Abstract
Centella asiatica is a medicinal plant used to enhance memory. We have previously shown that a water extract of Centella asiatica (CAW) attenuates β-amyloid (Aβ)-induced spatial memory deficits in mice and improves neuronal health. Yet the effect of CAW on other cognitive domains remains unexplored as does its In vivo mechanism of improving Aβ-related cognitive impairment. This study investigates the effects of CAW on learning, memory and executive function as well as mitochondrial function and antioxidant response in the 5×FAD model of Aβ accumulation.
Seven month old 5×FAD female mice were treated with CAW (2mg/mL) in their drinking water for two weeks prior to behavioral testing. Learning, memory and executive function were assessed using the object location memory task (OLM), conditioned fear response (CFR) and odor discrimination reversal learning (ODRL) test. Mitochondrial function was profiled using the Seahorse XF platform in hippocampal mitochondria isolated from these animals and tissue was harvested for assessment of mitochondrial, antioxidant and synaptic proteins.
CAW improved performance in all behavioral tests in the 5×FAD but had no effect on WT animals. Hippocampal mitochondrial function was improved and hippocampal and cortical expression of mitochondrial genes was increased in CAW-treated 5×FAD mice. Gene expression of the transcription factor NRF2, as well as its antioxidant target enzymes, was also increased with CAW treatment in both WT and 5×FAD mice. CAW treatment also decreased Aβ-plaque burden in the hippocampus of treated 5×FAD mice but had no effect on plaques in the cortex.
These data show that CAW can improve many facets of Aβ-related cognitive impairment in 5×FAD mice. Oral treatment with CAW also attenuates hippocampal mitochondrial dysfunction in these animals. Because mitochondrial dysfunction and oxidative stress accompany cognitive impairment in many pathological conditions beyond Alzheimer’s disease, this suggests potentially broad therapeutic utility of CAW.
Keywords: Executive function, antioxidant, mitochondrial function, beta amyloid
Introduction
Alzheimer’s disease (AD) is the most common form dementia affecting 5.5 million people in the United States age 65 and older, two-thirds of which are women (Association. 2017). The pathological hallmarks of the disease are the accumulation of amyloid-β (Aβ) plaques and neurofibrillary tangles which accompany synaptic dysfunction, neuronal loss and severe cognitive impairment (Baloyannis 2009, Mavroudis 2011). There is growing evidence that mitochondrial dysfunction and oxidative stress contribute to these deleterious physiological changes (Leuner K 2012). Alterations in mitochondrial bioenergetics as well as mitochondrial mass and enzyme expression in the brain are thought to precede, or even induce, cognitive decline in AD (Brown 2002, Manczak M 2004, Yao J 2009, Swerdlow 2018) and increased oxidative stress is similarly considered to be an early event in the brains of AD patients (Serrano 2004, Lovell 2007). These abnormalities have been observed in many mouse models of AD (Oakley 2006, Du H 2010, Cuadrado-Tejedor M 2013, Bilkei-Gorzo 2014).
The plant Centella asiatica (L) Urban (Apiaceae), also known as Gotu Kola, is used in traditional Chinese and Ayurvedic medicine to improve cognitive function and reverse cognitive impairments (Shinomol GK 2011). The neuroprotective and cognitive enhancing effects of Centella asiatica have been well-documented in in a variety of In vitro and In vivo models of neurodegenerative disease and in the setting of neurotoxic insults (Soumyanath 2005, Gupta 2006, Tabassum, Vaibhav et al. 2013, Lokanathan 2016). Studies from our own lab have shown that a water extract of Centella asiatica (CAW) attenuates Aβ-induced mitochondrial dysfunction and oxidative stress In vitro (Gray 2015, Gray 2017b) and can improve spatial memory in the animals (Soumyanath 2012). We have also recently found that CAW treatment can increase synaptic density and improve executive function in healthy aged mice (Gray 2018). Executive function includes behaviors like attentional selection, behavioral inhibition, cognitive flexibility, task switching, planning and decision-making and is an early marker of cognitive impairment in AD patients (Buckner 2004). Yet the effects of CAW on executive function have not been evaluated in Aβ -overexpressing mice nor have its In vivo effects on mitochondrial function, oxidative stress and synaptic endpoints been investigated in this pathological context. This study aims to address these gaps in understanding and provide evidence for the therapeutic utility of CAW by examining the effects of the extract in the 5×FAD mouse model of Aβ-accumulation.
Materials and Methods
CAW
Dried Centella asiatica aerial parts (lot number 170300206; country of origin India; harvest date 2015) was purchased from Oregon’s Wild Harvest, Redmond, OR. The water extract of Centella asiatica (CAW) was prepared as previously described (Gray 2016). Briefly, a total of 1200g raw plant material was refluxed with 15L of water for 1.5 hours, in several small batches. After filtering the extract to remove plant debris, the liquid was freeze dried to produce 245g of CAW powder. A voucher specimen of the plant material and the CAW are deposited in our laboratory and stored at −20C.
Animals
5×FAD mice were generated from a breeding pair obtained from The Jackson Laboratory. These mice overexpress human amyloid precursor protein (APP) and human presenilin 1 (PS1) with five mutations associated with familial Alzheimer’s Disease: the Swedish (K670N,M671L) Florida (1716V) and London (V717I) mutations in APP as well as two in PS1 (M146L and L286V) (Oakley 2006). Following weaning, litters were genotyped and group housed (4–5 per cage) until the commencement of experiments. Mice were maintained in a climate-controlled environment with a 12-hr light/12-hr dark cycle. Diet and water were supplied ad libitum, except during behavioral testing. All procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the institutional Animal Care and Use Committee of the Portland VA Medical Center.
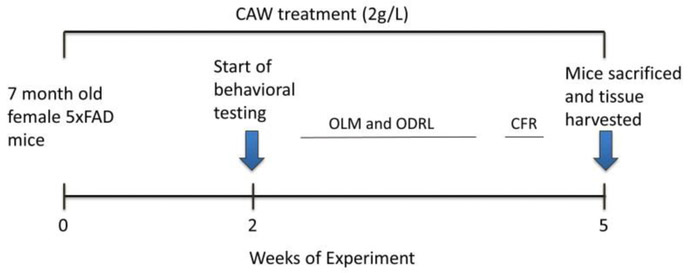
Seven month old female 5×FAD mice and their female WT littermates were either exposed to CAW in their drinking water at 2g/L or to untreated water for two weeks prior to the beginning of behavioral testing and throughout the tests. Water consumption was monitored throughout the experiment to ensure the addition of CAW did not affect overall water intake. Following 3 weeks of behavioral testing animals were sacrificed and tissue harvested as outlined in Figure 1.
Figure 1: Timeline of CAW treatment and behavioral assessment.
Mice were treated with CAW (2g/L) two weeks prior to the beginning of behavioral testing and treatment continued throughout the experiment. After testing, animals were sacrificed and tissue was harvested. CAW treatment lasted a total of 5 weeks.
Behavioral testing
Conditioned Fear Response (CFR): This test consists of three phases: habituation, conditioning and testing. During the habituation phase, animals are exposed to a 16×16×12 inch test chamber for 5 minutes. Immediately following habituation, 3 one-second shocks (0.7 mA) are randomly administered over a 3 minute period, with no more than one shock per minute. The test phase occurs twenty four hours later where animals are reintroduced to the test chamber for 5 minutes with no shock. Freezing behavior in the test phase is recorded. Baseline freezing, during the habituation phase, is subtracted.
Object Location Memory (OLM): This test also consists of three phases: habituation, training and testing. During habituation each mouse is placed in a square arena (38 × 38 × 64 cm high, constructed of white acrylonitrile butadiene styrene) for two 10 minute open field sessions on two consecutive days. During the training phase animals are exposed to two identical objects in fixed locations for 10 minutes, once an hour for 3 hours. The testing phase occurs two hours following the final training phase. In the testing one object is displaced to a novel spatial location and the mouse is allowed to explore the environment for 5 minutes. Time spent exploring the displaced and non-displaced objects is measured via a camera mounted above the arena, interfaced with ANYmaze video tracking system (Stoelting Co, Wood Dale, IL).
Odor discrimination reversal learning (ODRL): This test has three phases: shaping, acquisition and shift. In the shaping phase mice are introduced to the testing chamber and trained to dig for a food reward in lavender scented bedding material. Mice are presented with a single bowl containing the food reward that was progressively filled with bedding in five stages, 0%, 25%, 50%, 75% and 100% filled. The mouse advances to the subsequent training step when it has successfully retrieved the food reward 5 times in a row.
The acquisition phase begins after mice had completed the shaping phase. In this phase mice are presented with two cups one containing dried beans and the other string. In every trial one digging material has a vanilla odor and the other a mint odor and the odor and material pairings were randomly alternated between trials but balanced over the acquisition phase so that each mouse is exposed to roughly equal combinations of each odor and digging material. Whether the baited cup is presented on the right or left side of the apparatus is also balanced throughout testing. In the acquisition phase the mint-scented bowl is always baited regardless of digging material. Example trials are found in Table 1. Each trial is initiated by raising the divider and allowing access to both bowls. Mice are required to make 8 correct digs in any bout of 10 in order to reach criteria. Trials to reach criteria is recorded.
Table 1: Example of test pairings for Odor Discrimination Reversal Learning (ODRL) test.
Representative combinations of odor and digging material pairings during each phase of the ODRL. D1= dried bean, D2= string, O1=vanilla, O2=mint. Italicized indicates correct trial
| Right Position | Left Position | |
|---|---|---|
| Acquisition Phase | D1+O1 | D2+02 |
| D1+02 | D2+01 | |
| D2+01 | D1+02 | |
| D2+02 | D1+O1 | |
| Shift Phase | D1+O1 | D2+02 |
| D1+02 | D2+01 | |
| D2+01 | D1+02 | |
| D2+02 | D1+O1 |
After a mouse reaches criteria in the acquisition phase they immediately proceed to the shift phase. As in the previous phase in the shift phase mice are presented with two cups one containing dried beans and the other string. In every trial one digging material has the vanilla odor and the other the mint odor and again the odor + digging material pairings are balanced throughout the trial as is right/left location of the baited cup. In the shift phase however the cup with the dried beans is always baited regardless of odor. Again criteria is defined as 8 correct trials in any bout of 10 and trials to criteria are recorded. Mice are food restricted the night before each phase of the ODRL in order to motivate the animals.
Isolation of hippocampal mitochondria
Hippocampal mitochondria were isolated using a previously described protocol (Iuso 2017)with slight modifications. Briefly, isolated hippocampi were placed in cold isolation buffer containing 220 mM mannitol, 70 mM sucrose, 5 mM KH2P04, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA and 0.5% BSA (fatty acid free) and homogenized using an Arrow Engineering JR4000 homogenizer. Homogenate was centrifuged at 500g for 5 minutes at 4C. The supernatant fraction was then isolated and centrifuged at 14,000 g for 10minutes at 4C. Then the pellet fraction was resuspended in 12% Percoll and carefully layered on top of 24% Percoll and centrifuged at 16,000 g for 20 minutes at 4C. Protein concentration was assessed by a Bradford Assay.
Analysis of mitochondrial function
Mitochondrial function was assessed using the Seahorse Bioscience XF24 Extracellular Flux Analyzer. Isolated mitochondria were plated on Seahorse XF culture plates (Seahorse Bioscience) at a concentration of 2ug/well with 3-4 replicate wells per animal. The plate was centrifuged for 15 minutes at 2000xg and oxygen consumption rates (OCR) were measured under varying conditions using the MitoStress Kit as previously described (Iuso 2017). After three initial baseline measurements of OCR, a saturating concentration of ADP (2mM) was added to ensure maximum state III respiration and three subsequent measurements were taken. This was followed by the addition of the ATP synthase inhibitor oligomycin (2 μM) to induce state IV respiration and three additional measurements were taken. Next an electron transport chain (ETC) accelerator carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP at 4 μM), was added to induce maximal uncoupled (state IIIu) respiration and after 3 measurements were taken, mitochondrial inhibitors rotenone (1 μM) and antimycin (1 μM) were added, and three final measurements were taken.
Immunohistochemistry
Postmortem, the each hemisphere of the brain was separated for each animal. One hemisphere was fixed in 4% paraformaldehyde and then passed through a sucrose gradient, and frozen. Forty micron frozen coronal sections were then cut on a freezing microtome. Sections were incubated with agitation in blocking buffer (100 mM TBS, pH 8.0, 2 mg/ml bovine serum albumin, 2% horse serum, 0.5% Triton X-100) for 2h, then incubated overnight with primary antibody diluted 1:1000 in blocking buffer (rabbit polyclonal antibodies directed against either Aβ or human AβPP (Invitrogen). Sections were then incubated for 2 h with biotinylated secondary antibody (1:200, Vector Labs, Burlingame, CA), for 2 h with an avidin-linked peroxidase complex (ABC, Vector Labs), then developed with diaminobenzidine (DAB, Sigma) in PBS. Sections were washed, mounted in Permount (Fisher Scientific, Pittsburg, PA) and cover slipped. Protein expression was quantified in at least three coronal sections from each mouse, representing anterior, middle and posterior hippocampus and cortex. Hippocampal and cortical areas were traced using a computerized stage and stereo investigator software (Image J, Wayne Rasband, NIH, USA). Aβ levels were expressed as percentage of hippocampus or cortex occupied by these plaques. Mean values for each parameter were calculated from at least three sections per animal.
Gene Expression
Hippocampal, and cortical was homogenized and RNA was extracted using Tri-Reagent (Molecular Research Center). RNA was reverse transcribed with the Superscript III First Strand Synthesis kit (Invitrogen) to generate cDNA as per the manufacturer’s instructions. Relative gene expression was determined using TaqMan Gene Expression Master Mix (Invitrogen) and commercially available TaqMan primers (Invitrogen) for synaptophysin, post-synaptic density protein 95 (PSD95), mitochondrially encoded NADH dehydrogenase 1 (MtND1), mitochondrially encoded cytochrome B (Mt-CYB), mitochondrially encoded cytochrome c oxidase 1 (Mt-CO1), mitochondrially encoded ATP synthase 6 (Mt-ATP6), nuclear factor(erythroid-derived 2)-like 2 (NFE2L2; NRF2), NAD(P) H dehydrogenase-quinone oxidoreductase 1 (NQO1), glutamatecysteine ligase catalytic subunit (GCLC), heme oxygenase 1 (HMOX1), and glyceraldehyde-3phosphate dehydrogenase (GAPDH). Quantitative PCR (qPCR) was performed on a StepOne Plus Machine (Applied Biosystems) and analyzed using the delta-delta Ct method.
Graphs and Statistics
All bar graphs have error bars reflecting standard error of the mean. Statistical significance was determined using one- or two-way analysis of variance or with appropriate t-tests. Bonferroni post-hoc tests were also conducted. Significance was defined as p ≤0.05. Analyses were performed using Excel or GraphPad Prism 6.
Results
CAW attenuates Aβ-induced deficits in spatial memory
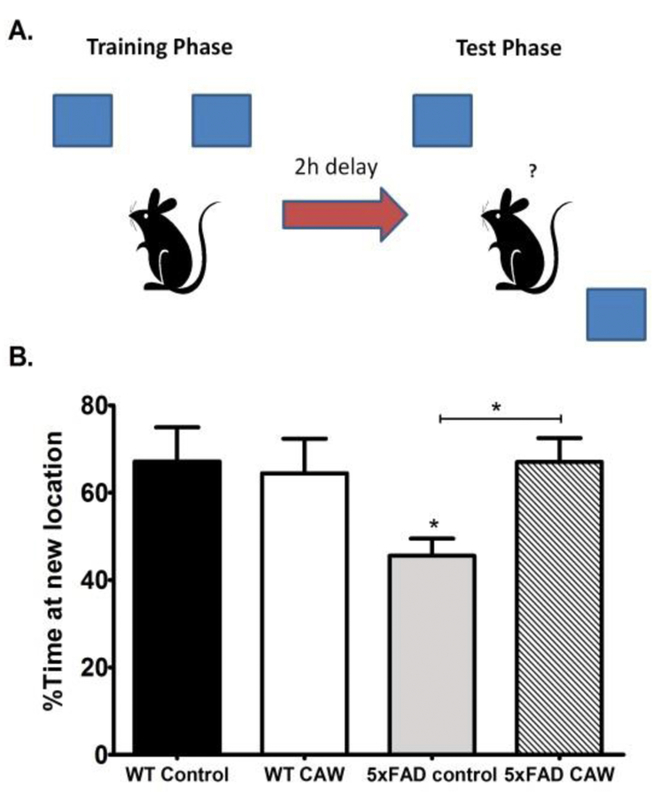
We have previously demonstrated the CAW improves spatial memory in aged (20 month old) mice, both in healthy WTs (Gray 2016) as well as the Tg2576 model of Aβ accumulation (Soumyanath 2012). To validate these findings in the 5×FAD mouse line we used the object location memory (OLM) test. The OLM is a test of spatial memory wherein the mouse using identical objects one of which is moved during testing to a location distinct from where it was during training (Figure 2A). If the mouse remembers the training location it should spend a greater amount of time with the object in the new location. We found that 5×FAD mice had significantly impaired spatial memory (p<0.05) but this deficit was attenuated by CAW treatment (p<0.05; Figure 2B). CAW treatment did not affect spatial memory in WT animals (Figure 2B).
Figure 2: CAW treatment improves object location memory retention in 5×FAD mice.
A) Schematic of the Object Location Memory task (OLM) set up.
B) At the 2h retention time point 5×FAD mice showed significantly reduced preference for the novel location. CAW-treatment attenuated this impairment in 5×FAD mice. n=5-7 mice in each group, *p<0.05
CAW improves contextual memory in Aβ overexpressing mice
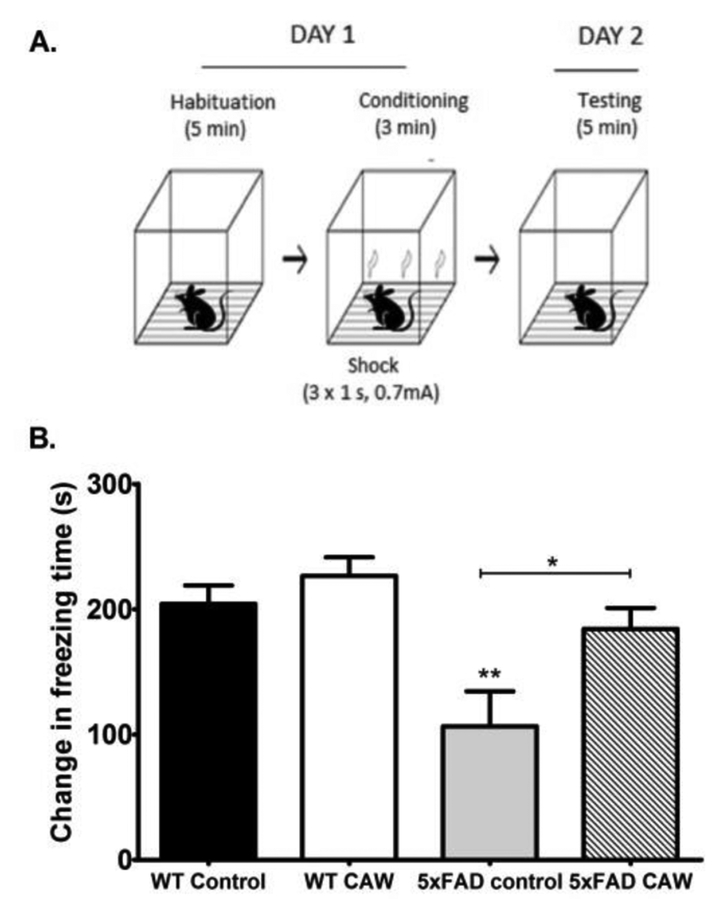
The conditioned fear response test (CFR) assesses contextual memory. In this test the amount of time a mouse freezes when placed in a chamber where it previously received a shock is measured. If the mouse remembers the association of the chamber with the foot shock it will freeze longer (Figure 3A). We observed that 5×FAD mice spent significantly less time freezing than their WT littermates (p<0.01). CAW treatment restored the freezing behavior in the 5×FAD mice (p<0.05) but had no effect on freezing in WT animals (Figure 3B).
Figure 3: CAW treatment improves contextual memory in 5×FAD mice.
A) Schematic of the conditioned fear response (CFR) testing set up.
B) 5×FAD mice froze significantly less in the testing phase than WT mice. CAW restored freezing behavior in 5×FAD mice to WT levels but had no effect on WT mice. Data is represented as the difference in freezing time between the test phase and the habituation phase, n=5-7 mice in each group, *p<0.05, **p<0.01.
CAW restores executive function in Aβ overexpressing mice
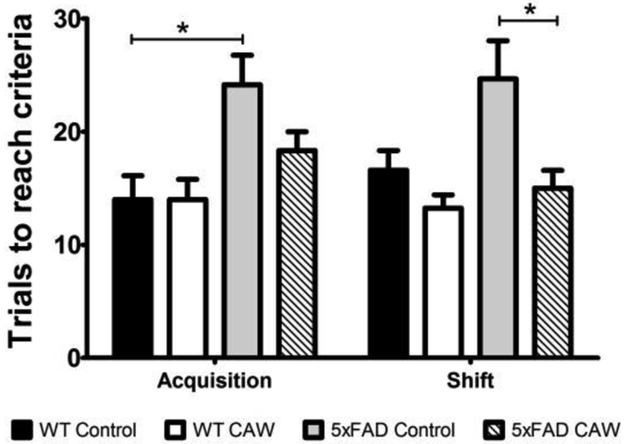
Executive function was assessed in the animals using the odor discrimination reversal learning (ORDL) test. The test occurs in two phases: acquisition and shift. The acquisition phase of the test evaluates learning while the shift phase targets executive function specifically. 5×FAD mice showed impairments in both the acquisition and shift phases of ODRL testing relative to their WT littermates, taking more trials to reach criteria in each phase of the test. Although the same trend was observed in each phase it was only statistically significant in the acquisition phase (p<0.05). CAW treatment appeared to attenuate these deficits in the 5×FAD mice, significantly so in the shift phase (p<0.05), but did not result in significant improvement in the WT animals (Figure 4).
Figure 4: CAW treatment improves executive function in 5×FAD mice.
In the acquisition phase of the ODRL the number of trials to reach criteria was significantly increased in 5×FAD mice. The same trend was evident in the shift phase. CAW treatment reduced the number of trials to reach criteria in both phases of the ODRL although this reduction only reached statistical significance in the shift phase. n=5–7 mice in each group, *p<0.05.
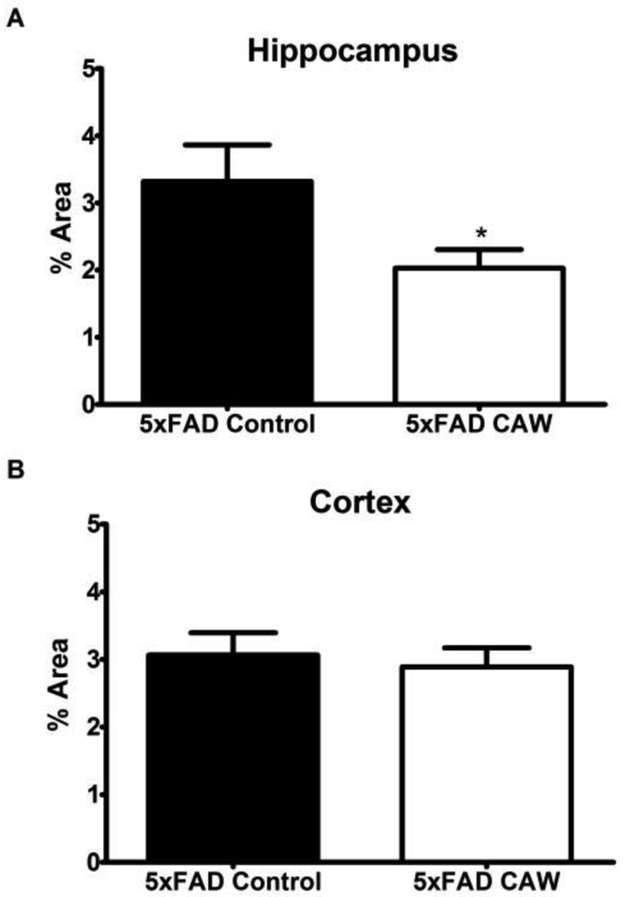
CAW reduces Aβ plaques in the hippocampus but not cortex of 5×FAD mice.
The plaque burden in the hippocampus of 5×FAD mice was significantly lower following CAW treatment (p<0.05; Figure 5A). Interestingly this decrease was not apparent in the cortex of these animals (Figure 5B). WT animals had no measurable Aβ accumulation in any brain region (data not shown).
Figure 5: CAW reduces Aβ accumulation in the hippocampus but not the cortex of 5×FAD mice.
A) CAW significantly reduced the Aβ plaque burden in the hippocampus of 5×FAD mice.
B) There was no effect of CAW treatment on Aβ accumulation in the cortex. n=10-14 mice each group, *p<0.05
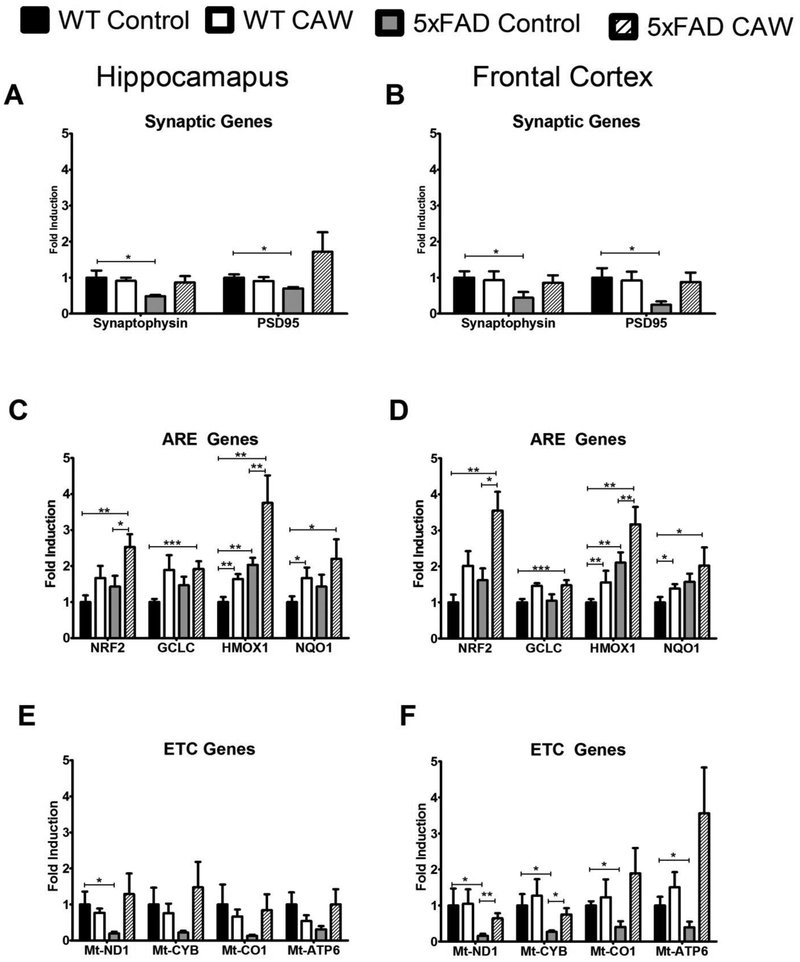
CAW treatment increases hippocampal and cortical expression of synaptic and antioxidant response genes.
5×FAD mice had significantly reduced synaptic gene expression in both the hippocampus and frontal cortex relative to WT mice (p<0.05). CAW treatment increased this expression in 5×FAD mice in both brain regions but had no effect on WT animals (Figure 6A and B).
Figure 6: CAW increases mitochondrial, antioxidant and synaptic proteins in the hippocampus and cortex of 5×FAD mice.
Synaptic gene expression was reduced in 5×FAD mice in the hippocampus (A) and frontal cortex (B) but this was attenuated by CAW treatment. CAW treatment also increased expression of NRF2 and its ARE-containing target genes in the hippocampus (C) and frontal cortex (D) of both 5×FAD and WT animals. 5×FAD mice had a reduction in expression of ETC genes in the hippocampus (E) and frontal cortex (F) and CAW treatment increased this expression in both brain regions. n=8-10, *p<0.05, **p<0.01, ***p<0.001.
There was no consistent significant genotype effect on antioxidant gene expression although there was a trend toward a coordinate increase in expression of NRF2 and its antioxidant response element (ARE)-containing target genes in both brain regions in the 5×FAD animals relative to WT littermates and a trend toward further increase in each genotype following CAW treatment (Figure 6C and 6D). CAW significantly increased HMOX1 and NQO1 in WT animals (p<0.01 and p<0.05 respectively) and increased the expression NRF2 and HMOX1 in 5×FAD mice (p<0.05 and p<0.01 respectively).
CAW attenuates deficits in mitochondrial gene expression and improves hippocampal mitochondrial function in Aβ overexpressing mice
There was a trend toward reduced expression of genes encoding proteins in the electron transport chain (ETC) in the hippocampus of 5×FAD mice (Figure 6E) and a significantly reduction in the frontal cortex of these animals (p<0.05; Figure 6F). CAW-treatment restored this expression to WT levels. WT animals were not affected by CAW treatment (Figure 6E and 6F).
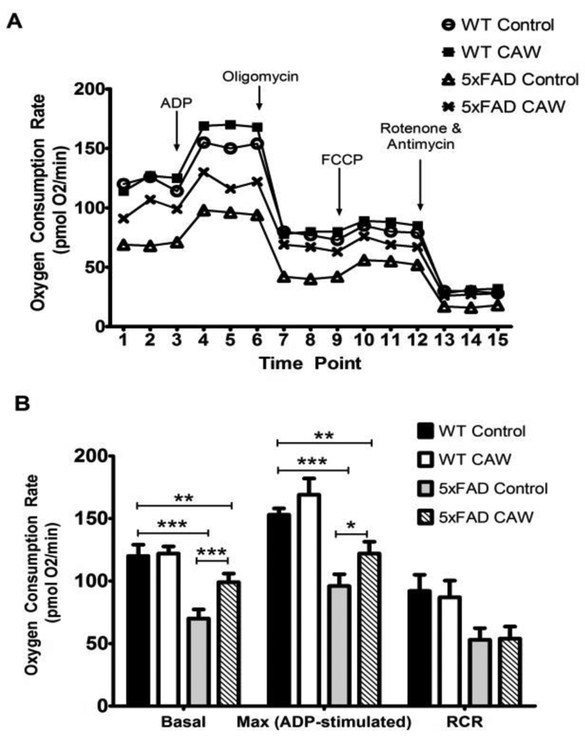
5×FAD mice also had a deficit in hippocampal mitochondrial bioenergetics that was likewise attenuated with CAW treatment (Figure 7A). CAW signficantly improved basal and ADP stimulated, state III, respiration in mitochondria isolated from 5×FAD mice (p<0.001 and p<0.05 respectively) but did not alter the respiratory control ratio (RCR, the difference between state III and oligomycin induced state IV respiration). Consistent with previous reports in isolated brain mitochondria there was only a modest increase in uncoupled or state IIIu respiration following FCCP stimulated in both genotypes. CAW did not affect the response to FCCP in either 5×FAD or WT animals. In fact CAW did not affect any of these respiratory parameters in mitochondria isolated from WT animals (Figure 7B).
Figure 7: CAW increases mitochondrial respiration in the hippocampus of 5×FAD mice.
A) The bioenergetic profile of 5×FAD mice, as determined by Seahorse XF analyzer, was impaired relative to WT mice and CAW treatment partially attenuated this impairment. B) CAW reduced the deficit in both basal and maximal respiration in hippocampal mitochondria isolated from 5×FAD mice but had no effect on RCR. n=4-5 per group, *p<0.05, **p<0.01, ***p<0.001.
Discussion
The cognitive-enhancing effects of Centella asiatica have been well documented in animal models as well as human trials (Gupta YK 2003, Veerendra Kumar MH 2003, Tiwari S 2008, Mato, Wattanathorn et al. 2011). Our lab has previously reported that CAW improves performance in the Morris Water Maze (MWM) in mice exposed to Aβ as well as healthy older mice (Soumyanath 2012, Gray 2016). In this study we further explore the effects of CAW on other domains of cognitive function as well as its mechanism of action.
We found that two weeks of CAW treatment prior to, and continuing throughout, behavioral testing (Figure 1) improved performance of 5×FAD mice in both the OLM and CFR tests. The OLM is a hippocampal dependent test of spatial memory (Cipolotti L 2006, Assini 2009) which is known to be impaired in AD patients (Kessels 2010, Moodley 2015). Contextual memory, which is assessed by CFR, is also hippocampally-mediated and is similarly impaired in AD patients (El Haj 2013). Animal models of AD recapitulate the deficits in both of these tasks (Good 2007, Dong 2008, Kim 2013, Yang 2018). The improvement in OLM and CFR seen in this study are consistent with previous reports showing that triterpenes, a class of compound abundant in Centella asiatica (Siddiqui 2007), can prevent deficits in the same tasks in of mouse models of AD as well as in healthy aged mice (Sirichoat 2015, Li 2016, Loganathan 2018). It is also in line with studies showing that both OLM and CFR performance by cognitively impaired mice is improved by treatment with polyphenols (de Oliveira 2014, Welbat 2016, Yimam 2016, Bassani 2017), a class of compounds which both Centella asiatica in general (Siddiqui 2007, Subban 2007) and CAW specifically are rich in(Soumyanath 2012, Gray 2014, Gray 2017a). It is likewise consistent with our lab’s previous finding that CAW improved performance in the MWM, another hippocampal test of spatial memory, in the Tg2576 model of Aβ accumulation (Soumyanath 2012).
While it has previously been shown that Tg2576 mice have impaired ODRL performance (Zhuo 2007, Zhuo 2008) to our knowledge this is the first report of executive function impairments in the 5×FAD mouse model and the first to report improvements in executive function with an intervention in any mouse model of Aβ accumulation. Executive function includes elements like impulse control, attenton, planning, cognitive flexibility and problem solving. It is mediated by the prefrontal cortex and is very sensitive to age-related decline (Buckner 2004, Raz 2006). The Wisconsin Card Sorting Test (WCST) is one of a number of widely used tests to assess executive function in humans. In this task subjects are required to adapt behavioral responses to choose the “correct” stimulus array based on sudden rule changes across multiple modalities (Eling 2008). Performance in this task declines with age and is impaired in AD (Silva-Filho 2007, Ashendorf 2008). The ODRL, also called attention set shifting task, is a parallel test that has been developed for rats and, more recently, mice (Birrell 2000, Garner 2006). Like the WCST the ODRL requires paying attention to relevant stimuli while ignoring irrelevant stimuli and subsequently shifting the attention, either within dimensions or between dimensions of the test stimuli (Birrell 2000). Also like the WCST performance in the ODRL declines with age and is impaired in models of AD (Barense 2002, Zhuo 2007, Young 2009, Marchese 2014).
We found that CAW treatment improved performance of 5×FAD mice in both the acquisition and shift phases of this test. The acquisition phase of the ODRL assesses classical learning and the improvement observed following CAW treatment is in line with our previous report of enhanced performance of CAW-treated Tg2576 mice in the hidden platform phase of the MWM. The shift phase of ODRL is the metric of cognitive flexibility. CAW treatment also attenuated impairments in this dimension of cognitive function in 5×FAD mice. This finding is consistent with a previous clinical trial showing that a Centella asiatica supplement improved executive function in healthy middle aged adults (Dev RDO 2009).
We also observed that CAW treatment significantly reduced the Aβ plaque burden in the hippocampus of 5×FAD animals. Notably this change was not seen in the cortex. Our previous report in Tg2576 mice found that Aβ levels were unchanged in the cortex of treated animals but did not evaluate hippocampal levels (Soumyanath 2012). It is interesting that there is a regionally specific effect of CAW on Aβ however the fact that CAW-induced behavioral improvements were seen in both hippocampal- and cortically-mediated tasks suggests that a reduction in Aβ is not necessary for the cognitive enhancing effects of the extract. Our synaptic gene expression data also supports this conclusion. We found synaptic gene expression was similarly increased in both the hippocampus and cortex following CAW treatment. Since increased synaptic density is known to correlate with improved cognitive function (Terry 1991) this is likely the physiological underpinning of the improvement in both the hippocampal- and cortically-mediated cognitive tests seen in this study.
Interestingly there was no effect of CAW on WT animals in any of the cognitive tests. This was somewhat surprising as we have recently reported that CAW can improve performance in OLM and ODRL in aged 20 month old WT animals (Gray 2018). It is possible that since the mice in this study were significantly younger, 7 months old, they do not have any cognitive deficits and therefore may already be maximally performing in these tasks. Indeed, this would be consistent with our previous report that CAW did failed to improve MWM behavior in young 2 month old WT mice (Gray 2016).
Increased mitochondrial dysfunction and oxidative stress are evident in the AD brain. Studies suggest that alterations in mitochondrial bioenergetics in the brain precede and may even induce cognitive decline in AD (Yao J 2009) and reduced neuronal mitochondrial function and number have been reported in AD patients (Hirai K, Vinters HV et al. 2001). These changes in mitochondrial function have been recapitulated in many mouse models of AD including the 5×FAD mice (Devi 2012, Wang 2016). In this study we found that oral CAW treatment can attenuate reductions in mitochondrial gene expression and improve mitochondrial function in the hippocampus of treated 5×FAD mice. To our knowledge this is the first report of impaired In vivo mitochondrial function in the hippocampus of these animals. Interestingly we found that CAW treatment increased basal and ADP-stimulated respiration in 5×FAD hippocampal mitochondria but had no effect on RCR. RCR is a measure of how tightly coupled respiration is to phosphorylation and decreases in RCR are associated with lower capacity for substrate oxidation and ATP turnover (Iuso 2017). The fact that CAW affects basal and maximal ADP-stimulated respiration but not RCR may reflect an increase in mitochondrial content following CAW treatment, rather than increased the activity or efficiency of individual ETC enzymes. The coordinate induction of mitochondrial genes by CAW seen in this study is also consistent with increased mitochondrial number; however further studies using electron microscopy are necessary to definitively confirm an effect of CAW on mitochondrial biogenesis. These studies are currently underway in our lab.
It would also be interesting in future experiments to profile the bioenergetics of mitochondria isolated from different brain regions. Our gene expression data suggests CAW has a global effect on mitochondrial gene expression but it is possible that it could have regionally specific effect on activity. If there is specificity to the activity change that would provide evidence that mitochondrial effect is independent of the cognitive enhancing effects of CAW as we did observe behavioral improvements in tasks mediated by multiple brain regions.
Antioxidant gene expression was also increased with CAW treatment. Both hippocampal and cortical expression of the transcription factor NRF2 and its target genes were all upregulated in the brains of treated WT and 5×FAD mice. NRF2 regulates the endogenous antioxidant response pathway by increasing the expression cytoprotective genes (Motohashi 2004). NRF2 expression and activity has been reported to be altered in AD (Ramsey 2007) and activation of NRF2 by various compounds has been shown to protect against Aβ-induced cell death (Kanninen K 2008) and improve mitochondrial function in healthy mice and human neuroblastoma cells (Hayashi 2017). It remains to be seen what role the activation of NRF2 plays in the cognitive enhancing and mitochondrial effects of CAW but experiments are underway in the lab to evaluate the effects of the extract on NRF2KO mice to address these questions.
Although women are disproportionately affected by AD, men do make up about one third of AD patient. Studies are underway in our lab to confirm the results seen in this study in male mice to inform the potential therapeutic utility of CAW in both genders. Additionally because CAW is a complex mixture it is of great interest to determine which compounds within the extract are responsible for its various effects. We have previously demonstrated that triterpenes and caffeoylquinic acids from CAW activate NRF2 and improve mitochondrial function in neuroblastoma cells exposed to Aβ (Gray 2015) and these same compounds increase synaptic density in neurons isolated from Aβ-overexpressing animals (Gray 2017a). Studies are ongoing to determine the cognitive and mitochondrial effects of these compounds In vivo.
Conclusions
This study further demonstrates the cognitive enhancing effects of CAW. Relatively short treatment with the extract increased the expression of synaptic genes and improved several different domains of cognitive performance in 5×FAD animals, including learning, memory and executive function. These cognitive-enhancing effects appear independent of Aβ plaque reduction because while CAW reduced the Aβ plaque burden in the hippocampus, it did not affect Aβ levels in the cortex, yet improvements were observed in both hippocampal and cortically mediated tasks. CAW treatment also improved hippocampal mitochondrial function and induced the expression of mitochondrial as well as antioxidant response genes in the brains of 5×FAD mice. While the exact relationship between these effects remains to be elucidated, as well as which compounds from the extract are responsible for each effect, the fact that synaptic dysfunction and cognitive impairment accompany oxidative stress and mitochondrial dysfunction in many neurological conditions as well as in healthy aging suggests a therapeutic potential for CAW beyond AD.
Highlights.
Centella asiatica improves spatial and contextual memory in Aβ-overexpressing mice
Centella asiatica also attenuates Aβ-induced deficits in executive function
Centella asiatica increases mitochondrial, antioxidant and synaptic gene expression
Oral treatment of Centella asiatica improves hippocampal mitochondrial function
Acknowledgements
This work was funded by NIH-NCCIH grant R00AT008831 (Gray), NIH-NCCIH grant R01AT008099 (Soumyanath), NIH-NCCIH T32 AT002688 (Wright) and a Department of Veterans Affairs Merit Review grant awarded to J. Quinn. The authors acknowledge Dr. Matthew Lattal and Dr. Gregory Peters for their assistance with the behavioral assays.
Abbreviations
- AD
Alzheimer’s Disease
- ADP
Adenosine diphosphate
- Aβ
β-amyloid
- CAW
Water extract of Centella asiatica
- CFR
Conditioned fear response
- ETC
Electron transport chain
- FCCP
Carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone
- GAPDH
Glyceraldehyde-3phosphate dehydrogenase
- GCLC
Glutamatecysteine ligase catalytic subunit
- HMOX1
Heme oxygenase 1
- Mt-ATP6
Mitochondrially encoded ATP synthase 6
- Mt-CO1
Mitochondrially encoded cytochrome c oxidase 1
- Mt-CYB
Mitochondrially encoded cytochrome B
- Mt-ND1
Mitochondrially encoded NADH dehydrogenase 1
- MWM
Morris water maze
- NQO1
NAD(P) H dehydrogenase-quinone oxidoreductase 1
- NRF2
nuclear factor(erythroid-derived 2)-like 2 (also called NFE2L2)
- OCR
Oxygen consumption rate
- ODRL
Odor discrimination reversal learning
- OLM
Object Location Memory
- PSD95
Post-synaptic density protein 95
- qPCR
Quantitative polymerase chain reaction
- RCR
Respiratory control ratio
- TBS
Tris buffered saline
- ATP
Adeonsine triphosphate
- WCST
Wisconsin card sorting test
- WT
Wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashendorf L, McCaffrey RJ. (2008). “Exploring age-related decline on the Wisconsin Card Sorting Test.” Clin Neuropsychol 22(2): 262–272. [DOI] [PubMed] [Google Scholar]
- Assini F, Duzzioni M, Takahashi RN. (2009). “Object location memory in mice: pharmacological validation and further evidence of hippocampal CA1 participation.” Behav Brain Res 204(1): 206–211. [DOI] [PubMed] [Google Scholar]
- Association., A. s. (2017). “Alzheimer’s disease facts and figures.” Alzheimer’s and Dementia 13: 325–373. [Google Scholar]
- Baloyannis SJ (2009). “Dendritic pathology in Alzheimer’s disease.” J Neurol Sci. 283(1–2): 153–157. [DOI] [PubMed] [Google Scholar]
- Barense M, Fox MT, Baxter MG. (2002). “Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats.” Learn Mem 9(4): 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani T, Turnes JM, Moura ELR, Bonato JM, Cóppola-Segovia V, Zanata SM, Oliveira RMMW, Vital MABF. (2017). “Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type.” Behav Brain Res 335: 41–54. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A (2014). “Genetic mouse models of brain ageing and Alzheimer’s disease.” Pharmacol Ther 142(2): 244–257. [DOI] [PubMed] [Google Scholar]
- Birrell J, Brown VJ (2000). “Medial frontal cortex mediates perceptual attentional set shifting in the rat.” J Neurosci 20(11): 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G, Borutaite V (2002). “Nitric oxide inhibition of mitochondrial respiration and its role in cell death.” Free Radic Biol Med 33: 1440–1450. [DOI] [PubMed] [Google Scholar]
- Buckner R (2004). “Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate.” Neuron 44(1): 195–208. [DOI] [PubMed] [Google Scholar]
- Cipolotti L BC (2006). “Amnesia and the hippocampus.” Curr Opin Neurol 19(6): 593–598. [DOI] [PubMed] [Google Scholar]
- Cuadrado-Tejedor M, C. J., Zamarbide M, Gómez-Isla T, Franco R, Perez-Mediavilla A. (2013). “Age-related mitochondrial alterations without neuronal loss in the hippocampus of a transgenic model of Alzheimer’s disease.” Curr Alzheimer Res 10(4): 390–405. [DOI] [PubMed] [Google Scholar]
- de Oliveira D, Zamberlam CR, Gaiardo RB, Rêgo GM, Cerutti JM, Cavalheiro AJ, Cerutti SM1. (2014). “Flavones from Erythrina falcata are modulators of fear memory.” BMC Complement Altern Med 15: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev RDO, M. S., Hambali Z, Samah BA (2009). “Comparison on cognitive effects of Centella asiatica in healthy middle aged female and male volunteers. .” Eur J Sci Res 31: 553–565. [Google Scholar]
- Devi L, Ohno M (2012). “Mitochondrial dysfunction and accumulation of the β-secretase-cleaved C-terminal fragment of APP in Alzheimer’s disease transgenic mice.” Neurobiol Pis 45(1): 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Coughlan C, Lewis B, Csernansky JG. (2008). “Effects of memantine on neuronal structure and conditioned fear in the Tg2576 mouse model of Alzheimer’s disease.” Neuropsychopharmacology 33(13): 3226–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H GL, Yan S, Sosunov AA, McKhann GM, Yan SS. (2010). “Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. .” Proc.Natl.Acad.Sci 107: 18670–18675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Haj M, Kessels RPC (2013). “Context Memory in Alzheimer’s Disease.” Dement Geriatr Cogn Dis Extra 3(1): 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eling P, Derckx K, Maes R (2008). “On the historical and conceptual background of the Wisconsin Card Sorting Test.” Brain Cogn 67(3): 247–253. [DOI] [PubMed] [Google Scholar]
- Garner J, Thogerson CM, Würbel H, Murray JD, Mench JA (2006). “Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice.” Behav Brain Res 173(1): 53–61. [DOI] [PubMed] [Google Scholar]
- Good M, Hale G (2007). “The “Swedish” mutation of the amyloid precursor protein (APPswe) dissociates components of object-location memory in aged Tg2576 mice.” Behav Neurosci 121(6): 1180–1191. [DOI] [PubMed] [Google Scholar]
- Gray N, Harris CJ, Quinn JF, Soumyanath A. (2016). “Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice.” J Ethnopharmacol 180: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N, Morré J, Kelley J, Maier CS, Stevens JF, Quinn JF, Soumyanath A (2014). “Caffeoylquinic Acids in Centella asiatica Protect against Amyloid-β Toxicity.” J Alzheimer’s Dis 40: 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N, Sampath H, Zweig JA, Quinn JF, Soumyanath A (2015). “Centella asiatica Attenuates Amyloid-β-Induced Oxidative Stress and Mitochondrial Dysfunction.” Journal of Alzheimer’s Disease 45(3): 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N, Zweig JA, Caruso M, Martin MD, Zhu JY, Quinn JF, Soumayanath A (2018). “Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice.” Brain and Behavior Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N, Zweig JA, Matthews DG, Caruso M, Quinn JF, Soumyanath A. (2017b). “Centella asiatica Attenuates Mitochondrial Dysfunction and Oxidative Stress in Aβ-Exposed Hippocampal Neurons.” Oxid Med Cell Longev 2017: 7023091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N, Zweig JA, Murchison C, Caruso M, Matthews DG, Kawamoto C, Harris CJ, Quinn JF, Soumyanath A. (2017a). “Centella asiatica attenuates Aβ-induced neurodegenerative spine loss and dendritic simplification.” Neursci Lett 646: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Flora SJ (2006).” Effect of Centella asiatica on arsenic induced oxidative stress and metal distribution in rats.” J Appl Toxicol 26: 21322. [DOI] [PubMed] [Google Scholar]
- Gupta YK, V. K. M., and Srivastava AK (2003). “Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats.” Pharmacology Biochemistry and Behavior 74(3): 579–585. [DOI] [PubMed] [Google Scholar]
- Hayashi G, Jasoliya M, Sahdeo S, Saccà F, Pane C, Filla A, Marsili A, Puorro G, Lanzillo R, Brescia Morra V, Cortopassi G. (2017). “Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans.” Hum.Mol.Genet 26(15): 2864–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, A. G., Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y,,T. M. Vinters HV, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen and P. G. RB, Smith MA. (2001). “Mitochondrial abnormalities in Alzheimer’s disease. .” J.Neurosci 21: 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuso A, Repp B, Biagosch C, Terrile C, Prokisch H. (2017). “Assessing Mitochondrial Bioenergetics in Isolated Mitochondria from Various Mouse Tissues Using Seahorse XF96 Analyzer.” Methods Mol Biol 1567: 217–230. [DOI] [PubMed] [Google Scholar]
- Kanninen K, M. T., Jyrkkänen HK, Goldsteins G, Keksa-Goldsteine V, Tanila H, Yamamoto M, Ylä-Herttuala S, Levonen AL, Koistinaho J. (2008). “Nuclear factor erythroid 2-related factor 2 protects against beta amyloid.” Mol Cell Neurosci 39(3): 302–313. [DOI] [PubMed] [Google Scholar]
- Kessels R, Rijken S, Joosten-Weyn Banningh LW, Van Schuylenborgh-VAN Es N, Olde Rikkert MG. (2010). “Categorical spatial memory in patients with mild cognitive impairment and Alzheimer dementia: positional versus object-location recall.” J Int Neuropsychol Soc 16(1): 200–204. [DOI] [PubMed] [Google Scholar]
- Kim C, Nam DW, Park SY, Song H, Hong HS, Boo JH, Jung ES, Kim Y, Baek JY, Kim KS, Cho JW, Mook-Jung I. (2013). “O-linked β-N-acetylglucosaminidase inhibitor attenuates β-amyloid plaque and rescues memory impairment.” Neurobiol Aging 34(1): 275–285. [DOI] [PubMed] [Google Scholar]
- Leuner K, M. W., Reichert AS. (2012). “From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease.” Mol Neurobiol 46(1): 186–193. [DOI] [PubMed] [Google Scholar]
- Li F, Wu X, Li J, Niu Q. (2016). “Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer’s disease model.” Mol Med Rep 13(6): 4904–4910. [DOI] [PubMed] [Google Scholar]
- Loganathan C, Thayumanavan P (2018). “Asiatic acid prevents the quinolinic acid-induced oxidative stress and cognitive impairment.” Metab Brain Dis 33(1): 151–159. [DOI] [PubMed] [Google Scholar]
- Lokanathan Y, Omar N, Ahmad Puzi NN, Saim A, Hj Idrus R. (2016). “Recent Updates in Neuroprotective and Neuroregenerative Potential of Centella asiatica.” Malays J MEd Sci 23(1): 4–14. [PMC free article] [PubMed] [Google Scholar]
- Lovell M, Markesbery WR (2007). “Oxidative damage in mild cognitive impairment and early Alzheimer’s disease.” J. Neurosci Res 85(2007): 3036–3040. [DOI] [PubMed] [Google Scholar]
- Manczak M, P. B., Jung Y, Reddy PH. (2004). “Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. .” Neuromolecular Med 5:147–162. [DOI] [PubMed] [Google Scholar]
- Marchese M, Cowan D, Head E, Ma D, Karimi K, Ashthorpe V, Kapadia M, Zhao H, Davis P, Sakic B (2014). “Autoimmune manifestations in the 3×Tg-AD model of Alzheimer’s disease.” J Alzheimers Dis 39(1): 191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato L, Wattanathorn J, Muchimapura S, Tongun T, Piyawatkul N, Yimtae K, Thanawirattananit P and Sripanidkulchai B (2011). “Centella asiatica Improves Physical Performance and Health-Related Quality of Life in Healthy Elderly Volunteer.” Evid Based Complement Alternat Med 2011: 579467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroudis I, Fotiou DF, Manani MG, Njaou SN, Frangou D, Costa VG, Baloyannis SJ. (2011). “Dendritic pathology and spinal loss in the visual cortex in Alzheimer’s disease: a Golgi study in pathology.” Int. J Neurosci 121(7): 347–354. [DOI] [PubMed] [Google Scholar]
- Moodley K, Minati L, Contarino V, Prioni S, Wood R, Cooper R, D’Incerti L, Tagliavini F, Chan D. (2015). “Diagnostic differentiation of mild cognitive impairment due to Alzheimer’s disease using a hippocampus-dependent test of spatial memory.” Hippocampus 25(8): 939–951. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M (2004). “Nrf2-Keap1 defines a physiologically important stress response mechanism.” Trends Mol Med 10(11): 549–557. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R (2006). “Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation..” J Neurosci 26(40): 10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey C, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL. (2007). “Expression of Nrf2 in neurodegenerative diseases.” J Neuropathol Exp Neurol 66(1): 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM (2006). “Differential aging of the brain: patterns, cognitive correlates and modifiers.” Neurosci Biobehav Rev 30(6): 730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano F, Klann E (2004). “Reactive oxygen species and synaptic plasticity in the aging hippocampus.” Ageing Res Rev 3(4): 431–443. [DOI] [PubMed] [Google Scholar]
- Shinomol GK, M., Bharath MM (2011). “Exploring the role of “Brahmi” (Bocopa monnieri and Centella asiatica) in brain function and therapy.” Recent Pat Endocr Metab Immune Drug Discov 5(1): 33–49. [DOI] [PubMed] [Google Scholar]
- Siddiqui BS, Aslam H, Ali ST, Khan S & Begum S, (2007). “Chemical constituents of Centella asiatica.” Journal of Asian natural products research 9(3–5): 407–414. [DOI] [PubMed] [Google Scholar]
- Silva-Filho J, Pasian SR, do Vale FAC. (2007). “Typical performance of elderlypatients with Alzheimer disease on the Wisconsin CardSorting Test (WCST).” Dement Neuropsychol 1(2): 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichoat A, Chaijaroonkhanarak W, Prachaney P, Pannangrong W, Leksomboon R, Chaichun A, Wigmore P, Welbat JU. (2015). “Effects of Asiatic Acid on Spatial Working Memory and Cell Proliferation in the Adult Rat Hippocampus.” Nutrients 7(10): 8413–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumyanath A, Zhong YP, Henson E, Wadsworth T, Bishop J, Gold BG, Quinn JF., (2012). “Centella asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer’s Disease: Investigation of a Possible Mechanism of Action. .” Int J Alzheimers Dis,: 381974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumyanath A, Zhong YP, Gold SA, Yu X, Koop DR, Bourdette D & Gold BG, (2005). “ Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in vitro.” Journal of Pharmacy & Pharmacology 57(9): 1221–1229. [DOI] [PubMed] [Google Scholar]
- Subban R, Veerakumar A, Manimaran R, Hashim KM, Balachandran I. (2007). “Two new flavonoids from Centella asiatica (Linn.).” J Nat Med 62(3): 369–373. [DOI] [PubMed] [Google Scholar]
- Swerdlow R (2018). “Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease.” J Alzheimers Dis 62(3): 1403–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassum R, Vaibhav K, Shrivastava P, Khan A, Ejaz Ahmed M, Javed H, Islam F, Ahmad S, Saeed Siddiqui M, Safhi M and Islam F (2013). “Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats. .” Neurol Sci 34: 925–933. [DOI] [PubMed] [Google Scholar]
- Terry R, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. (1991). “Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment.” Ann Neurol 30(4): 572–580. [DOI] [PubMed] [Google Scholar]
- Tiwari S, S. S., Patwardhan K, Gehlot S, Gambhir IS (2008). “Effect of Centella asiatica on mild cognitive impairment (MCI) and other common age-related clinical problems. .” Digest J Nanomat Biostructures 3: 215–220. [Google Scholar]
- Veerendra Kumar MH, G. Y. (2003). “Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. .” Clin Exp Pharmacol Physiol. 30(5–6): 336–342. [DOI] [PubMed] [Google Scholar]
- Wang L, Guo L, Lu L, Sun H, Shao M, Beck SJ, Li L, Ramachandran J, Du Y, Du H. (2016). “Synaptosomal Mitochondrial Dysfunction in 5×FAD Mouse Model of Alzheimer’s Disease.” PLoS One 11(3): e0150441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbat J, Chaisawang P, Chaijaroonkhanarak W, Prachaney P, Pannangrong W, Sripanidkulchai B, Wigmore P. (2016). “Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats.” Ann Anat 206: 7–13. [DOI] [PubMed] [Google Scholar]
- Yang X, Tohda C (2018). “Heat Shock Cognate 70 Inhibitor, VER-155008, Reduces Memory Deficits and Axonal Degeneration in a Mouse Model of Alzheimer’s Disease.” Front Pharmacol 9: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, I. R., Zhao L, Nilsen J, Hamilton RT, Brinton RD. (2009). “Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease.” Proc Natl Acad Sci 106(34): 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimam M, Burnett BP, Brownell L, Jia Q. (2016). “Clinical and Preclinical Cognitive Function Improvement after Oral Treatment of a Botanical Composition Composed of Extracts from Scutellaria baicalensis and Acacia catechu.” Behav Neurol 2016: 7240802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Sharkey J, Finlayson K (2009). “Progressive impairment in olfactory working memory in a mouse model of Mild Cognitive Impairment.” Neurobiol Aging 30(9): 1430–1443. [DOI] [PubMed] [Google Scholar]
- Zhuo J, Prakasam A, Murray ME, Zhang HY, Baxter MG, Sambamurti K, Nicolle MM (2008). “An increase in Abeta42 in the prefrontal cortex is associated with a reversal-learning impairment in Alzheimer’s disease model Tg2576 APPsw mice.” Curr Alzheimer Res 5(4): 385–391. [DOI] [PubMed] [Google Scholar]
- Zhuo J, Prescott SL, Murray ME, Zhang HY, Baxter MG, Nicolle MM. (2007). “Early discrimination reversal learning impairment and preserved spatial learning in a longitudinal study of Tg2576 APPsw mice.” Neurobiol Aging 28(8): 1248–1257. [DOI] [PubMed] [Google Scholar]