Abstract
Maintaining a healthy, anti-hypertrophic state in the heart prevents progression to cardiac failure. In humans, angiotensin II (AngII) indirectly and directly stimulates hypertrophy and progression, while estrogens acting through estrogen receptor beta (ERβ) inhibit these AngII actions. The KLF15 transcription factor has been purported to provide anti-hypertrophic action. In cultured neonatal rat cardiomyocytes, we found AngII inhibited KLF15, expression and nuclear localization, substantially prevented by estradiol (E2) or β-LGND2 (β-LGND), an ERβ agonist. AngII stimulation of transforming growth factor beta expression in the myocytes activated p38α kinase via TAK1 kinase, inhibiting KLF15 expression. All was comparably opposed by E2 or β-LGND. Knockdown of KLF15 in the myocytes induced myocyte hypertrophy and limited the anti-hypertrophic actions of E2 and β-LGND. Key aspects were confirmed in an in-vivo model of cardiac hypertrophy. Our findings define additional anti-hypertrophic effects of ERβ supporting testing specific receptor agonists in humans to prevent progression of cardiac disease.
Keywords: Estrogen, hypertrophy, transcription factor, angiotensin, transforming growth factor beta
1. Introduction
In humans, cardiac hypertrophy that results from pathological states is a major indication of the progression of heart disease toward eventual heart failure if untreated. One of the most important causes of cardiac hypertrophy and progression is the angiotensin II peptide (AngII) binding to its type I receptor (Zhou et al., 2017). Many therapies in humans target the formation of AngII or block its receptor functions. Cardiac hypertrophy often arises from poorly treated hypertension in humans (Blaustein, 2017), which is an indirect result of excessive AngII action in arterial blood vessels. This occurs in addition to the direct effects of AngII on the myocardium, acting at both myocytes to induce hypertrophy (Subramaniam and Lip, 2009), and cardiac fibroblasts that induces fibrosis and thus stiffening of the left ventricle (Ruiz-Ortega et al., 2007). Both functions accelerate the progression to cardiac ventricular dilation and apoptotic thinning, resulting in compromised ability to eject sufficient blood upon ventricular contraction.
As shown in rodent models, impaired calcium-regulated ventricular contraction, genetic hypertension, or aortic banding produces cardiomyocyte hypertrophy, cardiac fibrosis, and heart failure, mitigated by estrogen action in female mice and rats. In hypertensive women, hormone replacement after menopause lowers vascular resistance and decreases left ventricular hypertrophy (Light et al., 2001). From our previous studies, as well as those from other labs, estrogen acts through estrogen receptor beta (ERβ) to normalize blood pressure, inhibit hypertrophy, fibrosis, and progression to heart failure (Skavdahl et al., 2004;Babikar et al, 2006;Pedram et al., 2008, Thireau et al, 2010). Previously, we showed that a highly specific ERβ agonist, β-LGND2 (Yeperu et al., 2010), was comparable to estrogen in preventing these forms of cardiovascular disease in-vivo (Pedram et al., 2013a). Support for this comes from ERβ knockout mice that develop strong systolic and diastolic hypertension with aging, seen in both female and male mice (Zhu et al., 2002). The role of ERβ appears to arise mainly from the membrane receptor pool, as we reported estrogen or β-LGND actions occur from signal transduction, a major function of membrane-localized ER (Levin and Hammes, 2016).
The mechanisms by which AnglI and its receptor or other relevant stimuli produce cardiovascular dysfunction are complex and involve many targets. In this regard, we reported that E2/ERβ signaling in several organs opposes numerous hypertrophic mechanisms, resulting in the re-establishment of a homeostatic situation (Xue et al, 2008, 2013; Pedram et al., 2008). Here we focus on a transcription factor, KLF15, which has been reported to have important functions to oppose cardiac hypertrophy (Leenders et al, 2012). We tested the hypothesis that AngII stimulates the loss of KLF15, importantly underlying cardiomyocyte hypertrophy that is opposed by ERβ signaling. We identify new functions by which ERβ prevents cardiomyocyte hypertrophy through this transcription factor (TF) and confirm some important actions in an in-vivo model.
2. Materials and Methods
2.1. Reagents
Peptides Ang II, TGFβ (both from Sigma), and steroids E2 (Steraloids) were purchased from Tocris. Additional antibodies and phospho-specific antibodies used for immuno-blots were obtained from the followings: Cell Signaling Technology (Danvers, MA) TAK1 (D94D7) (#5206), Phospho-ATF-2 (Thr71) (#9221), Phospho-TAK1 (Thr187) (#4536); Santa Cruz, Biotechnology (Dallas, TX), KLF15 (A5) (SC-271675), GAPDH (0411) (sc-47724), MYH7 (A4.951) (sc-53090), Actin (2Q1055) (sc-58673), p38 Antibody (A-20) (sc-535), phospho-p38 (Thr 180/Tyr 182) (sc-17852-R); (Boster Biological Technology, Pleasanton, CA), ACTA2 (M01072–1). Detection antibodies conjugated with fluorophores FITC, or with phycoerythrin (PE) used in immunofluorescence imaging and flow cytometry (FACS) analysis, and FITC-conjugated wheat germ agglutinin used in imaging for cell size measurement were supplied by Invitrogen Molecular Probes, (Eugene, OR). Horseradish Peroxidase (HRP) secondary antibody used in western blot (WB) was from Santa Cruz, Biotechnology (Dallas, TX). All antibodies were used at dilutions 1/50 for fluorescence studies and 1/100 for immuno-blots.
Short interfering RNA used in knocked-down experiments were Stealth siRNAs (Set of 3) KLF15 RSS340443, RSS340444, RSS340445 and Stealth siRNA Negative Control Kit (Cat. 12935100) were from Invitrogen. A pool of 3 target-specific 19–25 Takl siRNAs siRNA (sc-155991), control siRNAs include products (sc-37007, sc-44230, sc-44231) and siRNA Transfection Reagent (sc-29528) were from Santa Cruz, Biotechnology (Dallas, TX). Rp-8-Br-cAMP and H-89 were from Cal Biochem and selective inhibitor of p38 MAPK, SB 203580, was from Tocris. Reagent concentrations used are identified in the figure legends. β-LGND is a strongly estrogen receptor beta selective agonist (Yepuru et al., 2010; Pedram et al., 2013) that was provided as a gift from the GTx corporation, Memphis, TN. Ampules of 16% or 32% formaldehyde (PFA) fixative solutions were obtained from Electron Microscopy Sciences (Fort Washington, PA) and used for up to 1 week after opening.
2.2. In-vivo model of cardiac hypertrophy
The Animal Care, and Research and Development Committees at the Department of Veterans Affairs Medical Center, Long Beach, California approved all rodent studies. Ten week old, ovariectomized female C57/BJ6 mice were obtained from Harlan/Sprague Dawley then housed in 12 hour on/off lighting at the VA animal facility and fed rodent chow devoid of soy or most plant products. Osmotic mini-pumps (Alzet, DURECT Corp, Cupertino, CA.) were filled with either AngII (0.7mg/kg/day) in saline, saline alone (control), AngII plus 100μl of an ERβ agonist, β-LGND (0.5mg) or β-LGND alone. Several laboratories extensively characterized the specificity of β-LGND for the ERβ isoform, in-vitro and invivo (Yepuru et al., 2010; Pedram et al., 2013). Each mouse had a mini-pump inserted subcutaneously under inhaled chlorofluorane anesthesia to provide 21-day infusion. In some mice, an E2 pellet (0.1 mg, 21-day release pellets, Innovative Research of America, Sarasota, Florida) was inserted under the skin and these mice did not receive β-LGND. The E2 pellet is well documented to produce physiological levels of E2 in the serum of mice, including our previous studies (Pedram et al, 2008). In all experiments, 5–6 mice were used per condition.
For comparison, to the ovariectomized female wild type mice, female, ovariectomized ERβ gene-deleted mice were obtained from Ken Korach, NIEHS (Zhu et al., 2002). These mice were subjected to the same conditions described involving administration of AngII ± ERβ agonist or E2 pellets, and all mice were identically housed and fed the same chow at the VA animal facility. At 21 days, the hearts were removed and weighed, and the left ventricle was dissected free (Pedram et al, 2008 and 2013a). The heart was then processed for ventricular protein and mRNA that was used in the studies described here. For relative protein detection, immunoblots were carried out on protein extracted from the left ventricles of mice from all conditions, following separation by SDS-PAGE and transfer to nitrocellulose. RT-PCR and densitometry of bands from gel separation was used to quantify mRNA expression. Extensive functional characterization of heart function prior to sacrifice in this model was previously reported (Pedram et al., 2008).
2.3. Isolation and experiments in cardiomyocytes
Neonatal rat cardiomyocytes were isolated from the hearts of one-to-three-day-old rats or from term pregnant female rats (Charles River) using a cardiomyocyte isolation kit (Worthington), according to the manufacturer’s instructions, as previously described (Pedram et al., 2008). The myocytes were incubated in DMEM/F12 supplemented with 10% fetal bovine serum, 1xITS (insulin-transferrin-selenium) (Sigma) antibiotic and antimycotic, and 10μg/ml fibronectin (to aid adherence) until 70% confluent. Cells were then incubated in in serum free DMEM for 16hrs prior to experimentation. Cells were then placed in medium containing very low serum (0.1%), and incubated for 24 hrs with TGFβ (10ng/ml) or AngII (100nM) with or without E2 (10nM) or β-LGND (10nM) for 24 hours. All conditions had DMSO at a final concentration of 0.03%. RNA and protein were extracted for various characterizations and actin and GAPDH were used as loading controls for protein and mRNA respectively. Data are SEM ± SD from 3 separate experiments combined and analyzed by ANOVA + Scheffe’s test, p<0.05 considered significantly different.
2.4. Gene expression by PCR
Total RNA was extracted using the Qiagen RNeasy Mini Kit. The RNA concentration was determined using spectrophotometer. 1μg total RNA/sample was used for cDNA synthesis using iScript cDNA synthesis Kit (Bio-Rad Laboratories). For qPCR analysis, the primers were designed using NCBI Primer-BLAST and were synthesized by Eurofins Genomics (Huntsville, AL). The 18S primers and GAPDH primers were also designed and used for normalization of qPCR. The PCR was performed using SsoFast EvaGreen Supermix (Bio-Rad) and thermocycling was carried out using CFX96 Real-Time PCR Detection System (Bio-rad). The qPCR cycling conditions were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 10 s, 56°C for 30 s. The melt curve followed from 55oC and 95°C. All samples were run in triplicate and relative quantification of gene expression was determined by 2−ΔΔCT (Pfaffi M, 2001; Livak KJ and Schmittgen TD, 2001). The following primers were synthesized by Uerfin (Germantown, MD):
Rat KLF15-Forward, 5’-TGACTCGCAAGCCTTCTGTT-3’; Reverse, 5’-CATCTCCGACACCTCCACTG-3’
ACTA2-Forward, 5’- CATCACCAACTGGGACGACA-3’; Reverse, 5’-TCCGTTAGCAAGGTCGGATG −3’
MYH7-Forward, 5’ - GCCTACCTCATGGGACTGAA-3’; Reverse, 5’-ACATTCTGCCCTTTGGTGAC-3’
18S primers: Forward 5’-CAGGATTGACAGATTGATAGCTCTT-3’; Reverse 5’-GAGTCT CGTTCGTT AT CGGAATTAA-3’
Rat GAPDH-Forward, 5’-ACTGAGCATCTCCCTCACAA-3’; Reverse, 5’-ACACTGCATTCACACACAAGA-3’
2.5. Small interfering RNA studies
Briefly, cardiomyocytes were seeded in 6-well plate and grown to 50–70% confluence. The KLF15, TAK1 or control siRNA constructs (80 pmols) were mixed with transfection reagent and applied to each well in a total volume of 1ml of transfection medium (Santa Cruz Biotech). After incubation for 5 hours, normal growth medium containing 2X serum and antibiotics (1ml) was added to transfected cells. Cells were then incubated for 18–24 hours, and then replaced with fresh normal medium and cultured for 48 hours. FACS or Immuno-blots were carried out 48 h after transfection to validate the protein knockdown or real time RT-PCR was used to verify specificity of the constructs. Only cells which had >70% knock down were used for experiments as described.
2.6. Western blot
Sub-confluent, cultured neonatal rat cardiomyocytes were serum deprived overnight, then incubated under various conditions for 30 min with kinase or cAMP inhibitors followed by 16 hrs additional treatments with 100 nM AngII or 10 ng/ml TGFβ ± 10nM E2 or βLGND. If the cells were also transfected with control or other siRNAs, then this occurred for 48 h prior to the addition of the peptides or steroids. Immuno-blots were carried out using standard techniques as we described (Pedram et al., 2008) using specific antibodies. Cells grown to ~70% confluency in a 100-mm petri dish, were rinsed with PBS, and scraped. Cells were lysed in buffer with a protease inhibitor and centrifuged, pre-cleared with IgG, and suspended in Protein A/G Agarose for 30 min. After pelleting, cell lysate was mixed with primary antibody agarose conjugate and incubated overnight at 4 C. A pellet was then made and resuspended for boiling, and electrophoretic separation. Upon transfer of the proteins to nitrocellulose, immuno-blotting of the proteins with specific first antibody was carried out in conditions that block nonspecific binding. Horseradish peroxidase-conjugated second antibody was used, followed by membrane washing, incubation with chemo-luminescence (ECL) reagents, and detected on the ChemoDoc system from BioRad. Band intensities are normalized for background and error bars are SEM from 3 separate experiments.
2.7. Kinase assays
P38α mitogen activated protein kinase activity was determined as we described (Kim et al, 2006). The invitro activity was quantified by immuno-precipitating p38α protein from cardiomyocytes treated under various conditions, and then equal amounts of p38α protein were separated by gel electrophoresis, then activity. An inhibitor of p38α, SB2036580 (Tocris) was used at a concentration of 100nM (IC50 inhibitory values are 50 and 500 nM for p38α and p38β respectively). TAK1 kinase was similarly detected under the same conditions and separation techniques, immuno-blotted with phosphor-TAK1 antibody. For in vitro kinase assays, the experimental cell lysates were immuno-precipitated with p38α antibody, then equal amounts of kinase protein were added to tubes containing ATF2 protein substrate and p32 as described previously (Razandi et al., 2000). The phosphorylated substrate was separated on 10% SDS-PAGE gel and detected by autoradiography, and the bands were quantitated by laser densitometry. Western blotting for total p38α was performed to demonstrate equal amounts of immuno-precipitated kinase protein.
2.8. In-vitro cardiomyocyte hypertrophy
Cardiomyocyte hypertrophy was determined as H3-leucine incorporation as we previously described (Pedram et al, 2013). Isolated cardiomyocytes were cultured on collagen-coated 48 well plates at a concentration of 5×104 (cells/well). Cells were serum-starved for 24hr in DMEM/F12 media supplemented with antibiotics, then were incubated under various treatment conditions in the presence of 3[H] Leucine (1μCi) for 24 h. Treatment conditions included medium containing E2 (10nM), β-LGND (10nM), and/or SB2036580 (1nM) for 2 hours prior to TGFβ−1 and ANGII addition to the media. Cells were treated for 16hrs, then collected by aspiration after exposure to 5% trichloroacetic acid (TCA) and centrifuged, pellets washed then dissolved in 0.5 M NaOH. Activity was measured in 4 ml of high ionic count scintillation fluid in a Beckman beta counter. Similar treatment conditions were used for cell size measurements of cardiac hypertrophy using method described in the immunofluorescence microscopy section.
2.9. Flow cytometry
Treated cardiomyocytes were prepared with the reagents and protocols from Santa Cruz Biotechnology (Dallas, TX). Versene detached cells were subjected to fixative, washed in ice-cold phosphate-buffered saline (PBS). The cells were permeabilized for 15 min on ice, washed and resuspended, then divided into 106 cell aliquots. Cells were incubated with 5 μl (1:200) of the primary antibody (1 mg/ml) for 1h or with an isotypic IgG antibody of the nonimmunized host respective to the primary antibody for 1 h. The primary antibodies included mouse monoclonal antibodies against KLF15, ACTA2, MHY7 antibody, phospho-p38α and phospho-TAK1 antibodies then detect with flouresence conjugated secondary or with isotypic control antibodies for one hour at room temperature. After washing, samples were analyzed with Bectin-Dickinson FACS Calibur flow cytometer (Mountain View, CA). Using FACS analysis, phospho-specific antibodies enable measurement of phosphorylation states in multiple proteins, consequently providing an effective method for analyzing signaling networks in thousands of single cells (Irish et al., 2004; Hoa et al., 2010). The data from 10,000 cells was collected. The bar graph is the average of three separate experiments.
2.10. Immunofluorescence Microscopy
Cardiomyocyte cells (3 × 105) were cultured on collagen (BD, Bedford, MA) coated glass cover slips. Twenty-four hours later cells were treated with various agents, washed with PBS, and fixed with 4% fresh paraformaldehyde for 10 min at room temperature. For cell size measurement experiment, fixed samples were incubated with 1 μg/ml fluorescence labeled wheat germ agglutinin (WGA-FITC) at room temperature for 1h, washed, and mounted antifade. For nuclear KLF15 quantification, fixed samples were washed, permeabilized with 0.2% Triton X-100 in PBS for 5 min, and washed 3X with PBS. Before antibody incubation, cells were blocked with serum from the same species as the secondary antibody or 2% bovine serum albumin (BSA) at room temperature for 30 min. Cells were incubated with KLF15 primary antibody (dilution 1:50 in 0.5% BSA-PBS) at room temperature for 2 hrs. The cells were then incubated for 1 h with FITC conjugated secondary antibody at 1μg/ml at room temperature in the dark. Cells were washed with PBS and the coverslips were mounted with antifade mounting medium. Images were acquired using the Spot Software with the SPOT FLEX camera (Diagnostic Instruments, Sterling Heights, MI) mounted on the Nikon Eclipse E600 Epi fluorescence microscope as previously described (Hoa et al., 2015). Fluorescence labeled samples was analyzed using the Image J Software with the MBF “ImageJ for Microscopy” plugins from NIH. To quantify KLF15 fluorescence in the nucleus from a single in focus plane-acquired image, ten random regions were defined using the Polygon or the Free hand selection tool for each of the treatments. The selected regions and background were measured for area, integrated density, and mean gray scale value. The relative level of fluorescence (au) was calculated as Integrated Density (McCloy et al., 2014) - (Area of selected region × Mean fluorescence of background readings). The data was analyzed using Prism Graph Software.
3. Results
3.1. AnglI and estrogenic compounds reciprocally regulate KLF15 expression.
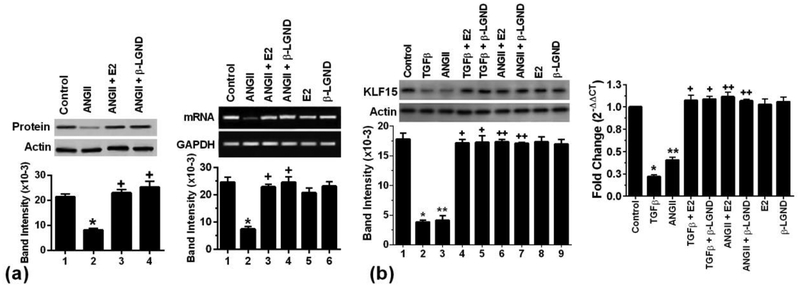
Since AnglI is an important hypertrophic peptide in humans, we examined its effects on KLF15 expression. In cultured neonatal rat cardiomyocytes, AngII exposure caused a significant inhibition of the anti-hypertrophic transcription factor mRNA and protein (Fig 1a). This was significantly reversed by coexposure of the myocytes to E2 or β-LGND. It has also been shown by several labs including ours that transforming growth factor beta (TGFβ) is stimulated by AngII, and underlies cardiomyocyte hypertrophy and fibroblast conversion to a myofibroblast. It is not clear whether the hypertrophic functions of AngII in cardiomyocytes are mainly mediated through TGFβ. To determine this, we compared the effects of equimolar AngII and TGFβ on KLF15 expression (Fig 1b). For both mRNA and protein, AngII and TGFβ effects were comparable, suggesting that most of the AngII function in this regard was mediated through TGFβ. Comparably, E2 and β-LGND opposed decreased KLF15 expression, as stimulated by AngII or TGFβ.
Fig 1. ERβ opposes ANGII suppression of KLF15 expression.
(a) AngII (100nM) inhibits and E2 (10nM) or β-LGND (10nM) prevents the inhibition of KLF15 protein (left), and mRNA (right) expression in cultured cardiomyocytes. Actin and GAPDH, respectively, are loading controls. Bar graph is mean± SD from 3 exps. p<0.05 vs control, +p<0.05 vs AngII alone from analysis by ANOVA + Schefe’s test. (b) Equimolar TGFβ (10ng/ml) or AngII represses KLF15 protein (left) and mRNA (right) that was significantly prevented by E2 or β-LGND. *p<0.05 or **p<0.05 vs control, +p<0.05 or ++p<0.05 vs AngII or TGFβ alone, n=3 exps.
3.2. TGFβ stimulates a TAK1-p38α kinase pathway to inhibit KLF15 expression.
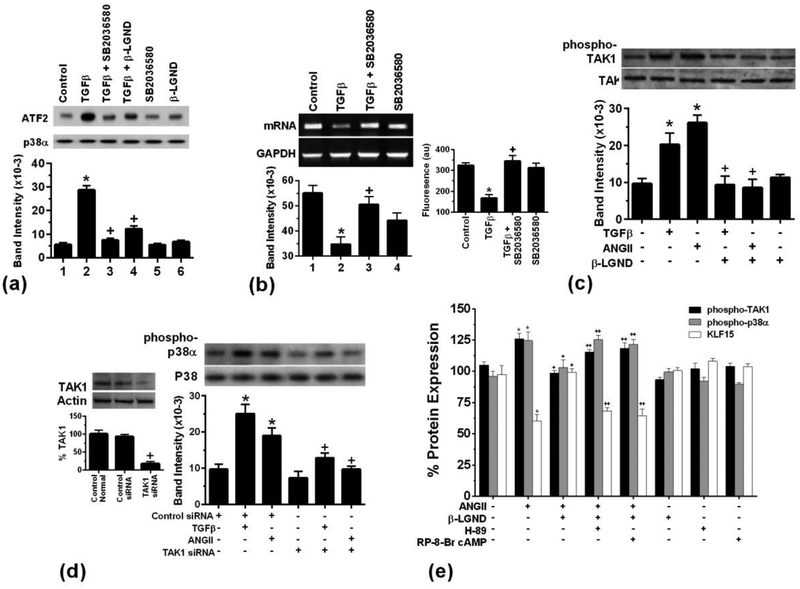
We then determined how TGFβ inhibited and ERβ opposed the decrease in KLF15 expression. TGFβ production and AngII have been reported to cause a p38α kinase-dependent suppression of KLF15 mRNA and protein, also stimulating cardiomyocyte hypertrophy (Leendoset et al., 2012). We previously published in a cardiomyocyte model of ischemia/reperfusion (I/R) injury, E2 rapidly inhibited p38α MAPK activation by I/R resulting in cardiomyocyte survival (Kim et al., 2006). We postulated that E2/ERβ inhibited TGFβ activation of p38α thereby preserving KLF15 production. Kinase activity was activated by TGFβ and a soluble inhibitor of p38 activity, SB20366580, strongly reversed this effect of TGFβ, thus validating the reagent (Fig 2a). β-LGND also significantly prevented p38α activation by TGFβ. Importantly, the SB compound reversed TGFβ-inhibition of KLF15 mRNA and protein (Fig 2b). These results indicate that β-LGND significantly reversed both TGFβ stimulation of p38α kinase activation and the resulting diminution of KLF15 (Figs 1 and 2).
Fig 2. Signaling to the regulation of KLF15 expression.
(a) TGFβ stimulates p38α activity, inhibited by SB2036580 or β-LGND. Bar graph is the mean±SD from 3 exps combined. *p < 0.05 vs. control, +p < 0.05 for TGFβ vs same + SB2036580 or β-LGND. (b) TGFβ inhibits KLF15 mRNA and protein in cardiomyocytes, blocked by the p38 antagonist SB2036580 (0.1μM) (c) TAK1 activating phosphorylation is stimulated by AngII or TGFβ, inhibited by β-LGND. *p<0.05 vs. control, + p<0.05 for TGFβ or AngII vs same plus β-LGND, n=3 exps. (d) TAK1 siRNA diminishes TGFβ or AngII-stimulated p38α activity. The latter was seen as phosphorylation at tyrosine182. *p<0.05 vs control, +p<0.05 for TGFβ or AngII vs same + β-LGND, n=3 exps. TAK1 siRNA validation is also shown. (e) Flow cytometry analysis of β-LGND inhibition of phospho-kinases due to cAMP/PKA. *p<0.05 for control vs. AngII-stimulated phospho-TAK1, phospho-p38α, or KLF15 proteins. +p<0.05 for AngII vs AngII + β-LGND, ++p<0.05 for AngII + β-LGND vs same + either H-89 (PKA inhibitor) or RP-8-Br-cAMP (cAMP inhibitor), n=3 exps.
To further support this pathway, we determined the effects of TGFβ and BLGND on the upstream kinase for p38α, TGFβ-activating kinase (TAK1). AngII and TGFβ each stimulated TAK1 kinase activity that was determined as phospho-TAK1, inhibited by β-LGND (Fig 2c). We also determined whether TAK1 was required for p38α activation by the hypertrophic peptides. The ability of AngII or TGFβ to stimulate p38α activation was strongly reversed by TAK1 siRNA (Fig 2d).
We then determined how ERβ inhibits this signaling pathway that results in KLF15 repression. We first found by flow cytometry that AngII caused ~a 25% increase in phosphorylated TAK1 or p38α proteins, while inhibiting KLF15 protein. All this was substantially reversed by β-LGND (Fig 2e). Furthermore, this occurred through ERβ stimulating a cAMP and protein kinase A (PKA)-dependent inhibition of TAK1 and p38α activity, using well validated inhibitors of cAMP and PKA. We previously published that ERβ stimulates PKA to inhibit TGFβ-induced signaling through c-Jun kinase to fibrosis in cardiac fibroblasts (Pedram et al., 2010). Here we implicate this important signal for ERβ to oppose AnglI/TGFβ action in the myocytes.
3.3. AngII and ERβ regulate the intracellular localization of KLF15
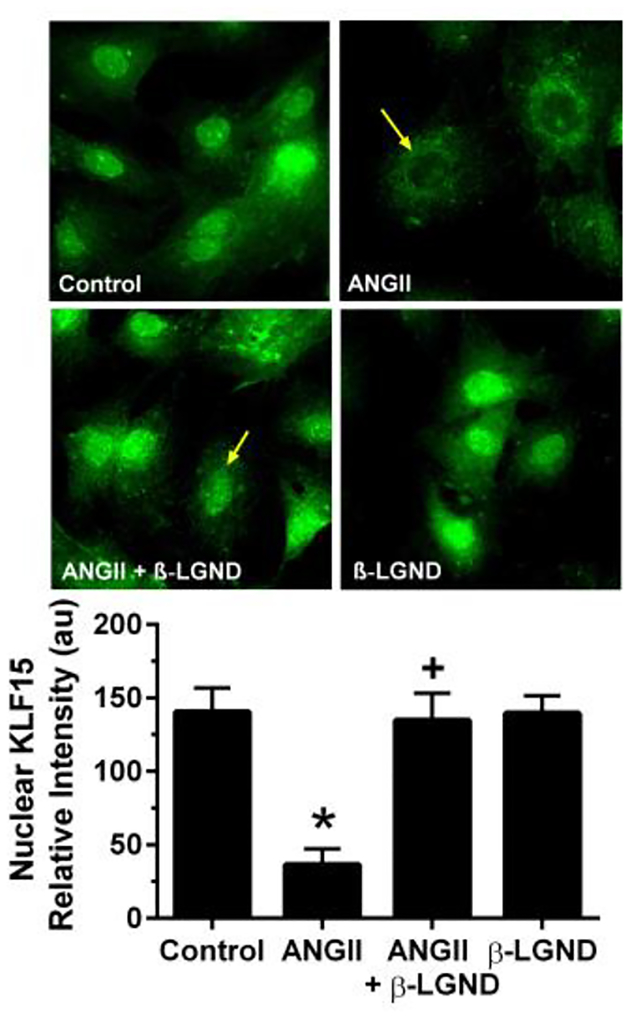
TFs mainly function in the cell nucleus to modulate gene expression. We therefore asked whether AngII and β-LGND modulated the cytoplasmic/nuclear ratio of KLF15. Using fluorescent microscopy to determine KLF15 protein localization in cardiomyocytes, AngII exposure limited both the overall protein abundance and the nuclear localization of this TF, compared to control (Fig 3). In contrast, β-LGND in the presence of the hypertrophic factor not only prevented the inhibition of protein abundance, but relocalized KLF-15 from extra-nuclear sites to the nucleus. This is necessary if, as we propose, ERβ restoration of the anti-hypertrophic state comparable to that seen under control conditions involves KLF15. Interestingly, in the absence of AngII, ERβ had little effect when compared to control levels of KLF15 abundance or cell localization.
Fig 3. AngII re-localizes KLF15 protein to the cytoplasm, opposed by β-LGND.
β-LGND coincubation blocks the AngII effect thereby restoring the control situation. Quantitation of KLF15 fluorescence is seen in the bar graph as mean±SD from counting cells per condition in duplicates from each of 3 exps combined. *p<0.05 for control vs AngII, +p<0.05 for AngII versus AngII + β-LGND.
3.4. KLF15 impacts the ability of ERβ to inhibit the hypertrophic effects of AngII,
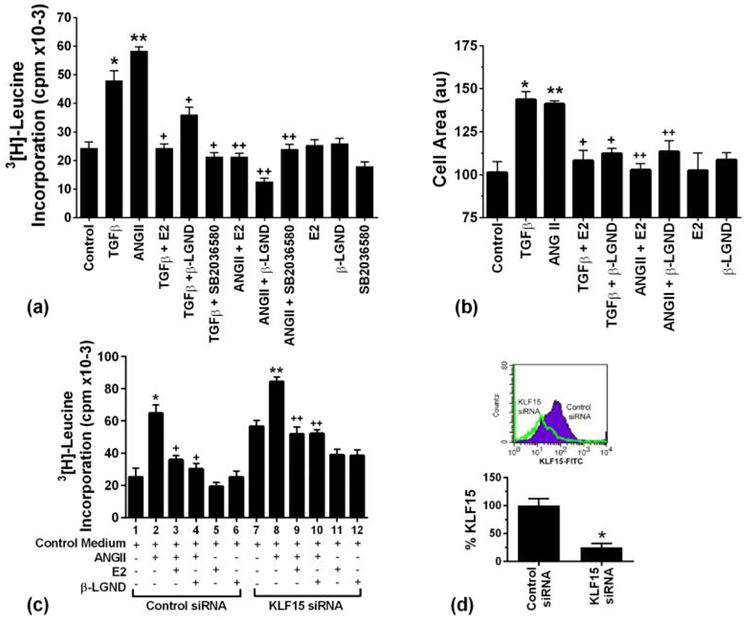
We then examined a functional role for KLF15 including the impact for regulation of cardiomyocyte hypertrophy by AngII and ERβ. We first found that AngII and TGFβ significantly stimulated leucine incorporation into myocyte protein, a marker of cell hypertrophy (Puhl et al, 2016) (Fig 4a). This was substantially limited by E2 or β-LGND. In addition, inhibiting p38α with the SB compound reversed these effects of the hypertrophic peptides.
Fig 4. Stimulation of cardiomyocyte hypertrophy by Ang II and TGFβ, opposed by E2 and β-LGND.
(a) Leucine incorporation into protein is stimulated by AngII and TGFβ, inhibited by estrogenic compounds. Data are from 3 exps. *p or **p < 0.05 vs. control, +p < 0.05 for TGFβ versus same + E2, β-LGND, or SB compound. ++p<0.05 for AngII vs. same + E2, β-LGND, or SB compound; (b) Cell area is increased in cardiomyocytes exposed to TGFβ or AngII, opposed by E2 or β-LGND. Data are combined from 3 exps. * or **p < 0.05 vs. control, +p < 0.05 for TGFβ versus same + E2 or β-LGND. ++p<0.05 for AngII vs same + E2 or β-LGND. (c) KLF15 siRNA stimulates cardiac hypertrophy, augments AngII effects, and partially reverses inhibition by E2 or β-LGND. Data are from 3 exps combined. *p<0.05 for control siRNA vs. AngII + control siRNA, **p<0.05 for control siRNA vs KLF15siRNA, +p<0.05 for AngII vs AngII + E2 or β-LGND under control siRNA conditions, ++p<0.05 for KLF15siRNA vs same +AngII, #p<0.05 for AngII + E2 or β-LGND + control siRNA vs AngII + E2 or β-LGND +KLF15 siRNA conditions; and (d) siRNA knockdown of KLF15. Results are cell expression of KLF15 mRNA (RT-PCR) or protein (flow cytometry), under KLF15 siRNA or control siRNA conditions. The bar graph is relative KLF15mRNA from 3 determinations, *p<0.05.
We also determined the surface area of the cardiomyocytes, and found comparable effects of prohypertrophic peptides and anti-hypertrophic steroid compounds on this measure of cell hypertrophy (Fig 4b). In both studies E2 or β-LGND effects alone were not different from control cells.
To support the importance of KLF15, we used siRNA to decrease the endogenous KLF15 in the cardiomyocytes that are then subjected to various culture conditions. As seen in Fig 4c, KLF15 knockdown by itself (lane 7) induced increased leucine incorporation compared to control siRNA (lane 1). These results indicate the importance of KLF15 in maintaining a basal state of anti-hypertrophy. In addition, the hypertrophic effects of AngII were augmented by KLF15 knockdown (lanes 2 and 8), presumably due to further decrease of this anti-hypertrophic peptide. In the setting of control siRNA, E2 or β-LGND each caused a comparable and significant decrease in AngII-stimulated leucine incorporation in cardiomyocytes (lanes 2–4). However, KLF15 siRNA diminished the abilities of E2 and β-LGND to oppose the hypertrophic action of AngII (lanes 3 and 4 vs lanes 9 and 10). This suggests that part of the mechanism by which ERβ inhibits cardiomyocyte hypertrophy depends on maintaining ample KLF15 expression to restore the anti-hypertrophic phenotype seen in the strictly control condition (lane 1). This concept is supported by the earlier data here showing the ERβ agonist or E2 restores normal expression of KLF15 to control levels (Fig 1). Validation of siRNA knockdown for KLF15 is seen in Fig 4d.
3.5. Downstream targets for KLF15 function
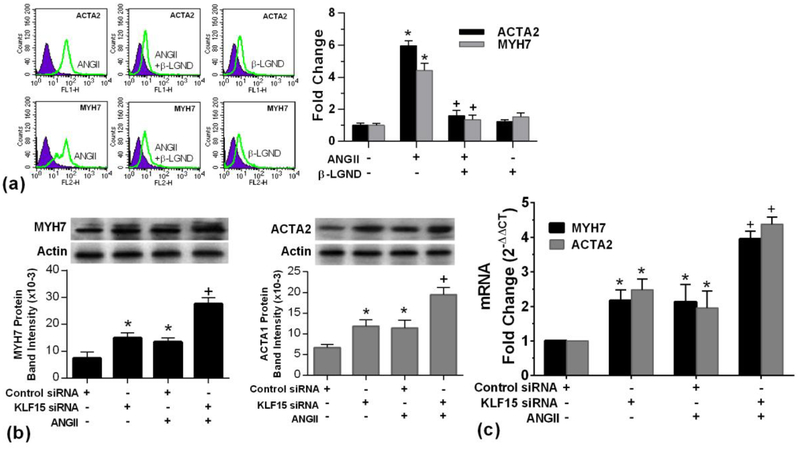
As an anti-hypertrophic TF, some hypertrophic genes should be targets for KLF15 action. We therefore determined the impact of KLF15 for the ability of AngII to regulate the expression of several known hypertrophic genes, MyH7 (also known as ß-MHC) (Yu et al., 2015) and ACTA2 (Tritsch et al., 2013). In cultured cardiomyocytes, we found that AngII significantly increased the mRNA and protein expressions of both hypertrophic genes (Fig 5a). Consistent with its anti-hypertrophic effects, inhibiting KLF15 with siRNA alone caused a significant up-regulation of these mRNAs and the resulting proteins (Figs.5b and 5c). Additionally, AngII stimulation of these targets was augmented by KLF15 knockdown. These results suggest that the demonstrated ability of AngII to inhibit this TF (Fig. 1) implicates KLF15-regulated transcriptional targets as a mechanism of AngII-induced cardiomyocyte hypertrophy. This would result also from AngII inhibiting KLF15 nuclear localization. KLF15siRNA alone also increased expression. *p<0.05 for control siRNA vs same + AngII or KLF15siRNA, +p<0.05 for AngII vs AngII + KLF15siRNA.
Fig 5. Hypertrophic gene and protein expression .
(a) flow cytometry analysis of ACTA2 (top) and MYH7 (bottom) expressions, bar graph data are from 3 exps. *p < 0.05 vs. control, +p < 0.05 for ANGII versus same + ß-LGND; (b) KLF15 protein and mRNA (c) were significantly stimulated by AngII that was augmented by KLF15siRNA, analyzed by western blot and real time PCR, respectively.
3.6. In-vivo confirmation of KLF15 regulation
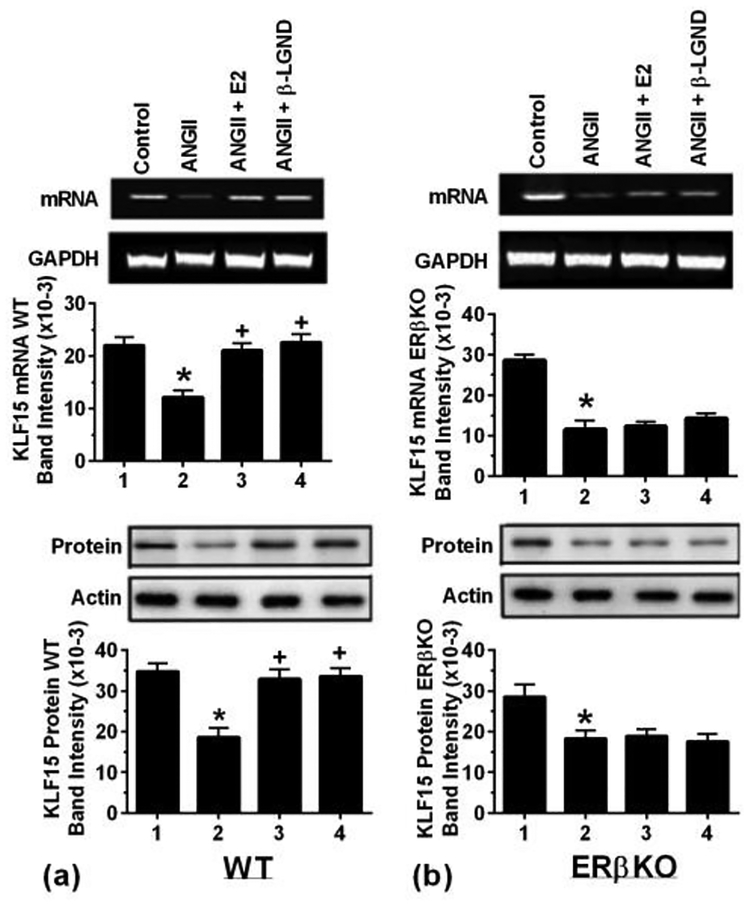
To confirm key aspects in-vivo, we utilized a model of ovariectomized female WT and ERβ-deleted mice, subcutaneously infusing AngII±E2 or βLGND or 0.1% saline alone as control over 4 weeks (5–7 mice per condition). Cardiac hypertrophy and progression to heart failure was seen from AngII alone, and we previously reported that both aspects were significantly prevented by co-infusion of either E2 or βLGND, only seen in the WT mice [Pedram et al., 2013a]. E2 or βLGND also prevented the AngII-stimulated deterioration of cardiac function in-vivo [Pedram et al., 2008 and 2013a] and cardiac fibrosis (Pedram et al., 2010 and 2016). We therefore determined effects on KLF15 expression at 2 weeks of infusion. AngII significantly suppressed KLF15 mRNA and protein in WT female mice, substantially reversed by co-infusion of either E2 or β-LGND (Fig 6a). By contrast, the suppressive effects of AngII on KLF15 expression were not affected by E2 or β-LGND in ERβKO mice (Fig 6b). Thus, ERβ mediates the effects of E2 on KLF15 in-vivo, thereby likely contributing to the anti-hypertrophic effects of this sex steroid on cardiomyocytes, as we showed in-vitro.
Fig 6-. In-vivo studies of AnglI-induced cardiac hypertrophy.
Ovexed, female mice were infused with saline or AnglI± β-LGND or E2 (pellet) for 4 weeks (4 mice per condition), and the hearts were processed for KLF15 mRNA and protein. Results are from pooled (a) WT mice and (b) ERβKO mice. Actin and GAPDH, respectively, are loading controls. Data are from 4 mice, pooled ventricles per condition for the Figs, each in WT and ERβKO mice. Bar graph data are analyzed by ANOVA and Scheffe’s test. *p < 0.05 vs. control, +p < 0.05 for AngII versus same + E2 or β-LGND.
4. Discussion
Uncontrolled hypertension or ischemia of the heart are common causes of the development of cardiac hypertrophy and progression to cardiac dysfunction, the most extreme form being heart failure. Key hypertrophic peptides are stimulated and secreted from several organs in the cardiovascular system in the hypertensive or ischemia state including AngII and ET-1. E2 replacement after menopause in women has been shown to have positive effects to prevent the development of progressive heart disease (Light et al., 2001). We previously showed that E2 acting through ERβ prevents the development of AngII-induced cardiomyocyte/heart hypertrophy and fibrosis of the heart (Pedram et al, 2010, 2013a). This is mediated through multiple described but also unknown mechanisms.
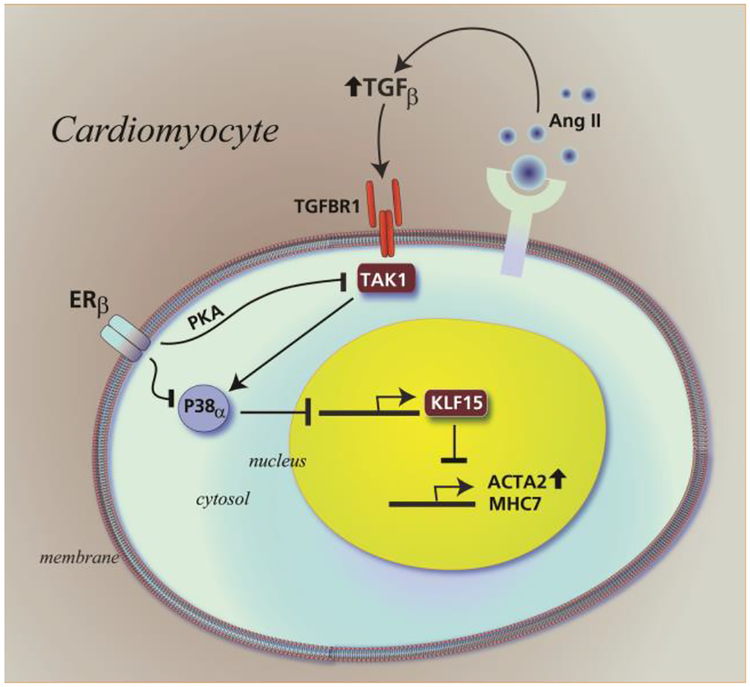
KLF15 has been reported to physically interact with and inhibit the myocardin protein function in the nucleus of cardiomyocytes, repressing heightened expression of pro-hypertrophic genes (Leenders et al., 2010, 2012). KLF15 is regulated by AngII, and overexpression of KLF15 blunts AngII-induced cardiomyocyte hypertrophy (Leenders et al., 2012). Here we establish a role for KLF15 as an anti-hypertrophic transcription factor (TF) that is reciprocally regulated by AngII/TGFβ and E2/β-LGND. Restoration of KLF15 abundance by ERβ contributed to inhibition by the sex steroid of the expression of several known hypertrophic genes, shown here to be stimulated by AngII. We also showed the novel ability of AngII to stimulate cardiomyocyte hypertrophy that in part reflected inhibition of KLF15 production. A summary cartoon is shown in Fig 7.
Fig 7-. Cartoon of regulation of KLF15 in cardiomyocytes to impact cardiac hypertrophy:
AngII acting through TGFβ stimulates a TAK1-p38α kinase axis that inhibits KLF15 expression and nuclear localization of the protein. This contributes to increased gene expression and cardiomyocyte hypertrophy. ERβ acting through protein kinase A opposes TAK1-p38α activation. This restores KLF15 abundance and nuclear localization, contributing in part to inhibition of AngII-induced gene expression and cardiomyocyte hypertrophy.
Inhibition of KLF15 mRNA and protein was also found here in the hearts of WT female mice exposed to low dose AngII infusion that we previously reported produced cardiac hypertrophy (Pedram et al, 2008). Here, E2 or an ERβ-selective agent, β-LGND, comparably reversed AngII-inhibition of the TF, restoring the levels to that seen in the control state, in-vitro and in-vivo. E2 had no comparable effects in the ERβ KO mouse, supporting the important role of the esr2 isoform as mediating the anti-hypertrophic function of the sex steroid, as reported by others (Skavdahl et al., 2004;Babikar et al, 2006, Thireau, 2010). The effects of E2 are also likely to occur in the vasculature, since AngII-induced hypertension is prevented by E2 or β-LGND (Pedram et al., 2013), and the ERβ KO mouse develops severe hypertension with aging (Zhu et al., 2002). However, we provide consistent evidence that there are also direct effects of E2/β-LGND on both isolated cardiomyocytes and fibroblasts to oppose direct effects of AngII that cause both hypertrophic and fibrotic phenotypes (Pedram et al., 2008,2010,2013, 2016). KLF15 has also been implicated in the regulation of cardiac progenitor cell fate in the postnatal heart (Noack et al., 2012), and in cardiac lipid metabolism (Prosdocimo et al., 2014), roles that could be important to E2 regulation of cardiac responses to stress.
We report that ERβ signaling through PKA inhibits the TAK1 and p38α kinase axis that is activated by AngII/TGFβ. Previous reports indicated that TGFβ stimulation of p38α inhibits KLF15 expression (Leender et al., 2010), here extended to include TAK1 signaling from AngII and TGFβ action. TAK1 activity is tightly regulated by complexing with the TAB family of proteins (TAB1–3), and all activities are modified by multiple post-translational modifications (Hirata et al., 2017). Thus, extensive studies will be needed to understand how PKA precisely regulates this upstream kinase, ultimately to diminish p38α activity.
Interestingly, E2 or β-LGND have little effect by themselves on KLF15 expression, These estrogenic compounds act in-vitro and in-vivo to restore the abundance of this TF to that seen under control conditions, in the setting of AngII suppressing this protein. This is consistent with our previous observations of various E2/ERβ actions, suggesting that E2 restores a default, anti-hypertrophic and anti-fibrotic system upon exposure of the heart to pathology-inducing peptides such as AngII. We conjecture that these functions of E2 promote a healthy cardiovascular environment in women during the reproductive years, to support optimal chances of conceiving and carrying a fetus to term.
Another mechanistic observation in the studies here is that E2/ERβ promotes the localization of the anti-hypertrophic TF into the nucleus of the cardiomyocyte, thus ensuring the inhibition of prohypertrophic genes that are stimulated by AngII. Previously, we reported that E2/ERβ inhibits the dephosphorylation of NFATC2, thereby sequestering this pro-hypertrophic TF in cytoplasm of cardiomyocytes to prevent collaboration with other TFs that induce the hypertrophic phenotype (Pedram et al, 2008). Therefore, it is not only the abundance but the cell localization of key transcription proteins that are important mechanistic targets for E2 regulation. This also occurs in cardiac fibroblasts, where membrane ERβ signals to the inhibition of AngII/TGFβ-induced phosphorylation of the Smad2 and 3 TFs, sequestering the SMADs in cytoplasm to prevent fibrosis-inducing gene expression (Pedram et al, 2010). We also conclude that membrane ERβ signaling is both required and perhaps sufficient to ensure these functions. Support for the latter concept arises from our studies in hepatocytes where membrane ERα is sufficient to signal to cytoplasmic sequestration of the Srebf1 TF, substantially reducing the expression of many genes that are important for lipids synthesis in this organ, as we also demonstrated invivo (Pedram et al, 2013).
Finally, we propose that an ERβ agonist as shown here could provide a degree of protection against cardiomyocyte hypertrophy in women who are at risk from poorly-controlled hypertension or other cardiac pathology-inducing states. Selective engagement of esr2 would not stimulate breast or uterine proliferation that is mediated by E2 acting at ERα and therefore would not be potentially problematic in these regards.
Acknowledgements
The work was mainly supported by a grant from the Research Service of the Department of Veteran’s Affairs, 1 IO1BX002316 to ERL. The funding agency had no role in the design or interpretation of study results. The authors have no conflicts of interest with the contents of this article.
Footnotes
Disclosure: HA, LG, KSK, and ERL have nothing to disclose and no conflicts of interest.
References
- Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, van Eys G, Grohé C, Doevendans PA 2006. Estrogen receptor protects the murine heart against left ventricular hypertrophy. Arterioscl. Thromb. Vasc. Biol 26, 1524–1530. [DOI] [PubMed] [Google Scholar]
- Blaustein MP 2017. How does pressure overload cause cardiac hypertrophy and dysfunction? High oubain affinity cardiac Na + pumps are crucial. Am. J. Physiol. Heart. Circ. Physiol doi: 10.1152/ajpheart.00131.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Takahashi M, Morishita T, Noguchi T, Matsuzawa A 2017. Post-Translational Modifications of the TAK1-TAB Complex. Int J. Mol. Sci 18, E205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa N, Ge L, Kuznetsov Y, McPherson A, Cornforth AN, Pham JT, Myers MP, Ahmed N, Salsman VS, Lamb LS Jr, Bowersock JE, Hu Y, Zhou YH, Jadus MR. 2010. Glioma cells display complex cell surface topographies that resist the actions of cytolytic effector lymphocytes. J Immunol 15;185(8):4793–803. [DOI] [PubMed] [Google Scholar]
- Hoa NT, Ge L, Erickson KL, Kruse CA, Cornforth AN, Kuznetsov Y, McPherson A, Martini F, Jadus MR 2015. Fascin-1 knock-down of human glioma cells reduce their microvilli/filopodia while improving their susceptibility to lymphocyte-mediated cytotoxicity. Am. J. Transl. Res 7, 271–284. [PMC free article] [PubMed] [Google Scholar]
- Irish JM, Hovland R, Krutzik PO, Perez OD, Bruserud Ø, Gjertsen BT, Nolan GP 2004. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 118, 217–228. [DOI] [PubMed] [Google Scholar]
- Kim JK, Pedram A, Razandi M,Levin ER 2006. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 isoforms. J. Biol. Chem 281, 67606767. [DOI] [PubMed] [Google Scholar]
- Leender JJ, Wijnen WJ, Hiller M, Van der Made, Lentink, Rick EW, Leeuwen, Herias V, Pokharel S, Heymans S, De Windt L, Haydal MA, Pinto YM, Creemers EE. 2010. Regulation of Cardiac Gene Expression by KLF15, a Repressor of Myocardin Activity. J. Biol. Chem 285,2744927456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders JJ, Wijnen WJ, van der Made I, Hiller M, Swinnen M, Vandendriessche T, Chuah M, Pinto YM, Creemers EE 2012. Repression of cariac hypertrophy by KLF15: underlying mechanisms and therapeutic implications. PLoS ONE 7, e36754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER, Hammes S 2016. Nuclear receptors outside the nucleus: extranuclear signaling by steroid receptors. Nat. Rev. Mol. Cell. Biol 17, 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KC, Hinderliter AL, West SG, Grewen KM, Steege JF, Sherwood A, Girdler SS 2001. Hormone replacement improves hemodynamic profile and left ventricular geometry in hypertensive and normotensive postmenopausal women. J. Hyperten 19, 269–278. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A 2014. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cyc. 13, 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack C, Zafiriou MP, Schaeffer HC, Renger A, Pavlova E, Dietz R, Zimmerman WH, Bergmann M, Zelarayan L. 2012. Kruppel-like factor 15 regulates Wnt/βcatenin transcription and controls cardiac progenitor cell fate in the postnatal heart, EMBO. Mol. Med 4, 992–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER 2008. Estrogen inhibits cardiac hypertrophy: Role of estrogen receptor beta to inhibit calcineurin. Endocrinol. 149, 3361–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi R, Aitkenhead M, Levin ER 2005. Estrogen inhibits cardiomyocyte hypertrophy in-vitro: Antagonism of calcineurin-related hypertrophy through Induction of MCIP1. J. Biol. Chem 280, 26339–26348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, O’Mahony F, Lubahn D, Levin ER 2010. Estrogen receptor beta prevents cardiac fibrosis. Mol. Endocrinol 24, 2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Korach KS, Narayanan R, Dalton JT, Levin ER 2013a. ERβ selective agonist inhibits angiotensin-induced cardiovascular pathology in female mice. Endocrinol 154, 4352–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, O’Mahony F, Harvey H, Harvey BJ, Levin ER 2013b. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Sci. Sig 6,RA36. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Narayanan R,Levin ER 2016. Estrogen receptor beta signals to inhibition of cardiac fibrosis. Mol. Cell. Endocrinol, 434,57–68. [DOI] [PubMed] [Google Scholar]
- Pfaffl M 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29,:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosdocimo DA, Anand P, Lia X, Zhu H, Shelkay S, Artero-Calderon P, Zhang L, Kirsh J, Moore D, Rosca MG, Vazquez E, Kemer J, Akat KM, Williams Z, Zhao J, Fuijoka H, Tuschi T, Bai X, Schulze PC, Hoppel CL, Jain MK, Haldar SM. 2014). Kruppel-like 15 is a critical regulator of cardiac lipid metabolism. J. Biol. Chem 289, 5914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl SL, Kazakov A, Muller A, Fries P, Wagner DR, Bohm M, Maack C, Devaux Y 2016. Adenosine A1 receptor activation attenuates cardiac hypertrophy and fibrosis in response to a1-adrenoreceptor stimulation in-vivo. Br. J. Pharmacol 173, 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, and Levin ER (2000) J. Biol. Chem 275, 38540–38546 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez, Carvajal G, Egido J. 2007. TGF-beta signaling in vascular fibrosis. Cardiovasc. Res 74, 196–206. [DOI] [PubMed] [Google Scholar]
- Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E 2004. Estrogen receptor-β mediates male-female differences in the development of pressure overload hypertrophy. Am. J. Physiol. Heart Circ. Physiol 288, H469–H476. [DOI] [PubMed] [Google Scholar]
- Subramaniam V, Lip GY 2009. Hypertension to heart failure: a pathophysiological spectrum relating blood pressure, drug treatments, and stroke. Expert Rev Cardiovasc Ther. 7,703–713. [DOI] [PubMed] [Google Scholar]
- Thireau J, Aimond F, Poisson D, Zhang B, Bruneval P, Eder V, Richard S, and Babuty D 2010. New insights into sexual dimorphism during progression of heart failure and rhythm disorders. Endocrinol. 151,1837–1845. [DOI] [PubMed] [Google Scholar]
- Tritsch E, Malkat Y, Lefebvre F, Diguet N, Escoubet B, Blanc J, De Windt LJ, Catalucci D, Vandecasteele G, Li Z, Mericskay M. 2013. An SRF/miR-1 axis regulates NCX1 and annexin A5 protein levels in the normal and failing heart. CardioVasc Res.3, 372–380. [DOI] [PubMed] [Google Scholar]
- Xue B, Zhao Y, Johnson AK, Hay M 2008. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am. J. Physiol. Heart. Circ. Physiol 3, 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Johnson AK, Hay M 2013. Sex differences in angiotensin II-induced hypertension: the central protective effects of estrogen. Am. J. Physiol. Regul. Comp. Physiol 305, R459–R463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, Veverka KA, Miller DD, Dalton JT, Narayanan R 2010. Estrogen receptor beta selective ligands alleviate high-fat diet and ovariectomy-induced obesity in mice. J. Biol. Chem 285, 31292–31303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BL, Xiang R, Peng DQ 2015. A novel MYH7 mutation in a family with cardiomyopathy presenting with restrictive physiology and varying degrees of left ventricle hypertrophy. Eur. Heart. J 36, 178. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wei SS, Wang H, Wang Q, LI W, Li G, Hou JW, Chen XM, Chen J, Xu WP, Li YG, Wang YP 2017. Crucial role of ROCK2- mediated phosphorylation and upregulation of FHOD3 in the pathogenesis of angiotensin II-Induced cardiac hypertrophy. Hypertension 69,1070–1083. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME 2002. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295, 505–508. [DOI] [PubMed] [Google Scholar]