Abstract
Most animals orient their bodies with respect to gravity to facilitate locomotion and perception. The neural circuits responsible for these orienting movements have long served as a model to address fundamental questions in systems neuroscience. Though postural control is vital, we know little about development of either balance reflexes or the neural circuitry that produces them. Recent work in a genetically and optically accessible vertebrate, the larval zebrafish, has begun to reveal the mechanisms by which such vestibular behaviors and circuits come to function. Here we highlight recent work that leverages the particular advantages of the larval zebrafish to illuminate mechanisms of postural development, the role of sensation for balance circuit development, and the organization of developing vestibular circuits. Further, we frame open questions regarding the developmental mechanisms for functional circuit assembly and maturation where studying the zebrafish vestibular system is likely to open new frontiers.
Introduction
Gravity is a pervasive force across Earth. Most animals learn to control their orientation with respect to gravity, engaging reflexive movements that correct and maintain posture and that also serve as organizing principles of locomotor behavior. The normal posture that results from these movements is vital for locomotion and facilitates perception by stabilizing gaze. Therefore, the development of gravity-related behaviors and their underlying neural circuitry is a general problem of vital importance.
Rigorous dissection of neural circuit function is only possible in the context of well-described behaviors, which dictate the constraints on the output of neural computations (Tytell et al., 2011; Krakauer et al., 2017). This poses a challenge for studies of posture in tetrapods, where a large number of muscles governing both limbs and trunk are engaged to maintain the animal’s orientation, defying experimental analysis of the motor output. Furthermore, behavioral capacity is constrained by the composition of the underlying neural circuits, which are built during early development and refined as animals mature. Here we focus on vestibular function in an animal with a simpler body plan, the larval zebrafish, that serves as a powerful proving ground for hypotheses about the functional development of neural circuits.
As a small model vertebrate, zebrafish have four features that uniquely facilitate the work on gravity-related behaviors and the responsible neural circuits. First, their simpler body plan facilitates decoding both destabilizing physical forces and the development of compensatory behaviors. Second, zebrafish are genetically accessible, with established mutant lines that disrupt balance and posture (Nicolson et al., 1998). Third, the external development of zebrafish embryos permits continuous access, whereas in amniotes much of the development takes place in ovo or in utero (Curthoys, 1979; Peusner, 2001; Fritzsch et al., 2014; Beraneck et al., 2014). Finally, the mostly transparent bodies of zebrafish larvae permit anatomical, electrophysiological, and optical (Favre-Bulle et al., 2017; Vanwalleghem et al., 2018) approaches during external development.
Vestibular circuits serve to transform sensed instability into corrective motor output. In order to produce rapid sensory-motor transformations for both posture and gaze stabilization, the nervous system relies predominantly on a short reflex arc (Szentágothai, 1964). In the inner ear, an otolith (or mass of otoconia, in later vertebrates) sits atop of hair cells. As the head tilts or translates, the otolith slides relative to the hair cells, which transduce this mechanical stimulus into electrical signals, releasing glutamate onto vestibular afferents of the eighth cranial nerve. Afferents relay this activity to central vestibular neurons, which encode head tilt in a variety of directions and project directly to motor centers, including cranial motor nuclei and the spinal cord. These feed-forward systems are remarkably well-conserved across vertebrates (Straka and Baker, 2013; Straka et al., 2014), reinforcing the generality of findings in the larval zebrafish.
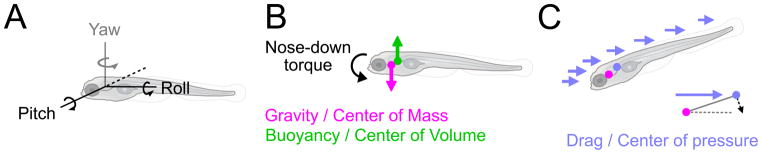
How zebrafish learn to stabilize posture
What physical challenges destabilize larval zebrafish (≤30 days old) and how do they learn to respond? Simliar to other highly maneuverable animals, zebrafish bodies are inherently unstable (Webb and Weihs, 2015). Vestibular-deficient animals often swim in corkscrew fashion or upside-down, suggesting instability in the roll axis (Figure 1A) (Whitfield et al., 1996). Anesthetized larvae are also unstable in the pitch axis, tipping headfirst (Figure 1A). Recent work offers a physical explanation for the nose-down torques that destabilize pitch (Ehrlich and Schoppik, 2017). In still water, the two main forces acting on zebrafish are gravity and buoyancy (Figure 1B). Gravity acts at a zebrafish’s center of mass to pull the fish down. Meanwhile, the buoyant force acts at the center of volume to push the zebrafish up. Because the center of mass for larval zebrafish sits rostral to the center of volume, fish are subject to a constant nose-down torque. Therefore, to maintain stability, fish must govern orientation in both the pitch and roll axes.
Figure 1. A diagram of forces that stabilize and destabilize larval zebrafish posture.
A) Three axes of rotation: Roll, Pitch (nose-up/nose-down) and Yaw (left/right turns). B) Forces in the pitch axis: the buoyant force acts at the center of volume (green circle) to elevate the fish; gravity acts at the center of mass (magenta circle) to pull the fish down. The center of mass sits forward of the center of gravity which leads to a nose-down torque that will rotate a passive fish nose-down. C) During forward translation, or in flow, the fish will rotate (shown here in the pitch axis) to align with the direction of drag (blue lines). The center of pressure (blue circle) is displaced caudally to the center of mass (pink circle) about which the fish rotates. This displacement acts as a moment arm, schematized in the corner, that generates stabilizing torque (black arrow) to align the body.
Zebrafish exhibit both active and passive mechanisms that govern orientation, with passive contributions mitigating some external destabilization and active components providing the remaining postural control. Larval zebrafish can actively rotate their bodies by selective contractions of the dorsal (epaxial) and ventral (hypaxial) trunk musculature. Differential contraction of the dorsal and ventral muscles on the right and left sides of the body subserves roll (Fig. 2a) (Bagnall and McLean, 2014). Conversely, contraction of dorsal or ventral muscles on the left and right sides in concert serves to rotate fish in the pitch axis, though the precise organization of this activity has not been described (Deliagina et al., 2007). Importantly, asymmetries in zebrafish morphology provide directional stability in both roll and pitch axes. Just as a weathervane rotates to adopt a stable orientation relative to the wind, fish orient in roll/pitch during forward translation (Figure 1C). Thus swimming and/or facing into flowing water passively stabilizes orientation.
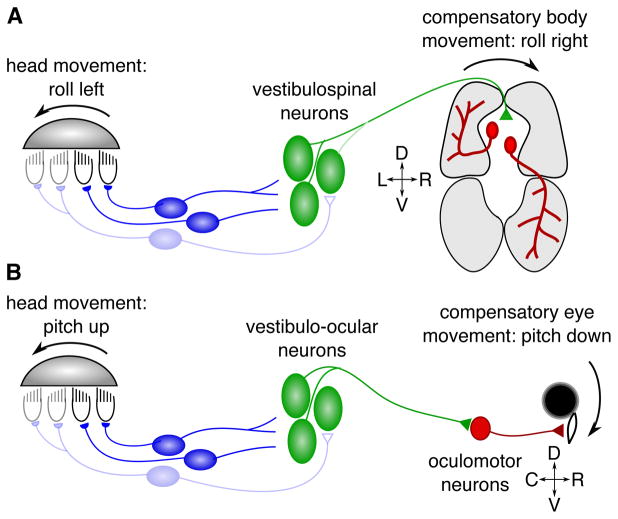
Figure 2. Schematic of vestibular circuits subserving posture and gaze stabilization.
A) At the onset of movement, the utricular otolith slides relative to the hair cells underneath, depolarizing some (black) and hyperpolarizing others (gray), depending on their ciliary orientation. Vestibular afferents (blue) relay hair cell signals to vestibulospinal neurons in the hindbrain (green). This vestibular drive sets up asymmetric activation of trunk musculature through as-yet unclear connectivity that is thought to rely both on direct synapses with motor neurons as well as indirectly via spinal premotor populations (Kasumacic et al., 2015; Murray et al., 2018). In this example, as the fish rolls to the left, stronger motor drive to ventral muscle on the right and dorsal muscle on the left (gray shaded regions) produces a self-righting torque (Bagnall and McLean, 2014). B) Equivalent schematic for vestibular-driven gaze stabilization. Here the brainstem vestibular nuclei are known to make direct connections to oculomotor neurons. Recent work has revealed that in pitch-related circuits, vestibulo-ocular neurons driving eye rotation downwards (active pathway, dark green) outnumber those driving upwards eye movements (inhibited pathway, pale green) by 6:1 (Schoppik et al., 2017).
Separating active and passive means of orientation enabled recent work showing that larval zebrafish learn to time their swimming to stabilize posture (Ehrlich and Schoppik, 2017). Larval zebrafish locomote in discrete swim bouts. Their bodies are so small that translation is constrained by viscous forces that minimize glide (Fuiman and Webb, 1988; McHenry, 2005; Voesenek et al., 2018). During the pause between swim bouts, larvae lose the stabilizing effects of flow and are instead pitched nose downwards by gravity. Subsequent swim bouts, made on average once a second, translate fish and correct this destabilization in concert with active reorienting movements (Figure 1C). At 4 days old, larvae time their swim bouts with comparable frequency at most postures. As they develop, the probability of making a bout becomes correlated with instability. As zebrafish rotate away too far or too quickly from their preferred posture, the probability of initiating a bout increases. Consequently, unlike younger larvae, older larvae specifically time bursts of swim bouts to compensate for instability. Emergent control of locomotor initiation thus underlies developmental improvements to postural stability.
Larval zebrafish reduce their density by inflating a gas-filled organ called the swim bladder; failure to do so is fatal. This early behavior requires larvae to reach the surface and gulp air (Goolish and Okutake, 1999). Unlike visual cues, vestibular cues are sufficient to orient and locomote to the surface. Normal larvae raised in the dark inflate their swim bladders properly, whereas fish missing their vestibular otoliths fail to inflate their swim bladder at a normal time, even when raised in the light (Riley and Moorman, 2000). Therefore to swim properly, larvae must use their vestibular system to orient and surface. (Lindsey et al., 2010).
Vestibular input is not required for vestibulospinal circuit development
The importance of locomotion for postural stability underscores the need for rapid functional development of the spinal circuits that generate axial swimming in response to vestibular input. In larval zebrafish, the spinal circuitry responsible undergoes a change in functional configuration from 1 to 3 days old, with a shift from a spontaneous coiling behavior to the beat-and-glide swim pattern. The spinal cord of the early embryo exhibits high levels of gap junctional coupling, which is thought to underlie coiling movements (Saint-Amant and Drapeau, 2001; Drapeau et al., 2002). During the transition from coiling to beat-and-glide swimming, there is a shift from reliance on electrical to chemical synapses (Knogler et al., 2014) and a concurrent transition in the ionic currents expressed in spinal cord premotor neurons (Tong and McDearmid, 2012). Additionally, the elements of spinal circuitry that produce fast swimming are largely in place by 2 days old, whereas the motor and premotor components of slower muscle control, which are thought to underlie most postural movements (Basaldella et al., 2015), develop around 2–4 days old (Kimura, 2006; McLean and Fetcho, 2009). Over the same time period, reticular circuits acquire sensitivity to vestibular and auditory input (Kohashi et al., 2012). Therefore spinal cord maturation, encompassing both neuronal differentiation and intrinsic and synaptic changes, prepares the circuit for appropriate motor outputs in response to newly functional vestibular signals around 3 days old.
Does patterned sensory activity play an instructive role in vestibular circuit development? Recent work in zebrafish has found that gravitational sensation is likely dispensable for this process. Both the utricular otolith and associated hair cells develop by 1 day old, well before postural behaviors emerge (Bever and Fekete, 2002; Tanimoto et al., 2011). The utricle is the only functional vestibular sensor in larval zebrafish (Riley and Moorman, 2000), due to the small size of the larval semicircular canals (Beck et al., 2004; Lambert et al., 2008). Mutant zebrafish lacking a functional otogelin allele exhibit delayed development of the utricular otolith to 2 weeks old, well after postural function normally develops. Intriguingly, once the utricular otolith is in place otogelin −/− larvae develop normal orientation to gravity, supporting the hypothesis that the underlying vestibulospinal circuit develops accurately in the absence of patterned sensory information (Roberts et al., 2017). Notably, the organization of the spinal circuit into distinct modules for differential control of dorsal and ventral musculature necessary for proper postural orientation persists in otogelin −/− larvae, even prior to the arrival of the otolith (Bagnall and McLean, 2014). Therefore spinal motor circuit assembly does not rely on patterned descending vestibular signals.
Organization of developing vestibular circuits that stabilize gaze and posture
Vestibular circuits rely on specific connectivity to route gravitational signals to appropriate motor outputs. Research on vestibular-driven postural circuits, which operate through the rich complexity of the spinal cord, has lagged behind that of vestibulo-ocular gaze-stabilizing circuits. There, eye movements are controlled through a highly conserved set of six muscles, and gaze-stabilizing behavior can be readily measured. In the gaze-stabilizing circuit, two major classes of central vestibular neurons are sufficient to elicit compensatory eye movements during pitch and roll (Boyle et al., 1992; Graf et al., 1997). One class is sensitive to nose-up body rotations, the other to nose-down, and both can respond during roll towards the ipsilateral ear (ipsiversive roll). During ipsiversive roll, both classes are active and the eyes rotate appropriately. To compensate eye position during pitch, the two classes have distinct effects on motor neurons: one class evokes torsional eye rotations that compensate nose-up pitch, whereas the other rotates the eyes to compensate nose-down pitch (Figure 2B). Recent work used the zebrafish to confirm an anatomical asymmetry among gaze-stabilizing neurons previously suggested in mammals (Iwamoto et al., 1990). Using single-cell tracing, lesions, and selective activation, it was demonstrated that vestibular neurons were distributed in a 6:1 nose-up to nose-down ratio (Schoppik et al., 2017).
Gaze stabilization in pitch and roll via the vestibulo-ocular reflex develops contemporaneously with consistent postural orientation (Bianco et al., 2012). At 3 days old, larval zebrafish show no measurable eye movements in response to body tilts in pitch or roll. 24 hours later, the responses are partially compensatory. By 10 days old, larval zebrafish can entirely compensate for ethologically-relevant body tilts by counter-rotating their eyes.
Gravity guides both postural and gaze stabilizing reflexes. Do neural circuits coordinate the two? Recent anatomical and lesion work supports a role for central vestibular neurons that perform the vertical/torsional vestibulo-ocular reflex. In addition to projections to the extraocular motoneurons that stabilize gaze, these vestibular neurons project to a set of reticulospinal neurons in the nucleus of the medial longitudinal fasciculus (Bianco et al., 2012). These midbrain reticulospinal neurons respond to visual cues, including moving gratings, and affect the speed and direction of locomotion (Thiele et al., 2014; Severi et al., 2014; Wang and McLean, 2014). Intriguingly, following early photoablation of these vestibular neurons larvae fail to inflate their swim bladders, indicating malfunction of righting behaviors (Schoppik et al., 2017). This work suggests a postural role for gaze-stabilizing vestibular neurons, evidence of coordinated processing of vestibular sensation for behavior.
Open questions well-suited to the zebrafish model
How do molecular cues coordinate sensorimotor circuit assembly? Proper vestibular circuit organization requires central vestibular neurons to coordinate information flow across two synapses: for example, peripheral afferents sensitive to nose-up pitch tilts must selectively innervate central vestibular neurons that project to nose-down effectors to stabilize gaze (Figure 2B). There are four possible substrates for the origin of developmental cues that govern this process. Utricular hair cells with opposite polarity are genetically differentiable (Jiang et al., 2017), raising the possibility that they express cell surface signaling molecules that trigger identity formation in afferents. Alternatively, central vestibular neurons may use a process such as Notch-mediated lateral inhibition to instruct fate, similar to the spinal cord (Appel et al., 2001). Thirdly, neuronal connectivity could be conferred retrogradely from the oculomotor neuron pools, which are segregated spatially during development (Greaney et al., 2016), as has been suggested for the chick (Glover, 1996) and shown for spinal interneurons (Baek et al., 2017). Finally, temporal cues could guide fate decisions: recent in vivo timelapse imaging demonstrated how peripheral afferents develop systematically in time (Zecca et al., 2015; Dyballa et al., 2017). Similar organization by birthdate has been demonstrated in extraocular motoneurons (Greaney et al., 2016) and spinal motor circuits (McLean and Fetcho, 2009) using genetically-encoded photoconvertible markers of post-mitotic neurons. Disentangling these competing hypotheses will require observing and perturbing developing circuits, experiments facilitated by the accessibility of the zebrafish preparation.
The cerebellum is well-known for its capacity to implement multisensory integration (Knogler et al., 2017) and drive motor learning (Harmon et al., 2017). Does it also serve to guide vestibular development? In zebrafish, Purkinje cells are functional by 5 days (Hsieh et al., 2014; Sengupta and Thirumalai, 2015), and in vivo imaging has revealed that a cerebello-vestibular projection develops around 5–7 days old (Bae et al., 2009; Hamling et al., 2015). This occurs after the early development of posture and gaze stabilization, but prior to refinements in bout timing and integration of sensory information from the semicircular canals. Thus, it should be possible to examine cerebellar contributions to vestibular circuit function by analysis of the ontogeny of postural behaviors relative to functional development of the Purkinje - vestibular synapse. Cerebellar signals could also direct the timing of swim bouts specifically to counter postural instability (Ehrlich and Schoppik, 2017), consistent with the known role of cerebellum in temporal precision (Kalmbach et al., 2011; Heiney et al., 2014).
Similarly, as larval zebrafish transition to juveniles and the semicircular canals begin to function, vestibular circuits must integrate these new signals without compromising behavior. The developmental processes that guide this integration are wholly unclear, but intriguing results in Xenopus point to a role for the semicircular canals in functional refinement of tuning (Branoner and Straka, 2014). The cerebellum is implicated in integration of canal and otolith signals (Angelaki et al., 2009), and might also govern their developmental alignment. Finally, the mature cerebellum uses visual feedback to tune the strength of the vestibulo-ocular reflex. In larval zebrafish, visual information can be appropriately integrated to augment gaze-stabilization (Bianco et al., 2012) Whether the cerebellum uses visual information to drive refinement of gaze-stabilizing circuits is an open research question well-suited to the zebrafish due to its external development.
Conclusions
Thanks to a set of unique advantages, the larval zebrafish model has shed light on a number of long-standing questions regarding vestibular circuit assembly, organization, and behavioral development. Recent work has shown that basic vestibular reflexes develop quite early, followed by later refinements that improve stability of posture and gaze. Mutant zebrafish with delayed peripheral development show normal circuitry, suggesting that gravity sensation may be dispensible. The relative simplicity of orienting underwater revealed that larval zebrafish learn to time their movements to control posture. Finally, recent work used the optical access to define the anatomy and function of a set of vestibular neurons responsible for both gaze stabilization and early postural behaviors. These findings demonstrate the burgeoning impact of larval zebrafish model for understanding vestibular development.
Excitingly, the developing zebrafish vestibular system permits investigation of oustanding questions regarding the roles of genetic signals, activity, and cerebellar feedback in directing neural circuit development. Does the sensory or motor periphery provide vital instructive cues governing central neuron identity / circuit connectivity? How are cerebellar and peripheral inputs — the hallmarks of mature vestibular circuits — incorporated during development without disrupting behavior? Studies of the mature vestibular system have informed our understanding of nearly every aspect of systems-level neuroscience (Goldberg et al., 2012). Studying vestibular circuit assembly and attendant behavior in the larval zebrafish stands to continue this trend, opening a new frontier to deepen understanding of functional neural development.
Highlights.
Vestibular circuits are vital to behavior, yet their development is poorly understood
Recent work demonstrates conservation of vertebrate vestibular principles in larval zebrafish
The experimental advantages of larval zebrafish permit analysis of vestibular development
Acknowledgments
M.W.B. is supported by NIH R00DC012536, R56DC016413, a Pew Scholar Award, McKnight Foundation Scholar Award, and Alfred P. Sloan Fellowship. D.S. is supported by National Institute on Deafness and Communication Disorders of the National Institutes of Health under award numbers R00DC012775, R56DC016316, and a Whitehead Fellowship. The authors wish to thank David Ehrlich for helpful comments on the manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Angelaki DE, Yakusheva TA, Green AM, Dickman JD, Blazquez PM. Computation of egomotion in the macaque cerebellar vermis. The Cerebellum. 2009;9:174–182. doi: 10.1007/s12311-009-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel B, Givan LA, Eisen JS. Delta-notch signaling and lateral inhibition in zebrafish spinal cord development. BMC Developmental Biology. 2001;1:13. doi: 10.1186/1471-213X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Bae YK, Kani S, Shimizu T, Tanabe K, Nojima H, Kimura Y, ichi Higashijima S, Hibi M. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Developmental Biology. 2009;330:406–426. doi: 10.1016/j.ydbio.2009.04.013. This work laid the foundation for study of cerebellum in zebrafish by defining the structures and transcription factors of cerebellar populations. [DOI] [PubMed] [Google Scholar]
- Baek M, Pivetta C, Liu JP, Arber S, Dasen JS. Columnar-intrinsic cues shape premotor input specificity in locomotor circuits. Cell Reports. 2017;21:867–877. doi: 10.1016/j.celrep.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Bagnall MW, McLean DL. Modular organization of axial microcircuits in zebrafish. Science. 2014;343:197–200. doi: 10.1126/science.1245629. This work demonstrated the motor output underlying rolling behaviors in larval fish, and revealed that the underlying spinal network is segregated into at least two largely distinct microcircuits for differential control of dorsal and ventral muscles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaldella E, Takeoka A, Sigrist M, Arber S. Multisensory signaling shapes vestibulo-motor circuit specificity. Cell. 2015;163:301–312. doi: 10.1016/j.cell.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Beck JC, Gilland E, Tank DW, Baker R. Quantifying the ontogeny of optokinetic and vestibuloocular behaviors in zebrafish, medaka, and goldfish. Journal of Neurophysiology. 2004;92:3546–3561. doi: 10.1152/jn.00311.2004. [DOI] [PubMed] [Google Scholar]
- •.Beraneck M, Lambert FM, Sadeghi SG. Development of Auditory and Vestibular Systems. Elsevier; 2014. Functional development of the vestibular system; pp. 449–487. An excellent review of vestibular development with focus on mammals. [Google Scholar]
- Bever MM, Fekete DM. Atlas of the developing inner ear in zebrafish. Developmental Dynamics. 2002;223:536–543. doi: 10.1002/dvdy.10062. [DOI] [PubMed] [Google Scholar]
- Bianco IH, Ma LH, Schoppik D, Robson DN, Orger MB, Beck JC, Li JM, Schier AF, Engert F, Baker R. The tangential nucleus controls a gravito-inertial vestibulo-ocular reflex. Current Biology. 2012;22:1285–1295. doi: 10.1016/j.cub.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle R, Goldberg J, Highstein S. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in squirrel monkey vestibular nuclei. iii. correlation with vestibulospinal and vestibuloocular output pathways. Journal of Neurophysiology. 1992;68:471fi484. doi: 10.1152/jn.1992.68.2.471. [DOI] [PubMed] [Google Scholar]
- ••.Branoner F, Straka H. Semicircular canal-dependent developmental tuning of translational vestibulo-ocular reflexes in Xenopus laevis. Developmental Neurobiology. 2014;75:1051–1067. doi: 10.1002/dneu.22234. This work shows compellingly that tuned input from the semicircular canals comes to refine the sensitivity of vestibular neurons that receive otolith inputs. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. The vestibulo-ocular reflex in newborn rats. Acta Oto-Laryngologica. 1979;87:484–489. doi: 10.3109/00016487909126456. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Beloozerova IN, Orlovsky GN. Nervous mechanisms controlling body posture. Physiology & Behavior. 2007;92:148–154. doi: 10.1016/j.physbeh.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Saint-Amant L, Buss RR, Chong M, McDearmid JR, Brustein E. Development of the locomotor network in zebrafish. Progress in Neurobiology. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Dyballa S, Savy T, Germann P, Mikula K, Remesikova M, Špir R, Zecca A, Peyriéras N, Pujades C. Distribution of neurosensory progenitor pools during inner ear morphogenesis unveiled by cell lineage reconstruction. eLife. 2017:6. doi: 10.7554/eLife.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Ehrlich DE, Schoppik D. Control of movement initiation underlies the development of balance. Current Biology. 2017;27:334–344. doi: 10.1016/j.cub.2016.12.003. The authors demonstrate that larval zebrafish learn to balance by preferentially initiating movements when unstable. They also define a mathematical framework for modeling the relationship between posture and locomotion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Favre-Bulle IA, Stilgoe AB, Rubinsztein-Dunlop H, Scott EK. Optical trapping of otoliths drives vestibular behaviours in larval zebrafish. Nature Communications. 2017:8. doi: 10.1038/s41467-017-00713-2. The authors present a novel technique for vestibular research in zebrafish: optical trapping of the otoliths to simulate movement in a head-fixed preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Kopecky BJ, Duncan JS. Development of Auditory and Vestibular Systems. Elsevier; 2014. Development of the mammalian ‘vestibular’ system; pp. 339–367. [Google Scholar]
- Fuiman LA, Webb PW. Ontogeny of routine swimming activity and performance in zebra danios (teleostei: Cyprinidae) Animal Behaviour. 1988;36:250–261. [Google Scholar]
- Glover JC. Development of second-order vestibular projections in the chicken embryo. Annals of the New York Academy of Sciences. 1996;781:13–20. doi: 10.1111/j.1749-6632.1996.tb15689.x. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Wilson VJ, Cullen KE, Angelaki DE, Broussard DM, Buttner-Ennever J, Fukushima K, Minor LB. The Vestibular System: A Sixth Sense. Oxford University Press; 2012. [Google Scholar]
- Goolish EM, Okutake K. Lack of gas bladder inflation by the larvae of zebrafish in the absence of an air-water interface. Journal of Fish Biology. 1999;55:1054–1063. [Google Scholar]
- Graf W, Spencer R, Baker H, Baker R. Excitatory and inhibitory vestibular pathways to the extraocular motor nuclei in goldfish. Journal of Neurophysiology. 1997;77:2765–2779. doi: 10.1152/jn.1997.77.5.2765. [DOI] [PubMed] [Google Scholar]
- Greaney MR, Privorotskiy AE, D’Elia KP, Schoppik D. Extraocular motoneuron pools develop along a dorsoventral axis in zebrafish, Danio rerio. Journal of Comparative Neurology. 2016;525:65–78. doi: 10.1002/cne.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamling KR, Tobias ZJ, Weissman TA. Mapping the development of cerebellar purkinje cells in zebrafish. Developmental Neurobiology. 2015;75:1174–1188. doi: 10.1002/dneu.22275. [DOI] [PubMed] [Google Scholar]
- Harmon TC, Magaram U, McLean DL, Raman IM. Distinct responses of purkinje neurons and roles of simple spikes during associative motor learning in larval zebrafish. eLife. 2017:6. doi: 10.7554/eLife.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiney SA, Kim J, Augustine GJ, Medina JF. Precise control of movement kinematics by optogenetic inhibition of purkinje cell activity. Journal of Neuroscience. 2014;34:2321–2330. doi: 10.1523/JNEUROSCI.4547-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Hsieh JY, Ulrich B, Issa FA, Wan J, Papazian DM. Rapid development of purkinje cell excitability, functional cerebellar circuit, and afferent sensory input to cerebellum in zebrafish. Frontiers in Neural Circuits. 2014:8. doi: 10.3389/fncir.2014.00147. This work demonstrates the onset of functionality of Purkinje cells in larval zebrafish including sensory responses, as early as 4 dpf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto Y, Kitama T, Yoshida K. Vertical eye movement-related secondary vestibular neurons ascending in medial longitudinal fasciculus in cat i. firing properties and projection pathways. Journal of Neurophysiology. 1990;63:902–917. doi: 10.1152/jn.1990.63.4.902. [DOI] [PubMed] [Google Scholar]
- ••.Jiang T, Kindt K, Wu DK. Transcription factor emx2 controls stereociliary bundle orientation of sensory hair cells. eLife. 2017:6. doi: 10.7554/eLife.23661. Mechanosensory hair cells in the otolith and elsewhere exhibit a variety of orientations. The authors demonstrate that a single transcription factor can govern which hair cells invert their orientation relative to default. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Voicu H, Ohyama T, Mauk MD. A subtraction mechanism of temporal coding in cerebellar cortex. Journal of Neuroscience. 2011;31:2025–2034. doi: 10.1523/JNEUROSCI.4212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Kasumacic N, Lambert FM, Coulon P, Bras H, Vinay L, Perreault MC, Glover JC. Segmental organization of vestibulospinal inputs to spinal interneurons mediating crossed activation of thoracolumbar motoneurons in the neonatal mouse. Journal of Neuroscience. 2015;35:8158–8169. doi: 10.1523/JNEUROSCI.5188-14.2015. Implementing a new imaging-based approach for circuit evaluation in rodent spinal cord, the authors show vestibulospinal input to commissural neurons important for hindlimb control of posture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y. alx, a zebrafish homolog of chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. Journal of Neuroscience. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knogler LD, Ryan J, Saint-Amant L, Drapeau P. A hybrid electrical/chemical circuit in the spinal cord generates a transient embryonic motor behavior. Journal of Neuroscience. 2014;34:9644–9655. doi: 10.1523/JNEUROSCI.1225-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knogler LD, Markov DA, Dragomir EI, Stih V, Portugues R. Sensorimotor representations in cerebellar granule cells in larval zebrafish are dense, spatially organized, and non-temporally patterned. Current Biology. 2017;27:1288–1302. doi: 10.1016/j.cub.2017.03.029. [DOI] [PubMed] [Google Scholar]
- Kohashi T, Nakata N, Oda Y. Effective sensory modality activating an escape triggering neuron switches during early development in zebrafish. Journal of Neuroscience. 2012;32:5810–5820. doi: 10.1523/JNEUROSCI.6169-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, Poeppel D. Neuroscience needs behavior: Correcting a reductionist bias. Neuron. 2017;93:480–490. doi: 10.1016/j.neuron.2016.12.041. [DOI] [PubMed] [Google Scholar]
- ••.Lambert FM, Beck JC, Baker R, Straka H. Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval xenopus. Journal of Neuroscience. 2008;28:8086–8095. doi: 10.1523/JNEUROSCI.1288-08.2008. This work demonstrated the minimal sizes of semicircular canals at which they become functional, including evidence from multiple species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey BW, Smith FM, Croll RP. From inflation to flotation: Contribution of the swimbladder to whole-body density and swimming depth during development of the zebrafish (danio rerio) Zebrafish. 2010;7:85–96. doi: 10.1089/zeb.2009.0616. [DOI] [PubMed] [Google Scholar]
- McHenry MJ. The mechanical scaling of coasting in zebrafish (danio rerio) Journal of Experimental Biology. 2005;208:2289–2301. doi: 10.1242/jeb.01642. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Spinal interneurons differentiate sequentially from those driving the fastest swimming movements in larval zebrafish to those driving the slowest ones. Journal of Neuroscience. 2009;29:13566–13577. doi: 10.1523/JNEUROSCI.3277-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Croce K, Belton T, Akay T, Jessell TM. Balance control mediated by vestibular circuits directing limb extension or antagonist muscle co-activation. Cell Reports. 2018;22:1325–1338. doi: 10.1016/j.celrep.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Nicolson T, Rüsch A, Friedrich RW, Granato M, Ruppersberg JP, Nüsslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron. 1998;20:271–283. doi: 10.1016/s0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- Peusner K. Development of the gravity sensing system. Journal of Neuroscience Research. 2001;63:103–108. doi: 10.1002/1097-4547(20010115)63:2<103::AID-JNR1001>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- •.Riley BB, Moorman SJ. Development of utricular otoliths, but not saccular otoliths, is necessary for vestibular function and survival in zebrafish. Journal of Neurobiology. 2000;43:329–337. doi: 10.1002/1097-4695(20000615)43:4<329::aid-neu2>3.0.co;2-h. The authors demonstrate that in larval zebrafish, the utricle but not the saccule is required for vestibular function. [DOI] [PubMed] [Google Scholar]
- Roberts R, Elsner J, Bagnall MW. Delayed otolith development does not impair vestibular circuit formation in zebrafish. Journal of the Association for Research in Otolaryngology. 2017;18:415–425. doi: 10.1007/s10162-017-0617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron. 2001;31:1035–1046. doi: 10.1016/s0896-6273(01)00416-0. [DOI] [PubMed] [Google Scholar]
- ••.Schoppik D, Bianco IH, Prober DA, Douglass AD, Robson DN, Li JM, Greenwood JS, Soucy E, Engert F, Schier AF. Gaze-stabilizing central vestibular neurons project asymmetrically to extraocular motoneuron pools. The Journal of Neuroscience. 2017;37:11353–11365. doi: 10.1523/JNEUROSCI.1711-17.2017. The authors revealed that an embedded circuit asymmetry in the vestibulo-ocular reflex facilitates downward eye movements, plausibly in service of the preponderance of pitch-up movements made by larval zebrafish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Sengupta M, Thirumalai V. AMPA receptor mediated synaptic excitation drives state-dependent bursting in purkinje neurons of zebrafish larvae. eLife. 2015:4. doi: 10.7554/eLife.09158. The authors demonstrate that larval Purkinje cells are active as early as 4 dpf and exhibit bistable up and down states, governed by synaptic input, in unanesthetized animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severi KE, Portugues R, Marques JC, O’Malley DM, Orger MB, Engert F. Neural control and modulation of swimming speed in the larval zebrafish. Neuron. 2014;83:692–707. doi: 10.1016/j.neuron.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka H, Baker R. Vestibular blueprint in early vertebrates. Frontiers in Neural Circuits. 2013:7. doi: 10.3389/fncir.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka H, Fritzsch B, Glover JC. Connecting ears to eye muscles: Evolution of a ‘simple’ reflex arc. Brain, Behavior and Evolution. 2014;83:162–175. doi: 10.1159/000357833. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. The Oculomotor System. Hoeber Medical Division, Harper and Row; 1964. Pathways and synaptic articulation patterns connecting vestibular receptors and oculomotor nuclei; pp. 205–223. [Google Scholar]
- Tanimoto M, Ota Y, Inoue M, Oda Y. Origin of inner ear hair cells: Morphological and functional differentiation from ciliary cells into hair cells in zebrafish inner ear. Journal of Neuroscience. 2011;31:3784–3794. doi: 10.1523/JNEUROSCI.5554-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TR, Donovan JC, Baier H. Descending control of swim posture by a midbrain nucleus in zebrafish. Neuron. 2014;83:679–691. doi: 10.1016/j.neuron.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, McDearmid JR. Pacemaker and plateau potentials shape output of a developing locomotor network. Current Biology. 2012;22:2285–2293. doi: 10.1016/j.cub.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytell E, Holmes P, Cohen A. Spikes alone do not behavior make: why neuroscience needs biomechanics. Current Opinion in Neurobiology. 2011;21:816–822. doi: 10.1016/j.conb.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanwalleghem GC, Ahrens MB, Scott EK. Integrative whole-brain neuroscience in larval zebrafish. Current Opinion in Neurobiology. 2018;50:136–145. doi: 10.1016/j.conb.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Voesenek CJ, Muijres FT, van Leeuwen JL. Biomechanics of swimming in developing larval fish. The Journal of Experimental Biology. 2018;221:jeb149583. doi: 10.1242/jeb.149583. [DOI] [PubMed] [Google Scholar]
- Wang WC, McLean DL. Selective responses to tonic descending commands by temporal summation in a spinal motor pool. Neuron. 2014;83:708–721. doi: 10.1016/j.neuron.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb PW, Weihs D. Stability versus maneuvering: Challenges for stability during swimming by fishes. Integrative and Comparative Biology. 2015;55:753–764. doi: 10.1093/icb/icv053. [DOI] [PubMed] [Google Scholar]
- Whitfield T, Granato M, van Eeden F, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg C, Jiang Y, Kane D, Kelsh R, Mullins M, Odenthal J, Nusslein-Volhard C. Mutations affecting development of the zebrafish inner ear and lateral line. Development. 1996;123:241–254. doi: 10.1242/dev.123.1.241. [DOI] [PubMed] [Google Scholar]
- Zecca A, Dyballa S, Voltes A, Bradley R, Pujades C. The order and place of neuronal differentiation establish the topography of sensory projections and the entry points within the hindbrain. Journal of Neuroscience. 2015;35:7475–7486. doi: 10.1523/JNEUROSCI.3743-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]