cytokines.
| GAPDH | Forward | GAG GTG ACC GCA TCT TCT TG |
| Reverse | CCG ACC TTC ACC ATC TTG TC | |
| IL-1β | Forward | CCC TGA AGG ATG TGA TCA TTG |
| Reverse | GGC AAA GGG TTT CTC CAC TT | |
| IL-6 | Forward | AAG ACC CAA GCA CCT TCT TT |
| Reverse | AGA CAG CAC GAG GCA TTT TT | |
| IL-10 | Forward | TGT ACC TTA TCT ACT CCC AGG TTC TCT |
| Reverse | GTG TGG GTG AGG AGC ACG TA | |
| TNF | Forward | TGT ACC TTA TCT ACT CCC AGG TTC TCT |
| Reverse | GTG TGG GTG AGG AGC ACG TA |
2.3. Does intra-PAG administration of M6G eliminate sex differences in analgesia?
To determine if M6G produces equipotent analgesia in males and females, thermal nociception was assessed using the paw thermal stimulator immediately following a single injection of M6G (0.2 μg or 0.7 μg) into the vlPAG [51]. PWL was measured every 10 minutes for 120 minutes following injection. A separate group of animals received a single injection of morphine as a positive control (7 μg/0.25 μl/side) [47], or saline as a negative control (0.25 ul/side), resulting in 4 groups: saline (n=9 males, 7 females), morphine (n= 8 males, 16 females), M6G low-dose (n=9 males, 10 females), M6G high dose (n=10 males).
To confirm that M6G analgesia was mediated by μ opioid receptor and not TLR4, animals received a single injection of M6G at the effective dose (0.2 μg in females, 0.7 μg in males), followed by a single subcutaneous injection of the MOR and TLR4 antagonist (−)-naloxone (3.7 mg/kg) [52]] or the TLR4-specific antagonist (+)-naloxone (8.0 mg/kg). This resulted in 4 additional groups: saline + (−)-naloxone (n= 6 males, 4 females), M6G + (−)-naloxone (n= 7 males, 5 females), saline + (+)-naloxone (n= 5 males, 5 females), and M6G + (+)-naloxone (n= 7 males, 6 females). Animals treated with saline + (−)-naloxone or saline + (+)-naloxone are not statistically different from saline-only animals; therefore, these groups are pooled for analysis and presentation.
At the end of behavioral testing, brain tissue was collected for analysis of inflammatory cytokines using qPCR to determine if M6G activates microglia in a TLR4 dependent manner.
2.3.1. Data analysis and presentation.
Paw withdrawal latency was normalized to %MPE and analyzed using Repeated Measures ANOVA (R) for main effects of sex and treatment across time; Greenhouse-Geisser correction was applied when the assumption of sphericity was violated. Tukey’s post-hoc analysis was conducted when appropriate. Pre-planned comparisons of group differences in %MPE at 40 min post-morphine was analyzed using t-Test. Values of p≤0.05 were considered statistically significant.
qPCR data are presented as the normalized ratio of the target gene relative to the GAPDH control gene using ΔCq. Data shown represent normalized values obtained using 2−(ΔCq). The impact of sex and treatment on cytokine mRNA expression were analyzed by two-way ANOVAs, followed by Tukey’s post-hoc analysis when appropriate. Tissue from saline treated animals from the M6G and M3G studies were pooled. PCR data are presented as 2−(ΔCq) normalized means ±SEM; p≤0.05 was considered significant.
2.4. Does intra-PAG M3G alter morphine analgesia in a sex-dependent manner?
To determine if PAG TLR4 activation with M3G impacts morphine analgesia to a comparable degree in males and females, rats were implanted with bilateral cannula aimed at the vlPAG. On the day of testing, animals received a single intra-PAG injection of M3G (0.075 μg/0.25 μl/side) or saline (0.25 μl/side). Comparable doses have been shown to increase glial activation and cytokine expression when administered intrathecally [29]. Approximately 45 minutes following the initial injection of M3G, animals received cumulative doses of morphine to determine the ED50. Briefly, animals received an injection of morphine every 20 minutes, resulting in cumulative doses of 1.8, 3.2, 5.6, 8.0, 10.0, and 18 mg/kg [16, 47, 79]. Control animals received repeated saline injections (1ml/kg; s.c). This resulted in four treatment groups: saline + saline (n=5 males, 5 females), M3G + saline (n=8 males, 7 females), saline + morphine (n=5 males, 7 females), and M3G + morphine (n=9 males, 7 females).
At the end of behavioral testing, brain tissue was collected for analysis of inflammatory cytokines using qPCR to determine if M3G sex-specifically alters glia activation via TLR4.
2.4.1. Data analysis and presentation
Half-maximal antinociceptive effect (ED50) and 95% confidence intervals (CI) were calculated from dose–response curves from morphine-treated animals using Graph-Pad PRISM software. To generate curves, data were normalized such that each individual animal’s baseline PWL score = 0% and 20s = 100% [46, 53, 54]. Repeated measures ANOVA was used to assess for significant effects of treatment and sex, with Tukey’s post hoc tests where appropriate. GraphPad PRISM does not generate exact p-values, therefore these values are presented as p≤ or ≥0.05; values of p≤0.05 were considered statistically significant.
3. Results
3.1. M6G is More Effective in Females Than in Males
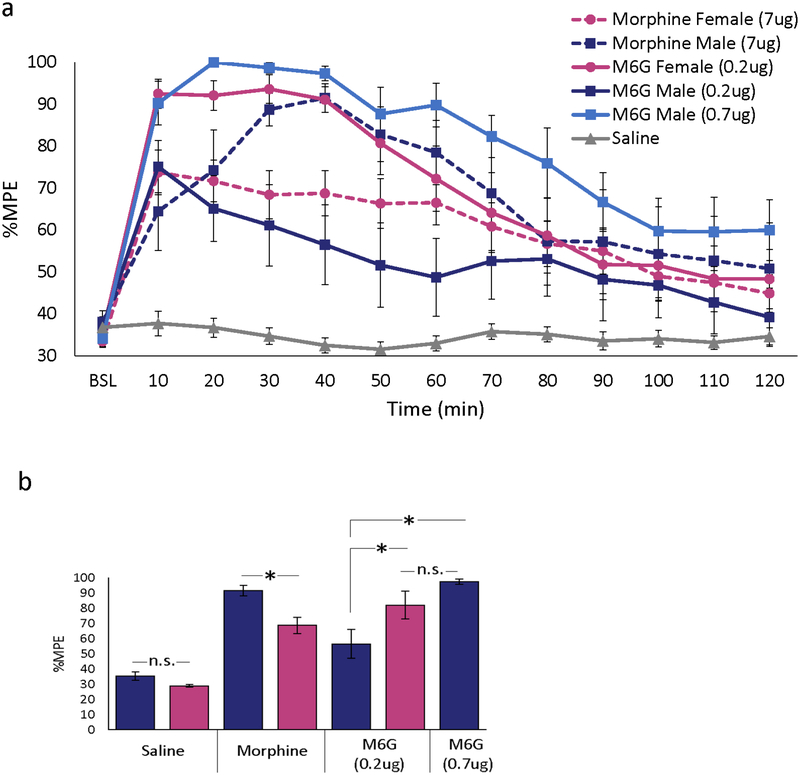
Exogenous M6G produces robust analgesia and is reported to have greater potency than morphine, presumably due to its exclusive action at MOR and therefore bypassing the opposing effects mediated by TLR4 signaling [31, 34, 55]. To test the hypothesis that M6G activation of MOR (and not TLR4), results in equipotent analgesia between both sexes, animals received a single injection of M6G (0.2ug), morphine (7.0ug) or saline directly into the vlPAG and PWLs recorded for 120 min post-injection.
Intra-PAG morphine significantly increased PWL in both males (p=0.002) and females (p<0.001) compared with saline controls (Figure 1a). Morphine-induced antinociception was significantly greater in males than in females at 30–60 min post-injection, with %MPE at 40 min post-injection of 93% in males versus 69% in females (p=0.04; Figure 1b). In contrast to morphine, intra-PAG administration of M6G to females resulted in robust analgesia (mean %MPE for 40 min post-injection of 91% vs 69% following morphine; p=0.009). Interestingly, M6G (0.2ug) was significantly more effective in females than in males throughout the first 60 min (p= 0.007). Although males receiving low-dose M6G had a trend for greater analgesia than saline-treated males, these groups were not statistically different (p>0.05). To determine if the sex difference in M6G analgesia was due to drug potency, a higher dose of M6G (0.7ug) was also administered. Intra-PAG administration of M6G (0.7ug) produced maximal analgesia in males that was not significantly different from females receiving low-dose M6G (p=0.99) or from males receiving morphine (p=0.46). High-dose M6G was fatal in females (n=6), and this dose was discontinued. Together, these data indicate that, although intra-PAG morphine produced significantly greater analgesia in males than females, administration of the metabolite M6G produced significantly greater analgesia in females than males.
Figure 1. Intra-PAG M6G produces robust analgesia in both sexes, but is more potent in females.
(a) Intra-PAG morphine (7 μg) produced a greater analgesic response in males in comparison to females. By contrast, intra-PAG M6G (0.2μg) was effective in females but not males. In males, intra-PAG administration of the higher dose of M6G (0.7 μg) produced analgesia that was comparable to females receiving the 0.2μg dose. Repeated measures analysis showed significant main effect of treatment [F(2,54)=36.73, p<0.001], sex [F(1,54)=5.83, p=0.019], time [F(12,630)=40.94, p<0.001], and treatment by time [F(24, 630)=9.30, p<0.001]. As no differences were noted in %MPE for intra-PAG saline treated males and females, these data are combined and shown as one group. All injections were given intra-PAG in a volume of 0.25μl/side. (b) At 40 min post-morphine, %MPE was significantly higher in males than females [t(22)=−2.73, p=0.012]. In females, %MPE following intra-PAG M6G (0.2μg) was significantly higher than morphine alone [t(22)=−2.85, p=0.009]. In males, response to M6G (.2μg) was not significantly different from saline [t(16)=2.03, p=.059]. In contrast, M6G (0.7μg) produced a response that was not significantly different from morphine [t(16)=1.52, p=0.148]. Data are plotted as mean %MPE + S.E.M. *, p≤0.01; n.s., not significant.
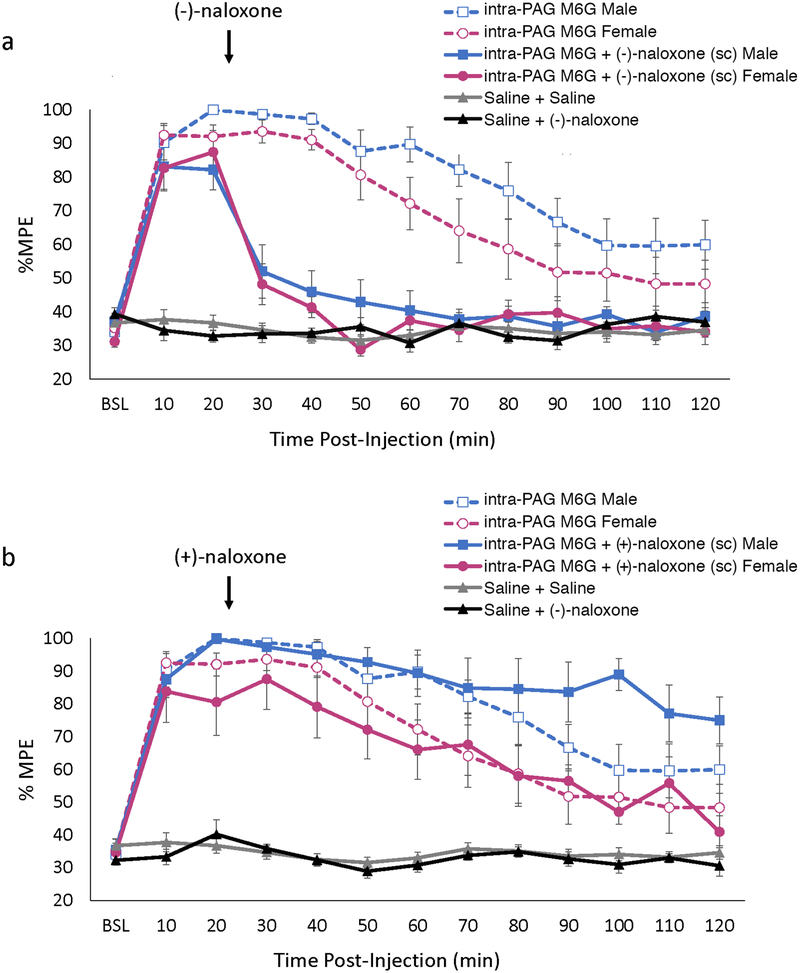
To determine if M6G was acting via MOR (and not TLR4), the MOR selective antagonist (−)-naloxone was co-administered with M6G. Administration of (−)-naloxone significantly and completely antagonized the analgesic effects of M6G in both males (p<0.001) and females (p<0.001; Figure 2a). Indeed, within 20 minutes of (−)-naloxone administration, PWLs were not significantly different from saline in males (p=0.54) or females (p=0.49). By contrast, administration of the TLR4 antagonist (+)-naloxone had no effect on M6G-induced analgesia in either males (p=0.90) or females (p=0.99; Figure 2b). Administration of either (−)-naloxone or (+)-naloxone had no effect on PWL latency alone in males (p>0.99 and p>0.99) or females (p>0.99 and p>0.99, respectively). Together, these results suggest that the analgesic effects of M6G are mediated via an action at MOR, and not TLR4.
Figure 2. Receptor mechanisms underlying intra-PAG M6G analgesia.
(a) Inhibition of mu opioid receptor with (−)-naloxone (3.7 mg/kg; s.c.) results in a complete blockade of intra-PAG M6G analgesia (dosage: males, 0.7μg; females, 0.2 μg; see Figure 1). As no differences were noted in %MPE for males and females treated with either Saline + Saline or Saline + Naloxone, data for each sex are combined and shown as one group. (b) In contrast, inhibition of TLR4 with (+)-naloxone (8.0 mg/kg; s.c.) has no effect on the analgesia produced by the intra-PAG administration of M6G (dosage: males, 0.7μg; females, 0.2 μg; see Figure 1). Data are plotted as mean %MPE + S.E.M.
3.2. M6G Does Not Alter Cytokine Profiles
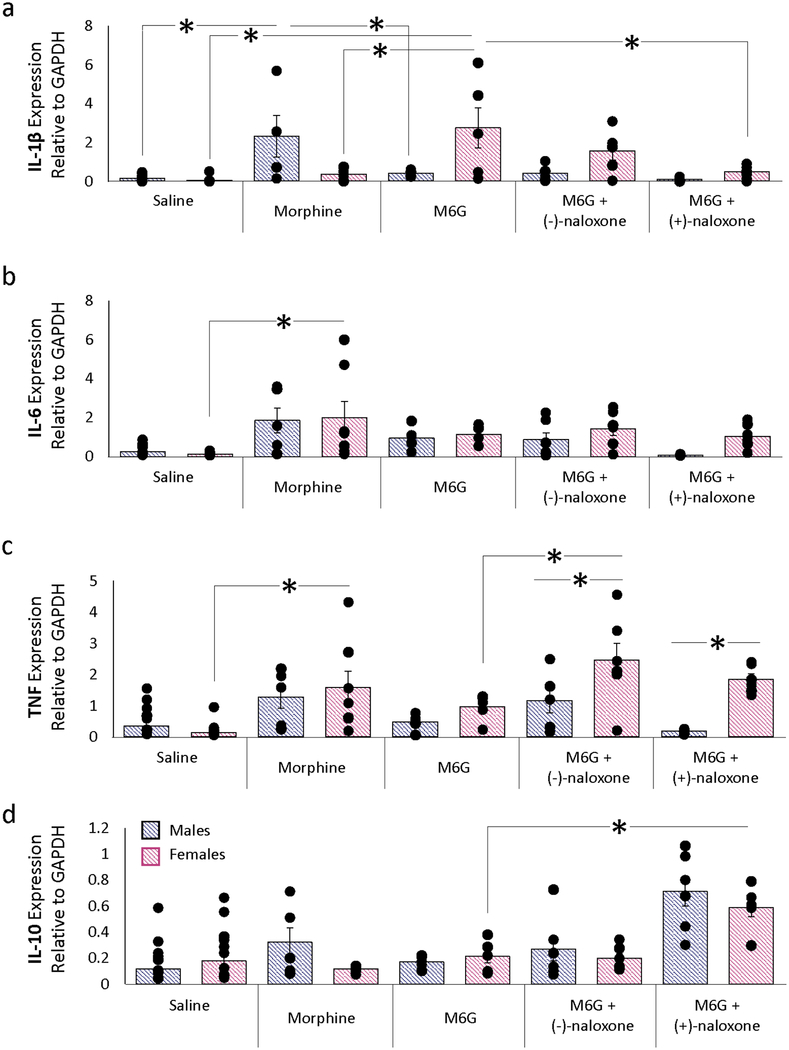
Our next series of experiments used qPCR to determine if acute morphine or M6G altered cytokine expression in the PAG in a sex-dependent manner. We have previously reported that chronic morphine administration increases PAG cytokine expression (IL-1β, IL-6 and TNF) in males and that this local neuroinflammatory response actively opposes the analgesic effects of morphine [24]. However, the impact of acute morphine administration on PAG cytokine levels in males and females is not known. M6G has been previously shown to have immunomodulatory effects on peripheral immune function, including reduced cytokine production, decreased B cell and lymphocyte proliferation, and reduced natural killer cell activity [43, 56].
Intra-PAG morphine significantly increased the expression levels of IL-6 and TNF in females relative to saline (p=0.004 and p=0.005, respectively; Figure 3); this finding is consistent with the reduced analgesic response observed following administration. Paradoxically, morphine significantly increased IL-1β levels in males relative to saline (p=0.002). In males, intra-PAG M6G (0.7 ug) did not significantly alter cytokine expression levels, a finding consistent with the potent analgesia observed. In contrast, M6G increased IL-1β in females relative to morphine (p=0.001). Despite completely reversing M6G-induced analgesia, co-administration of M6G + (−)-naloxone significantly increased TNF in females (p=0.009); a similar effect was observed with (+)-naloxone (p=0.003). IL-10 levels in females were also increased by M6G + (+)-naloxone (p=0.04).
Figure 3. M6G immunomodulation in the PAG.
Changes in (a) IL-1β, (b) IL-6, (c) TNF, and (d) IL-10 were assessed using qPCR following opioid administration. Increased expression levels of IL-6 and TNF were observed following intra-PAG morphine in females relative to saline. In males, morphine significantly increased IL-1β levels relative to saline. In males, intra-PAG M6G (0.7 μg) did not significantly alter cytokine expression levels. Co-administration of intra-PAG M6G with systemic (−)-naloxone significantly increased TNF in females (p=0.009); a similar effect was observed with systemic (+)-naloxone (p=0.003). IL-10 levels in females were also increased by intra-PAG M6G + systemic (+)-naloxone (p=0.04). PCR data are presented as 2−(ΔCq) normalized means ±SEM; *, p≤0.05.
3.3. M3G Attenuates Morphine Analgesia in Males Only
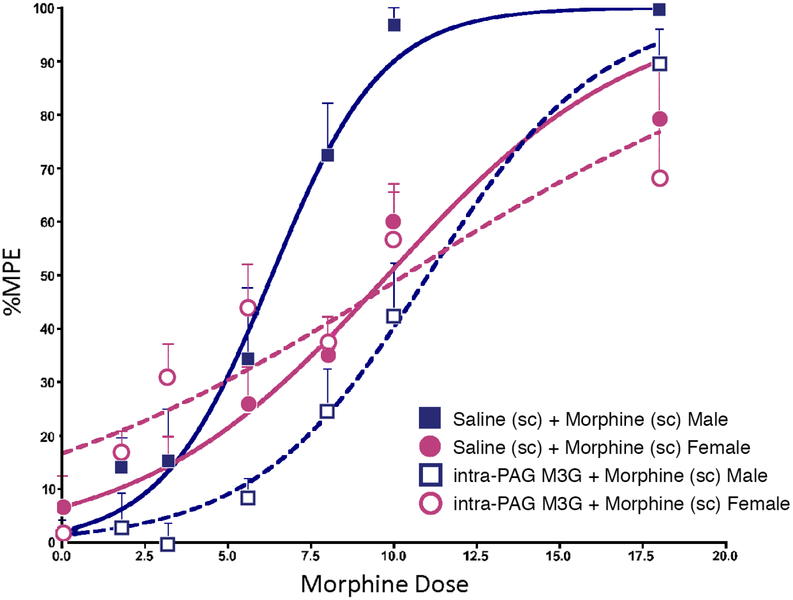
We have previously reported that PAG microglia show a more ‘activated’ phenotype in females compared to males [16]. As M3G has been reported to oppose the analgesic effects of morphine and M6G via an action at microglia TLR4 [39–42, 57, 58], we next determined if intra-PAG M3G opposition to morphine was greater in females than in males. Male and female rats received a single intra-PAG injection of M3G (0.075ug, or saline) 45 min prior to receiving cumulative injections of morphine (or saline). Consistent with our previous studies, morphine efficacy was significantly greater in males than females (ED50= 5.00 versus 7.81, p<0.05; Figure 4). In males, pre-treatment with M3G resulted in a significant rightward shift in the morphine dose response curve (p<0.01) that completely abolished the sex difference in morphine response (ED50= 10.28 in males versus 8.82 in females, p>0.05; Figure 4a). Interestingly, M3G had no effect on morphine analgesia in females (ED50 7.81 versus 8.82 with morphine alone). In control rats, M3G alone did not significantly alter response latencies from saline in males (p=0.99) or females (p=0.94) groups (data not shown).
Figure 4. M3G attenuates morphine analgesia in male, but not female, rats.
a) Intra-PAG administration of M3G (0.075 μg/0.25 μl/side) significantly attenuates systemic morphine analgesia in males, but not females. Administration of M3G+Saline or saline alone had no impact on PWLs (data not shown). Data are plotted as mean %MPE + S.E.M.; *, p≤0.05.
3.4. M3G Alters Cytokine Profiles
The results of the behavioral studies above suggest that increased concentrations of M3G in the PAG are sufficient to attenuate morphine antinociception in males. As M3G is known to induce pro-inflammatory responses that oppose the analgesic effects of morphine [29, 30, 37, 59], we next used qPCR to determine if M3G increased cytokine expression in the PAG of males and females.
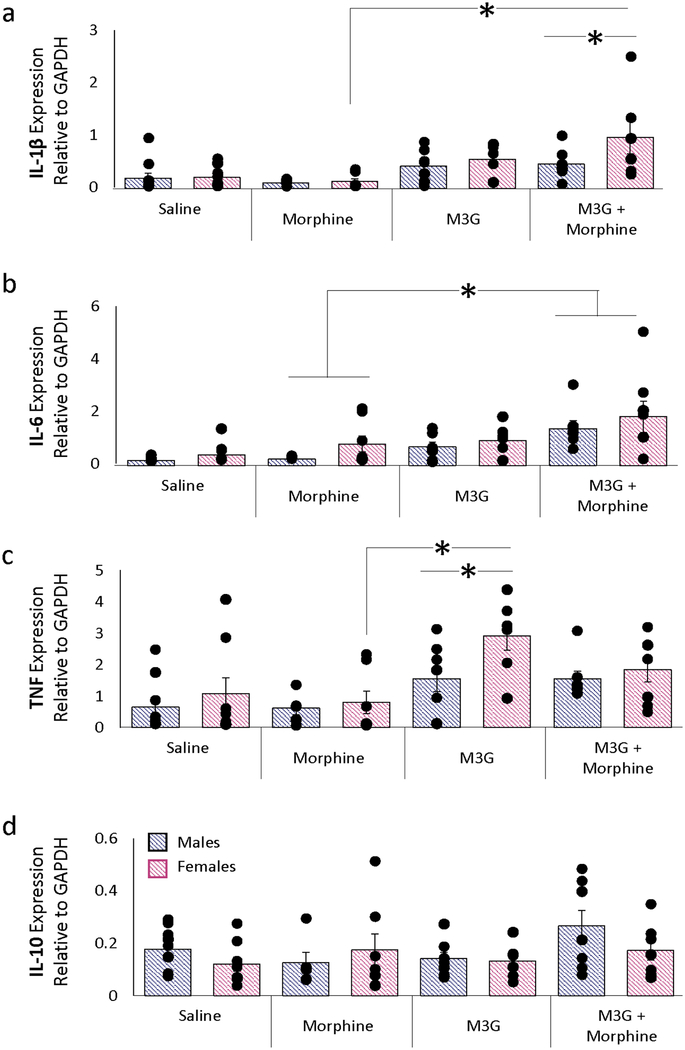
Administration of M3G or M3G+morphine significantly increased PAG expression levels of IL-1β (p=0.009) and TNF (p=0.002) in females, and IL-6 in both sexes (p=0.019) relative to morphine treatment alone (Fig. 5). IL-10 levels were remarkably stable regardless of treatment or sex. In contrast to the changes in cytokine levels induced by intra-PAG morphine (Figure 3), systemic morphine administration was insufficient in altering PAG cytokine levels.
Figure 5. M3G immunomodulation in the PAG.
a) IL-1β expression significantly increased in female rats treated with intra-PAG M3G and systemic morphine relative to systemic morphine-only controls. b) Similar to IL-1β, IL-6 expression significantly increased in intra-PAG M3G and systemic morphine treated females relative to morphine-only controls. c) TNF expression significantly increased in intra-PAG M3G females relative to systemic morphine females as well as intra-PAG M3G males. d) No significant changes in IL-10 expression were observed between any treatment groups. PCR data are presented as 2−(ΔCq) normalized means ±SEM; *, p≤0.05.
4. Discussion
The present study examined both the behavioral and immunomodulatory effects of the morphine metabolites, M3G and M6G. Intra-PAG administration of M6G resulted in significantly greater analgesia in females than in males. Importantly, M6G resulted in near maximal analgesia in females, a 26% increase over the maximum response observed following morphine. M6G analgesia was reversed with (−)-naloxone, and unchanged with (+)-naloxone, consistent with previous studies showing that the effects of M6G are mediated via MOR and not glial TLR4 [19, 34, 35]. In contrast, M3G administration, which acts via TLR4, resulted in a significant rightward shift in the morphine dose response curve in males only. Pretreatment with M3G in females did not alter the response to morphine. Given our previous studies reporting that PAG microglia show a more ‘activated’ phenotype in females, this suggests that the lack of impact of M3G on morphine is due to a ceiling effect. Despite the large and significant shifts in pain sensitivity observed following M6G administration in males and females, no reliable shift in PAG proinflammatory cytokine concentrations were observed. In contrast, pro-inflammatory cytokines were significantly elevated in male and/or female rats treated with M3G alone or in combination with morphine. Together, these data indicate that M3G may contribute to the attenuation of morphine analgesia observed in females, as administration of M6G, but not M3G and/or morphine results in significantly greater analgesia relative to males. These studies further suggest that the immunomodulatory effects of morphine and its metabolites result in sex-dependent effects on pain modulation, and have far-reaching implications for the use of opioids to treat pain in women.
4.1. Sex Differences in Pharmacokinetics
Previous dogma held that there were no sex differences in morphine metabolism, likely due to a history of conflicting HPLC results in human studies showing both the presence [60] and absence [61, 62] of sex differences in M3G or M6G concentrations following morphine treatment. However, pre-clinical studies in rats have consistently reported significant sex differences in metabolite concentrations using HPLC [44, 45, 63]. To date, few studies have examined the effect of endogenous morphine metabolites on pain modulation—and to our knowledge, the present experiments are the first to demonstrate sex-specific causal relationships.
Results from pharmacokinetic studies also support sex-specific differences in morphine metabolism. In humans, morphine is metabolized by isozymes in the uridine 5’-diphosphoglucuronosyltransferase (UGT) 1 and 2 subfamilies, with almost all preferentially synthesizing M3G over M6G (~45–55% and 15% of metabolized product, respectively; [64]). Importantly, sex differences in the expression of UGT1 and 2 subclasses of enzymes have been reported in humans [65] and rats [66]. Further, these enzymes metabolize, and are directly influenced by, steroid hormones [67]. Although no studies have examined the effects of sex or gonadal hormones on the expression of these isozymes in brain tissue, in rats gonadectomy significantly decreases the M3G:morphine ratio in females only, suggesting the involvement of steroid hormones in mediating sex differences in morphine metabolism [44].
4.2. Immunomodulatory Effects of Morphine Metabolites
M6G is a known immunomodulator, with predominantly anti-inflammatory effects [43, 56]. Here, M6G increased expression of IL-1β, but did not change expression of IL-6, TNF, and IL-10 relative to saline controls. Antinociceptive and immunomodulatory effects of M6G have been classically attributed to neuronal MOR [43, 68]; however, no studies have investigated the possibility of M6G exerting its effects via MOR on CNS glial cells, which may account for the discrepancies in immune modulation between previous and present studies [69].
Inflammatory mechanisms of M3G have been established, as M3G significantly increases pro-inflammatory IL-1β mRNA in BV-2 microglia cultures [29]. TLR4 activation by M3G is modest relative to TLR4’s natural agonist lipopolysaccharide (LPS; [19]), and may not induce robust increases in cytokine concentrations in vivo [29, 59]. In the present study, we report that M3G, administered in combination with morphine, increased the expression of IL-1β and IL-6 in a sex dependent manner relative to saline-treated controls. With the exception of TNF, M3G alone was not sufficient to alter cytokine expression in the PAG, suggesting that, in vivo, central M3G does not produce measurable immune activation relative to peripheral LPS, which has robust sex-specific effects on cytokine expression in the PAG [17].
Overall, patterns of cytokine expression observed following M3G or M6G treatment were equivocal, and not consistently reversed with (+)-naloxone or (−)-naloxone. Similar inconsistencies have been reported, suggesting a complicated role for morphine metabolites and their relative contributions to immune modulation following morphine [56, 70]. Estrous cycle was monitored in the present experiments, however, stage of estrous did not correlate with the observed variability in qPCR results, although this study was not powered to examine estrous cycle effects. A number of other factors may play a role; for example, route of drug administration [intracerebroventricular vs. subcutaneous; [71]], and duration of administration [acute vs. chronic; [72] have been shown to alter metabolite-induced, immune-related activity. Indeed, it may be that although acute morphine and/or metabolite administration is sufficient to induce changes in behavior that are likely driven by cytokine release, the changes in mRNA levels are below the level of reliable detection. Clearly, immune modulation by M3G and M6G remains vastly understudied, and further experiments comparing brain cytokine concentrations using various doses, time-points, and routes of administration will be useful to understand how M3G and M6G each contribute to immune modulation.
4.3. Behavioral Effects of Morphine-6-Glucuronide
In the present study, we hypothesized that in the absence of immune activation by M3G or morphine, M6G would produce equipotent analgesia in males and females. This is supported by a study in healthy human subjects demonstrating no differences in analgesic responses to M6G between males and females [62]. Surprisingly, we found that exogenous, intra-PAG administration of M6G is more potent in females than in males. Along with our previous data demonstrating sex differences in the phenotype of PAG microglia, this finding provides a new converging line of evidence to support our hypothesis that TLR4 is a primary contributor to sex differences in morphine action. It also initiates exciting and important questions regarding the mechanisms of opioid analgesia; specifically, why and how does M6G produce robust analgesia in females compared with many other opioids that produce more potent analgesia in males [73–77]?
One possible explanation for the reversal of sex differences observed with M6G is that M6G induces a more robust physiological response. The PAG sends dense projections to the rostral ventromedial medulla (RVM), which together with descending projections to the spinal cord dorsal horn, constitute the endogenous descending analgesia circuit. Previous anatomical studies in our lab have reported that the density of PAG-RVM output neurons is significantly greater in females compared with males. However, despite this difference in the density of projection neurons, the percent of PAG-RVM neurons activated by morphine is significantly greater in males (20% vs 50%) [48, 78]. Based on the results of the present study, we would predict that M6G activates a greater proportion of PAG-RVM neurons than morphine in females, resulting in improved analgesia. Further investigation of the binding properties of M6G in males and females is clearly warranted.
4.4. Behavioral Effects of Morphine-3-Glucuronide
The present study shows that injection of M3G into the vlPAG prior to morphine administration causes a significant attenuation of morphine analgesia in males only. It has been previously reported that female rats produce approximately 2–3 times more M3G than their male counterparts following a single systemic injection of morphine [44, 45]. M3G levels are significantly higher in females following morphine; therefore, we suspect that the lack of behavioral effect observed in females is due to saturation of M3G at TLR4 (i.e., a ceiling effect of M3G). This interpretation is consistent with our hypothesis that increased M3G reduces morphine’s effects, and may contribute to sexually dimorphic responses to morphine.
The sex-specific effects we observe here with M3G have broad implications that apply to other opioids that create 3-glucuronide metabolites. Glucuronidation at the 3-site of the substrate molecule is associated with glial activation and neuronal excitability; for example, morphine-3-glucuronide [29] and estradiol-3-glucuronide [79] both activate glial cells in a TLR4-dependent manner, increasing the release of pro-inflammatory mediators, and ultimately resulting in increased neuronal excitation. Hydromorphone-3-glucuronide [80] and normorphine-3-glucuronide [81] have also been shown to increase neuronal excitability, likely through the same mechanisms. Interestingly, exogenous opioids with the greatest sex difference in ED50 in rats (oxymorphone, hydromorphone, and morphine; [75] all produce 3-glucuronide metabolites by Phase II metabolism via UGTs. In contrast, drugs producing comparable ED50’s in males and females (codeine, oxycodone, fentanyl) undergo Phase I metabolism by cytochrome P450 (CYP) enzymes, and therefore do not produce 3-glucuronide metabolites on their first pass [82–84]. More research is needed to understand how metabolism and elimination of these drugs may differ in males and females, and how 3-glucuronide metabolites impact analgesia.
5. Conclusions
Together, these data demonstrate an important proof of principle: that in the absence of TLR4 signaling, opioid analgesia is equally effective—if not more effective—in females than in males. Historically, M6G has not been used for the treatment of clinical pain in humans. This is perhaps due to its “low and slow” blood brain barrier permeability, high variability in the doses of M6G required to induce analgesia (depending on the type of pain and method of administration), and tendency to accumulate in plasma in patients with impaired renal function [see [55, 85] for review]. However, clinical trials of M6G demonstrate comparable analgesia to morphine at appropriate doses, while reducing the negative side effects typically associated with morphine, such as nausea and sedation, in both men and women [62, 86–88]. Clearly, further research is required to address the relevance of treatment with M6G, as these studies may provide insight into improved treatment strategies for pain management in females.
Highlights:
Sex-specific effects of morphine glucuronide metabolites are proposed.
Morphine-6-glucuronide (M6G) analgesia was more potent in females than in males.
Morphine-3-glucuronide (M3G) reduced morphine effective dose in males only.
Opioids bypassing innate immune receptor activation are effective in females.
Acknowledgements:
(−)-Morphine sulfate, Morphine-3-β-D-glucuronide, Morphine-6-β-D-glucuronide, (−)-naloxone and (+)-naloxone were kindly provided by the National Institute on Drug Abuse drug supply program. We thank Dr. Hasse Walum for assistance with the statistical analysis.
Funding:
This work was supported by National Institutes of Health Grant [DA16272 and DA041529].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Dowell D, Haegerich TM Using the CDC Guideline and Tools for Opioid Prescribing in Patients with Chronic Pain. Am Fam Physician. 2016,93:970–2. [PMC free article] [PubMed] [Google Scholar]
- [2].Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000,24:375–89. [DOI] [PubMed] [Google Scholar]
- [3].Loyd DR, Wang X, Murphy AZ Sex Differences in Mu Opioid Receptor Expression in the Rat Midbrain Periaqueductal Gray are Essential for Eliciting Sex Differences in Morphine Analgesia. . J Neurosci. 2008,28:14007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Craft RM Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain. 2003,19:175–86. [DOI] [PubMed] [Google Scholar]
- [5].Dawson-Basoa MB, Gintzler AR 17-Beta-estradiol and progesterone modulate an intrinsic opioid analgesic system. Brain Res. 1993,601:241–5. [DOI] [PubMed] [Google Scholar]
- [6].Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology (Berl). 2001,158:154–64. [DOI] [PubMed] [Google Scholar]
- [7].Terner JM, Lomas LM, Picker MJ Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain. 2005,6:372–83. [DOI] [PubMed] [Google Scholar]
- [8].Stoffel EC, Ulibarri CM, Craft RM Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003,103:285–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lane DA, Patel PA, Morgan MM Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005,135:227–34. [DOI] [PubMed] [Google Scholar]
- [10].Loyd DR, Morgan MM, Murphy AZ Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience. 2007,147:456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bobeck EN, McNeal AL, Morgan MM Drug dependent sex-differences in periaqueducatal gray mediated antinociception in the rat. Pain. 2009,147:210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Krzanowska EK, Bodnar RJ Morphine antinociception elicted from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999,821:224–30. [DOI] [PubMed] [Google Scholar]
- [13].Commons KG, Aicher SA, Kow LM, Pfaff DW Presynaptic and postsynaptic relations of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol. 2000,419:532–42. [DOI] [PubMed] [Google Scholar]
- [14].Commons KG, van Bockstaele EJ, Pfaff DW Frequent colocalization of mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. J Comp Neurol. 1999,408:549–59. [PubMed] [Google Scholar]
- [15].Wang H, Wessendorf MW Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience. 2002,109:619–34. [DOI] [PubMed] [Google Scholar]
- [16].Bernal SA, Morgan MM, Craft RM PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behav Brain Res. 2007,177:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doyle HH, Eidson LN, Sinkiewicz DM, Murphy AZ Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. The Journal of Neuroscience. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, et al. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010,167:880–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010,24:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bonizzi G, Karin M The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004,25:280–8. [DOI] [PubMed] [Google Scholar]
- [21].Doyle HH, Murphy AZ Sex differences in innate immunity and its impact on opioid pharmacology. J Neurosci Res. 2017,95:487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ogoshi F, Yin HZ, Kuppumbatti Y, Song B, Amindari S, Weiss JH Tumor necrosis-factor-alpha (TNF-alpha) induces rapid insertion of Ca2+-permeable alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Exp Neurol. 2005,193:384–93. [DOI] [PubMed] [Google Scholar]
- [24].Stellwagen D, Beattie EC, Seo JY, Malenka RC Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005,25:3219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ Toll-like Receptor 4 Mediates Morphine-Induced Neuroinflammation and Tolerance via Soluble Tumor Necrosis Factor Signaling. Neuropsychopharmacology. 2017,42:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yan X, Yadav R, Gao M, Weng HR Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2014,62:1093–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Franchi S, Moretti S, Castelli M, Lattuada D, Scavullo C, Panerai AE, et al. Mu opioid receptor activation modulates Toll like receptor 4 in murine macrophages. Brain Behav Immun. 2012,26:480–8. [DOI] [PubMed] [Google Scholar]
- [28].Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007,7:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010,165:569–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Grace PM, Ramos KM, Rodgers KM, Wang X, Hutchinson MR, Lewis MT, et al. Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae. Neuroscience. 2014,280:299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Christrup LL Morphine metabolites. Acta anaesthesiologica Scandinavica. 1997,41:116–22. [DOI] [PubMed] [Google Scholar]
- [32].Coughtrie MW, Ask B, Rane A, Burchell B, Hume R The enantioselective glucuronidation of morphine in rats and humans. Evidence for the involvement of more than one UDP-glucuronosyltransferase isoenzyme. Biochemical pharmacology. 1989,38:3273–80. [DOI] [PubMed] [Google Scholar]
- [33].Togna AR, Antonilli L, Dovizio M, Salemme A, De Carolis L, Togna GI, et al. In vitro morphine metabolism by rat microglia. Neuropharmacology. 2013,75:391–8. [DOI] [PubMed] [Google Scholar]
- [34].Abbott FV, Palmour RM Morphine-6-glucuronide: analgesic effects and receptor binding profile in rats. Life sciences. 1988,43:1685–95. [DOI] [PubMed] [Google Scholar]
- [35].Wittwer E, Kern SE Role of morphine’s metabolites in analgesia: concepts and controversies. The AAPS journal. 2006,8:E348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Loser SV, Meyer J, Freudenthaler S, Sattler M, Desel C, Meineke I, et al. Morphine-6-O-beta-D-glucuronide but not morphine-3-O-beta-D-glucuronide binds to mu-, delta- and kappa- specific opioid binding sites in cerebral membranes. Naunyn-Schmiedeberg’s archives of pharmacology. 1996,354:192–7. [DOI] [PubMed] [Google Scholar]
- [37].Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, et al. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J Neuroinflammation. 2012,9:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Juni A, Klein G, Kest B Morphine hyperalgesia in mice is unrelated to opioid activity, analgesia, or tolerance: evidence for multiple diverse hyperalgesic systems. Brain research. 2006,1070:35–44. [DOI] [PubMed] [Google Scholar]
- [39].Ekblom M, Gardmark M, Hammarlund-Udenaes M Pharmacokinetics and pharmacodynamics of morphine-3-glucuronide in rats and its influence on the antinociceptive effect of morphine. Biopharmaceutics & drug disposition. 1993,14:1–11. [DOI] [PubMed] [Google Scholar]
- [40].Bartlett SE, Cramond T, Smith MT The excitatory effects of morphine-3-glucuronide are attenuated by LY274614, a competitive NMDA receptor antagonist, and by midazolam, an agonist at the benzodiazepine site on the GABAA receptor complex. Life sciences. 1994,54:687–94. [DOI] [PubMed] [Google Scholar]
- [41].Angst MS, Clark JD Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006,104:570–87. [DOI] [PubMed] [Google Scholar]
- [42].Smith MT, Watt JA, Cramond T Morphine-3-glucuronide--a potent antagonist of morphine analgesia. Life sciences. 1990,47:579–85. [DOI] [PubMed] [Google Scholar]
- [43].Carrigan KA, Lysle DT Morphine-6 beta-glucuronide induces potent immunomodulation. Int Immunopharmacol. 2001,1:821–31. [DOI] [PubMed] [Google Scholar]
- [44].Baker L, Ratka A Sex-specific differences in levels of morphine, morphine-3-glucuronide, and morphine antinociception in rats. Pain. 2002,95:65–74. [DOI] [PubMed] [Google Scholar]
- [45].South SM, Edwards SR, Smith MT Antinociception versus serum concentration relationships following acute administration of intravenous morphine in male and female Sprague-Dawley rats: differences between the tail flick and hot plate nociceptive tests. Clinical and experimental pharmacology & physiology. 2009,36:20–8. [DOI] [PubMed] [Google Scholar]
- [46].Eidson LN, Murphy AZ Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. 2013,33:15952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Loyd DR, Wang X, Murphy AZ Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008,28:14007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Loyd DR, Morgan MM, Murphy AZ Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci. 2008,27:1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hargreaves K, Dubner R, Brown F, Flores C, Joris J A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988,32:77–88. [DOI] [PubMed] [Google Scholar]
- [50].Wang X, Traub RJ, Murphy AZ Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006,291:R300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mathes WF, Kanarek RB Chronic running wheel activity attenuates the antinociceptive actions of morphine and morphine-6-glucouronide administration into the periaqueductal gray in rats. Pharmacology, biochemistry, and behavior. 2006,83:578–84. [DOI] [PubMed] [Google Scholar]
- [52].Wu D, Kang YS, Bickel U, Pardridge WM Blood-brain barrier permeability to morphine-6-glucuronide is markedly reduced compared with morphine. Drug metabolism and disposition: the biological fate of chemicals. 1997,25:768–71. [PubMed] [Google Scholar]
- [53].Morgan MM, Fossum EN, Stalding BM, King MM Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006,7:358–66. [DOI] [PubMed] [Google Scholar]
- [54].Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ Toll-Like Receptor 4 Mediates Morphine-Induced Neuroinflammation and Tolerance via Soluble Tumor Necrosis Factor Signaling. Neuropsychopharmacology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kilpatrick GJ, Smith TW Morphine-6-glucuronide: actions and mechanisms. Med Res Rev. 2005,25:521–44. [DOI] [PubMed] [Google Scholar]
- [56].Thomas PT, Bhargava HN, House RV Immunomodulatory effects of in vitro exposure to morphine and its metabolites. Pharmacology. 1995,50:51–62. [DOI] [PubMed] [Google Scholar]
- [57].Smith GD, Smith MT Morphine-3-glucuronide: evidence to support its putative role in the development of tolerance to the antinociceptive effects of morphine in the rat. Pain. 1995,62:51–60. [DOI] [PubMed] [Google Scholar]
- [58].Yaksh TL, Harty GJ, Onofrio BM High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: clinical and theoretic implications. Anesthesiology. 1986,64:590–7. [DOI] [PubMed] [Google Scholar]
- [59].Xie N, Gomes FP, Deora V, Gregory K, Vithanage T, Nassar ZD, et al. Activation of μ-opioid receptor and Toll-like receptor 4 by plasma from morphine-treated mice. Brain, behavior, and immunity. 2017,61:244–58. [DOI] [PubMed] [Google Scholar]
- [60].Murthy BR, Pollack GM, Brouwer KL Contribution of morphine-6-glucuronide to antinociception following intravenous administration of morphine to healthy volunteers. Journal of clinical pharmacology. 2002,42:569–76. [DOI] [PubMed] [Google Scholar]
- [61].Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000,93:1245–54; discussion 6A. [DOI] [PubMed] [Google Scholar]
- [62].Romberg R, Olofsen E, Sarton E, den Hartigh J, Taschner PE, Dahan A Pharmacokinetic-pharmacodynamic modeling of morphine-6-glucuronide-induced analgesia in healthy volunteers: absence of sex differences. Anesthesiology. 2004,100:120–33. [DOI] [PubMed] [Google Scholar]
- [63].South SM, Wright AW, Lau M, Mather LE, Smith MT Sex-related differences in antinociception and tolerance development following chronic intravenous infusion of morphine in the rat: modulatory role of testosterone via morphine clearance. The Journal of pharmacology and experimental therapeutics. 2001,297:446–57. [PubMed] [Google Scholar]
- [64].De Gregori S, De Gregori M, Ranzani GN, Allegri M, Minella C, Regazzi M Morphine metabolism, transport and brain disposition. Metabolic brain disease. 2012,27:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gallagher CJ, Balliet RM, Sun D, Chen G, Lazarus P Sex differences in UDP-glucuronosyltransferase 2B17 expression and activity. Drug metabolism and disposition: the biological fate of chemicals. 2010,38:2204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Iwano S, Higashi E, Miyoshi T, Ando A, Miyamoto Y Focused DNA microarray analysis for sex-dependent gene expression of drug metabolizing enzymes, transporters and nuclear receptors in rat livers and kidneys. The Journal of toxicological sciences. 2012,37:863–9. [DOI] [PubMed] [Google Scholar]
- [67].Strasser SI, Smid SA, Mashford ML, Desmond PV Sex hormones differentially regulate isoforms of UDP-glucuronosyltransferase. Pharmaceutical research. 1997,14:1115–21. [DOI] [PubMed] [Google Scholar]
- [68].Lysle DT, Carrigan KA Morphine-6beta-glucuronide modulates the expression of inducible nitric oxide synthase. Inflammation. 2001,25:267–75. [DOI] [PubMed] [Google Scholar]
- [69].Gessi S, Borea PA, Bencivenni S, Fazzi D, Varani K, Merighi S The activation of mu opioid receptor potentiates LPS-induced NF-kB promoting an inflammatory phenotype in microglia. FEBS Lett. 2016,590:2813–26. [DOI] [PubMed] [Google Scholar]
- [70].Hashiguchi S, Morisaki H, Kotake Y, Takeda J Effects of morphine and its metabolites on immune function in advanced cancer patients. J Clin Anesth. 2005,17:575–80. [DOI] [PubMed] [Google Scholar]
- [71].Hashiguchi Y, Molina PE, Boxer R, Naukam R, Abumrad NN Differential responses of brain, liver, and muscle glycogen to opiates and surgical stress. Surg Today. 1998,28:471–4. [DOI] [PubMed] [Google Scholar]
- [72].Eckhardt K, Nevo I, Levy R, Mikus G, Eichelbaum M, Vogel Z Morphine-related metabolites differentially activate adenylyl cyclase isozymes after acute and chronic administration. FEBS Lett. 2000,470:309–14. [DOI] [PubMed] [Google Scholar]
- [73].Barrett AC, Smith ES, Picker MJ Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. European journal of pharmacology. 2002,452:163–73. [DOI] [PubMed] [Google Scholar]
- [74].Terner JM, Lomas LM, Smith ES, Barrett AC, Picker MJ Pharmacogenetic analysis of sex differences in opioid antinociception in rats. Pain. 2003,106:381–91. [DOI] [PubMed] [Google Scholar]
- [75].Peckham EM, Traynor JR Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. The Journal of pharmacology and experimental therapeutics. 2006,316:1195–201. [DOI] [PubMed] [Google Scholar]
- [76].Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain. 2005,6:261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bai X, Zhang X, Li Y, Lu L, Li B, He X Sex differences in peripheral mu-opioid receptor mediated analgesia in rat orofacial persistent pain model. PLoS One. 2015,10:e0122924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Loyd DR, Murphy AZ Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. The Journal of comparative neurology. 2006,496:723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lewis SS, Hutchinson MR, Frick MM, Zhang Y, Maier SF, Sammakia T, et al. Select steroid hormone glucuronide metabolites can cause toll-like receptor 4 activation and enhanced pain. Brain, behavior, and immunity. 2015,44:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Smith MT Neuroexcitatory effects of morphine and hydromorphone: evidence implicating the 3-glucuronide metabolites. Clinical and experimental pharmacology & physiology. 2000,27:524–8. [DOI] [PubMed] [Google Scholar]
- [81].Smith GD, Prankerd RJ, Smith MT Biochemical synthesis, purification and preliminary pharmacological evaluation of normorphine-3-glucuronide. Life sciences. 1997,61:95–104. [DOI] [PubMed] [Google Scholar]
- [82].Smith HS Opioid metabolism. Mayo Clinic proceedings. 2009,84:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chan S, Edwards SR, Wyse BD, Smith MT Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clinical and experimental pharmacology & physiology. 2008,35:295–302. [DOI] [PubMed] [Google Scholar]
- [84].Holtman JR Jr., Wala EP Characterization of the antinociceptive effect of oxycodone in male and female rats. Pharmacology, biochemistry, and behavior. 2006,83:100–8. [DOI] [PubMed] [Google Scholar]
- [85].Lotsch J, Geisslinger G Morphine-6-glucuronide: an analgesic of the future? Clin Pharmacokinet. 2001,40:485–99. [DOI] [PubMed] [Google Scholar]
- [86].Cann C, Curran J, Milner T, Ho B Unwanted effects of morphine-6-glucoronide and morphine. Anaesthesia. 2002,57:1200–3. [DOI] [PubMed] [Google Scholar]
- [87].Hanna MH, Elliott KM, Fung M Randomized, double-blind study of the analgesic efficacy of morphine-6-glucuronide versus morphine sulfate for postoperative pain in major surgery. Anesthesiology. 2005,102:815–21. [DOI] [PubMed] [Google Scholar]
- [88].Dahan A, van Dorp E, Smith T, Yassen A Morphine-6-glucuronide (M6G) for postoperative pain relief. Eur J Pain. 2008,12:403–11. [DOI] [PubMed] [Google Scholar]