Abstract
Background:
While in humans vertical sleeve gastrectomy (VSG) is fashioned by applying multiple staple loads, rodent VSG is generally created through a single staple load application.
Objectives:
To investigate the impact of a two-staple load VSG rat model more closely resembling the multi-staple load operation done in humans on weight, metabolic outcomes, and the microbiome and how these compare to those obtained with the standard one-staple load model.
Setting:
University research facility, United States.
Methods:
High-fat diet-induced obese male rats were randomized to single-staple load VSG (VSG1), two-staple load VSG (VSG2), or sham operation (Sham). Outcomes included body weight and composition, food intake, glucose metabolism, lipids, bile acids, and intestinal microbiome. Statistical comparisons were performed using analysis of variance.
Results:
Both procedures resulted in substantial weight and body fat loss compared to Shamtreated animals. Weight loss was modestly greater for VSG2 compared to VSG1. Food intake was reduced in both procedures and accounted for the observed weight reduction. Glucose tolerance and plasma and hepatic lipid profiles were improved comparably in VSG1 and VSG2 relative to Sham. Bile acids were higher for VSG2 compared with Sham but not significantly different between VSG1 and VSG2. Neither procedure impacted intestinal microbiome richness and diversity compared to Sham across multiple intestinal sections. Colonic Actinobacteria was more abundant in VSG2 than in Sham. Relative abundances of bacterial phyla did not differ between VSG1, VSG2, and Sham across the remaining intestinal sections.
Conclusions:
Although VSG1 or VSG2 offer effective and overall comparable platforms for the study of obesity, VSG2 resulted in superior weight loss.
Keywords: Sleeve gastrectomy, vertical sleeve gastrectomy, obesity surgery, metabolic surgery, bariatric surgery, weight loss surgery, surgical technique, animal experiment, glucose metabolism, lipid metabolism, intestinal microbiome
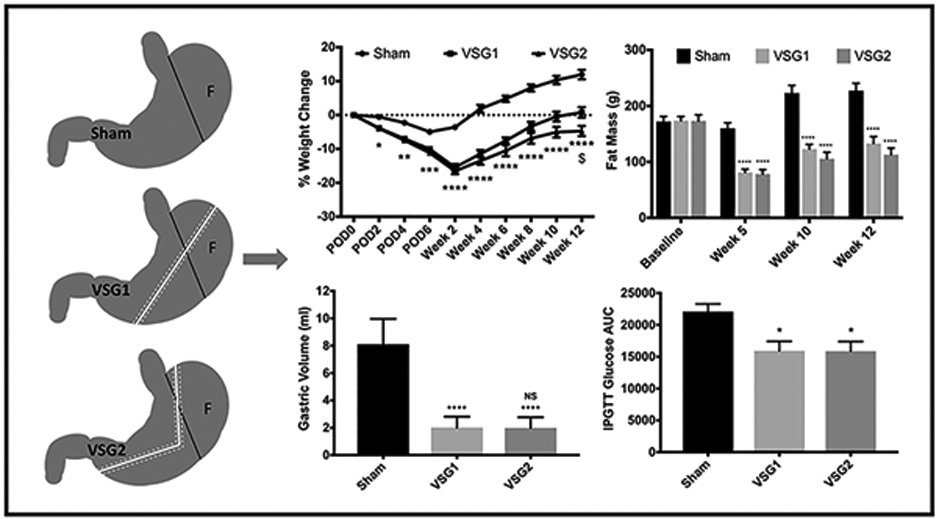
Graphical Abstract Legend
Comparable weight loss, fat mass reduction, and improvement in glucose tolerance for both vertical sleeve gastrectomy models compared with Sham. All graphs include the entire cohort (Sham n=10; VSG1 n=12; VSG2 n=12). Analyses performed by Tukey’s multiple comparison test applied to values obtained from one-way analysis of variance (ANOVA). VSG1, single-staple load vertical sleeve gastrectomy; VSG2, two-staple load vertical sleeve gastrectomy; F, fundus; IPGTT, Intraperitoneal glucose tolerance test; AUC, Area under curve; *, statistical significance between Sham and VSG groups; $, statistical significance between VSG groups; NS, non-significance between VSG groups; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; $, p <0.05.
Introduction
Metabolic surgery is the most effective therapy for obesity and related comorbidities. Several randomized clinical trials have proven the long-lasting benefits and superiority of multiple bariatric operations over intense medical management and lifestyle interventions.(1,2) While versions of these procedures have been in use for decades, how these surgeries work remains elusive. A key strategy to produce a deeper mechanistic understanding of these procedures is to model them in rodents that enable a wide range of experiments simply not feasible in humans. However, producing rodent models that accurately recapitulate these operations done in humans has a number of challenges including the small size of the rodent’s gastrointestinal tract and potentially important anatomic differences.
Vertical sleeve gastrectomy (VSG) is the most commonly performed metabolic operation in the United States and accounted for 37% of all bariatric procedures worldwide in 2013.(3,4) The prominent role of VSG within the spectrum of metabolic surgery can be explained by its lower technical complexity, similar effectiveness, and lower complication rate when compared to other bariatric operations. These characteristics, particularly its comparative technical ease, makes VSG an attractive operation to explore in rodent models where the small size creates a number of technical challenges. In humans, the sleeve is created by removing about 80% of the stomach including most of the fundus by sequentially applying multiple staple loads while taking care to avoid narrowing at the pylorus, incisura, and angle of His.(5) In rodents, VSG is generally created through a single staple load application. (6,7) In this approach, the entirety of the stomach to be resected must be brought into the single staple load while balancing the extent of resection, hence the effectiveness, with avoiding narrowing at the pylorus, incisura, and angle of His. This in turn may limit the extent of fundal resection compared to the procedure performed in humans where multiple staple loads allow for better angulation and safe approximation of the staple line cephalad to the angle of His.
In this report, we sought to investigate the impact of a multi-staple load operation by comparing a novel two-staple load rat VSG (VSG2) to the standard one-staple load model (VSG1). VSG2 should provide similar technical flexibility when creating the sleeve as that observed with VSG done in patients as it allows for multiple staple load applications and potentially more complete fundal resection without compromising patency at the angle of His. Similarly, the anatomic result of the gastric resection obtained in patients can be better approximated by resecting the antrum and body with the second staple load while retaining appropriate width at the pylorus and incisura. We report on the differences on weight, body composition, metabolic outcomes including glucose and lipid metabolism, bile acids, and the intestinal microbiome between VSG1 and VSG2. In addition, outcomes from VSG1 and VSG2 are compared to those obtained from a group undergoing sham operation (Sham).
Materials and Methods
Animals
Male Long-Evans rats (250–300 g) were purchased from Harlan Laboratories (Indianapolis, IN) and housed in individual cages in rooms maintained at 25°C temperature, 50–60% humidity, and a 12:12-h light-dark cycle. The animals were maintained on a high-fat diet (HFD) pre- and post-operatively. Thirty-six animals were randomly assigned to either VSG1 (n=13), VSG2 (n=13), or Sham (n=10). There were two surgical deaths. One animal in the VSG1 group was euthanized on postoperative day (POD) 3 secondary to rapid shallow breathing and lack of righting reflex. One animal in the VSG2 group was euthanized on week 5 secondary to a contained gastric leak presenting in the form of an approximately 4 cm abscess. Data collected from these animals were excluded from analyses. All procedures for animal use were approved by the Institutional Animal Care and Use Committee.
Diet
All animals had ad libitum access to food and water throughout the experiment, except where noted. Animals were placed on Tso’s 40% high-fat butter diet (Research Diets, New Brunswick, NJ) on arrival and for 8 weeks thereafter. Three days before operation food was removed and the animals provided with Osmolite 1 Cal (Abbott Laboratories, Columbus, OH). One day before operation, Osmolite was removed. Osmolite was given back on PODO immediately after operation. On POD3, HFD was re-introduced and used throughout the duration of the study. On POD4, the Osmolite liquid diet was removed.
Surgery
Operations were performed under isoflurane, and the VSG and Sham surgeries were performed technically, as described previously. (6) Pictures of the VSG1 and VSG2 surgeries are depicted in Figure 1. For VSG1, approximately 80% of the stomach was resected along the greater curvature with a single application of an ETS-FLEX 35-mm staple gun (Ethicon Endo-Surgery, Cincinnati, OH). In VSG2, two applications of the ETS-FLEX 35-mm staple gun were used to create the sleeve.
Figure 1.
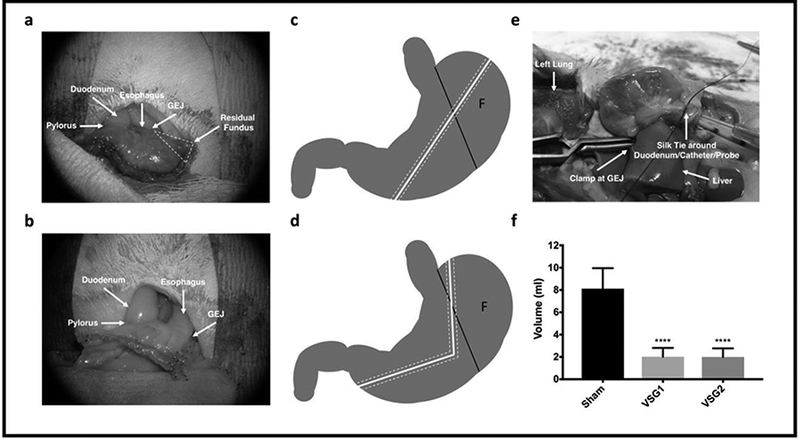
Surgical Procedures and Gastric Volume. VSG1 (a) and VSG2 (b). Schematic representation of VSG1 (c) and VSG2 (d) where F indicates fundus and dashed white tracks signify staple lines. Gastric volume procedure (e) and analysis (Sham n=10; VSG1 n=12; VSG2 n=12) (f). Analysis performed by Tukey’s multiple comparison test applied to values obtained from one-way analysis of variance (ANOVA). GEJ signifies gastroesophageal junction. *, denotes statistical significance between Sham and VSG groups. ****, p < 0.0001.
Postoperative Care
All rats were given meloxicam 0.5 mg/kg of body weight once daily and buprenorphine hydrochloride 0.03 mg/kg twice daily for 3 days, and 10 ml of warm saline twice daily on POD0 and once on POD1. Food intake and body weight were monitored at baseline, daily for 1 week after surgery, and weekly thereafter. Food intake was calculated by weighting unconsumed food pellets and subtracting this value from the starting food weight for a given period and for each animal. Cumulative food intake was then obtained by adding food intake values from all individual time periods encompassing the entire duration of the study for each animal. Nuclear magnetic resonance was used to assess lean and fat mass (Echo MRI: Echo Medical Systems, Houston, TX) at baseline and at weeks 5, 10, and 12 after operation. This was accomplished by placing live rats in their corresponding holder according to weight, introducing the holder containing the rodent into the body composition analyzer designated slot, and generating the average from two separate measurements of both fat and lean mass in grams for each animal.
Glucose Tolerance Test (GTT)
Five-weeks after surgery, 6-hrs fasted rats were injected with 2g/kg 25% dextrose (Phoenix Pharmaceutical, St. Joseph, MO) intraperitoneally (IP). Blood glucose was measured at baseline (0), 15, 30, 45, 60 and 120-min using Accu-chek glucometers and test strips (Roche, Indianapolis, IN). All blood samples were obtained from the tip of the tail vein of freely moving rats.
Tissue Collection and Assays
All animals were sacrificed at the end of week 12 after an overnight fast. Whole blood was collected via a cardiac puncture and cold-centrifuged to obtain plasma which was stored at −80 °C. Insulin sensitivity was assessed from samples obtained at sacrifice by measuring glucose with Rat Glucose Assay Kit (Crystal Chem, Elk Grove Village, IL) and insulin with Ultra Sensitive Rat Insulin Enzyme-linked Immunosorbent Assay (ELISA) Kit (Crystal Chem, Elk Grove Village, IL) values. The homeostatic model assessment for insulin resistance (HOMA-IR) was then calculated. Plasma triglycerides were analyzed by the Diabetes Research Center Chemistry Laboratory by a GPO-PAP method run on a Randox RX Series Daytona chemistry analyzer. Total bile acid data were obtained by colorimetric analysis with the Total Bile Acids Assay Kit (BQ Kits, San Diego, CA). Laparotomy was performed and 25–50 mg of liver obtained and flash-frozen in liquid nitrogen. Liver sample analysis for fatty acid profile was performed by The Division of Metabolism Endocrinology and Diabetes by extraction of liver tissues with chloroform-methanol followed by derivatization of fatty acids with BF3-methanol reagent, purification on thin layer chromatographic plate, and identification and quantification of fatty acids by gas chromatography on Agilent GC model 6890N with Agilent HP-88 column. Intestinal contents for microbiome analysis were collected by milking duodenal, jejunal, and ileal contents, sampling cecal fecal matter, and a colonic pellet all of which were flash-frozen.
Gastric Volume Measurements
Gastric volume measurements were obtained at sacrifice using pressure probe and transducing equipment provided by Ethicon Endo-Surgery (Cincinnati, OH) by transecting the duodenum at 3 mm from pylorus, dividing the diaphragm, and identifying and clamping the intrathoracic esophagus at the gastroesophageal junction. The probe and catheter were introduced through the pylorus and secured in place by tying a single snug 4–0 silk ligature around duodenum/catheter/probe. The recorded volume represents the volume in milliliters at which a stable reading of 20 mmHg was obtained following gastric infusion of saline (Figure 1).
Microbiome
Microbiome samples were processed by the Microbiome and Metabolomics Core with methods described elsewhere.(8) Briefly, after DNA isolation, the V4 region of the 16S rRNA gene was amplified and sequenced. Sequences were processed and analyzed using the software package Mothur (v.1.38.0) and analysis of molecular variance (AMOVA).
Statistical Analysis
GraphPad Prism statistical software 7.0c (La Jolla, CA) was used to analyze data. The results are expressed as means ± SE, and one-way and two-way independent repeated-measures analysis of variance (ANOVA) were applied where appropriate. A Tukey’s multiple comparison test was performed to further analyze significant interactions. A P value <0.05 was considered to be statistically significant. Linear regression was used to correlate cumulative food intake and percentage weight change for the entire cohort (n=34). Data for microbiome and liver and plasma lipid analyses were generated for 6 randomly-selected rodents per group (Sham n=6; VSG1 n=6; VSG2 n=6). All other analyses and corresponding graphs included the entire cohort (Sham n=10; VSG1 n=12; VSG2 n=12).
Results
Both VSG groups maintained significantly lower body weight when compared with Sham. Lower body weight in VSG1 and VSG2 relative to Sham resulted from decreased body fat while lean mass remained similar between groups. VSG2 exhibited slightly lower, albeit significant, body weight when compared to VSG1. Gastric volume was significantly reduced in VSG1 and VSG2 relative to Sham. VSG1 and VSG2 showed lower cumulative food intake when compared with Sham. Reductions in food intake for VSG1 and VSG2 occurred during the initial 5 weeks of the study after which intake remained comparable to that of the Sham group. Percent weight loss was greatly explained by cumulative food intake (Figures 1 and 2).
Figure 2.
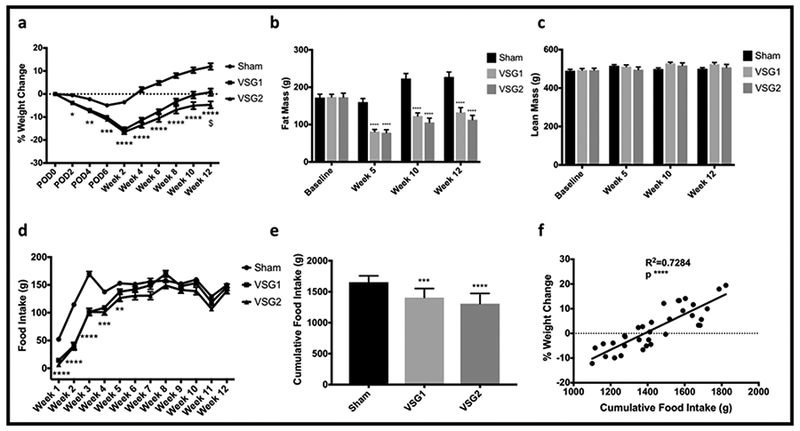
Body Weight, Body Composition, and Food Intake. Percentage weight change over time by group (a). Fat mass over time by group (b). Lean mass over time by group (c). Mean weekly food intake by group (d). Cumulative food intake by group (e). Linear regression of percent weight change and cumulative food intake for the entire cohort (n=34) (f). All graphs include the entire cohort (Sham n=10; VSG1 n=12; VSG2 n=12). Analyses (a-e) performed by Tukey’s multiple comparison test applied to values obtained from one-way analysis of variance (ANOVA). *, denotes statistical significance between Sham and VSG groups. $ indicates statistical significance between VSG groups. **** p < 0.0001; *** p < 0.001; $ p < 0.05.
Challenge with intraperitoneal dextrose 2g/kg revealed significantly superior glycemic tolerance in VSG1 and VSG2 compared with Sham. Both VSG models had comparable and significantly lower baseline glucose, insulin, and HOMA-IR values relative to Sham (Appendix). Both VSG groups tended to have higher total plasma bile acids levels compared with Sham, though the difference was only significant for VSG2 compared with Sham. Plasma triglycerides and all classes of hepatic fatty acids were lower for VSG1 and VSG2 relative to Sham (Figure 3).
Figure 3.
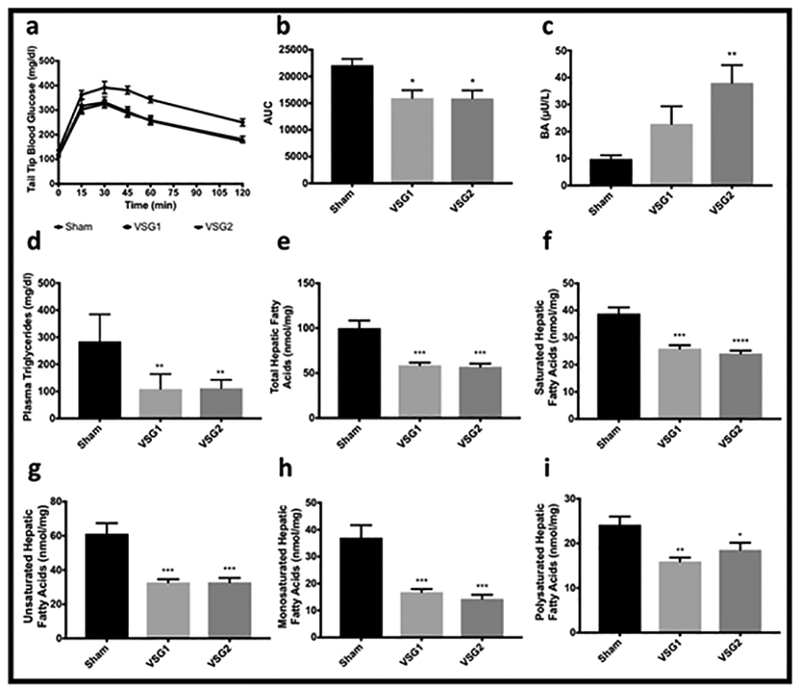
Glucose Handling, Bile Acids, and Plasma and Hepatic Lipids. Glucose levels at baseline and at different time points after injection of intraperitoneal dextrose 2g/kg (a). Area under the curve (AUC) for glucose levels over time obtained shown in a (b). Total fasted bile acids (c). Fasting plasma triglycerides by group (d). Total (e), saturated (f), unsaturated (g), monosaturated (h), and polysaturated (i) hepatic fatty acids by group (e). Graphs a-c include the entire cohort (Sham n=10; VSG1 n=12; VSG2 n=12). Graphs d-i include 6 randomly-selected rodents per group (Sham n=6; VSG1 n=6; VSG2 n=6). Analyses performed by Tukey’s multiple comparison test applied to values obtained from one-way analysis of variance (ANOVA). *, denotes statistical significance between Sham and VSG groups. ****, p < 0.0001; ***, p < 0.001; **, p<0.01; *, p<0.05.
Postsurgical indices of microbial richness and diversity in the duodenum, jejunum, ileum, cecum, and colon did not differ between groups. When looking at phyla, a higher relative abundance of colonic Actinobacteria was appreciated for VSG2 compared to Sham although the absolute contribution of Actinobacteria to the overall colonic microbial community was small. Further analyses with AMOVA revealed no significant differences between the bacterial communities of sham, VSG1 and VSG2 groups in the duodenum, jejunum, ileum, cecum or colon (Figure 4).
Figure 4.
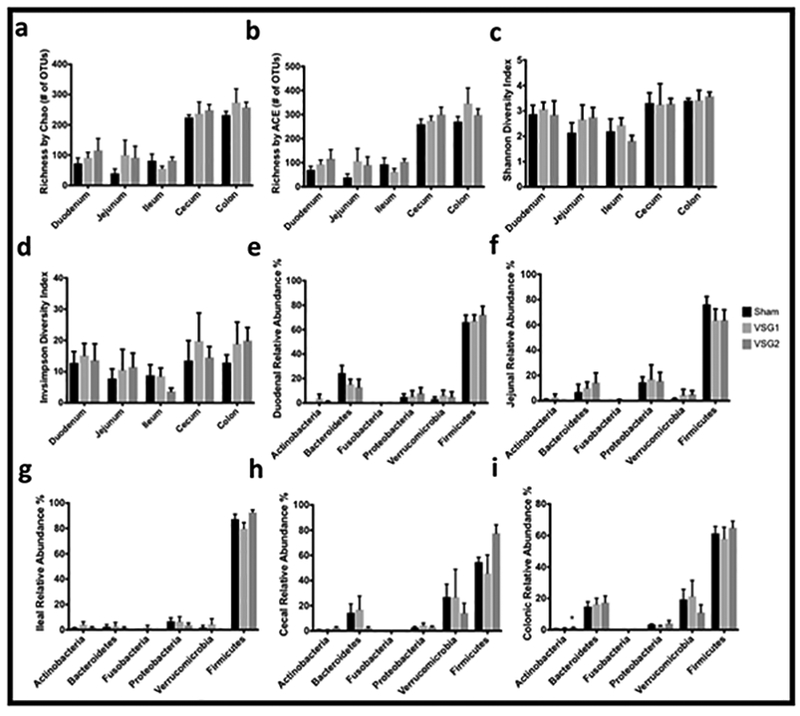
Microbial Community Richness and Diversity and Relative Abundances of Bacterial Phyla by Intestinal Section. Microbial community richness by Chao (a) and ACE (b). Microbial community diversity by Shannon (c) and Invsimpson (d). Relative abundances of duodenal (e), jejunal (f), ileal (g), cecal (h), and colonic (i) bacterial phyla. Graphs a-i include 6 randomly-selected rodents per group (Sham n=6; VSG1 n=6; VSG2 n=6). Analyses performed by Tukey’s multiple comparison test applied to values obtained from one-way analysis of variance (ANOVA). *, denotes statistical significance between Sham and VSG groups. *, p < 0.05.
Discussion
VSG2 offers an alternative rodent model more closely resembling the multi-staple load VSG performed in patients. This novel model resulted in superior weight loss and otherwise overall comparable outcomes compared to the standard one-staple load VSG1. Sustained reduction in body weight secondary to lower body fat were appreciated in VSG1 and VSG2 relative to Sham (Figure 2a-b). Several mechanisms have been proposed whereby the resection of about 80% of the stomach, including most of the fundus, results in such profound effects. Accelerated gastric emptying has been extensively documented after VSG in both patients and murine models and proposed as a mechanism whereby VSG exerts its effects. (9,10) Fundal resection accounts for most of the accelerated gastric emptying appreciated after VSG. (9) Data derived from clinical and animal studies signal to a potential role of accelerated gastric emptying in mediating some of the metabolic benefits observed following VSG via hormonal changes such as nutrient-stimulated increases in GLP-1. (11,12) However, with exception of limited clinical data showing no correlation between POD1 gastric emptying and weight loss up to 12 months the role of accelerated gastric emptying on weight loss following VSG remains largely unexplored. (13)
Interestingly, by week 12 VSG2 animals had slightly, yet significant, lower body weight than animals in the VSG1 group (Figure 2a). Moreover, this difference in body weight between VSG1 and VSG2 occurred independently of sleeve volume which was similar for both groups (Figure 1f). Instead, lower body weight in VSG2 relative to VSG1 could have resulted, at least partially, from lower, non-statistically significant, cumulative food intake in VSG2 compared with VSG1. Although lower body weight in VSG2 did not translate into a significant reduction in fat mass relative to VSG1, given that the non-significant gap in the latter parameter widened over time (Figure 2b), it is plausible that a significant difference could have been appreciated had the experiment been carried out for a longer period of time. Our two-staple load model allows for more complete and precise proximal fundal resection via the first staple load application (Figure 1a-d). This in turn may have resulted in lower body weight in VSG2 relative to VSG1 derived from comparatively lower food intake in the former group. Overall, these findings underscore the importance of deliberate surgical technique, emphasizing not only adequate gastric volume removal but also accurate and complete gastric fundal resection, to produce optimal outcomes. Although the mechanisms whereby more complete fundal resection may translate into greater weight loss are not fully understood, clinical data shows that revisional resection of residual fundus or neofundus following primary VSG results in notable additional weight loss. (14,15)
In this cohort, both VSG models showed lower cumulative food intake, resulting from reductions during the initial 5 weeks of the study, relative to Sham (Figure 2d-e). Moreover, most of the effects on body weight and composition were accounted for by reductions in cumulative food intake (Figure 2f). Reduction in food intake after VSG has been extensively documented in animal and clinical studies. (16,17) Transient reduction in food intake rather than changes in energy expenditure may explain a significant portion of the weight loss appreciated after this operation. (18) While the underlying mechanisms whereby VSG results in diminished food intake remain largely unknown, reduction of nutrient consumption is an important determinant of weight loss after VSG.
This study shows comparable improvement in glucose and lipid metabolism for VSG1 and VSG2 which were superior compared with Sham (Figures 3a-b, d-i). Significant improvements in glucose metabolism have been extensively documented following VSG. (1,19,20) Improvements in glucose metabolism after this metabolic procedure largely occurs independently of weight loss. (19,20) Similarly, significant improvements in lipid metabolism has been widely demonstrated after VSG. (1,21) Likewise, the beneficial effects of VSG on lipid metabolism have been reported to take place in a weight-independent manner. (21) Our results are consistent with these data. Interestingly, others have shown that when fundal resection was added to the Roux-en Y gastric bypass (RYGB) patients experienced lower postprandial glucose and fasting ghrelin levels and higher postprandial peptide YY, GLP-1, and insulin responses compared to patients undergoing the standard RYGB without resection of the fundus. (22) This signals to a potential association between fundal resection and optimal metabolic benefit following bariatric surgery in humans, possibly mediated by neuroendocrine mechanisms. However, important differences exist between the gastric fundus of humans and rats. (23) Case in point, while the stomach fundus in rats is non-glandular and lined by keratinized stratified squamous epithelium, the gastric fundus in humans is glandular and lined by columnar epithelia. Moreover, whereas the non-glandular fundus in rats may function mostly as a food reservoir, the glandular fundus in humans also modulates neuroendocrine pathways via secretion of peptide hormones. Therefore, that we report comparable metabolic outcomes independently from the extent of fundal resection in rodents could be attributed to well-established morphologic and physiologic differences between human and rat gastric fundi.
Our data show that fasted plasma bile acids increased in VSG1 and VSG2 relative to Sham although this difference was only statistically significant for VSG2 (Figure 3c). Several reports have documented increase in bile acids following VSG. (21,24) This interesting finding has prompted a variety of investigations exploring the mechanisms whereby bile acids may influence weight loss and metabolic benefit after VSG identifying FXR and TGR5 as potential mediators. (25,26)
The intestinal microbiome and its changes after metabolic surgery is a current area of intense investigation. Herein we report on the intestinal microbiome, across all intestinal sections, following VSG. With the exception of colonic Actinobacteria, which was more abundant in VSG2 compared with Sham, relative abundances of bacterial phyla did not differ between VSG1, VSG2, and Sham across the remaining intestinal sections (Figures 4e-i). Furthermore, indices of microbial richness and diversity as well as analyses with AMOVA revealed no significant differences between the bacterial communities of sham, VSG1 and VSG2 groups in the duodenum, jejunum, ileum, cecum or colon (Figure 4a-d). At least one other report observed higher relative abundance of Actinobacteria after VSG.(27) However, based on our data, the contribution of this phylum to the overall colonic bacterial community was small and unlikely to account for the substantial weight changes and metabolic improvement resulting from VSG. Clinical studies have reported conflicting changes in the microbiome including changes in Proteobacteria, Firmicutes, Bacteroidetes, and Roseburia, after VSG.(28–30) Animal data is similarly inconsistent and identify changes in Proteobacteria, Akkermansia, Gammaproteobacteria, Desulfovibrionaceae, Cyanobacteria, Actinobacteria, Turicibacteraceae, Adlercreutzia, Enterococcus, Porphyromonadaceae, and Roseburia as potentially associated with the changes appreciated after VSG. (25–27,31) The mechanisms whereby these shifts may modulate the weight loss and metabolic benefits observed after VSG remain speculative. Nevertheless, in the current experiment, there appears to be no meaningful association between changes to the intestinal microbiome, sampled from intestinal contents across all intestinal sections, and the beneficial effects on weight and metabolism seen following VSG in Long-Evans rats.
Several limitations should be considered when interpreting our data and conclusions. Diminished Pertinent to body weight and composition, animals were only followed for 12 weeks and no conclusions can therefore be made regarding outcomes at later time points. However, metabolic outcomes including glucose tolerance and lipid profiles occur early and independently of weight loss. Furthermore, the length of this experiment is equivalent to most published animal studies. 20,21 Relevant to the intestinal microbiome, animals were kept on HFD pre- and post-operatively which may have altered intestinal microbial communities beyond changes associated with the surgical procedure. Moreover, changes in the intestinal microbiome after VSG may also be species-dependent. Lastly, although comparable to most animal experiments in metabolic surgery, surgical groups are composed of small number of animals which could potentially result in type II error.
Conclusions
While overall similar outcomes were appreciated for both rodent models of VSG relative to Sham, superior weight loss was seen after VSG2 compared to VSG1. Significant and sustained reduction in body weight secondary to decreased fat mass was noted in VSG1 and VSG2 compared with Sham. More complete and precise fundal resection accomplished through the application of two staple loads in VSG2 could have accounted for the small, yet significant body weight reduction observed in this model relative to VSG1. This highlights complete fundal resection as a key surgical step when performing the VSG in order to attain best outcomes. Diminished food intake appears to be an important mediator of VSG-induced changes in body weight and fat mass as these were strongly and directly correlated with cumulative food intake. However, the mechanisms whereby VSG results in food intake reduction and how this association may translate into such profound body weight and composition effects are not fully understood. Measures of glucose and lipid metabolism improved for VSG1 and VSG2 relative to Sham. Whereas we acknowledge, based on the existing literature, the potential role of microbial intestinal communities in mediating some effects of the VSG, our data show no significant association between the weight loss and metabolic benefits seen with this operation and the microbiome in Long-Evans rats on HFD. Though one- or two-staple load VSG in rats offer effective and overall comparable platforms for the study of obesity and related comorbidities, the difference in weight loss noted between these two models warrants further exploration.
Supplementary Material
Highlights.
Comparable reduction in fat mass in VSG1 and VSG2 relative to Sham
Superior weight loss in VSG2 relative to VSG1
Decreased food intake accounts for the majority of reduction in body weight
Both VSG models resulted in improved glucose and lipid metabolism relative to Sham
The intestinal microbiome did not differ between VSG1, VSG2, and Sham
Acknowledgments
Disclosures
One of the authors receives financial research support from Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, Janssen/Johnson & Johnson, Zafgen, MedImmune, Sanofi, and Kallyope and serves as a consultant for Ethicon Endo-Surgery/Johnson & Johnson, Orexigen, Novo Nordisk, Daiichi Sankyo, Janssen/Johnson & Johnson, Novartis, Paul Hastings Law Firm, Kallyope, and Scohia. The remaining authors have no commercial associations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes-5-Year Outcomes. N Engl J Med. 2017;376(7):641–651. doi:10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet Lond Engl. 2015;386(9997):964–973. doi:10.1016/S0140-6736(15)00075-6 [DOI] [PubMed] [Google Scholar]
- 3.English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. December 2017. doi:10.1016/j.soard.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 4.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25(10):1822–1832. doi:10.1007/s11695-015-1657-z [DOI] [PubMed] [Google Scholar]
- 5.Bellanger DE, Greenway FL. Laparoscopic sleeve gastrectomy, 529 cases without a leak: short-term results and technical considerations. Obes Surg. 2011;21(2):146–150. doi:10.1007/s11695-010-0320-y [DOI] [PubMed] [Google Scholar]
- 6.Stefater MA, Pérez-Tilve D, Chambers AP, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138(7):2426–2436, 2436.e1–3. doi:10.1053/j.gastro.2010.02.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson-Pérez HE, Chambers AP, Ryan KK, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380–2385. doi:10.2337/db12-1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenigsknecht MJ, Theriot CM, Bergin IL, Schumacher CA, Schloss PD, Young VB. Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect Immun. 2015;83(3):934–941. doi:10.1128/IAI.02768-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni BV, LaSance K, Sorrell JE, et al. The role of proximal versus distal stomach resection in the weight loss seen after vertical sleeve gastrectomy. Am J Physiol Regul Integr Comp Physiol. 2016;311(5):R979–R987. doi:10.1152/ajpregu.00125.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah S, Shah P, Todkar J, Gagner M, Sonar S, Solav S. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying halftime in morbidly obese patients with type 2 diabetes mellitus. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2010;6(2):152–157. doi:10.1016/j.soard.2009.11.019 [DOI] [PubMed] [Google Scholar]
- 11.Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306(4):E424–432. doi:10.1152/ajpendo.00469.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sista F, Abruzzese V, Clementi M, Carandina S, Cecilia M, Amicucci G. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2017;13(1):7–14. doi:10.1016/j.soard.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 13.Parikh M, Eisner J, Hindman N, Balthazar E, Saunders JK. Tests of correlation between immediate postoperative gastroduodenal transit times and weight loss after laparoscopic sleeve gastrectomy. Surg Endosc. 2012;26(12):3548–3551. doi:10.1007/s00464-012-2352-y [DOI] [PubMed] [Google Scholar]
- 14.Silecchia G, De Angelis F, Rizzello M, Albanese A, Longo F, Foletto M. Residual fundus or neofundus after laparoscopic sleeve gastrectomy: is fundectomy safe and effective as revision surgery? Surg Endosc. 2015;29(10):2899–2903. doi:10.1007/s00464-014-4017-5 [DOI] [PubMed] [Google Scholar]
- 15.Iannelli A, Schneck AS, Noel P, Ben Amor I, Krawczykowski D, Gugenheim J. Re-sleeve gastrectomy for failed laparoscopic sleeve gastrectomy: a feasibility study. Obes Surg. 2011;21(7):832–835. doi:10.1007/s11695-010-0290-0 [DOI] [PubMed] [Google Scholar]
- 16.Verger EO, Aron-Wisnewsky J, Dao MC, et al. Micronutrient and Protein Deficiencies After Gastric Bypass and Sleeve Gastrectomy: a 1-year Follow-up. Obes Surg. 2016;26(4):785–796. doi:10.1007/s11695-015-1803-7 [DOI] [PubMed] [Google Scholar]
- 17.Schneck A-S, Iannelli A, Patouraux S, et al. Effects of sleeve gastrectomy in high fat diet-induced obese mice: respective role of reduced caloric intake, white adipose tissue inflammation and changes in adipose tissue and ectopic fat depots. Surg Endosc. 2014;28(2):592–602. doi:10.1007/s00464-013-3211-1 [DOI] [PubMed] [Google Scholar]
- 18.Bielohuby M, Stemmer K, Berger J, et al. Carbohydrate content of post-operative diet influences the effect of vertical sleeve gastrectomy on body weight reduction in obese rats. Obes Surg. 2012;22(1):140–151. doi:10.1007/s11695-0U-0528-5 [DOI] [PubMed] [Google Scholar]
- 19.Kadera BE, Portenier DD, Yurcisin BM, Demaria EJ, Gaddor MM, Jain-Spangler K. Evidence for a metabolic mechanism in the improvement of type 2 diabetes after sleeve gastrectomy in a rodent model. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2013;9(3):447–452. doi:10.1016/j.soard.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 20.Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–958. doi:10.1053/j.gastro.2011.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings BP, Bettaieb A, Graham JL, et al. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153(8):3620–3632. doi:10.1210/en.2012-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chronaiou A, Tsoli M, Kehagias I, Leotsinidis M, Kalfarentzos F, Alexandrides TK. Lower ghrelin levels and exaggerated postprandial peptide-YY, glucagon-like peptide-1, and insulin responses, after gastric fundus resection, in patients undergoing Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg. 2012;22(11):1761–1770. doi:10.1007/s11695-012-0738-5 [DOI] [PubMed] [Google Scholar]
- 23.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16(5):351–380. [DOI] [PubMed] [Google Scholar]
- 24.Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obes Silver Spring Md. 2014;22(2):390–400. doi:10.1002/oby.20548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. doi:10.1038/nature13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGavigan AK, Garibay D, Henseler ZM, et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2017;66(2):226–234. doi:10.1136/gutjnl-2015-309871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y, Liu C-Q, Shan C-X, et al. Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: Increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab Res Rev. August 2016. doi:10.1002/dmrr.2857 [DOI] [PubMed] [Google Scholar]
- 28.Medina DA, Pedreros JP, Turiel D, et al. Distinct patterns in the gut microbiota after surgical or medical therapy in obese patients. PeerJ. 2017;5:e3443. doi:10.7717/peerj.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes Surg. October 2016. doi:10.1007/s11695-016-2399-2 [DOI] [PubMed] [Google Scholar]
- 30.Damms-Machado A, Mitra S, Schollenberger AE, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. BioMed Res Int. 2015;2015:806248. doi:10.1155/2015/806248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao Y, Ding R, Xu B, et al. Alterations of Gut Microbiota After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in Sprague-Dawley Rats. Obes Surg. 2017;27(2):295–302. doi:10.1007/s11695-016-2297-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


