To the Editor:
It is well recognized that chronic rhinosinusitis with nasal polyps (CRSwNP) is characterized by type 2 inflammation with eosinophilia and high levels of IL-5 and IL-13 in Western countries.1 In addition, several studies suggest that the inflammatory endotypes of CRSwNP in Asian countries are shifting towards type 2 inflammation from non-eosinophilic inflammation and that 50% or more of nasal polyps (NPs) currently show tissue eosinophilia in these countries.1 However, the mechanisms of induction of type 2 inflammation in NPs are still largely unclear. Recent evidence suggests that the epithelial-derived cytokines IL-25, IL-33 and thymic stromal lymphopoietin (TSLP) may be important initiators of innate type 2 immunity and amplifiers of adaptive type 2 inflammatory responses in many type 2 inflammatory diseases.1 In the case of CRS, recent studies, including our own, found that TSLP is upregulated in NPs (Table S1).1,2 However, our initial studies suggested that IL-25 and IL-33 were not elevated in NP tissues compared to control sinus tissues.3,4 Although we concluded that TSLP may significantly contribute to type 2 inflammation in NPs, the role of IL-25 and IL-33 remains uncertain. In contrast to our study, other recent studies have shown that IL-25 is significantly elevated in NPs (Table S2). In the case of IL-33, published studies showed mixed results in NP tissue (Table S3). Therefore the importance of IL-25 and IL-33 in CRSwNP is still largely unclear.
To revisit the validity of our earlier studies of IL-25 and IL-33, we collected control ethmoid sinus tissues and NPs from patients with aspirin-tolerant CRSwNP and Aspirin-exacerbated respiratory disease (AERD, which has a triad of symptoms; CRSwNP, asthma and aspirin (or other NSAID) sensitivity) from a new cohort at Northwestern (Table S4 and S5) and carefully determined the presence of IL-25 and IL-33 by four different methods; real-time RT-PCR, Luminex, western blot and immunohistochemistry. Detailed methods are given in the Supporting Information. First, we analyzed the expression of IL-25. To ensure the validity of our results, we purchased primer/probe sets for IL-25 from two different companies and performed real-time RT-PCR. We found that the levels of mRNA for IL-25 were near or below the detection limit of PCR and there was no significant difference between controls and NPs (Figure 1AB). Since IL-25 is known to be expressed in the epithelial cells and the amount of epithelium might be much diluted in RNA from tissue biopsy samples, we also performed real-time RT-PCR on epithelial scrapings. However, IL-25 mRNA was still almost undetectable and there was no significant difference between control sinus tissues and NPs (Figure S1). In contrast, we observed that total TSLP mRNA (both short and long variants) was significantly higher in NP tissue than control tissue (Figure 1C), which confirmed our previous study.2 We also found that mRNA for a long variant of TSLP which has cytokine activity was also significantly elevated in NPs (Figure 1D). Levels of TSLP mRNA was similar between NPs from CRSwNP and AERD (Figure 1CD). We next analyzed IL-25 protein in tissue homogenates by Luminex from 26 controls and 37 NPs and found that IL-25 was below the detection limit in all samples (not shown).
Figure 1. IL-25 was not elevated in NPs.
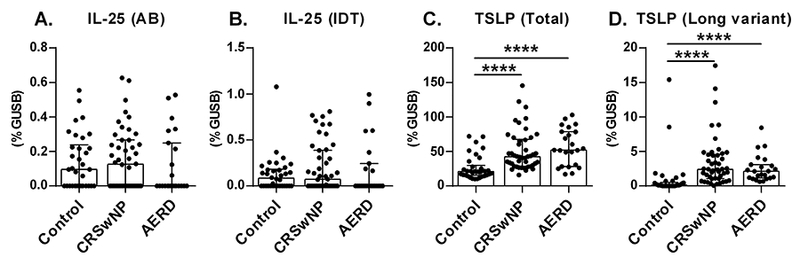
Total RNA was extracted from whole control ethmoid sinus tissue (Control; n = 34) and NP tissue from patients with CRSwNP (n = 46) and AERD (n = 23). We obtained primer/probe sets for IL-25 from two different companies, Applied Biosystems (AB) and Integrated DNA Technologies (IDT), and expression of mRNAs for IL-25 and TSLP (total and a long variant) was analyzed by real-time RT-PCR. Gene expression was normalized to a housekeeping gene, β-glucuronidase (GUSB), and expression levels were shown as % expression of GUSB. Results are shown as median with interquartile range. ****P < 0.0001 by the 1-way ANOVA.
Although several groups recently published that IL-25 is highly upregulated in NP tissue, our data clearly showed that the level of IL-25 in our cohort at Chicago, Illinois was very low and was not elevated in NPs (Figure 1). To identify potential reasons for this discrepancy, we searched for IL-25 related manuscripts. We found 11 manuscripts of which 8 showed that IL-25 was elevated in NPs (Table S2). Interestingly, most of the positive results came from Asian countries including Korea, China and Japan, except for one manuscript from Australia. In contrast, the three published negative results for IL-25 were studies from the USA, Australia and Turkey. This suggests that the mechanisms of type 2 inflammation may be different between Asian and Western countries, and that IL-25 may only be involved in Asian NPs. Future studies will be required to determine whether genetic and/or environmental factors elevate expression of IL-25 in NPs in Asian countries.
We next evaluated the presence of IL-33. IL-33 is a nuclear protein that is released from the nucleus as a cytokine during cell death and by other unknown mechanisms. Among the splice variants of IL-33 identified in airway epithelium by Gordon et al., two variants, that had deletions of exons 3 and 4 (IL-33∆exon3,4) or IL-33∆exon3,4 with partial deletion of exon 5 (IL-33∆exon3,4s5), were able to move into the cytosol and be secreted as active cytokines without cell death.6 Interestingly, they also found that the expression of IL-33∆exon3,4 was strongly associated with airway type 2 inflammation, although full-length IL-33 was not.6 We therefore tested samples for the presence of mRNAs of full-length IL-33, IL-33∆exon3,4 and IL-33∆exon3,4s5 by RNase H-dependent quantitative RT-PCR using the published protocol.6 We found that mRNAs for full-length IL-33, IL-33∆exon3,4 and IL-33∆exon3,4s5 were present but there were no differences between controls and NPs (Figure 2A-C). We next evaluated the presence of IL-33 protein in tissue extracts by Luminex and again found that IL-33 protein was present but not elevated in NP tissues, even in NPs from AERD patients (Figure 2D). It has been established that IL-33 is cleaved by proteases and cleaved IL-33 shows higher activity than pro-IL-33.5 However, it is not clear whether Luminex and commercial ELISA kits are able to detect both cleaved and splice variants of IL-33 proteins, and this may lead to inconsistent results. To determine the presence of pro (full-length), cleaved and splice variants of IL-33 proteins in tissue, we performed western blots of IL-33 with three commonly used antibodies. However, we still could not detect major differences between control sinus tissues and NP tissues (Figure 2E). We also quantified the IL-33 bands by densitometry and found that there was no significant difference between controls (n=12) and NPs (n=11) (not shown). Finally, we evaluated the presence of IL-33 protein by immunohistochemistry and found that IL-33 was localized mainly in the nuclei of basal epithelial cells and endothelial cells but there was no significant difference between controls (n=10) and NPs (n=10) (Figure 2F and not shown).
Figure 2. IL-33 was not elevated in NPs.
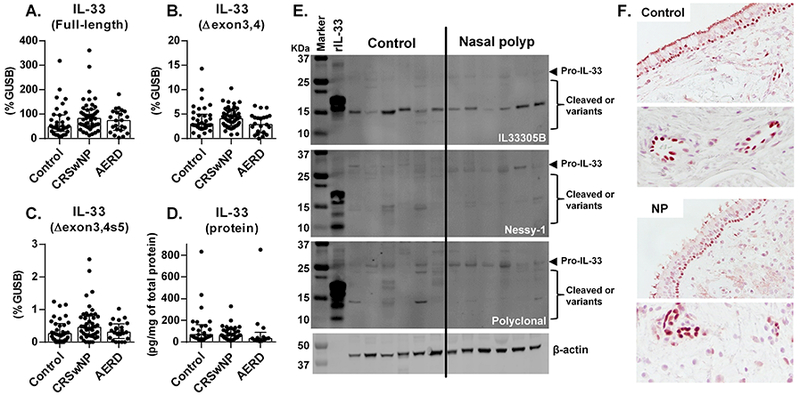
Expression of mRNAs for full-length IL-33 (A), IL-33∆exon3,4 (B) and IL-33∆exon3,4s5 (C) was determined by RNase H-dependent quantitative RT-PCR in control ethmoid sinus tissue (Control; n = 34) and NPs from CRSwNP (n = 46) and AERD (n = 23). IL-33 protein in tissue extracts was determined by Luminex (D, control (n = 26), CRSwNP NP (n = 37) and AERD NP (n = 13)) and Western blot (E). IL-33 protein concentration was normalized to the concentration of total protein (D). The results are representative of 2 separate experiments with separate donors (control (n = 12) and NP (n = 11)) (E). Representative immunostaining for IL-33 is shown in a control ethmoid tissue and an NP tissue (F). Magnification; ×400 (upper) and ×1200 (lower). Results are shown as median with interquartile range.
Thus, in contrast to IL-25, we detected mRNA and protein for IL-33 but IL-33 was not elevated in NPs in our cohort (Figure 2).4 Although 19 manuscripts have evaluated the presence of IL-33 in NPs, these studies have reported mixed results. Eleven showed elevation of IL-33 in NPs and 8 showed negative results (Table S3). Interestingly, unlike IL-25, the study locations did not clearly correlate with the findings (Table S3). In the case of the United States, Liu et al. reported that IL-33 was elevated in NPs from patients with AERD compared to NPs from aspirin-tolerant CRSwNP patients.7 However, we could not find a difference in IL-33 between AERD NP and non-AERD NP (Figure 2) which confirmed our previous finding.4 Although possible, we think that it is unlikely that the presence and degree of transcriptional regulators of IL-33 in NPs differ between Chicago, Illinois and Boston, Massachusetts, leaving this disparity unexplained. Shaw et al. found that IL-33 was not elevated in NP tissue but reported that epithelial cells from patients with CRSwNP expressed more IL-33 than cells from patients with non-polypoid CRS after stimulation with Aspergillus fumigatus.8 Colonization by Staphylococcus aureus is known to occur in some cases of CRSwNP and the presence of Staphylococcus aureus enterotoxin B–specific IgE is associated with severe type 2 inflammation in NPs.1,9 Staphylococcus aureus-derived products are known to induce IL-33 in mice.9 However, the presence of Aspergillus fumigatus and Staphylococcus aureus enterotoxin B–specific IgE in NPs in our cohort in Chicago was rare (not shown). These results suggest that induction of IL-33 in NPs might be strongly controlled by geographically variable environmental factors, including infectious conditions, and IL-33 might not be consistently elevated in NP around the world.
Since several patients received corticosteroids before surgery which may affect the expression of IL-25 and IL-33, and may induce discrepancies in the study, we evaluated the effect of corticosteroid. However, we found no significant difference in levels of IL-25 and IL-33 in NPs based on the status of steroid treatment (Figure S2 and not shown).
In conclusion, we report that IL-25 is almost absent, and IL-33 is present but not elevated in NP tissue in our US-based population. We have confirmed earlier reports by our group and others that TSLP is elevated in CRSwNP. We suggest that these findings primarily implicate TSLP as the major epithelial derived cytokine that skews adaptive immune responses toward type 2 in CRSwNP. Although IL-25 and IL-33 have been considered to be therapeutic targets for treatment of CRSwNP, this strategy may not always fit in all international regions and may require careful investigation in geographic area before starting clinical trials.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported in part by NIH grants, R01 AI104733, R37 HL068546 and U19 AI106683 and by a grant from the Ernest S. Bazley Foundation.
We would like to gratefully acknowledge Ms. Lydia Suh, Mr. James Norton, Mr. Roderick Carter, Ms. Caroline P.E. Price Ms. Julia H. Huang and Ms. Kathleen E. Harris (Northwestern University Feinberg School of Medicine) for their skillful technical assistance.
Funding: This research was supported in part by NIH grants, R01 AI104733, R37HL068546 and U19 AI106683 and by a grant from the Ernest S. Bazley Foundation.
Abbreviations
- AERD
Aspirin-exacerbated respiratory disease
- CRS
Chronic rhinosinusitis
- CRSwNP
CRS with nasal polyps
- ILC
Innate lymphoid cell
- ILC2
Group 2 innate lymphoid cell
- NP
Nasal polyp
- TSLP
Thymic stromal lymphopoietin
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest as to the interpretation and presentation of this manuscript
AUTHOR CONTRIBUTIONS
AK designed the study. NO, JAP, AIK and AK performed the experiments. NO and AK analyzed the data. BKT, KEH, WWS, ATP, LCG, KCW, SSS, DBC, RCK and RPS helped in sample collection and evaluation. AK and NO wrote the manuscript. All authors have read and approved the final form of the manuscript.
REFERENCES
- 1.Kato A Immunopathology of chronic rhinosinusitis. Allergology international : official journal of the Japanese Society of Allergology. 2015;64(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. The Journal of allergy and clinical immunology. 2013;132(3):593–600 e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters AT, Kato A, Zhang N, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. The Journal of allergy and clinical immunology. 2010;125(2):397–403 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. American journal of respiratory and critical care medicine. 2015;192(6):682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefrancais E, Duval A, Mirey E, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(43):15502–15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon ED, Simpson LJ, Rios CL, et al. Alternative splicing of interleukin-33 and type 2 inflammation in asthma. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(31):8765–8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Kanaoka Y, Barrett NA, et al. Aspirin-Exacerbated Respiratory Disease Involves a Cysteinyl Leukotriene-Driven IL-33-Mediated Mast Cell Activation Pathway. J Immunol. 2015;195(8):3537–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw JL, Fakhri S, Citardi MJ, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. American journal of respiratory and critical care medicine. 2013;188(4):432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teufelberger AR, Nordengrun M, Braun H, et al. The IL-33/ST2 axis is crucial in type 2 airway responses induced by Staphylococcus aureus-derived serine protease-like protein D. The Journal of allergy and clinical immunology. 2018;141(2):549–559 e547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


