SUMMARY
Lipotoxic cardiomyopathy (LCM) is characterized by abnormal myocardial accumulation of lipids, including ceramide; however, the contribution of ceramide to the etiology of LCM is unclear. Here, we investigated the association of ceramide metabolism and ceramide-interacting proteins (CIPs) in LCM in the Drosophila heart model. We find that ceramide feeding or ceramide-elevating genetic manipulations are strongly associated with cardiac dilation and defects in contractility. High ceramide-associated LCM is prevented by inhibiting ceramide synthesis, establishing a robust model of direct ceramide-associated LCM, corroborating previous indirect evidence in mammals. We identified several CIPs from mouse heart and Drosophila extracts, including caspase activator Annexin-X, myosin chaperone Unc-45, and lipogenic enzyme FASN1, and remarkably, their cardiac-specific manipulation can prevent LCM. Collectively, these data suggest that high ceramide-associated lipotoxicity is mediated, in part, through altering caspase activation, sarcomeric maintenance, and lipogenesis, thus providing evidence for conserved mechanisms in LCM pathogenesis in mammals.
In Brief
Lipotoxic cardiomyopathy (LCM) is characterized by abnormal myocardial accumulation of lipids, including ceramide. Here, Walls et al. find that ceramide feeding or ceramide-elevating genetic manipulations induce LCM. They identified several ceramide-interacting proteins, whose subsequent cardiac-specific manipulation can prevent LCM by altering caspase activation, sarcomeric maintenance, and lipogenesis.
Graphical Abstract

INTRODUCTION
The global obesity epidemic is a growing healthcare crisis; currently, more than 1.9 billion adults are overweight, 600 million of who are considered clinically obese (Haemmerle et al., 2011; Shabana and Hasnain, 2016). Importantly, obesity is a major risk factor for a variety of human disorders, including diabetes and heart disease. Obesity-related heart disease is characterized by a signature set of cardiac defects generally referred to as lipotoxic cardiomyopathy (LCM). Classic hallmarks of LCM recapitulated in animal models include left ventricular hypertrophy, diastolic (with or without systolic) dysfunction, contractile defects, and cardiac steatosis (Goldberg et al., 2012). In humans, cardiac steatosis is characterized by abnormal accumulation of a range of lipids, including triacylglycerides (TAG), fatty acids, and the sphingolipid ceramide.
Elevated cardiac ceramide levels have also been observed in a variety of obesity models, including genetic models of ectopic lipid accumulation such as db/db and ob/ob, diacylglycerol acyl-transferase (DGAT)- or adipose triglyceride lipase (ATGL)-deficient mice (Demarco et al., 2013; Dobrzyn et al., 2015; Gao et al., 2015; Liu et al., 2014), and mice overexpressing peroxi-some proliferator-activated receptor α/γ(PPARα/γ ) or fatty acid transport protein 1 (FATP1) (Baranowski et al., 2007; Chui et al., 2005; Finck et al., 2003). However, the potential role of ceramide in the pathophysiology of LCM is poorly understood, in part because of the complexity of mammalian metabolism. In a mouse model of LCM, administration of myriocin, which blocks de novo ceramide synthesis by inhibiting serine palmi-toyltransferase (SPT), lowered ceramide levels, abolished cardiac dilation, and improved cardiac contractile function (Park et al., 2008a), suggesting that ceramide plays a critical role in LCM. Moreover, ATGL knock down in cardiomyocytes decreased PPARα/δ and PPARγ coactivator 1-α (PGC-1α/γ) activity (Haemmerle et al., 2011), resulting in elevated intracellular free fatty acid (FFA) levels and accumulation of ceramide (Gao et al., 2015). Conversely, loss of stearoyl-CoA desaturase 1 prevents cardiac dysfunction in genetically obese ob/ob mice, possibly by reducing ceramide levels (Dobrzyn et al., 2010). These results provide strong but indirect evidence that reduction of ceramide levels can attenuate LCM. However, it remains to be determined whether direct elevation of ceramide levels—by genetic or dietary manipulation—is sufficient to induce LCM, and, if so, through which mechanisms.
The development of standardized Drosophila heart function assays (Fink et al., 2009) has enabled several fly models of LCM to be established, including LCM induced by high-fat diet (Birse et al., 2010), high-sugar diet (Na et al., 2013), ATGL or PGC-1 deficiency (Diop et al., 2015), and perturbation of SREBP processing (Lim et al., 2011). These models suggested that the metabolic pathways and regulatory mechanisms implicated in LCM are highly conserved between flies and mammals, allowing Drosophila to serve as a facile model to study the genetic mechanisms underlying the associated cardiac abnormalities (Birse and Bodmer, 2011; Diop and Bodmer, 2012),
Here, we interrogated the role of ceramide metabolism and accumulation as potentially causal factors in the development of LCM in Drosophila. We found that systemic, adipose-specific, or heart-specific attenuation of ceramide degradative gene expression was sufficient to induce symptoms of LCM, as was direct ceramide feeding. Interestingly, inhibition of ceramide biosynthesis gene expression also caused heart dysfunction, but in opposite direction (leading to cardiac constriction as opposed to dilation), suggesting that ceramide homeostasis in cardio-myocytes is critical for optimal heart function. Using a proteomics approach, we identified several putative ceramide-inter-acting proteins (CIPs) that might underlie the etiology of ceramide-associated LCM. Of these, Annexin X, Unc-45, and fatty acid synthase 1 (FASN1) were found to play critical roles in high ceramide-associated heart dysfunction, and when manipulated could prevent LCM. In addition, cardiac-specific expression of dIAP, a potent caspase inhibitor, also prevented LCM. Our findings suggest that genetic and dietary interventions that cause excess ceramide accumulation leads to cardiomyopathy through mechanisms involving sarcomeric and lipogenic dysregulation and caspase activation.
RESULTS
Global Inhibition of Ceramide Degradation Induces LCM-like Phenotypes
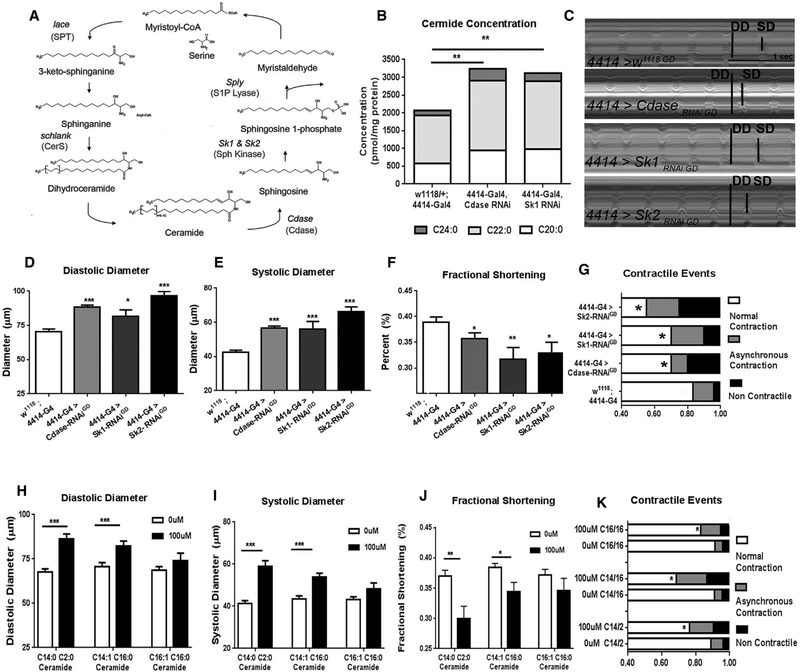
To determine whether modulating ceramide metabolism plays a causal role in LCM in Drosophila, we assessed several classic hallmarks of mammalian LCM; namely heart chamber dilation, contractile dysfunction, and loss of pumping power, in flies carrying mutations in enzymes in the sphingolipid biosynthetic pathway (Figure 1A). We previously showed that knockdown (KD) of sphingosine kinase 2 (Sk2) in Drosophila promoted ceramide and lipid/TAG accumulation through dysregulation of fat storage, lipogenic gene programs, and caloric uptake (Walls et al., 2013). Using the GAL-4/UAS system, we found that global KD of two additional other genes in the ceramide degradative pathway, Cdase (ceramidase) and Sk1 (sphingosine kinase 1), also caused elevated levels of ceramide (amide bond linked fatty acid: C24, C22, and C20) and TAG (Figures 1B and S1A) compared with levels in control flies (w1118, 4414-Gal4).
Figure 1. Ceramide Accumulation Induces LCM.
(A) Ceramide metabolism in the sphingolipid pathway.
(B–G) Analysis of 3-week-old flies with global RNAi-mediated knock down of Cdase, Sk1, or Sk2 (using the ubiquitous Act5 driver [4414-Gal4]). (B) Whole-fly ceramide content (C14:1 sphingoid backbone with C20, C22, or C24 secondary fatty acyl chains), (C) representative M-modes (DD, diastolic diameter; SD, systolic diameter), (D) diastolic diameter, (E) systolic diameter, (F) fractional shortening, and (G) contractile events.
(H–K) Wild-type w1118 flies were fed either 100μM C14:1/C2:0, C14:1/C16:0, or C16:1/C16:1 ceramides from day 1 and analyzed at 3 weeks of age relative to 0 μM (vehicle-fed) controls. (H) Diastolic diameter, (I) systolic diameter, (J) fractional shortening, and (K) contractile events. G/K: *p < 0.05 (χ2 test); for all other panels, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 (Student’s t test). Error bars represent SEM.
We next investigated the consequences of whole-organism genetic inhibition of ceramide degradation—causing ceramide accumulation (Walls et al., 2013)—on heart function in 3-weekold females by using SOHA, a semi-intact specimen preparation combined with high-speed video microscopy (Fink et al., 2009; Ocorr et al., 2009). Representative M-mode traces are shown in Figure 1C. Compared with the 4414-Gal4 or UAS-Cdase -RNAi control flies, Cdase, Sk1, and Sk2 KD flies exhibited wider diastolic and systolic diameters (Figures 1D, 1E, S1B, and S1C), resulting in reduced fractional shortening (Figures 1F and S1D) and contractile defects such as non-contractile regions and asynchronous beating (Figures 1G and S1E) (Diop et al., 2015). Of note, because global Sk1 and Sk2 KD also induced fat accumulation (Figure S1B) (Walls et al., 2013), these data suggest that these sphingosine kinases are required systemically for optimal overall lipid homeostasis and heart function.
Dietary Administration of Ceramide Induces LCM-like Phenotypes
Because high-fat diets are associated with ceramide elevation and cardiomyopathy, we asked whether dietary administration of ceramide would recapitulate the cardiac defects observed in the genetic experiments. To test this hypothesis, we added either 100 μM C14:1/2:0, C14:1/C16:0, or C16:1/C16:0 synthetic ceramide to the diet of wild-type w1118 flies for 3 weeks and compared their heart function to vehicle-fed flies. Diastolic and systolic diameters were increased (Figures 1H–1I) resulting in reduced fractional shortening (Figure 1J). Higher incidence of contractile dysfunctions was also observed in these flies (Figure 1K). Notably, in C14:1/2:0 ceramide-fed flies, increased whole-fly ceramide and TAG concentrations (Figures S1F and S1G) were observed. Additionally, similar, but less pronounced defects in cardiac function were observed when feeding only 10 μM ceramide for 3 weeks (Figures S1H–S1L) or after only 1 week of 100 μM C14:1/C2:0 ceramide feeding (Figure S2). Flies fed the C16:1 sphingoid base ceramide exhibited similar trends but did not reach statistical significance (Figures 1H–1K). Taken together, these data suggest that dietary ceramide led to LCM-like phenotypes in wild-type flies, similar to ceramide degradative gene KD.
Global Inhibition of De novo Ceramide Synthesis Induces Cardiac Defects
The finding that dietary or genetic augmentation of ceramide levels is associated with cardiac defects suggests that ceramide homeostasis needs to be tightly controlled. We there-fore analyzed flies in which we genetically inhibited de novo ceramide synthesis. In Drosophila, lace encodes SPT, which catalyzes the rate limiting step of de novo ceramide synthesis (Adachi-Yamada et al., 1999), while schlank encodes ceramide synthase, which catalyzes the addition of a second fatty acid chain to sphingosine to form ceramide (Bauer et al., 2009) (Figure 1A). Previous work showed that global KD of lace induces a lean phenotype and reduces both ceramide and TAG levels, primarily due to increased TAG utilization and deactivation of a lipogenic gene program (Walls et al., 2013). A similar pheno-type (reduction in ceramide and TAG levels) is observed in schlank-deficient flies, where the reduction in lipids was partially attributed to suppression of SREBP processing, which is a major lipogenic regulatory pathway (Bauer et al., 2009).
To test the effects of blocking de novo ceramide synthesis on cardiac function, we examined lace and schlank KD flies, as well as wild-type flies administered myriocin, an SPT inhibitor with known efficacy in flies (Figure 2A) (Hebbar et al., 2015). In contrast to the Cdase and SK1/2 RNAi lines that have a dilated cardiac phenotype, flies with global KD of lace and schlank exhibited a constricted heart, with reduced diastolic and systolic diameters but unchanged fractional shortening (Figures 2B–2D and S1B–S1D). Of note, global KD of lace, but not schlank, caused an increase in asynchronous contractions and non-contractile regions of the heart (Figure 2E). A similar phenotype was observed in wild-type flies fed for 3 weeks with 100 μM myriocin, which decreased diastolic and systolic diameters but had no effect on fractional shortening (Figures 2F–2H). Like lace KD, 100 μM myriocin administration also increased the frequency of contractile dysfunction events (Figure 2I). These data suggest that optimal heart function requires well-balanced ceramide metabolism, with genetic or pharmacological manipulations to either increase or decrease ceramide levels manifesting a close association with cardiac dysfunction.
Figure 2. Inhibition of Ceramide Synthesis Confers a Constricted Phenotype.
(A) De novo ceramide synthesis was blocked genetically via knock down of lace (SPT) or schlank (CerS) or pharmacologically via inhibition of SPT by myriocin.
(B–E) Analysis of flies with global knock down of lace or schlank. (B) Diastolic diameter, (C) systolic diameter, (D) fractional shortening, and (E) contractile events.
(F–I) Wild-type w1118 flies were fed from day 1 of adulthood with myriocin (100 μM) plus 0 (vehicle), 10, or 100 μM C14:1/C2:0 ceramide and analyzed 3 weeks later. (F) Diastolic diameter, (G) systolic diameter, (H) fractional shortening, and (I) contractile events. For (E) and (I) *p < 0.05 (χ2 test); for all other panels, *p ≤ 0.05, (**p ≤ 0.01, ***p ≤ 0.001 (Student’s t test). Error bars represent SEM.
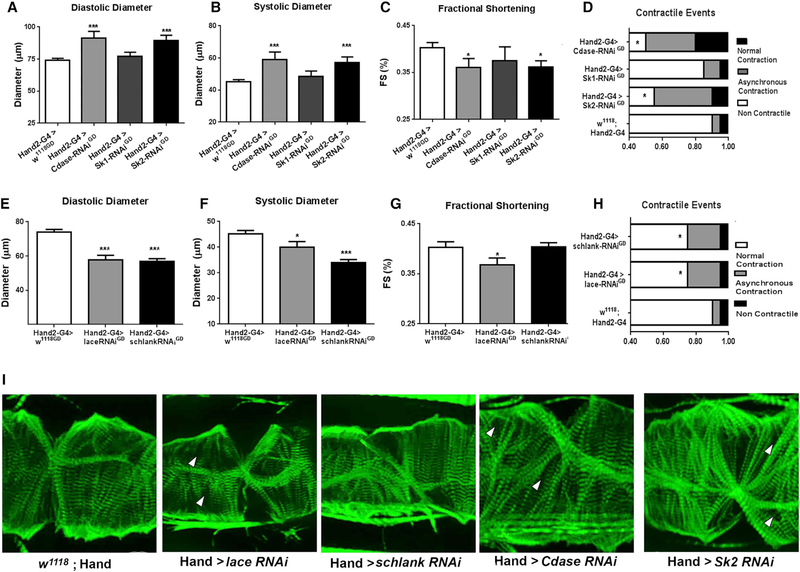
Heart-Specific Modulation of Ceramide Metabolic Genes Causes LCM
Because global changes in ceramide metabolism may indirectly affect heart function, we next sought to determine if heart-specific modulation of ceramide metabolic genes was sufficient to induce LCM-like phenotypes. Indeed, heart-specific KD of Cdase and Sk2 using the Hand2 Gal4 driver increased diastolic and systolic diameters, substantially reduced fractional shortening, and increased contractile defects (Figures 3A–3D and S1B–S1D). Flies with cardiac-specific KD of Cdase and Sk2 also exhibited less tightly organized myofibrillar structure, as shown by F-actin staining (Figure 3I). However, heart-specific KD of Sk1 KD did not induce the cardiac phenotype observed in flies with global Sk1 KD (compare Figures 2A–2D with Figures 1B–1G), which was not unexpected because Sk1 is not expressed at significant levels in the Drosophila heart (Chintapalli et al., 2007; dos Santos et al., 2015). Collectively, these observations imply that ceramide and TAG accumulation in non-cardiac tissue can induce LCM-like phenotypes via systemic actions.
Figure 3. Heart-Specific Modulation of Ceramide Metabolism Induces Cardiac Defects.
(A–D) Analysis of flies with heart-specific knock down of Cdase, Sk1, or Sk2 (using by the Hand2 driver). (A) Diastolic diameter, (B) systolic diameter, (C) fractional shortening, and (D) contractile defects.
(E–H) Analysis of flies with heart-specific knock down of lace or schlank. (E) Diastolic diameter, (F) systolic diameter, (G) fractional shortening, and (H) contractile events.
(I) F-actin staining (green) of the flies analyzed in (A)–(H) showing differences in diameters and irregularities in circumferential myofibril structure and arrangement. Gaps between myofibrils are indicated by arrowheads. Scale bar, 10 μm. White arrows denote gaps. (D) and (H) *p < 0.05 (χ2 test); all other panels, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 (Student’s t test). Error bars represent SEM.
Next, we examined whether heart-specific KD of ceramide synthetic genes lace and schlank could recapitulate the constricted heart phenotype observed after global KD. Notably, heart-specific KD of either gene decreased diastolic and systolic diameters and fractional shortening and increased asyn-chronous contractions and contractile defects (Figures 3E–3H). Loss of fractional shortening and increased cardiac defects correlated well with changes in cardiac morphology. Of note, cardiac-specific lace, Cdase, and Sk2 KD fly hearts showed discernable gaps in myofibril patterning within the cardiomyocytes. Cdase and Sk2 hearts also exhibited increased disorganization across circumferential fibers. Importantly, heart-specific KD did not lead to detectable changes in global TAG storage (Figure S3). Taken together, these data provide evidence that ceramide homeostasis in the heart itself is critical to preserving its structure and function.
Fat Body (Adipose)-Specific Inhibition of Ceramide Degradation Induces LCMin a Non-autonomous Manner
A recent study showed that targeted induction of ceramide degradation in mice improved metabolism and reduced hepatic steatosis through cross-talk between liver and adipose tissue (Xia et al., 2015). In Drosophila, the fat body plays a primary role in lipid storage and lipogenesis, with overlapping functions comparable to those of mammalian adipose tissue and liver. Therefore, we hypothesized that KD of ceramide degradation genes in the fat body may induce LCM-like phenotypes in the heart (Figure S4A). Indeed, fat body-specific KD of Cdase, Sk1, and Sk2 increased whole fly ceramide and TAG levels (Figures S4B and S4C) and also increased diastolic and systolic diameters, with a concomitant reduction in fractional shortening (Figures S4D–S4F). Of note, fat body-specific KD of Sk1 leads to cardiac dilation nearly identical to that observed after global Sk1 KD, supporting the possibility that the LCM-like phenotypes resulting from global Sk1 KD may be primarily attributed to its effects on the fat body.
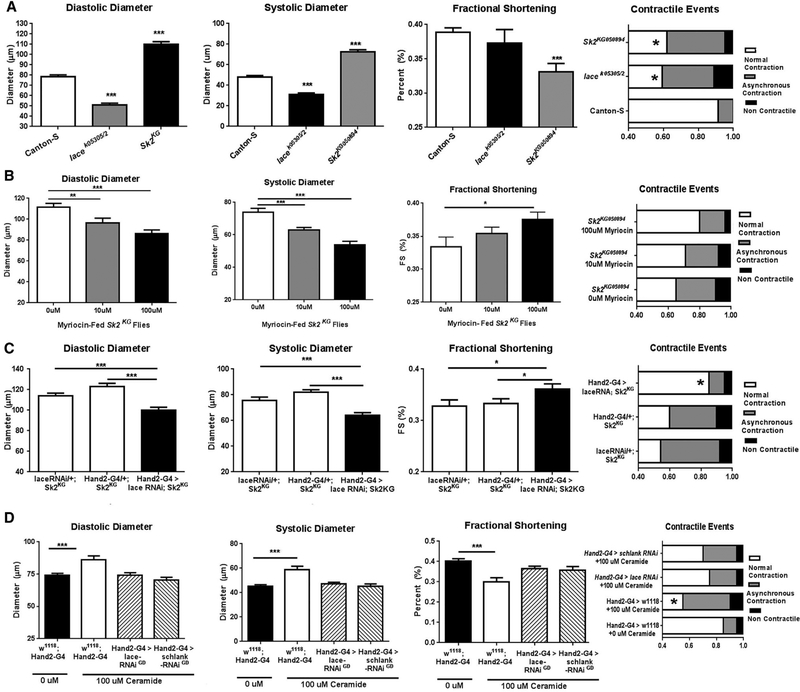
Inhibition of De Novo Ceramide Synthesis Counters LCM Induced by Manipulations that Augment Ceramide Levels
Because global and cardiac-specific dysregulation of ceramide metabolism has both beneficial and detrimental effects on heart function, we asked whether the systemic effects of interventions that cause high ceramide levels could be countered by blocking de novo ceramide synthesis globally or in the heart alone. To test this, we used a combination of genetic and pharmacological approaches. Three-week-old Sk2KG mutant flies, like the global Sk2 RNAi line, exhibit dilated diastolic and systolic diameters, loss of fractional shortening, and contractile defects (Figure 4A). Notably, administration of myriocin to Sk2KG flies, to block global de novo ceramide synthesis, resulted in a dose-dependent suppression of cardiac dysfunction (Figure 4B). In addition, excess ceramide accumulation in Sk2KG mutant flies was reversed to normal (Figure S5A).
Figure 4. Targeted Inhibition of DeNovo Ceramide Synthesis Prevents Ceramide-Associated LCM.
(A) Compared to control Canton-S flies, lace transheterozygotes and Sk2 hypomorphic mutants exhibit cardiac defects.
(B) Administration of myriocin (100 μM for 3 weeks) prevents LCM phenotypes in Sk2 hypomorphic mutants.
(C) Heart-specific inhibition of de novo ceramide synthesis by lace RNAi prevents ceramide-associated LCM in Sk2 hypomorphic mutants.
(D) Control flies fed with 100 μM ceramide from day 1 of adulthood for 3 weeks exhibit LCM, which is prevented by heart-specific knock down of lace or schlank. Far right panels: *p < 0.05 (χ2 test); all other panels, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 (Student’s t test). Error bars represent SEM.
Similar results were obtained when de novo ceramide syn-thesis was inhibited in the heart alone. Heart-specific lace KD in the Sk2KG mutants, significantly reduced dilation, improved fractional shortening, and reduced the frequency of contractile defects compared to the Sk2KG mutant controls (Figure 4C). Heart-specific repression of de novo ceramide synthesis by lace or schlank KD also prevented the cardiac defects induced by ceramide feeding of wild-type flies (Figure 4D), substantiating the findings in the genetic mutants. Taken together, these data confirm that suppression of de novo ceramide synthesis prevents the LCM-inducing effects of genetic augmentation of ceramide levels, providing support for a possible direct role for ceramide in the pathology of LCM.
Identification of Ceramide-Interacting Proteins
We used a proteomics approach to identify CIPs that might mediate ceramide-associated LCM. A pull-down assay was performed by incubating protein extracts from whole flies with an immobilized ceramide trap (biotinylated synthetic ceramide coupled to streptavidin-coated beads). SDS-PAGE of the isolated material identified putative CIPs as unique protein bands absent or less abundant from the post-pull-down supernatant and biotin/BSA controls (Figure 5A). We then performed 1D tandem mass spectrometry (MS/MS) proteomics analysis on the isolated proteins, which identified 217 putative CIPs from Drosophila (Figure 5B; Table S1). To narrow our search to CIPs most likely to be relevant to ceramide-associated LCM in both Drosophila and mammals, we performed pull-downs using mouse heart extracts and identified 125 putative CIPs (Figure 5B; Table S2). Of the 217 CIPs, 193 have putative mouse orthologs, 90 of which are expressed in the Drosophila heart (Cammarato et al., 2011). We identified 20 fly-mouse CIP orthologs based on DIOPT score for evolutionary conservation (Marygold et al., 2016) that were pulled down in both Drosophila and mouse heart extracts (Figures 5B and 5C), and the orthologs of all 20 are known to be expressed in the Drosophila heart (Cammarato et al., 2011).
Figure 5. The Ceramide-Protein Interactome.
(A) Silver-stained SDS-PAGE gel of CIPs pulled down from wild-type flies or mouse heart using a biotinylated ceramide–streptavidin bead trap. Lanes are: LD,ladder; whole extract, mouse heart; PDSN, post-pull-down supernatant (unbound proteins); CIPs, proteins bound to beads; biotin + BSA, biotinylated SA control.
(B) Venn diagram showing orthologous CIPs pulled down in whole-fly extracts and mouse heart extracts.
(C) Uniprot names of orthologous fly and mouse CIPs.
(D) Cytoscape BiNGO analysis showing enrichment of fly and mouse CIPs in overlapping gene ontology categories for biological process, molecular function, andcellular compartment.
Comparative gene ontology and network analysis in both whole fly and mouse heart revealed that the CIPs were enriched for involvement in specific biological processes, including actin filament organization, protein localization, and caspase activity; in molecular functions such as actin and GTP binding; and in diverse cellular compartments, but particularly mitochondria, myofibrils, and the actin cytoskeleton (Figure 5D). From the 20 orthologous CIPs, we assessed the potential LCM-related roles of 10 candidates across ontological categories, including apoptosis (Annexin 9, Annexin 10), sarcomeric maintenance and function (Unc-45, Hsp22, Klc, Zasp67), and lipid metabolism (kdn, Fasn1, Acsl).
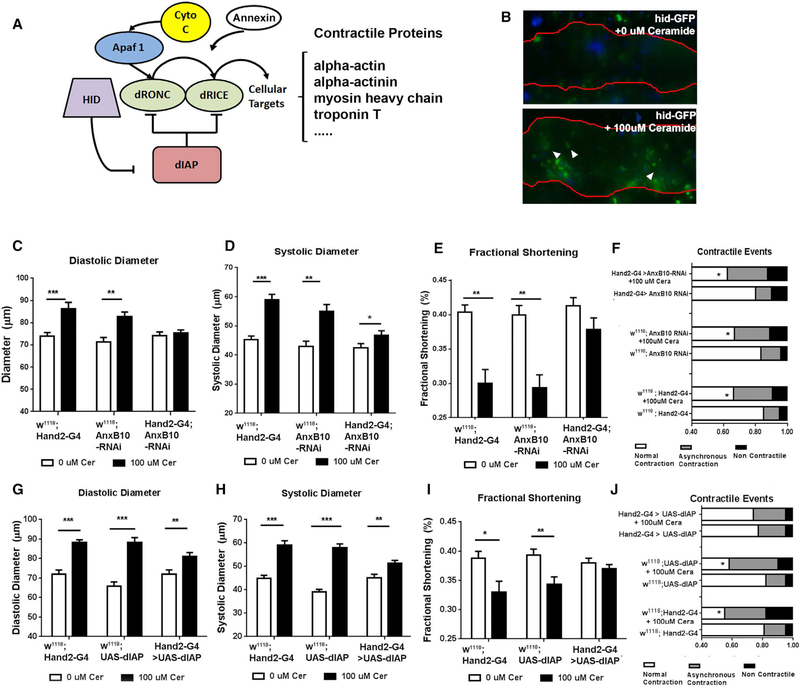
Heart-Specific Inhibition of Caspase Activation Attenuates Ceramide-Associated LCM
Late-stage LCM and subsequent heart failure is in part characterized by dilated cardiomyopathy, with gradual loss of cardio-myocyte function and eventual cell death (Feuerstein, 1999; Merkle et al., 2007; Yang et al., 2013). Ceramide is an established inducer of the caspase-dependent intrinsic apoptotic pathway, and contractile and structural cardiac proteins are known cas-pase targets (Fischer et al., 2003). To determine whether ceramide-associated loss of cardiac function is associated with increased caspase activation in the fly heart, we fed wild-type flies ceramide and examined activation of apoptosis. Under physiological conditions, Drosophila Inhibitor of Apoptosis (dIAP) suppresses the activity of the Drosophila caspases Dronc and Drice (Figure 6A) (Steller, 2008). However, when ceramide levels are elevated, the dIAP involution defective (hid) is transcriptionally activated, thereby allowing caspase activation via inhibition of dIAP (Fischer et al., 2003; Steller, 2008). Using a hid reporter line (Tanaka-Matakatsu et al., 2009), in which GFP expression is under the control of the hid promoter, we found that flies fed with 100 μM ceramide exhibited a >2-fold increase in GFP expression compared with vehicle-fed flies (Figure 6B), suggesting that caspases could be activated in the cardiomyocytes of ceramide-fed flies. Thus, dietary ceramide may induce a pre-apoptotic condition that contributes to compromised function such as cardiac dilation and reduced fractional shortening.
Figure 6. Ceramide-Associated Pathogenesis of LCM-like Phenotypes Is Caspase-Dependent.
(A) The intrinsic apoptotic pathway in Drosophila, which is highly conserved in mammals.
(B) Flies expressing GFP driven by the hid promoter were fed from day 1 of adulthood with vehicle or 100 μM ceramide and analyzed 3 weeks later. Red line indicates outline of cardiac chamber, arrows indicate GFP expression in the cardiomyocyte.
(C–F) Analysis of flies with heart-specific knockdown of Anx (Annexin X) fed from day 1 of adulthood with vehicle or 100 μM ceramide and analyzed 3 weeks later. (C) Diastolic diameter, (D) systolic diameter, (E) fractional shortening, and (F) contractile events.
(G–J) Analysis of flies with heart-specific overexpression of dIAP fed from day 1 of adulthood with vehicle or 100 μM ceramide and analyzed 3 weeks later. (G) Diastolic diameter, (H) systolic diameter, (I) fractional shortening, and (J) contractile events. (F) and (J) *p < 0.05 (χ2 test); all other panels, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 (Student’s t test). Error bars represent SEM.
Annexins have been reported to preferentially bind ceramide-rich domains and promote caspase activation via calcium-dependent signaling processes (Babiychuk et al., 2008; Debret et al., 2003; Solito et al., 2001). We therefore tested whether KD of the putative CIP Annexin X in Drosophila heart might prevent LCM. Indeed, heart-specific KD of d Annexin X was able to protect from ceramide-associated cardiac dilation and restore fractional shortening. However, other contractile defects were not rescued (Figures 6C–6F), suggesting that ceramide-associated LCM is only partially prevented. Of note, heart-specific KD of Annexin B9 also prevented ceramide-associated chamber dilation, but not loss of fractional shortening, and the prevalence of contractile defects was high even in vehicle-fed flies (Figures S6A–S6D).
In a complementary approach, we examined the effect of caspase inhibition on ceramide-associated LCM by overexpressing dIAP1 in the heart. Remarkably, this attenuated the increase in diastolic and systolic diameters and loss of fractional shortening in ceramide-fed flies (Figures 6G–6I). Unlike Annexin X KD, dIAP overexpression also rescued the remaining contractile defects (Figure 5J). Thus, inhibition of caspase activity by Annexin X KD or overexpression of dIAP1 in the heart was able to attenuate the ceramide-associated LCM-like phenotypes.
Cardiac Expression of the CIP Unc-45, a Myosin Chaperone, Attenuates Ceramide-Associated LCM
Unc-45 deficiency has previously been reported to cause extensive myosin loss associated with cardiac chamber dilation, loss of pumping power, and severe contractile defects in flies (Melkani et al., 2011). These defects are qualitatively similar to but more severe than those induced by manipulation of ceramide biosynthesis. Unc-45 is a myosin chaperone that translocates from the lipid-rich Z-line (Takahashi et al., 2001) to the A-band during muscle maintenance and/or stress (Lee et al., 2014). We speculated that ceramide accumulation in the Z-band might prevent Unc-45 translocation to the A-band, leading to loss of sarcomere integrity and induction of cardiac defects.
In order to address this hypothesis, we performed a protein-lipid overlay assay to validate the putative ceramide-binding activity of Unc-45. We found that Drosophila Unc-45 (dUnc-45) exhibited direct binding to membrane immobilized C6 biotin ceramide, validating the binding observed in the ceramide pull-down (Figure 7A). We also observed dUnc45 binding with C14:1/C16:0 ceramide—a naturally occurring ceramide species in fruit flies. Notably, promiscuous binding to other ceramide species was also observed, with presumably preferential binding to phosphorylated and saturated ceramide species (Figure S5B). However, dUnc-45 did not bind to sphingomyelin or sphingo-sine, nor to other membrane lipids (Figures S5B and S5C).
Figure 7. Ceramide-Associated LCM Is CIPDependent.
(A) Membranes were spotted with 1 μg, 0.5 μg, and 0.1 μg of either C6 biotin, C14:1, or C18:1 ceramide as indicated on the blot. Membranes were incu-bated with purified dUnc45 (top two) or hFASN (bottom two).
(B) Bound CIPs were detected by incubation withrespective antibodies.
(C) Relative cardiac dUnc45 and dFASN1 mRNAexpression under normal (white) and high ceramide (black) feeding conditions was measured by qPCR.
(D–G) Analysis of cardiac parameters in flies with heart-specific overexpression of Unc-45 (D), heart-specific knock down of FASN1 (E), and in combination (F) fed from day 1 of adulthood with vehicle or 100 μM C14:1/C2:0 ceramide and analyzed 3 weeks later. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. (G) Contractile events in the flies described in (B)–(D), (χ2 test, *p < 0.05). Error bars represent SEM. Sph, sphingosine; SM, sphingomyelin; Cer1P, ceramide 1-phosphate; C6/C16Cer, C6/C16 fatty acyl chain on a C18 sphingoid backbone; DH, dihydro derivatives.
Interestingly, ceramide feeding induced transcriptional activation of dUnc-45 with a nearly 2-fold increase in cardiac dUnc-45 mRNA relative to normal fed flies (Figure 7B). This is similar to findings in heart failure patients, where Unc-45b abundance was shown to be elevated, possibly as an insufficient compensatory response (Stanley et al., 2007). If so, ectopic Unc-45 overexpression might overcome this insufficiency, and prevent ceramide-associated LCM. Indeed, flies with heart-specific Unc-45 overexpression showed significant reversal of the cardiac defects, with diastolic and systolic diameters reduced to normal and restoration of normal cardiac pumping power, as reflected by fractional shortening (Figure 7C). Only a partial prevention of ceramide-associated contractile defects relative to w1118;Hand2-Gal4 was observed. However, relative to vehicle-fed flies, where baseline contractile defects were slightly elevated, virtually no additional contractile defects were observed upon ceramide feeding (Figure S7C, 3rd and 4th bar from top).
We also investigated the contribution of other sarcomere-associated CIPs to ceramide-associated LCM. We found that dilation was abolished by heart-specific overexpression of kinesin light chain, Klc, a protein involved in microtubule motor activity (Ali et al., 2008), and Zasp67, a Z-band protein involved in myofibril assembly (Katzemich et al., 2013), but not of Hsp22, a heat-shock/CRYAB-like protein (Figures S6A and S6B). How-ever, loss of fractional shortening was not prevented in these flies (Figure S6C) and large increases in asynchronous beating and non-contractile regions were observed in both ceramide and vehicle-fed flies (Figure S6). Thus, these CIPs appear to be important for cardiac function under both physiological conditions and ceramide stress. Future studies will determine the basis for the protective versus detrimental roles of sarcomere-associated CIPs in heart function.
Heart-Specific Knockdown of the Lipogenic CIP FASN1 Prevents LCM
Fatty acid synthase is a multi-enzyme protein involved in fatty acid synthesis, whose main function is the production of palmitate from malonyl-CoA and acetyl-CoA (Singh et al., 1984). Drosophila have three FASN encoding genes, where FASN1 is the predominant isoform expressed in the heart (FlyBase Con-sortium, 1998). We previously reported that KD of Drosophila FASN1 (termed dFASN from here on) in Drosophila protects against high fat diet-induced LCM (Birse et al., 2010; Diop et al., 2015) and that global dFASN expression is increased in ceramide-accumulating Sk2 mutant flies (Walls et al., 2013). Moreover, abnormal lipid accumulation, both systemically and specifically in the heart, is a defining feature of LCM, and ceramide accumulation has been noted in several heart failure models (Baranowski and Go´rski, 2011; Demarco et al., 2013; Goldberg et al., 2012). Consistent with our CIP pull-down as-says, we found that recombinant human dFASN binds directly to C6-biotin-ceramide (Figure 7A). Additionally, human FASN1 (hFASN) also binds C18:1/C16:0 ceramide—a naturally occur-ring ceramide in humans. (Figure 7A). We also observed some binding affinity of FASN to dihydroceramide, but not other sphingolipid species including sphingosine (Sph), sphingomyelin (SM) or ceramide phosphates (C1P, DHC1P) (Figure S5B), nor other membrane lipids (Figure S5C).
Consistent with related findings, ceramide fed flies exhibited over a 4-fold increase in cardiac dFASN expression (Figure 7B). Because fatty acids are required for de novo synthesis of ce-ramides and TAGs, we hypothesized that cardiac-specific KD of dFASN, should protect against ceramide-associated LCM. This was indeed the case. We found that ceramide-associated cardiac phenotypes were partially prevented by dFASN KD (Figure 7D), similar to the effects of Unc-45 overexpression (Figure 7C). Of note, KD of two additional CIPs associated with lipid metabolism; kdn (citrate synthase) and Acsl (longchain-fatty-acid-CoA ligase), caused a moderate loss of fractional shortening and increase in contractile defects, but did not induce dilation, in both vehicle- and ceramide-fed flies (Figure S6). This suggests that the lipogenesis-associated CIPs, like the sarcomeric CIPs, play a dual role in ceramide-associated LCM.
Of note, combinatorial dUnc-45 over-expression and FASN KD resulted in nearly complete rescue of the ceramide-associated LCM phenotype, including events of contractile dysfunction, under high ceramide feeding (Figures 7E and Figure S7C, top two bars). This correlated with a 7-fold increase in cardiac dUnc-45 expression and ~70% decrease in cardiac FASN expression under high ceramide feeding (Figures S7A and S7B). Taken together, these data suggest that multiple CIPs are involved in both overlapping and parallel processes to mediate the cardiotoxic effects associated with ceramide accumulation and sphingolipid metabolism in the heart.
DISCUSSION
The multifaceted nature of lipotoxic cardiomyopathy makes it difficult to investigate the role of individual molecules in LCM pathogenesis (Baranowski and Go´rski, 2011; Drosatos and Schulze, 2013; Jiang et al., 2011; Park and Goldberg, 2012). In the present study, we provide evidence for a direct role of ceramide in the induction and progression of LCM-like pheno-types in the Drosophila heart. Our experiments suggest that both the spatial (systemic, fat body [adipose]) and temporal (duration, age) regulation of ceramide metabolism is important for the onset and progression of the hallmarks of LCM. We also identified a number of CIPs through which ceramide or metabolites closely tracking with ceramide levels may mediate its effects on heart function. Collectively, our data provide evidence that the onset and progression of LCM is dependent, at least in part, on caspase activation, suppression of sarcomeric maintenance, and promotion of excessive lipogenesis in the heart.
We also found that disruption of ceramide degradation in the fat body (adipose) adversely affects heart function. However, it is not yet known whether the cardiac dysfunction is secondary to manipulation of ceramide metabolism in the fat body or due to increased production of circulating lipids (e.g., ceramide, TAG) by the fat body that leads to the heart defects. Our evidence that heart-specific inhibition of ceramide synthesis prevents ceramide feeding-induced LCM supports the latter possibility.
Interestingly, heart structure and function was compromised not only by inhibition of ceramide catabolism or direct ceramide feeding, but also by inhibition of de novo ceramide synthesis. Thus, KD of the ceramide synthetic genes lace and schlank or administration of the SPT inhibitor myriocin resulted in a constricted heart phenotype. Importantly, severe cardiac defects, including loss of fractional shortening, cardiac wall thinning/dilation, and other markers of cardiac failure, have been reported in mice with cardiomyocyte-specific deletion of the murine lace ortholog Sptlc-2, (Lee et al., 2012).
Taken together, these data provide direct evidence that cardiac homeostasis is tightly associated with ceramide levels and critically depends on the sphingolipid biosynthetic network through both heart-autonomous and systemic mechanisms (Figure 7). This is reminiscent of the need to maintain reactive oxygen species (ROS) levels within a narrow range to ensure robust cardiac function, and that perturbations that raise or lower ROS levels were detrimental to heart function (Lim et al., 2014).
Our genetic and pharmacological evidence that inhibition of de novo ceramide synthesis prevents ceramide-associated LCM is consistent with a previous finding that inhibition of de novo ceramide synthesis by myriocin reduces elevated ceramide levels and ameliorates LCM (Park et al., 2008a). How-ever, in the latter study, it was unclear whether the effect could be directly attributed to ceramide. Our work presented here clearly demonstrates that genetic, pharmacological or dietary manipulation aimed at altering ceramide levels in the heart, likely acting via a number of CIPs, elicits cardiac defects associated with LCM. Inhibition of ceramide synthesis has been reported to have beneficial effects in several models related to metabolic syndrome and lipotoxicity, including obesity (Yang et al., 2009), diabetes and insulin resistance (Kurek et al., 2015; Ussher et al., 2010), non-alcoholic fatty liver disease (Kasumov et al., 2015; Kurek et al., 2015), and atherosclerotic plaques (Park et al., 2008b).
We provide insights into the potential mechanisms by which manipulation of ceramide levels and sphinolipid metabolism induces cardiac abnormalities that are also observed in mammalian systems (Goldberg et al., 2012). Moreover, our results corroborate a recent report that cardiomyocyte hypertrophy may be linked to ceramide accumulation in ATGL-deficient mice (Gao et al., 2015). We have also addressed two important but previously unresolved issues linking ceramide to LCM (Chaurasia and Summers, 2015), by demonstrating that (1) genetic manipulation of ceramide metabolism in the heart is sufficient to induce LCM-like phenotypes, and (2) ceramides, generated systemically or in cardiomyocytes, are closely associated with cardio-lipotoxicity, which includes caspase activation, sarcomeric dysregulation, and excessive lipogenesis.
While ceramide modulation closely tracts cardiac pathology in our model, we cannot rule out the potential contributions of other ceramide-based, complex sphingolipids, such as ceramide 1-phosphate (C1P) or glycosphingolipids. We have previously reported that dCERK (ceramide kinase) mutants exhibit a dilated LCM-like phenotype (Nirala et al., 2013), as we observe here in Sk2 and Ceramidase-deficient flies (Figures 1D–1G, 3A–3D, and 4A–4D). dCERK mutant flies exhibit ceramide accumulation and reduced C1P levels. Thus, C1P modulation is unlikely responsible. In flies, glycosylated ceramides have been identified in embryos, but not in larva, prepupa, or across multiple studies in adults (Guan et al., 2013). However, our results do suggest that the observed cardiac pathologies are dependent upon ceramide biosynthetic and degradative path-ways, implicating ceramide or potentially other ceramide metabolites that are directly dependent upon the ceramide metabolic pathway.
Model for Ceramide Modulation of LCM Pathogenesis
We identified numerous CIPs that are candidates for mediating the pathological effects of ceramide-associated LCM in the heart, including candidates that are involved in apoptotic, lipo-genic, and myofibrillar maintenance processes. Direct interactions with ceramide-enriched membrane domains are likely to modulate CIP activity, stability, and/or subcellular localization, thereby eliciting the pathophysiological events that underlie the cardiac dysfunction. In the case of FASN, we postulate that inter-action with ceramide-enriched domains, possibly in the lipid droplet membrane, might protect FASN from degradation in the cytoplasm and promote fatty acid synthesis and storage. This hypothetical model is supported by previous work showing that ceramide accumulation is associated with upregulation of FASN expression and obesity (Abdel-Magid, 2015; Berndt et al., 2007; Boizard et al., 1998; Kim et al., 2007; Menendez et al., 2009; Walls et al., 2013).
Alternatively, CIP activity might be suppressed, rather than enhanced, by ceramide binding. We speculate that ceramide accumulation in the Z-band might prevent translocation of Unc-45 from the Z-band to the A-band during myosin maintenance and stress (Figure S8), leading to the observed contractile dysfunctions and cardiomyopathy. Indeed, Unc-45 deficiency has previously been shown to cause severe dilated cardiomyopathy in the fly model (Melkani et al., 2011).
Lastly, we found that activation of caspases via ceramide, HID, and dIAP may also play a key role in ceramide-associated LCM. Cardiomyocyte apoptosis has long been a therapeutic area of interest for a variety of cardiac ailments, including myocardial infarction and ischemia reperfusion injury (Fauvel et al., 2001; Feuerstein, 1999; Narula et al., 1999), and caspase activity is known to play a key role in heart failure (Merkle et al., 2007; Yang et al., 2013). Here, we showed that activation of proapoptotic caspases mediates ceramide-associated LCM prior to detectable loss of cardiomyocytes. This suggests that moderate levels of caspase activation may be related to defective cardiac remodeling before cardiomyocyte death, which is generally associated with late-stage cardiac failure (de Jonge et al., 2003; Kostin, 2011; Lu et al., 2013) and mortality in patients with chronic heart failure (Yu et al., 2015). Indeed, rats fed a high-fat diet show a gradual accumulation of ceramide and the LCM pathogenesis follows a progressive course (Harasim et al., 2015).
In mammals, there is strong evidence to support that myocardial steatosis and subsequent cardiac remodeling is inducible upon exposure to high caloric diets, but also reversible (Szczepaniak et al., 2007; Unger and Orci, 2001; Utz et al., 2011). Our data demonstrate that modulation of ceramide/sphingolipid metabolism in the heart is sufficient to induce LCM, this process is also reversible, using genetic or pharmacological intervention to modulate ceramide metabolism or CIPs. In this regard, evolutionary conservation of ceramide metabolism and CIPs across flies and mammals establish the Drosophila heart as a robust, genetic model to elucidate lipid-protein interactions underlying lipotoxic cardiomyopathies.
EXPERIMENTAL PROCEDURES
Drosophila Stocks and Husbandry
Drosophila stocks were reared on a standard laboratory diet consisting of yeast, corn starch, and molasses. Experiments were performed on 3-weekold flies unless specified. For ceramide supplementation experiments, flies were fed with standard diet containing 0 (xx vehicle), 10 μM, or 100 μM C14:1/C2:0 ceramide from day 1 of adulthood and analyzed 1 or 3 weeks later. The following lines were acquired from the Bloomington Drosophila Stock Center (https://bdsc.indiana.edu/): Canton-S, w1118 y[1] w[*]; Pw[+mC] = Act5C-GAL4 25FO1/CyO, y[+] (4414), w*,PUAS-DIAP1.H3 (6657), w[*]; Pw [+mC] = hid-EGFP.5’F-WT2 (50750), w[1118];Pw[+mC] = Cg-GAL4.A2 (11393), lacek05305 (12176), lace2 (3156), and Sk2KG00894 (14133). The following UAS-RNAi transgenic lines were acquired from the Vienna Drosophila Resource Center (http://stockcenter.vdrc.at/control/main): laceGD-RNAi (21805), schlankGD-RNAi (41114), Cdase GD-RNAi (30190), Sk1GD RNAi (32932), Sk2GD RNAi (41905).
Analysis of Cardiac Parameters Using the Semiautomatic Optical
Heartbeat Analysis Assay
Analysis of cardiac contractile parameters was performed on surgically exposed fly hearts using high-resolution video microscopy, as previously described (Fink et al., 2009). Briefly, flies were dissected to expose the heart within the abdomen and were kept in oxygenated artificial hemolymph. Sub-merged dissected hearts were oxygenated for 15 min at room temperature to equilibrate. High-speed digital movies were made with a Leica DM-LFSA microscope with a 10 3 water immersion lens, a Hamamatsu EM-CCD camera, and HCI image capture software (Hamamatsu). High-speed digital movies were recorded and analyzed using the semiautomatic optical heartbeat analysis (SOHA) software developed in-house. Fractional shortening (FS), commonly used by cardiologists as an important parameter of contractility, is calculated from diastolic diameter (DD) and SD (FS = DD-DS/DD).
Pharmacological Experiments
Myriocin (CAS: 35891–70-4) was acquired from Cayman Chemical, and N-acetyl-D-erythro-sphingosine (C14 sphingoid base) was acquired from Matreya. Stock solutions of lipids were prepared in methanol and mixed into standard fly food to achieve the desired final concentrations. Controls were fed with standard fly food mixed with an equivalent volume of vehicle.
Isolation of CIPs by Protein Pull-Down Assays
Pull-down assays were performed in triplicate as previously described (Kota et al., 2012). In brief, proteins were extracted from C57BL/6 mouse hearts or whole Drosophila (100 mg of whole flies) in 1.0 mL of RIPA buffer. Extracts were incubated with biotinylated C6-ceramide bound to streptavidin-coated M260 Dynabeads for 2 hr. After washing, CIPs attached to the beads were eluted by magnet and used for proteomics analysis.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software). Data are presented as the mean ± SEM. p values were calculated using Student’s t test, with the exception of contractile event data, which were evaluated using a Chi-square test after combining asynchronous and non-contractile parameters.
Supplementary Material
Highlights.
Dietary ceramide is associated with lipotoxicity in the Drosophila heart model
Genetic modulation of cardiac ceramide metabolism induces or prevents lipotoxicity
Ceramide interacts with conserved proteins (CIPs) in mouse hearts and Drosophila
Modulating the CIPs, Unc-45, Annexin10, and FASN prevents lipotoxic heart disease
ACKNOWLEDGMENTS
S.M.W. was supported by an NIH/NHLB NRSA postdoctoral fellowship (1F32HL124966), a Rees-Stealy Research Foundation Fellowship, an ARCS Foundation Scholarship, and an American Heart Association Predoctoral Fellowship. K.O. was supported by NIH (R01 HL132241). G.H. was supported by NIH (R21 HL80811). R.B. was supported by NIH (R01 HL054732, P01 AG033561, and P01 HL098053).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.02.034.
REFERENCES
- Abdel-Magid AF (2015). Fatty acid synthase (FASN) inhibitors as potential treatment for cancer, obesity, and liver related disorders. ACS Med. Chem.Lett. 6, 838–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi-Yamada T, Gotoh T, Sugimura I, Tateno M, Nishida Y, Onuki T, and Date H (1999). De novo synthesis of sphingolipids is required for cell survival by down-regulating c-Jun N-terminal kinase in Drosophila imaginal discs. Mol. Cell. Biol. 19, 7276–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MY, Lu H, Bookwalter CS, Warshaw DM, and Trybus KM (2008). Myosin V and Kinesin act as tethers to enhance each others’ processivity. Proc. Natl. Acad. Sci. USA 105, 4691–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk EB, Monastyrskaya K, and Draeger A (2008). Fluorescent annexin A1 reveals dynamics of ceramide platforms in living cells. Traffic 9, 1757–1775. [DOI] [PubMed] [Google Scholar]
- Baranowski M, and Go´rski J (2011). Heart sphingolipids in health and disease. Adv. Exp. Med. Biol. 721, 41–56. [DOI] [PubMed] [Google Scholar]
- Baranowski M, Bachnio A, Zabielski P, and Go´rski J (2007). PPARalpha agonist induces the accumulation of ceramide in the heart of rats fed high-fat diet. J. Physiol. Pharmacol. 58, 57–72. [PubMed] [Google Scholar]
- Bauer R, Voelzmann A, Breiden B, Schepers U, Farwanah H, Hahn I, Eckardt F, Sandhoff K, and Hoch M (2009). Schlank, a member of the ceramide synthase family controls growth and body fat in Drosophila. EMBO J. 28, 3706–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt J, Kovacs P, Ruschke K,Klo¨ ting N, Fasshauer M, Scho¨ n MR, Ko¨ rner A, Stumvoll M, and Blu€her M. (2007). Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia 50, 1472–1480. [DOI] [PubMed] [Google Scholar]
- Birse RT, and Bodmer R (2011). Lipotoxicity and cardiac dysfunction in mammals and Drosophila. Crit. Rev. Biochem. Mol. Biol. 46, 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, and Oldham S (2010). High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 12, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boizard M, Le Liepvre X, Lemarchand P, Foufelle F, Ferre P, and Dugail I (1998). Obesity-related overexpression of fatty-acid synthase gene in adipose tissue involves sterol regulatory element-binding protein transcription factors. J. Biol. Chem. 273, 29164–29171. [DOI] [PubMed] [Google Scholar]
- Cammarato A, Ahrens CH, Alayari NN, Qeli E, Rucker J, Reedy MC, Zmasek CM, Gucek M, Cole RN, Van Eyk JE, et al. (2011). A mighty small heart: the cardiac proteome of adult Drosophila melanogaster. PLoS ONE 6, e18497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia B, and Summers SA (2015). Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 26, 538–550. [DOI] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, and Dow JA (2007). Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39, 715–720. [DOI] [PubMed] [Google Scholar]
- Chui PC, Guan HP, Lehrke M, and Lazar MA (2005). PPARgamma regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J. Clin. Invest. 115, 2244–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge N, van Wichen DF, van Kuik J, Kirkels H, Lahpor JR, GmeligMeyling FH, van den Tweel JG, and de Weger RA (2003). Cardiomyocyte death in patients with end-stage heart failure before and after support with a left ventricular assist device: low incidence of apoptosis despite ubiquitous mediators. J. Heart Lung Transplant. 22, 1028–1036. [DOI] [PubMed] [Google Scholar]
- Debret R, El Btaouri H, Duca L, Rahman I, Radke S, Haye B, Sallenave JM, and Antonicelli F (2003). Annexin A1 processing is associated with caspase-dependent apoptosis in BZR cells. FEBS Lett. 546, 195–202. [DOI] [PubMed] [Google Scholar]
- Demarco VG, Ford DA, Henriksen EJ, Aroor AR, Johnson MS, Habibi J, Ma L, Yang M, Albert CJ, Lally JW, et al. (2013). Obesity-related alterations in cardiac lipid profile and nondipping blood pressure pattern during transition to diastolic dysfunction in male db/db mice. Endocrinology 154, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop SB, and Bodmer R (2012). Drosophila as a model to study the genetic mechanisms of obesity-associated heart dysfunction. J. Cell. Mol. Med. 16, 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop SB, Bisharat-Kernizan J, Birse RT, Oldham S, Ocorr K, and Bodmer R (2015). PGC-1/Spargel counteracts high-fat-diet-induced obesity and cardiac lipotoxicity downstream of TOR and Brummer ATGL lipase. Cell Rep. https://doi.org/10.1016/j.celrep.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzyn P, Dobrzyn A, Miyazaki M, and Ntambi JM (2010). Loss of stearoyl-CoA desaturase 1 rescues cardiac function in obese leptin-deficient mice. J. Lipid Res. 51, 2202–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzyn P, Bednarski T, and Dobrzyn A (2015). Metabolic reprogramming of the heart through stearoyl-CoA desaturase. Prog. Lipid Res. 57, 1–12. [DOI] [PubMed] [Google Scholar]
- dos Santos G, Schroeder AJ, Goodman JL, Strelets VB, Crosby MA, Thurmond J, Emmert DB, and Gelbart WM; FlyBase Consortium (2015). FlyBase: introduction of the Drosophila melanogaster Release 6 reference genome assembly and large-scale migration of genome annotations. Nucleic Acids Res. 43, D690–D697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosatos K, and Schulze PC (2013). Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr. Heart Fail. Rep. 10, 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvel H, Marchetti P, Chopin C, Formstecher P, and Nevie` re R (2001). Differential effects of caspase inhibitors on endotoxin-induced myocardial dysfunction and heart apoptosis. Am. J. Physiol. Heart Circ. Physiol. 280, H1608–H1614. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ (1999). Apoptosis in cardiac diseases–new opportunities for novel therapeutics for heart diseases. Cardiovasc. Drugs Ther. 13, 289–294. [DOI] [PubMed] [Google Scholar]
- Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, and Kelly DP (2003). A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc. Natl. Acad. Sci. USA 100, 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Callol-Massot C, Chu A, Ruiz-Lozano P, Izpisua Belmonte JC, Giles W, Bodmer R, and Ocorr K (2009). A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques 46, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Ja¨nicke RU, and Schulze-Osthoff K (2003). Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 10, 76–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium FlyBase (1998). FlyBase: a Drosophila database. Nucleic Acids Res. 26, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Feng XJ, Li ZM, Li M, Gao S, He YH, Wang JJ, Zeng SY, Liu XP, Huang XY, et al. (2015). Downregulation of adipose triglyceride lipase promotes cardiomyocyte hypertrophy by triggering the accumulation of ceramides. Arch. Biochem. Biophys. 565, 76–88. [DOI] [PubMed] [Google Scholar]
- Goldberg IJ, Trent CM, and Schulze PC (2012). Lipid metabolism and toxicity in the heart. Cell Metab. 15, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XL, Cestra G, Shui G, Kuhrs A, Schittenhelm RB, Hafen E, van der Goot FG, Robinett CC, Gatti M, Gonzalez-Gaitan M, and Wenk MR (2013). Biochemical membrane lipidomics during Drosophila development. Dev. Cell 24, 98–111. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Bu€ttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, et al. (2011). ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-a and PGC-1. Nat. Med. 17, 1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasim E, Ste˛ pek T, Konstantynowicz-Nowicka K Baranowski M Go´rski J, and Chabowski A (2015). Myocardial lipid profiling during time course of high fat diet and its relationship to the expression of fatty acid transporters.Cell. Physiol. Biochem. 37, 1147–1158. [DOI] [PubMed] [Google Scholar]
- Hebbar S, Sahoo I, Matysik A, Argudo Garcia I, Osborne KA, Papan C, Torta F, Narayanaswamy P, Fun XH, Wenk MR, et al. (2015). Ceramides and stress signalling intersect with autophagic defects in neurodegenerative Drosophila blue cheese (bchs) mutants. Sci. Rep. 5, 15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XC, Goldberg IJ, and Park TS (2011). Sphingolipids and cardiovascular diseases: lipoprotein metabolism, atherosclerosis and cardiomyopathy. Adv. Exp. Med. Biol. 721, 19–39. [DOI] [PubMed] [Google Scholar]
- Kasumov T, Li L, Li M, Gulshan K, Kirwan JP, Liu X, Previs S, Willard B, Smith JD, and McCullough A (2015). Ceramide as a mediator of nonalcoholic fatty liver disease and associated atherosclerosis. PLoS ONE 10, e0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzemich A, Liao KA, Czerniecki S, and Scho¨ ck F. (2013). Alp/Enigma family proteins cooperate in Z-disc formation and myofibril assembly. PLoS Genet. 9, e1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Kleman AM, and Ronnett GV (2007). Fatty acid synthase gene regulation in primary hypothalamic neurons. Neurosci. Lett. 423, 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostin S (2011). Types of cardiomyocyte death and clinical outcomes in patients with heart failure. J. Am. Coll. Cardiol. 57, 1532–1534. [DOI] [PubMed] [Google Scholar]
- Kota V, Szulc ZM, and Hama H (2012). Identification of C(6) -ceramide-interacting proteins in D6P2T Schwannoma cells. Proteomics 12, 2179–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek K,Mik1osz A,qukaszuk B,Chabowski A,Go´rski J, and Z_ endzianPiotrowska M (2015). Inhibition of ceramide de novo synthesis ameliorates diet induced skeletal muscles insulin resistance. J. Diabetes Res. 2015, 154762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Kim JR, Hu Y, Khan R, Kim SJ, Bharadwaj KG, Davidson MM, Choi CS, Shin KO, Lee YM, et al. (2012). Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. J. Biol. Chem. 287, 18429–18439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CF, Melkani GC, and Bernstein SI (2014). The UNC-45 myosin chaperone: from worms to flies to vertebrates. Int. Rev. Cell Mol. Biol. 313, 103–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Wang W, Wessells RJ, Ocorr K, and Bodmer R (2011). Phospholipid homeostasis regulates lipid metabolism and cardiac function through SREBP signaling in Drosophila. Genes Dev. 25, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Wang W, Chen J, Ocorr K, and Bodmer R (2014). ROS regulate cardiac function via a distinct paracrine mechanism. Cell Rep. 7, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Trent CM, Fang X, Son NH, Jiang H, Blaner WS, Hu Y, Yin YX, Farese RV Jr., Homma S, et al. (2014). Cardiomyocyte-specific loss of diacylglycerol acyltransferase 1 (DGAT1) reproduces the abnormalities in lipids found in severe heart failure. J. Biol. Chem. 289, 29881–29891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu FH, Fu SB, Leng X, Zhang X, Dong S, Zhao YJ, Ren H, Li H, Zhong X, Xu CQ, and Zhang WH (2013). Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell. Physiol. Biochem. 31, 728–743. [DOI] [PubMed] [Google Scholar]
- Marygold SJ, Crosby MA, and Goodman JL; FlyBase Consortium (2016). Using FlyBase, a Database of Drosophila Genes and Genomes. Methods Mol. Biol. 1478, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkani GC, Bodmer R, Ocorr K, and Bernstein SI (2011). The UNC-45 chaperone is critical for establishing myosin-based myofibrillar organization and cardiac contractility in the Drosophila heart model. PLoS ONE 6, e22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Vazquez-Martin A, Ortega FJ, and Fernandez-Real JM (2009). Fatty acid synthase: association with insulin resistance, type 2 diabetes, and cancer. Clin. Chem. 55, 425–438. [DOI] [PubMed] [Google Scholar]
- Merkle S, Frantz S, Scho¨ n MP, Bauersachs J Buitrago M Frost RJ, Schmitteckert EM, Lohse MJ, and Engelhardt S (2007). A role for caspase-1 in heart failure. Circ. Res. 100, 645–653. [DOI] [PubMed] [Google Scholar]
- Na J, Musselman LP, Pendse J, Baranski TJ, Bodmer R, Ocorr K, and Cagan R (2013). A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 9, e1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, et al. (1999). Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc. Natl. Acad. Sci. USA 96, 8144–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirala NK, Rahman M, Walls SM, Singh A, Zhu LJ, Bamba T, Fukusaki E, Srideshikan SM, Harris GL, Ip YT, et al. (2013). Survival response to increased ceramide involves metabolic adaptation through novel regulators of glycolysis and lipolysis. PLoS Genet. 9, e1003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Fink M, Cammarato A, Bernstein S, and Bodmer R (2009). Semi-automated optical heartbeat analysis of small hearts. J. Vis. Exp. (31) https://doi.org/10.3791/1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, and Goldberg IJ (2012). Sphingolipids, lipotoxic cardiomyopathy, and cardiac failure. Heart Fail. Clin. 8, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, and Goldberg IJ (2008a). Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49, 2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Rosebury W, Kindt EK, Kowala MC, and Panek RL (2008b). Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol. Res. 58, 45–51. [DOI] [PubMed] [Google Scholar]
- Shabana, and Hasnain S (2016). Obesity, more than a ‘cosmetic’ problem. Current knowledge and future prospects of human obesity genetics. Biochem. Genet. 54, 1–28. [DOI] [PubMed] [Google Scholar]
- Singh B, Stakkestad JA, Bremer J, and Borrebaek B (1984). Determination of malonyl-coenzyme A in rat heart, kidney, and liver: a comparison between acetyl-coenzyme A and butyryl-coenzyme A as fatty acid synthase primers in the assay procedure. Anal. Biochem. 138, 107–111. [DOI] [PubMed] [Google Scholar]
- Solito E, de Coupade C, Canaider S, Goulding NJ, and Perretti M (2001). Transfection of annexin 1 in monocytic cells produces a high degree of spontaneous and stimulated apoptosis associated with caspase-3 activation. Br. J. Pharmacol. 133, 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BA, Shaw J, Arab S, Liu P, Kirshenbaum LA, and Van Eyk JE (2007). Abstract 870: UNC-45B exhibits increased abundance in heart failure patients and functions as a novel dual regulator of myosin heavy chain transcription and assembly. Circulation 116, II_170. [Google Scholar]
- Steller H (2008). Regulation of apoptosis in Drosophila. Cell Death Differ. 15, 1132–1138. [DOI] [PubMed] [Google Scholar]
- Szczepaniak LS, Victor RG, Orci L, and Unger RH (2007). Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ. Res. 101, 759–767. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Shimada K, Ahn DH, and Ji JR (2001). Identification of lipids as the main component of skeletal muscle Z-discs. J. Muscle Res. Cell Motil. 22, 353–360. [DOI] [PubMed] [Google Scholar]
- Tanaka-Matakatsu M, Xu J, Cheng L, and Du W (2009). Regulation of apoptosis of rbf mutant cells during Drosophila development. Dev. Biol. 326, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, and Orci L (2001). Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 15, 312–321. [DOI] [PubMed] [Google Scholar]
- Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM, and Lopaschuk GD (2010). Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59, 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz W, Engeli S, Haufe S, Kast P, Hermsdorf M, Wiesner S, Pofahl M, Traber J, Luft FC, Boschmann M, et al. (2011). Myocardial steatosis, cardiac remodelling and fitness in insulin-sensitive and insulin-resistant obese women. Heart 97, 1585–1589. [DOI] [PubMed] [Google Scholar]
- Walls SM Jr., Attle SJ, Brulte GB, Walls ML, Finley KD, Chatfield DA, Herr DR, and Harris GL (2013). Identification of sphingolipid metabolites that induce obesity via misregulation of appetite, caloric intake and fat storage in Drosophila. PLoS Genet. 9, e1003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, Sifuentes AJ, McDonald JG, Gordillo R, and Scherer PE (2015). Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, and Samad F (2009). Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 297, E211–E224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Ye D, and Wang Y (2013). Caspase-3 as a therapeutic target for heart failure. Expert Opin. Ther. Targets 17, 255–263. [DOI] [PubMed] [Google Scholar]
- Yu J, Pan W, Shi R, Yang T, Li Y, Yu G, Bai Y, Schuchman EH, He X, and Zhang G (2015). Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can. J. Cardiol. 31, 357–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.