Abstract
Thrombosis is the most common underlying pathology responsible for morbidity and mortality in cardiovascular disease (CVD). Platelet adhesion, activation, and aggregation play central roles in hemostasis; however, the same process may also cause thrombosis and vessel occlusion at the site of ruptured atherosclerotic lesions leading to heart attack and stroke. ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) are an essential component of the platelet phospholipid membrane and play a major role in many aspects of platelet function. Dietary supplementation of ω-3 and ω-6 PUFAs has long been used to slow the progression of CVD and to prevent acute cardiovascular events. Despite this, the role of ω-3 and ω-6 PUFAs and their oxylipin metabolites in platelet function remains controversial due to the lack in our understanding of the mechanistic regulation controlling platelet reactivity in vitro and substantial evidence for PUFA regulation of thrombotic events in vivo. In this review, we will outline the role of platelet physiology in hemostasis and the effect of ω-3 and ω-6 PUFAs on platelet function, with special emphasis on in vivo effects on hemostasis and thrombosis due to the role of PUFAs and their bioactive lipids in circulation. Further, recent mechanistic insights and evidence for cardio-protective effects of PUFAs and their bioactive lipids will be discussed.
Keywords: ω-3 PUFAs, ω-6 PUFAs, platelet function, hemostasis, thrombosis
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death globally. Thrombosis is the most common underlying pathology of ischemic heart disease, ischemic stroke, and venous thromboembolism (VTE), the three major cardiovascular disorders. Platelet adhesion and aggregation at the site of vascular injury are key events required for formation of a platelet plug and the arrest of bleeding (hemostasis). However, this process may also have pathological consequences (i.e., thrombosis). Antiplatelet therapy has been widely used in the prevention and management of occlusive thrombosis in atherosclerotic blood vessels, the main cause of ischemic events.[1–4]
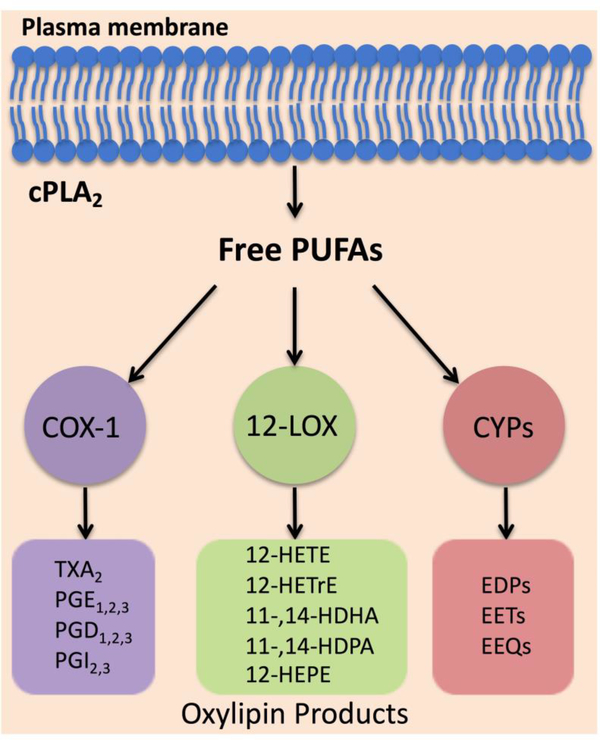
Successful development and wide use of anti-platelet drugs have substantially decreased mortality and morbidity of cardiovascular disease.[5] Aspirin, a cyclooxygenase-1 (COX-1) inhibitor, has been shown to reduce the risk of major cardiovascular events by approximately 25% in primary prevention and to reduce morbidity and mortality rates by up to 50% in patients with acute coronary syndrome, suggesting that oxylipins, the bioactive metabolites of polyunsaturated fatty acids (PUFAs), play an important role in regulation of platelet activation.[2] Upon cellular activation, PUFAs are released from the embedded phospholipid bilayer membrane and are oxygenated by three families of enzymes COX, lipoxygenase (LOX), and cytochrome P450 (CYP) into distinct classes of oxylipins (Figure 1).[6] Oxylipins are potent bioactive lipid mediators but with short half-lives, therefore they are not stored but rather are synthesized de novo from PUFAs by oxygenases in tightly regulated manner.[6] Upon cellular activation, cPLA2 hydrolyzes PUFAs from the lipid membrane generating free PUFAs. Oxylipins can diffuse through the plasma membrane and bind GPCRs in the local environment. Additionally, select oxylipins can activate the transcription factor PPAR.[6] Early studies showed that exogenously added oxylipins could become esterified into membrane phospholipids of cells, but it was uncovered more recently that such esterified oxylipins were formed endogenously by multiple types of cells, including platelets.[7] Despite the proven life-saving clinical benefits of inhibiting platelets by aspirin and other antiplatelet agents, these therapies are associated with an increased risk of bleeding.[8] Thus, the burden of thrombotic complications of CVD remains high.
Figure 1. Biosynthesis of oxylipins.
Upon cellular activation, cPLA2 hydrolyzes polyunsaturated fatty acids (PUFAs) from the lipid membrane generating free PUFAs. Oxygenases (COX, LOX, and CYP) metabolize free PUFAs into distinct oxylipins.
While the health benefits of ω-3 and ω-6 PUFAs have been the focus of many reviews, in the present review we will discuss the effects of the main ω-3 and ω-6 PUFAs and their respective oxylipin metabolites on platelet function and the regulation of thrombosis and hemostasis in vivo.
2. Role of platelets in hemostasis and thrombosis
Platelets are small anucleated cells (1–2 microns in diameter) circulating in the blood, first discovered in 1874 by Osler.[9] Derived from megakaryocytes in bone marrow, platelets circulate on average 7 to 10 days after they enter into blood circulation, maintain a concentration of 150 – 400 × 109/L in healthy humans and are the second most prevalent blood cell after red blood cells.[10] Platelets have long been recognized for their critical role in hemostasis, an important physiological process to aid in the cessation of bleeding.[11, 12] Growing evidence from studies on platelet function over the past few decades has shown that the importance of platelet function goes far beyond their primary role in hemostasis.[10]
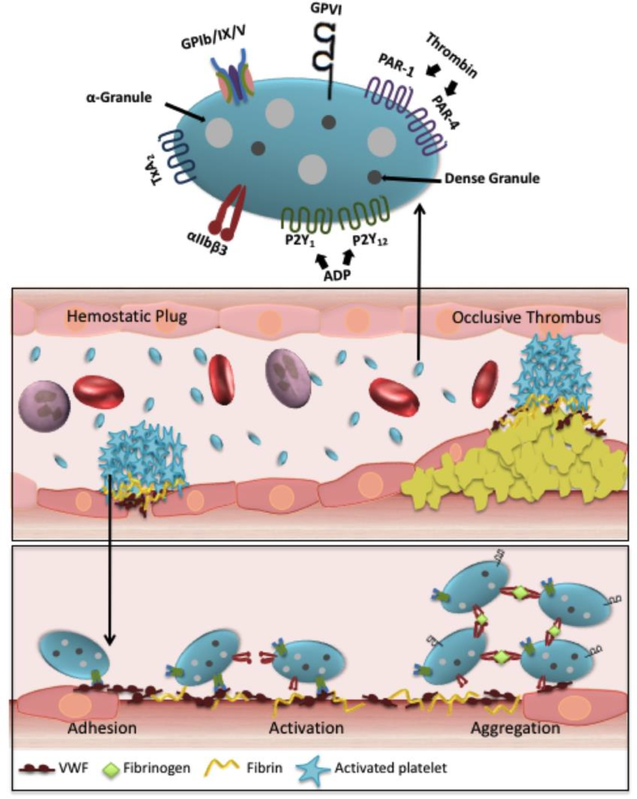
Platelets are actively involved in a number of physiologic and pathologic processes, such as inflammation[13], anti-microbial host defense[14], aspects of the immune response[15], tumor growth and metastasis[16, 17], angiogenesis[18], lymphatic vessel development[19], and atherosclerosis.[20] Therefore, platelets are emerging as a hot research topic in many different physiologic and pathologic processes. Under normal circumstances, circulating platelets are in a resting state resembling a discoid shape in the bloodstream but always ready to safeguard the vascular integrity by responding to any disruption of the vascular endothelial lining. In the event of vascular injury, endothelial cells in vessel walls are disrupted and expose subendothelial matrix proteins. This process initiates platelet adhesion and subsequent platelet activation and aggregation at the site of vascular injury, which is a key event required for the formation of the platelet plug (referred to as primary hemostasis) to stop bleeding (Figure 2).[21] Activated platelets also provide procoagulant cell surface membranes to activate the coagulation cascade, a series of enzymatic reactions to generate thrombin ultimately leading to the formation of a fibrin clot (referred to as secondary hemostasis) to seal the vessel blood leakage.[22, 23] Thus, platelets not only play a well-established role in primary hemostasis (platelet adhesion, activation, and aggregation); they also actively contribute to the generation of thrombin, the most potent platelet agonist known to amplify the process of secondary hemostasis.[23]
Figure 2. Role of platelets in hemostasis and thrombosis.
After vascular injury, subendothelial proteins (primarily collagen and VWF) become exposed, initiating platelets in circulation to tether and adhere to the site of vascular injury. The process of platelet adhesion is mediated by the binding of platelet surface GPIb-IX-V receptors to immobilized VWF on collagen on the injured vessel wall. This results in the activation phase in which αIIbβ3 undergoes a conformation change to its active form and the release of the soluble agonists (ADP and TxA2) from platelet dense and α-granules. Platelet aggregation is mediated by αIIbβ3 binding to fibrinogen and/or other ligands in plasma. Simultaneously with platelet aggregation, coagulation is initiated to ensure stable hemostatic clot formation. The process of platelet adhesion and aggregation also leads to the formation of occlusive thrombi which results in vessel occlusion at the site of ruptured plaque in pathological conditions.
The process of platelet interaction with the vessel wall and subendothelial matrix is accomplished through the involvement of many platelet receptors and their corresponding ligands (Figure 2).[24] Major platelet surface receptors include the GPIb-IX-V complex, GPVI, integrins αIIbβ3 (also known as GPIIbIIIa), α2β1, α5β1, α6β1, thrombin receptors PAR1 and PAR4 (protease activated receptors), ADP receptors (P2Y1, P2Y12 and P2X1) and thromboxane A2 receptors (TP).[24] Platelet adhesion: Platelet-vessel wall interaction, the tethering and adhesion of platelets to the injured site, is primarily mediated by platelet surface GPIbα receptor in GPIb-IX-V complex which binds to the A1 domain of von Willebrand factor (VWF) immobilized on collagen upon vascular injury, particularly at high shear stress.[25] Subsequent stable platelet adhesion is further mediated by other integrin ligands with their corresponding ligands such as integrin αIIbβ3 bound to fibronectin and fibrinogen/fibrin, α5β1 to fibronectin or collagen, and α2β1 to collagen.[26] Platelet activation: Following initial tethering and adhesion of circulating resting platelets at the site of vascular injury, platelets rapidly undergo a well-defined signaling cascade (Ca2+ influx, degranulation, phosphatidylserine exposure, etc.) that leads to platelet shape change facilitating further platelet activation and platelet granule release.[27] Platelet α-granule (P-selectin[28], VWF[29], fibrinogen (Fg)[28], fibronectin[30], vitronectin[31], multimerin[32], platelet factor 4[33], and many other proteins) and dense granule (adenosine di-phosphate (ADP), polyphosphates and other) contents are secreted and, in turn, amplify the activation process of integrin αIIbβ3 inside-out signaling, leading to further platelet aggregation.[34] GPIba-VWF interaction is also shown to play an important role in platelet adhesion at low shear conditions.[35] Platelet aggregation: Activation of platelets is a critical step for aggregation. Platelet activation results in a conformational change in the αIIbβ3 receptor on the platelet surface making it available to bind to its major ligand fibrinogen or other alternative ligand(s).[24] This process allows for cross-linking to adjacent activated platelets resulting in platelet aggregation and hemostatic plug formation. Despite the dogma of VWF and Fg interactions thought to be required for platelet adhesion and aggregation for decades, platelet aggregation and thrombus formation is still successful in mice lacking von Willebrand factor and fibrinogen, even after depletion of plasma fibronectin.[30] This observation indicates that other known or unknown ligands of αIIbβ3 could mediate platelet aggregation independent of VWF and Fg.[30] Interestingly, αIIbβ3 is indispensable for the platelet aggregation process as no thrombus formation is detected in mice lacking αIIbβ3 under intravital microscopy.
After the initial platelet plug formation, the second mechanism required to stop bleeding is the coagulation cascade via extrinsic or intrinsic pathways. Activation of the coagulation system leads to the generation of thrombin, which converts fibrinogen to fibrin.[23] There are many interactions between these two mechanisms, the initial platelet adhesion and aggregation and coagulation system that lead to clotting. For example, activated platelets accelerate coagulation by providing a negatively charged phosphatidylserine-rich membrane surface that enhances cell-based thrombin generation. Conversely, thrombin generation leads to further platelet activation and is crucial for platelet adhesion and aggregation and polymerized fibrin. Fibrin operates to stabilize the platelet plug or hemostatic clot (physiologic).
Although these are important steps for hemostasis, the same processes can also lead to the development of arterial thrombosis and vessel occlusion when the integrity of the vessel wall is compromised by rupture of an atherosclerotic plaque (Figure 2).[36] Excessive platelet activation, aggregation, and blood coagulation may lead to the formation of occlusive thrombi resulting in severe consequences such as myocardial infarction, ischemic stroke, and pulmonary embolism, which are the predominant causes of morbidity and mortality worldwide. Intravascular thrombosis is the cause of myocardial infarction (MI) and stroke as well as venous thrombosis (VT), which often leads to venous thromboembolism (VTE) and pulmonary embolism (PE).[37] Structurally, arterial and venous thrombi are distinct. Arterial thrombi are platelet-rich thrombi forming at the side of atherosclerotic plaques. Unstable angina and myocardial infarction and ischemic stroke are typically the result of excessive platelet adhesion, aggregation, and vessel occlusion formed at ruptured atherosclerotic lesions under high shear flow rates (arterial: 300–800 s−1 and stenotic vessels 800–10,000 s−1) in coronary and cerebral arteries.[36] Platelets also contribute to the orchestration of venous thrombi consisting mainly of fibrin and red blood cells under low shear rate (20–200 s−1).[35] In venous thrombosis, endothelial activation causes adhesion of platelets and leukocytes. In turn, adhered leukocytes become activated and initiate expression of tissue factor and lead to the activation of the coagulation cascade. Recent studies also indicate the potential role of other factors such as platelets-neutrophils aggregates and neutrophil extracellular trap (NET) in the pathogeneses and development of both arterial and venous thrombosis.[37]
3. Anti-platelet therapy in cardiovascular disease
Platelet activation and aggregation play a central role in arterial thrombus formation, which results in acute thrombotic events, such as myocardial infarction and ischemic stroke. Antiplatelet drugs are the frontline treatment of cardiovascular diseases for both the prevention and treatment of thrombotic events. Current antiplatelet therapies inhibit platelet function by targeting platelet enzymes (phosphodiesterase, cyclooxygenase), receptors (purinergic, prostaglandins, protease-activated receptors, thromboxane), and glycoproteins (αIIbβ3, GPVI, VWF, GPIb).[38, 39] Aspirin is by far the most widely used antiplatelet therapy and operates by irreversibly inhibiting platelet COX-1 to block platelet thromboxane A2 (TXA2); however, it does not prevent platelet activation occurring via various signaling pathways that are independent of TXA2. Therefore, a number of other antiplatelet reagents have been developed to overcome the limitations of aspirin.
More recently, 12-lipoxygenase (12-LOX), an oxygenase predominantly expressed in human platelets, is emerging as a potential anti-platelet target.[40] Earlier studies showed 12-LOX utilizes arachidonic acid (AA) released from the phospholipids as a substrate to form bioactive metabolites 12-(S)-hydroperoxyeicosatetraenoic acid (12-HPETE) and 12-Hydroxyeicosatetraenoic acid (12-HETE) which regulate a number of biological processes such as integrin activation, vascular hypertension, and progression of certain types of cancer.[41, 42] Metabolic products of 12-LOX formed during platelet activation have been shown to play a role in platelet activation, thrombin generation, and granule secretion in vitro and ex vivo suggesting a role of 12-LOX in regulating platelet function, as well as hemostasis and thrombus formation in vivo.[43] Furthermore, using a newly developed highly selective platelet 12-LOX inhibitor, ML355, we demonstrated 12-LOX plays an important role in the regulation of human platelet function in vitro and ex vivo flow conditions.[44, 45] Orally administered 12-LOX inhibitor in mice showed dose-dependent inhibition of thrombus formation while minimally impairing hemostasis in preclinical animal models of thrombosis and hemostasis.[46] Thus, pharmacologically targeting 12-LOX may be a viable anti-platelet approach although future clinical studies are needed to validate this hypothesis.
Other potent novel antithrombotic agents, such as αIIbβ3 inhibitors, have been developed and clinically proven to effectively inhibit platelet aggregation and thrombus formation. However, studies have shown that aggressive inhibition of platelet function by platelet αIIbβ3 inhibitors is associated with increased risk of bleeding and only modestly improved mortality.[47] Despite the effectiveness of current antiplatelet therapies, the irreversibility and continued use of these agents is associated with severe bleeding complications.[48] Therefore, an adequate level of suppression of platelet function is critical to maintaining the balance between hemostasis and pathologic thrombosis to prevent adverse events.
Another potential therapeutic treatment to influence platelet function is PUFAs. Fatty acids are hydrocarbon chains with a carboxyl group at one end and a methyl group at the other. The two most common families of PUFAs are classified as omega-3 (ω-3) and omega-6 (ω-6) based on the location of the last double bond relative to the terminal methyl end of the molecule. Dietary supplementation with ω-3 and ω-6 PUFAs has long been used to slow the progression of CVD and to prevent acute cardiovascular events. Furthermore, alteration of the ratio of ω-3 and ω-6 in diets is associated with the pathogenesis of cardiovascular disease and its complications.[49, 50] While the cardioprotective benefits of ω-3 and ω-6 PUFAs remains debatable, ω-3 and ω-6 PUFA supplementation is widely used in both the primary and secondary prevention of CVD.
4. The effects of PUFAs on platelet function
The platelet membrane, like other mammalian cell membranes, is a lipid bilayer composed of an outer leaflet that contains cholinephospholipids, primarily phosphatidylcholine and sphingomyelin, and an inner leaflet comprised of the negatively charged aminophospholipids phosphatidylethanolamine (PE) and phosphatidylserine (PS).[51, 52] The platelet phospholipid membrane plays a major role in many aspects of platelet function.[53] Asymmetric distribution of the membrane phospholipids is well regulated by a number of calcium and adenosine triphosphate (ATP) dependent enzymes.[51] Upon platelet activation, this asymmetric orientation of membrane phospholipids is disrupted resulting in calcium-dependent exposure PS on the platelet surface. It is well known that PS surface exposure is a major component of normal hemostasis because it supports platelet procoagulant functions. Formation of the prothrombonase complex on platelet membrane surface further enhances platelet activation by facilitating conversion of prothrombin to thrombin.[43, 54]
Upon cellular activation, cytoplasmic phospholipase A2 (cPLA2) hydrolyzes PUFAs from the lipid membrane generating free PUFAs. Liberation of PUFAs from the platelet membrane is achieved in an agonist dependent manner. Platelet stimulation by agonists including thrombin, ADP, and collagen cause the translocation of cPLA2α to the plasma membrane where it cleaves PUFAs. A study (reference) shows that the most abundant phospholipid in the platelet is phosphatidylcholine, containing a number of PUFAs including arachidonic acid (AA) (13.5%), linoleic acid (LA) (7.9%), dihomo-γ-linolenic acid (DGLA) (2.1%), Eicosadienoic acid (EDA) (0.6%), docosahexaenoic acid (DHA) (0.6%), eicospentaenoic acid (EPA) (0.2%), Alpha-linolenic acid (ALA) (0.1%), and γ -linolenic acid (GLA) (0.07%).[55] This study also shows that supplementation with ω-6 PUFAs affects the phospholipid composition of the platelet plasma membrane[55]. Additionally, studies comparing individuals from inland and coastal communities in Europe show that differences in diet (i.e. consumption of fish products) affect both the phospholipid composition of the platelet plasma membrane and platelet function.[56–58] AA is a major component of membrane phospholipids which is required for prostaglandin synthesis and is also responsible for accelerating some phases of the coagulation process.[59] The composition of the phospholipids in the platelet membrane is dynamic in nature, and because many of the fatty acids that make up the phospholipid bilayer are not produced in the body, their content is primarily regulated by dietary intake.[60] Therefore, polyunsaturated fatty acid content in the platelet membranes varies, depending on diet.
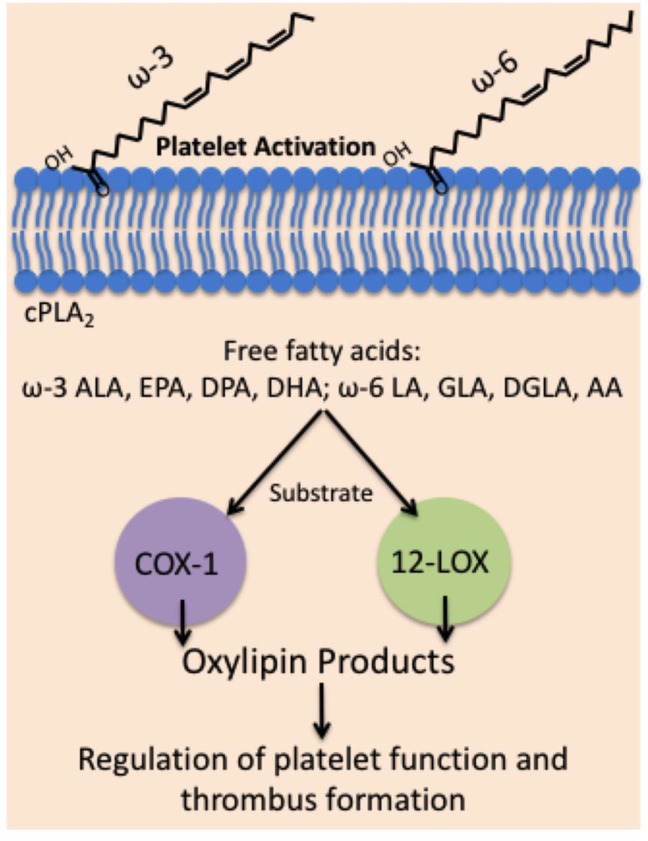
The effects of dietary intake on primary prevention of coronary heart disease have been a long-standing interest. Early studies have shown dietary supplementation of fatty acids increase fatty acid incorporation into the platelet lipid membrane.[55] Recent research work has revealed a number of lipid products, derived from ω-3 or ω-6 PUFAs, regulate and alter platelet function (Figure 3).[6, 40, 61, 62] Understanding how these newly identified lipids regulate platelet function will aid in our understanding of how diet alteration or fatty acid supplementation affect platelet function and modulate hemostasis and thrombosis in the vessel.
Figure 3. Regulation of platelet function and thrombosis by ω-3 and ω-6 PUFAs.
PUFAs are an essential component of the platelet phospholipid membrane with the main ω-3 and ω-6 PUFAs being oxygenated by two important oxygenases (COX-1 and 12-LOX) to produce oxylipins in platelets. ω-3 and ω-6 PUFA oxylipin metabolites will further contribute to the regulation of platelet function in hemostasis and thrombosis.
4.1. Omega-3 fatty acids and platelet function
ω-3 PUFAs are a class of essential fatty acids that are an integral part of cell membranes throughout the body and affect the function of the cell receptors in these membranes. Epidemiological evidence suggests that a ω-3 fatty acid-rich diet promotes beneficial cardiovascular, neurological, and anti-inflammatory health effects.[63–67] However, the underlying biochemical mechanisms facilitating these beneficial effects are yet to be fully elucidated. Unlike most types of fats that can be made from other fats or raw materials in the human body, ω-3 PUFAs must be obtained from diet.[68] There are three main ω-3 PUFAs obtained from food: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) come mainly from fish while alpha-linolenic acid (ALA), the most common omega-3 fatty acid in most human diets, is found in vegetable oils and nuts (especially walnuts), flax seeds and flaxseed oil, leafy vegetables, and some animal fat, especially in grass-fed animals.[69] Despite the sources of these PUFAs being known, it can be challenging to get the appropriate amount of EPA and DHA through diet alone. Dietary Guidelines for Americans states that an average daily consumption of 250 mg of EPA and DHA is associated with reduced cardiac deaths among individuals with and without preexisting CVD.[70] Studies suggest that ALA is mainly used for energy and its conversion into EPA and DHA is very limited.[71] We and others report that ω-3 fatty acids act on the platelet membrane to reduce platelet aggregation and thromboxane release by acting on COX-1 and 12-LOX, the two important oxygenases involved in metabolizing fatty acids into oxylipins in platelets.(Figure 3)[61] Our earlier studies comparing the reactivity of six essential fatty acids with 12-LOX show that EPA, AA, and DGLA react comparably, ALA reacts more slowly, and EDA and LA do not react at all.[43] It has been reported that exogenous addition of DHA and EPA inhibits human platelet aggregation in vitro but DHA seems to have a much more potent effect on human platelet function.[72] Even with the known effect, the mechanism by which supplementation with ω-3 PUFAs decreases platelet aggregation is remains unknown. It has been reported that ω-3 PUFAs incorporate into platelet membrane phospholipids, leading to a concomitant reduction of ω-6 PUFAs along with an increase in EPA.[73] EPA can then compete with AA and inhibit the cyclooxygenase-1 pathway. The decrease of platelet aggregation by ω-3 PUFAs has also been attributed to a decrease in thromboxane A2 and an increase in prostaglandins, thromboxanes[74], and the synthesis of nitric oxide in endothelial cells.[75] A recent study also showed ω-3 PUFA–derived lipid metabolites can originate from the crosstalk between the endocannabinoid and cytochrome P450 (CYP) epoxygenase metabolic pathways. ω-3 endocannabinoid epoxides epoxyeicosatetraenoic acid-ethanolamide (EEQ-EA) and epoxydocosapentaenoic acid-ethanolamide (EDP-EA) derived from DHA and EPA, respectively, exert anti-inflammatory, vasodilatory, and reciprocally modulate platelet aggregation.[76]
The evidence of cardioprotection in published studies is derived from many sources, including epidemiological studies analyzing populations with high dietary ω-3 PUFAs intake.[77] The strongest evidence for a beneficial effect of ω-3 fats is mostly related to the heart.[78] Supplementation with ω-3 PUFAs has been reported to have several beneficial effects including reducing cardiovascular mortality[79, 80], improved lipid profile[81, 82], anti-inflammatory effects[83], reducing cardiac arrhythmias [82], vasodilatory mechanisms[84] and anti-platelet effects.[85] However, pinpointing the cardioprotective therapeutic effects of ω-3 PUFAs remains challenging, as large clinical trials predominantly demonstrated neutral effects of ω-3 PUFAs in placebo-controlled studies. The OPERA trial showed no alterations in the number or severity of atrial fibrillation events or incidents of myocardial infarction or stroke.[86] Furthermore, the Risk and Prevention Study[65] and the Alpha Omega trials[87] showed no alterations in cardiovascular-derived incidents of mortality, and the OMEGA-PAD I trial[88] showed no alterations in vascular endothelial cell functions. Several interventional studies were reviewed by Begg et al. and largely concluded that ω-3 PUFAs may provide a clinical benefit, but the degree and nature of ω-3 PUFA cardioprotection remains unclear.[89] Thrombosis, like many of the other endpoints examined in ω-3 PUFA-oriented clinical trials, also shows mixed results[86, 90–93]. Although supplementation of ω-3 PUFAs has been shown to reduce platelet aggregation and activation in healthy subjects, a higher than recommended dose of ω-3 PUFAs may be needed due to platelet hyperactivite prothrombotic conditions such as in CVD.
It is worthwhile to mention that the prevalence of CVD is closely associated with diet.[94] Anthropological and epidemiological studies as well as studies at the molecular level indicate that human beings evolved on a diet with a ratio of omega-6 to omega-3 essential fatty acids (EFA) of ~1, whereas in Western diets the ratio is anywhere between 15/1 and 16.7/1.[95] Some studies suggest that this alteration of the ratio of ω-6 to ω-3 essential fatty acids in Western diets promotes the pathogenesis of many diseases including cardiovascular disease, cancer, and inflammatory and autoimmune diseases, whereas increased levels of ω −3 PUFA (a lower ω-6/ω-3 ratio), exert suppressive effects.[49, 50] Studies also compared the effects of ω-3 PUFAs on platelet activity in men and women. Study results suggested that men are more likely to benefit from supplementation with EPA, whereas women are more responsive to DHA.[96] While these observations are interesting, the effects of EPA and DHA supplementation on individuals of different ages and sexes should be investigated in future studies.
4.2. Omega-6 fatty acids and platelet function
ω-6 PUFAs are another class of essential fatty acids that are abundant components of cell membranes and serve as precursors to bioactive lipid mediators. ω-6 PUFAs need to be obtained from food because humans are not able to synthesize them. ω-6 PUFAs are a major component of the Western diet, derived from poultry, nuts, seeds and vegetable oils.[97] Linoleic acid (LA) is a precursor to the variety of ω-6 series of fatty acids including AA, gamma-linolenic acid (GLA), and dihomo-γ-linolenic acid (DGLA).[97, 98] LA is by far the most common fatty acid found in the human food supply. It is a primary fatty acid found in cooking oils, including vegetable, corn, canola, peanut, soybean, safflower, and sunflower oils. GLA is present in less commonly known oils—mainly borage, black currant seed, and evening primrose. AA is found in red meat, eggs, and poultry products. AA is one of the most abundant fatty acids in platelet membranes, granules, and soluble fractions. [99] AA is the precursor of thromboxane and prostacyclin, two of the most active compounds related to platelet function. The role of AA in regulation of platelet function has been extensively studied for decades. It is well demonstrated that COX-1 oxidizes AA to generate prostanoids (prostaglandins (PGs) and thromboxanes (TXs)) series to regulate platelet function.[6, 61, 62] Released TXA2 acts as a soluble agonist like adenosine diphosphate (ADP) to amplify platelet activation through its thromboxane receptor (TPα) on platelets and exerts prothrombotic properties.[61] In contrast, PGI2 (prostacyclin), a well-characterized vasodilator[100], has been shown to activate adenylate cyclase in the platelet via the prostacyclin (IP) receptor and in turn antagonizes platelet aggregation.[101]
ω-6 PUFA, DGLA, has been shown to play a role in inhibiting platelet aggregation ex vivo, although the exact oxylipin products by cyclooxygenase-1 (COX-1) or platelet 12-lipoxygenase responsible for the inhibitory effects of DGLA on platelet function remain unclear (Figure 3).[61, 102] [62] For a long time, the antiplatelet effects of DGLA have been primarily attributed to the COX-1–derived prostanoid metabolites (TXA1 and prostaglandin E1), although the DGLA-derived products of COX-1 are produced in low amounts in platelets.[61, 62, 102, 103]. In our earlier study, we show that the bioactive lipid products resulting from 12-LOX oxidation of DGLA, 12-(S)-hydroperoxy-8Z,10E,14Z-eicosatrienoic acid [12(S)-HPETrE], and its reduced product, 12(S)-HETrE, resulted in significant attenuation of agonist-mediated platelet aggregation, granule secretion, αIIbβ3 activation, Rap1 activation, and clot retraction in ex vivo.[43]. This observation was further confirmed by our recently published study showing that ω-6 PUFA, DGLA, exhibits cardioprotective properties through its reduced oxidized lipid form 12(S)-HETrE by inhibiting platelet activation and thrombosis in vivo via a Gαs-linked GPCR-dependent manner.[62] These results strongly indicate that as DGLA may be competitively oxidized by both COX-1 or 12-LOX pathways, its inhibitory effect on platelet function may be dictated by the downstream oxylipin products from these two pathways.
5. In vivo effect of omega 3 and 6 PUFAs on arterial thrombosis in preclinical animal models
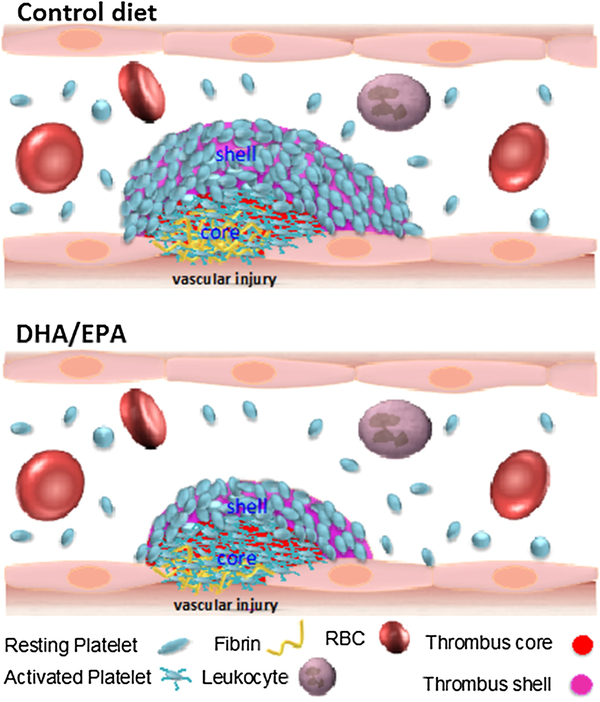
Despite the lack of mechanistic and in vivo evidence of anti-thrombotic benefits of ω-3 and ω-6 PUFAs, dietary supplementation with PUFAs is commonly used for their potential cardioprotective effects, including their antiplatelet effects. It is difficult to reconcile the observed antiplatelet effects of ω-3 and ω-6 PUFAs or oxylipin products in vitro with clinically relevant study results.[98, 104] Early studies using animal models of arterial or venous thrombosis indicate PUFA supplementation in diet may alter thrombosis.[105–107] However, results from those studies are not conclusive. In the last decade there have been significant advances in preclinical in vivo animal models of thrombosis using real-time intravital microscopy.[30, 31, 46] Recently, we reported the effects of ω-3 PUFA supplementation on platelet function in vivo in mice after 6 weeks of DHA/EPA-enriched diet using two well-established intravital microscopy murine models of thrombosis (Figure 4).[108] In a laser-induced cremaster arteriole thrombosis model, we found that platelet accumulation and fibrin formation in thrombi were attenuated in the DHA/EPA-fed mice compared to control diet. Intriguingly, the level of P-selectin positive platelets that accumulated at the thrombus core was similar between the two groups of mice as observed under confocal intravital microscopy. Vessel occlusion was significantly delayed in mice on a DHA/EPA diet in a FeCI3-induced carotid artery thrombosis model. Despite this, DHA/EPA had relatively minimal effects on agonist-induced secretion, aggregation, and adhesion. DHA/EPA was observed to modulate platelet-mediated thrombin generation in vitro. Taken together, these study results support a role for ω-3 PUFA supplementation as a means to achieve cardioprotective effects by attenuating platelet function resulting in modulation of thrombus formation and vessel occlusion.
Figure 4. Attenuation of thrombosis following the DHA/EPA rich-diet.
Represented figure shows the thrombus formation in response to vessel injury in individual on a control diet or EPA/DPA-rich diet. Compared to the thrombi in the control diet (upper panel), thrombus formation is attenuated in the DHA/EPA-rich diet. DHA/EPA-rich diet resulted in a decrease of platelet accumulation on outer layers (shell region) of thrombi without affecting the overall platelet accumulation at the center (core region) of thrombi.
More intriguing in vivo evidence and mechanistic insights were obtained recent study on the 12-LOX-derived oxylipin of omega-6 PUFA DGLA, 12(S)-HETrE.[62] 12-HETrE, a DGLA-derived metabolite of 12-LOX, reduces thrombus growth in a laser-induced injury model of thrombosis, but the inhibitory effects of DGLA on platelet-mediated thrombus formation are 12-LOX dependent. Despite the strong antithrombotic effects, DGLA did not impair hemostasis. This study provided the first evidence of a 12-LOX oxylipin regulating platelet function in a Gs α subunit–linked G-protein–coupled receptor–dependent manner. Furthermore, the antiplatelet effects of 12-HETrE are partially dependent on IP signaling providing further insight into the mechanism by which DGLA supplementation inhibits platelets function.
Taken together, results from these preclinical studies provided important mechanistic insights on how ω-3 and ω-6 PUFAs regulate platelet function and modulate thrombosis in vivo.
6. Conclusions
Adequate platelet reactivity is required for platelet adhesion and aggregation at the site of vascular injury to maintain hemostasis. However, excessive platelet reactivity can lead to the formation of occlusive thrombi, the predominate underlying cause of myocardial infarction and stroke. ω-3 and ω-6 polyunsaturated fatty acids are an essential component of the platelet phospholipid membrane and play a major role in regulation of platelet function. Dietary supplementation with ω-3 or ω-6 PUFAs may alter platelet lipid membrane phospholipid composition and affect platelet function, which, in turn, may alter the progression and thrombotic complications of cardiovascular disease. Therefore, mechanistic insights into how PUFAs and metabolites affect platelet function could have therapeutic potential.
While current anti-platelet therapies are successful in reducing the mortality rate associated with CVD, thrombotic complications of CVD remain a challenge and there is a great need to develop new therapies. This review has discussed the role of platelets in hemostasis and in the pathophysiology of CVD. We overviewed the effect of ω-3 and ω-6 PUFAs on platelet function with special emphasis on the effects on thrombus formation in vivo, including recent mechanistic insights and evidence for their cardioprotective effects. With an overwhelming amount of controversial data on the potential therapeutic effects of ω-3 and ω-6 PUFAs on platelet function, it is important to elucidate the specific factors contributing to the overall inhibition of unwarranted platelet activation leading to thrombosis. Very few studies to date have investigated the individual efficacy of the major ω-3 and ω-6 PUFAs and their oxylipin metabolites on platelet thrombosis.
In the future it would be beneficial to evaluate the changes of the levels of PUFAs in the platelet membrane following supplementation or diet change and further elucidate the specific contributions of each individual fatty acid in the regulation of platelet function. It is imperative to uncover the underlining mechanisms of PUFAs in the regulation of platelets to reduce morbidity and mortality associated with cardiovascular disease.
Highlights.
Dietary supplementation of ω-3 and ω-6 PUFAs has long been used to slow the progression of CVD and to prevent acute cardiovascular events. In this review, we will outline the role of platelet physiology in hemostasis and the effect of ω-3 and ω-6 PUFAs on platelet function, with special emphasis on in vivo effects on hemostasis and thrombosis due to the role of PUFAs and their bioactive lipids in circulation. Further, recent mechanistic insights and evidence for cardio-protective effects of PUFAs and their bioactive lipids will be discussed.
Acknowledgments
We thank Benjamin Tourdot, Madeline Jackson, Jennifer Yeung and Izabela Holinstat for carefully reviewing the article and helpful suggestions.
Sources of Funding
This study was supported by the National Institute of Health Grants GM105671 (M. Holinstat) and HL114405 (M. Holinstat).
Footnotes
Conflicts of interest:
None of the authors had a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].I.S.C.f.W.T. Day, Thrombosis: a major contributor to the global disease burden, J Thromb Haemost 12(10) (2014) 1580–90. [DOI] [PubMed] [Google Scholar]
- [2].Jackson SP, Schoenwaelder SM, Antiplatelet therapy: in search of the ‘magic bullet’, Nat Rev Drug Discov 2(10) (2003) 775–89. [DOI] [PubMed] [Google Scholar]
- [3].Coller BS, Historical perspective and future directions in platelet research, J Thromb Haemost 9 Suppl 1 (2011) 374–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mackman N, Triggers, targets and treatments for thrombosis, Nature 451(7181) (2008) 914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Papp J, Kenyeres P, Toth K, Clinical importance of antiplatelet drugs in cardiovascular diseases, Clin Hemorheol Microcirc 53(1–2) (2013) 81–96. [DOI] [PubMed] [Google Scholar]
- [6].Tourdot BE, Ahmed I, Holinstat M, The emerging role of oxylipins in thrombosis and diabetes, Front Pharmacol 4 (2014) 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hammond VJ, O’Donnell VB, Esterified eicosanoids: generation, characterization and function, Biochim Biophys Acta 1818(10) (2012) 2403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Serebruany VL, Malinin AI, Eisert RM, Sane DC, Risk of bleeding complications with antiplatelet agents: meta-analysis of 338,191 patients enrolled in 50 randomized controlled trials, Am J Hematol 75(1) (2004) 40–7. [DOI] [PubMed] [Google Scholar]
- [9].O. W., An account of certain organisms occurring in the liquor sanguinis [J] Proc R Soc Lond. 1874:391–398. [Google Scholar]
- [10].Xu XR, Zhang D, Oswald BE, Carrim N, Wang X, Hou Y, Zhang Q, Lavalle C, McKeown T, Marshall AH, Ni H, Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond, Crit Rev Clin Lab Sci 53(6) (2016) 409–30. [DOI] [PubMed] [Google Scholar]
- [11].B. G., Su di un nuovo elemento morfologico del sangue dei mammiferi e della sua importanza nella trombosi e nella coagulazione [J] L’Osservatore. 1881:785–787. [Google Scholar]
- [12].W. J., The origin and nature of blood platelets [J] Boston Med Surg J., ACS Nano (1906:643–645.). [Google Scholar]
- [13].von Hundelshausen P, Petersen F, Brandt E, Platelet-derived chemokines in vascular biology, Thromb Haemost 97(5) (2007) 704–13. [DOI] [PubMed] [Google Scholar]
- [14].Klinger MH, Jelkmann W, Role of blood platelets in infection and inflammation, J Interferon Cytokine Res 22(9) (2002) 913–22. [DOI] [PubMed] [Google Scholar]
- [15].Semple JW, Italiano JE Jr., Freedman J, Platelets and the immune continuum, Nat Rev Immunol 11(4) (2011) 264–74. [DOI] [PubMed] [Google Scholar]
- [16].Labelle M, Hynes RO, The initial hours of metastasis: the importance of cooperative hosttumor cell interactions during hematogenous dissemination, Cancer Discov 2(12) (2012) 1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Franco AT, Corken A, Ware J, Platelets at the interface of thrombosis, inflammation, and cancer, Blood 126(5) (2015) 582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Italiano JE Jr., Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL, Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released, Blood 111(3) (2008) 1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, Herzog B, Lu M, Nieswandt B, Oliver G, Makinen T, Xia L, Kahn ML, Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life, J Clin Invest 124(1) (2014) 273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Murphy AJ, Bijl N, Yvan-Charvet L, Welch CB, Bhagwat N, Reheman A, Wang Y, Shaw JA, Levine RL, Ni H, Tall AR, Wang N, Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis, Nat Med 19(5) (2013) 586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jackson SP, The growing complexity of platelet aggregation, Blood 109(12) (2007) 5087–95. [DOI] [PubMed] [Google Scholar]
- [22].Berndt MC, Metharom P, Andrews RK, Primary haemostasis: newer insights, Haemophilia 20 Suppl 4 (2014) 15–22. [DOI] [PubMed] [Google Scholar]
- [23].Monroe DM, Hoffman M, Roberts HR, Platelets and thrombin generation, Arterioscler Thromb Vasc Biol 22(9) (2002) 1381–9. [DOI] [PubMed] [Google Scholar]
- [24].Ni H, Freedman J, Platelets in hemostasis and thrombosis: role of integrins and their ligands, Transfus Apher Sci 28(3) (2003) 257–64. [DOI] [PubMed] [Google Scholar]
- [25].Dopheide SM, Maxwell MJ, Jackson SP, Shear-dependent tether formation during platelet translocation on von Willebrand factor, Blood 99(1) (2002) 159–67. [DOI] [PubMed] [Google Scholar]
- [26].Savage B, Almus-Jacobs F, Ruggeri ZM, Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow, Cell 94(5) (1998) 657–66. [DOI] [PubMed] [Google Scholar]
- [27].Ruggeri ZM, Mechanisms initiating platelet thrombus formation, Thromb Haemost 78(1) (1997) 611–6. [PubMed] [Google Scholar]
- [28].Yang H, Lang S, Zhai Z, Li L, Kahr WH, Chen P, Brkic J, Spring CM, Flick MJ, Degen JL, Freedman J, Ni H, Fibrinogen is required for maintenance of platelet intracellular and cell-surface P-selectin expression, Blood 114(2) (2009) 425–36. [DOI] [PubMed] [Google Scholar]
- [29].Ikeda Y, Handa M, Kawano K, Kamata T, Murata M, Araki Y, Anbo H, Kawai Y, Watanabe K, Itagaki I, et al. , The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress, J Clin Invest 87(4) (1991) 1234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reheman A, Yang H, Zhu G, Jin W, He F, Spring CM, Bai X, Gross PL, Freedman J, Ni H, Plasma fibronectin depletion enhances platelet aggregation and thrombus formation in mice lacking fibrinogen and von Willebrand factor, Blood 113(8) (2009) 1809–17. [DOI] [PubMed] [Google Scholar]
- [31].Reheman A, Gross P, Yang H, Chen P, Allen D, Leytin V, Freedman J, Ni H, Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation, J Thromb Haemost 3(5) (2005) 875–83. [DOI] [PubMed] [Google Scholar]
- [32].Reheman A, Tasneem S, Ni H, Hayward CP, Mice with deleted multimerin 1 and alphasynuclein genes have impaired platelet adhesion and impaired thrombus formation that is corrected by multimerin 1, Thromb Res 125(5) (2010) e177–83. [DOI] [PubMed] [Google Scholar]
- [33].Kowalska MA, Rauova L, Poncz M, Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis, Thromb Res 125(4) (2010) 292–6. [DOI] [PubMed] [Google Scholar]
- [34].Li Z, Delaney MK, O’Brien KA, Du X, Signaling during platelet adhesion and activation, Arterioscler Thromb Vasc Biol 30(12) (2010) 2341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cosemans JM, Schols SE, Stefanini L, de Witt S, Feijge MA, Hamulyak K, Deckmyn H, Bergmeier W, Heemskerk JW, Key role of glycoprotein Ib/V/IX and von Willebrand factor in platelet activation-dependent fibrin formation at low shear flow, Blood 117(2) (2011) 651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ruggeri ZM, Platelets in atherothrombosis, Nat Med 8(11) (2002) 1227–34. [DOI] [PubMed] [Google Scholar]
- [37].Koupenova M, Kehrel BE, Corkrey HA, Freedman JE, Thrombosis and platelets: an update, Eur Heart J 38(11) (2017) 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yeung J, Holinstat M, Newer agents in antiplatelet therapy: a review, J Blood Med 3 (2012) 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Holinstat M, Reheman A, Dual antiplatelet therapy for PCI: Are we tailored to all?, Thromb Res 135(6) (2015) 1045–6. [DOI] [PubMed] [Google Scholar]
- [40].Tourdot BE, Holinstat M, Targeting 12-Lipoxygenase as a Potential Novel Antiplatelet Therapy, Trends Pharmacol Sci 38(11) (2017) 1006–1015. [DOI] [PubMed] [Google Scholar]
- [41].Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, Sigman CC, Kelloff GJ, Lipoxygenase inhibitors as potential cancer chemopreventives, Cancer Epidemiol Biomarkers Prev 8(5) (1999) 467–83. [PubMed] [Google Scholar]
- [42].Ghosh J, Myers CE, Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells, Proc Natl Acad Sci U S A 95(22) (1998) 13182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR, Holinstat M, Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation, J Lipid Res 53(12) (2012) 2546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yeung J, Apopa PL, Vesci J, Stolla M, Rai G, Simeonov A, Jadhav A, FernandezPerez P, Maloney DJ, Boutaud O, Holman TR, Holinstat M, 12-lipoxygenase activity plays an important role in PAR4 and GPVI-mediated platelet reactivity, Thromb Haemost 110(3) (2013) 569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kenyon V, Rai G, Jadhav A, Schultz L, Armstrong M, Jameson JB 2nd, Perry S, Joshi N, Bougie JM, Leister W, Taylor-Fishwick DA, Nadler JL, Holinstat M, Simeonov A, Maloney DJ, Holman TR, Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase, J Med Chem 54(15) (2011) 5485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Adili R, Tourdot BE, Mast K, Yeung J, Freedman JC, Green A, Luci DK, Jadhav A, Simeonov A, Maloney DJ, Holman TR, Holinstat M, First Selective 12-LOX Inhibitor, ML355, Impairs Thrombus Formation and Vessel Occlusion In Vivo With Minimal Effects on Hemostasis, Arterioscler Thromb Vasc Biol 37(10) (2017) 1828–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Subban V, Sarat Chandra K, Glycoprotein IIb-IIIa inhibitors - do we still need them?, Indian Heart J 65(3) (2013) 260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Berger PB, Bhatt DL, Fuster V, Steg PG, Fox KA, Shao M, Brennan DM, Hacke W, Montalescot G, Steinhubl SR, Topol EJ, Investigators C, Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial, Circulation 121(23) (2010) 2575–83. [DOI] [PubMed] [Google Scholar]
- [49].Simopoulos AP, The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases, Exp Biol Med (Maywood) 233(6) (2008) 674–88. [DOI] [PubMed] [Google Scholar]
- [50].Simopoulos AP, The omega-6/omega-3 fatty acid ratio, genetic variation, and cardiovascular disease, Asia Pac J Clin Nutr 17 Suppl 1 (2008) 131–4. [PubMed] [Google Scholar]
- [51].Fadeel B, Xue D, The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease, Crit Rev Biochem Mol Biol 44(5) (2009) 264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chap H, Forty five years with membrane phospholipids, phospholipases and lipid mediators: A historical perspective, Biochimie 125 (2016) 234–49. [DOI] [PubMed] [Google Scholar]
- [53].Hemker HC, van Rijn JL, Rosing J, van Dieijen G, Bevers EM, Zwaal RF, Platelet membrane involvement in blood coagulation, Blood Cells 9(2) (1983) 303–17. [PubMed] [Google Scholar]
- [54].Hoffman M, Monroe DM 3rd, A cell-based model of hemostasis, Thromb Haemost 85(6) (2001) 958–65. [PubMed] [Google Scholar]
- [55].Barre DE, Holub BJ, The effect of borage oil consumption on the composition of individual phospholipids in human platelets, Lipids 27(5) (1992) 315–20. [DOI] [PubMed] [Google Scholar]
- [56].Simonsen T, Vartun A, Lyngmo V, Nordoy A, Coronary heart disease, serum lipids, platelets and dietary fish in two communities in northern Norway, Acta Med Scand 222(3) (1987) 237–45. [DOI] [PubMed] [Google Scholar]
- [57].Vidgren HM, Agren JJ, Schwab U, Rissanen T, Hanninen O, Uusitupa MI, Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men, Lipids 32(7) (1997) 697–705. [DOI] [PubMed] [Google Scholar]
- [58].Vognild E, Elvevoll EO, Brox J, Olsen RL, Barstad H, Aursand M, Osterud B, Effects of dietary marine oils and olive oil on fatty acid composition, platelet membrane fluidity, platelet responses, and serum lipids in healthy humans, Lipids 33(4) (1998) 427–36. [DOI] [PubMed] [Google Scholar]
- [59].Morin RJ, The role of phospholipids in platelet function, Ann Clin Lab Sci 10(6) (1980) 463–73. [PubMed] [Google Scholar]
- [60].Skeaff CM, Hodson L, McKenzie JE, Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course, J Nutr 136(3) (2006) 565–9. [DOI] [PubMed] [Google Scholar]
- [61].Yeung J, Hawley M, Holinstat M, The expansive role of oxylipins on platelet biology, J Mol Med (Berl) 95(6) (2017) 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yeung J, Tourdot BE, Adili R, Green AR, Freedman CJ, Fernandez-Perez P, Yu J, Holman TR, Holinstat M, 12(S)-HETrE, a 12-Lipoxygenase Oxylipin of Dihomo-gamma-Linolenic Acid, Inhibits Thrombosis via Galphas Signaling in Platelets, Arterioscler Thromb Vasc Biol 36(10) (2016) 2068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].von Schacky C, Harris WS, Cardiovascular benefits of omega-3 fatty acids, Cardiovasc Res 73(2) (2007) 310–5. [DOI] [PubMed] [Google Scholar]
- [64].Breslow JL, n-3 fatty acids and cardiovascular disease, Am J Clin Nutr 83(6 Suppl) (2006) 1477S–1482S. [DOI] [PubMed] [Google Scholar]
- [65].R.a.P.S.C. Group, n-3 fatty acids in patients with multiple cardiovascular risk factors, N Engl J Med 368(19) (2013) 1800. –8. [DOI] [PubMed] [Google Scholar]
- [66].Guasch-Ferré M, Babio N, Martínez-González MA, Corella D, Ros E, Martín-Peláez S, Estruch R, Arós F, Gómez-Gracia E, Fiol M, Santos-Lozano JM, Serra-Majem L, Bulló M, Toledo E, Barragán R, Fitó M, Gea A, Salas-Salvadó J, Investigators PS, Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease, Am J Clin Nutr 102(6) (2015) 1563–73. [DOI] [PubMed] [Google Scholar]
- [67].Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D, A.H.A.N.C.o.t.C.o.L.a.C. Health, C.o.E.a. Prevention, C.o.C.D.i.t. Young, C.o.C.a.S. Nursing, a.C.o.C. Cardiology, Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association, Circulation 135(15) (2017) e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Benatti P, Peluso G, Nicolai R, Calvani M, Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties, J Am Coll Nutr 23(4) (2004) 281–302. [DOI] [PubMed] [Google Scholar]
- [69].McEwen BJ, Morel-Kopp MC, Chen W, Tofler GH, Ward CM, Effects of omega-3 polyunsaturated fatty acids on platelet function in healthy subjects and subjects with cardiovascular disease, Semin Thromb Hemost 39(1) (2013) 25–32. [DOI] [PubMed] [Google Scholar]
- [70].F.a.N.B.D.r.i.f.e. Institute of Medicine, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academy Press;, (2005). [Google Scholar]
- [71].Swanson D, Block R, Mousa SA, Omega-3 fatty acids EPA and DHA: health benefits throughout life, Adv Nutr 3(1) (2012) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Larson MK, Tormoen GW, Weaver LJ, Luepke KJ, Patel IA, Hjelmen CE, Ensz NM, McComas LS, McCarty OJ, Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation, Am J Physiol Cell Physiol 304(3) (2013) C273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lev EI, Solodky A, Harel N, Mager A, Brosh D, Assali A, Roller M, Battler A, Kleiman NS, Kornowski R, Treatment of aspirin-resistant patients with omega-3 fatty acids versus aspirin dose escalation, J Am Coll Cardiol 55(2) (2010) 114–21. [DOI] [PubMed] [Google Scholar]
- [74].Wander RC, Patton BD, Comparison of three species of fish consumed as part of a Western diet: effects on platelet fatty acids and function, hemostasis, and production of thromboxane, Am J Clin Nutr 54(2) (1991) 326–33. [DOI] [PubMed] [Google Scholar]
- [75].Abeywardena MY, Head RJ, Longchain n-3 polyunsaturated fatty acids and blood vessel function, Cardiovasc Res 52(3) (2001) 361–71. [DOI] [PubMed] [Google Scholar]
- [76].McDougle DR, Watson JE, Abdeen AA, Adili R, Caputo MP, Krapf JE, Johnson RW, Kilian KA, Holinstat M, Das A, Anti-inflammatory omega-3 endocannabinoid epoxides, Proc Natl Acad Sci U S A 114(30) (2017) E6034–E6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fleming JA, Kris-Etherton PM, The evidence for alpha-linolenic acid and cardiovascular disease benefits: Comparisons with eicosapentaenoic acid and docosahexaenoic acid, Adv Nutr 5(6) (2014) 863S–76S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Duda MK, O’Shea KM, Stanley WC, omega-3 polyunsaturated fatty acid supplementation for the treatment of heart failure: mechanisms and clinical potential, Cardiovasc Res 84(1) (2009) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F, G.I.-P. Investigators, Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione, Circulation 105(16) (2002) 1897–903. [DOI] [PubMed] [Google Scholar]
- [80].Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, Gissi HFI, Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial, Lancet 372(9645) (2008) 1223–30. [DOI] [PubMed] [Google Scholar]
- [81].Bays HE, Tighe AP, Sadovsky R, Davidson MH, Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications, Expert Rev Cardiovasc Ther 6(3) (2008) 391–409. [DOI] [PubMed] [Google Scholar]
- [82].Singer P, Wirth M, Can n-3 PUFA reduce cardiac arrhythmias? Results of a clinical trial, Prostaglandins Leukot Essent Fatty Acids 71(3) (2004) 153–9. [DOI] [PubMed] [Google Scholar]
- [83].Calder PC, The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability, Mol Nutr Food Res 56(7) (2012) 1073–80. [DOI] [PubMed] [Google Scholar]
- [84].Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ, Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men, Circulation 102(11) (2000) 1264–9. [DOI] [PubMed] [Google Scholar]
- [85].Gajos G, Rostoff P, Undas A, Piwowarska W, Effects of polyunsaturated omega-3 fatty acids on responsiveness to dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: the OMEGA-PCI (OMEGA-3 fatty acids after pci to modify responsiveness to dual antiplatelet therapy) study, J Am Coll Cardiol 55(16) (2010) 1671–8. [DOI] [PubMed] [Google Scholar]
- [86].Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, Latini R, Libby P, Lombardi F, O’Gara PT, Page RL, Tavazzi L, Tognoni G, O. Investigators, Fish oil and postoperative atrial fibrillation: the Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial, JAMA 308(19) (2012) 2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kromhout D, Giltay EJ, Geleijnse JM, A.O.T. Group, n-3 fatty acids and cardiovascular events after myocardial infarction, N Engl J Med 363(21) (2010) 2015–26. [DOI] [PubMed] [Google Scholar]
- [88].Grenon SM, Owens CD, Nosova EV, Hughes-Fulford M, Alley HF, Chong K, Perez S, Yen PK, Boscardin J, Hellmann J, Spite M, Conte MS, Short-Term, High-Dose Fish Oil Supplementation Increases the Production of Omega-3 Fatty Acid-Derived Mediators in Patients With Peripheral Artery Disease (the OMEGA-PAD I Trial), J Am Heart Assoc 4(8) (2015) e002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Begg A, Connolly S, Halcox J, Kaba A, Main L, Purcell H, Williams H, Yellon D, Omega-3 fatty acids in cardiovascular disease: re-assessing the evidence, Br J Cardiol 19 (2012) 79–84. [Google Scholar]
- [90].Thorngren M, Gustafson A, Effects of 11-week increases in dietary eicosapentaenoic acid on bleeding time, lipids, and platelet aggregation, Lancet 2(8257) (1981) 1190–3. [DOI] [PubMed] [Google Scholar]
- [91].Lorenz R, Spengler U, Fischer S, Duhm J, Weber PC, Platelet function, thromboxane formation and blood pressure control during supplementation of the Western diet with cod liver oil, Circulation 67(3) (1983) 504–11. [DOI] [PubMed] [Google Scholar]
- [92].Gibney MJ, Bolton-Smith C, The effect of a dietary supplement of n-3 polyunsaturated fat on platelet lipid composition, platelet function and platelet plasma membrane fluidity in healthy volunteers, Br J Nutr 60(1) (1988) 5–12. [DOI] [PubMed] [Google Scholar]
- [93].Wachira JK, Larson MK, Harris WS, n-3 Fatty acids affect haemostasis but do not increase the risk of bleeding: clinical observations and mechanistic insights, Br J Nutr 111(9) (2014) 1652–62. [DOI] [PubMed] [Google Scholar]
- [94].Buttar HS, Li T, Ravi N, Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation, Exp Clin Cardiol 10(4) (2005) 229–49. [PMC free article] [PubMed] [Google Scholar]
- [95].Simopoulos AP, Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases, Biomed Pharmacother 60(9) (2006) 502–7. [DOI] [PubMed] [Google Scholar]
- [96].Phang M, Lincz LF, Garg ML, Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women, J Nutr 143(4) (2013) 457–63. [DOI] [PubMed] [Google Scholar]
- [97].Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C, Health implications of high dietary omega-6 polyunsaturated Fatty acids, J Nutr Metab 2012 (2012) 539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Russo GL, Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention, Biochem Pharmacol 77(6) (2009) 937–46. [DOI] [PubMed] [Google Scholar]
- [99].Marcus AJ, The role of lipids in platelet function: with particular reference to the arachidonic acid pathway, J Lipid Res 19(7) (1978) 793–826. [PubMed] [Google Scholar]
- [100].Mahmud I, Smith DL, Whyte MA, Nelson JT, Cho D, Tokes LG, Alvarez R, Willis AL, On the identification and biological properties of prostaglandin J2, Prostaglandins Leukot Med 16(2) (1984) 131–46. [DOI] [PubMed] [Google Scholar]
- [101].Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA, Role of prostacyclin in the cardiovascular response to thromboxane A2, Science 296(5567) (2002) 539–41. [DOI] [PubMed] [Google Scholar]
- [102].Farrow JW, Willis AL, Proceedings: Thrombolytic and anti-thrombotic properties of dihomo-gamma-linolenate in vitro, Br J Pharmacol 55(2) (1975) 316P–317P. [PMC free article] [PubMed] [Google Scholar]
- [103].Srivastava KC, Metabolism of arachidonic acid by platelets: utilization of arachidonic acid by human platelets in presence of linoleic and dihomo-gamma-linolenic acids, Z Ernahrungswiss 17(4) (1978) 248–61. [DOI] [PubMed] [Google Scholar]
- [104].Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico, Lancet 354(9177) (1999) 447–55. [PubMed] [Google Scholar]
- [105].Lenox CE, Bauer JE, Potential adverse effects of omega-3 Fatty acids in dogs and cats, J Vet Intern Med 27(2) (2013) 217–26. [DOI] [PubMed] [Google Scholar]
- [106].Gong Y, Lin M, Piao L, Li X, Yang F, Zhang J, Xiao B, Zhang Q, Song WL, Yin H, Zhu L, Funk CD, Yu Y, Aspirin enhances protective effect of fish oil against thrombosis and injury-induced vascular remodelling, Br J Pharmacol 172(23) (2015) 5647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hornstra G, Lussenburg RN, Relationship between the type of dietary fatty acid and arterial thrombosis tendency in rats, Atherosclerosis 22(3) (1975) 499–516. [DOI] [PubMed] [Google Scholar]
- [108].Adili R, Voigt EM, Bormann JL, Foss KN, Hurley LJ, Meyer ES, Veldman AJ, Mast KA, West JL, Whiteheart SW, Holinstat M, Larson MK, In vivo modeling of docosahexaenoic acid and eicosapentaenoic acid-mediated inhibition of both platelet function and accumulation in arterial thrombi, Platelets (2017) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]