Introduction
The lateral skull base anatomy is complex and rich with essential neurovascular structures. Surgical dissection in the lateral skull base requires a firm understanding of the anatomical relationships to ensure optimal outcomes. Dissection requires careful identification of known landmarks to generate a mental image of the relative location of these structures. Safety and efficiency of dissection increases with experience as the surgeon’s 3D mental map of the anatomy improves. Technology that improves visualization of anatomic structures and their spatial relationship would significantly improve the surgeon’s operative experience.
Extended reality (ExR) describes the spectrum, or “virtuality continuum” of technology from Virtual Reality (VR) where the user is immersed in the digital world to Augmented Reality (AR)1, which allows the user to overlay digital data or images over their environment. Recent technology advancements2 have allowed for ExR Head Mounted Display (HMD) devices. These wearable devices make application of ExR much more accessible and applicable to everyday uses including architecture3, healthcare4, simulation5 and education6. Current headsets produced by Oculus, HTC, Sony and Google create fully immersive VR environments. In contrast, Google Glass is an example of an AR headset that can be used to display pertinent healthcare data during a medical encounter6. Mixed Reality (MR) refers to interactive digital data displayed over the native environment.
A MR HMD that projects images onto a transparent lens over the user’s visual field. The MR HMD has 3 key features ideal for image-guided navigation: 1) MR view, 2) spatial mapping, and 3) interactive hands-free interface.
Mixed Reality:
The MR headset projects images onto a transparent lens overlying the user’s visual field utilizing stereoscopy to create the perception of depth for 3D models. Due to the transparency, the user maintains view of the external environment with a holographic overlay, allowing the user to maintain focus on the actual field by displaying data in a head-up manner. Current intraoperative navigation systems require the surgeon to fix their attention on a separate screen to check the relative position of a probe or instrument placed onto a point of interest. With the HMD display, the surgeon does not need to break view of the actual surgical field.
Spatial Mapping
The MR HMD employs spatial mapping technology to allow images to be anchored to specific points in the environment. The headset contains an infrared projector and camera that map the user’s 3D environment when first turned on. 3D images displayed in the headset stay fixed relative to the mapped physical space while the user moves. In an operative setting, a 3D image-based model could be tethered to the patient while allowing the surgeon freedom of movement. Current intraoperative surgical navigation systems rely on co-registration of 3D data points generated from radiographic data and physical data points on the patient7. The system then co-registers these two data sets and aligns them to allow navigation. MR allows visual registration and adjustment of the 3D model as physical structures are identified.
Interactive hands-free interface
The MR headset features a multidimensional, hands-free interface ideal for use in a surgical setting. The MR headset contains a motion detection camera, gyrometer and microphone. Thus, the user can control the headset by gesture, gaze and voice commands. Application windows appear as holographs in the user’s field of view similar to that of a personal computing desktop. A dot in the center of the screen acts as a cursor that can be moved around the holographic environment with head movement and gaze. Hand gestures can be used to “click” on objects. The headset also responds to voice commands after a voice prompt.
The objectives of this study are 1) to develop an MR platform for visualization of temporal bone structures 2) to measure the accuracy of the platform as an image guidance system.
Materials and Methods
The Human Research Protection Office at the study institution reviewed this study and determined that the study was exempt from Institutional Review Board review.
Image acquisition and 3D hologram generation
Fig. 1 demonstrates our workflow for image acquisition and 3D holographic generation. First, 0.6 mm slice thickness, high resolution, two-dimensional computed tomography (CT) scans (Siemens Biograph, Siemens Healthcare, Erlangen, Germany) of cadaveric temporal bones and heads were obtained. Digital imaging and communications in medicine (DICOM) data generated from the CT scans were imported into an open-source software ITK-SNAP8, where a combination of semi-automatic and manual segmentation was employed to generate three-dimensional (3D) reconstruction images. Stereolithiography (STL) files of the 3D reconstructed images were then imported into an open-source system, MeshLab9. Depending upon the complexity of the model, STL files can be comprised of thousands of raw, triangular meshes that can require substantial processing time in 3D platforms. In addition, the processing power of the headset limits the number of displayed triangular meshes. Meshlab allows for the simplification of triangular meshes while preserving geometrical detail and surface contouring. Furthermore, STL files can be converted to OBJ files in Meshlab, the latter of which is readily displayed with the MR platform described below.
Figure 1.
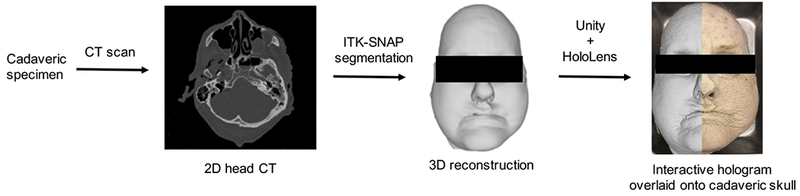
Work flow demonstrating generation of 3D interactive hologram and projection onto cadaveric specimen as seen through the Microsoft HoloLens.
Development of mixed reality (MR) platform
To view and interact with 3D images on the Microsoft HoloLens(Washington, Seattle), an MR platform must be built to allow for image release into the headset in the form of a Visual Studio project (Version 15.6;Microsoft, 2017). We developed a MR platform specific to our use in Unity®(Version 3.1;Unity, 2017), a gaming platform for 3D animation and hologram manipulation. C# scripts were used to generate interactive OBJ files that could be manipulated in the xyz dimensions. The images generated in Unity were then visualized in the HMD. Using C# programming in Unity and Visual Studio, code was written to allow for user-friendly voice, gaze, and gesture commands.
Surface registration and accuracy measurements
Prior to image deployment, the HMD was calibrated each time for each specific user’s interpupillary distance. Once the 3D holographic image was displayed in the HMD, soft-tissue surface anatomy was used to align the holographic images over the physical objects. A combination of voice and hand gestures were employed to manipulate the holographic images in space to overlay the physical objects.
To verify the accuracy of our system, target registration error (TRE) was measured. TRE is defined as the displacement between an image (is) and physical space (ps) location of a specific point, and is calculated using the following formula: ([Xis − Xps]2 + [Yis − Yps]2 + [Zis − Zps]2)1/2. We used a 3D holographic image of a cadaveric skull overlayed on a 3D printed model of the same specimen to perform our accuracy measurements. 7 landmarks, 5 external and 2 internal structures, were chosen.
Our approach to error measurement is shown in Fig 2. First, a rigid four-ball passive probe was registered with an Optitrack camera bar (NaturalPoint, Coravllis, Oregon). Using infrared(IR) cameras, the Optitrack bar allows for tracking of marked rigid bodies. We reassigned the registration point on the passive probe from its center of mass to the tip to facilitate TRE measurement. Motive, an open-source Unity plug-in made available through Optitrack, allowed positional data of the location of the passive probe to be streamed real-time into Unity, allowing us to simultaneously record x,y, and z coordinates.
Figure 2.
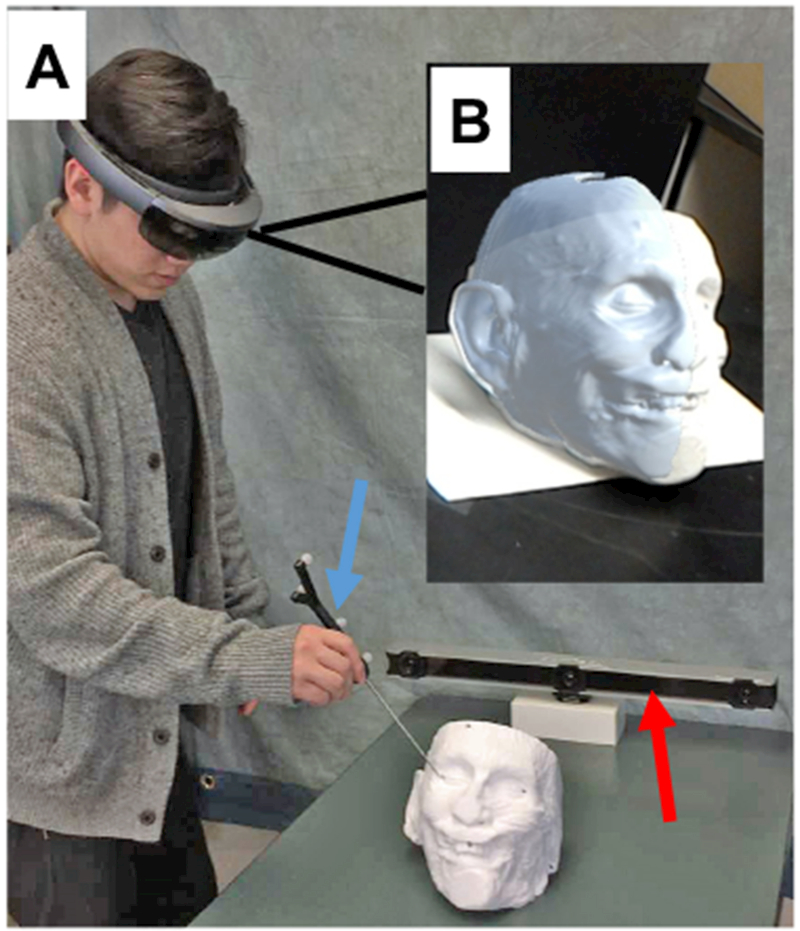
Demonstration of measurement of target localization error (TLE). Panel A) Observer using 4-point passive probe (blue arrow) to measure TLE of selected landmarks with Optitrack camera bar (red arrow); Panel B) View through the HoloLens during measurement.
Results
3D hologram generation and registration for the MR HMD
Using our novel MR platform, we were able to successfully display interactive holograms of soft tissue, bony anatomy, and internal ear structures onto cadaveric and 3D printed models.
For cadaveric skull and temporal bones, the following structures were generated as interactive holograms: skin, bone, and inner ear anatomy including the facial nerve, cochlea, semicircular canals, sigmoid sinus, and internal carotid artery. We were able to successfully use voice, gesture, and gaze commands to align our holograms onto our physical objects using surface registration (Fig. 3).
Figure 3.

3D interactive holograms superimposed on cadaveric temporal bone as seen through the HoloLens. Panel A) Skin and bone; Panel B) Bone only; Panel C) Internal Ear structures including jugular bulb (blue), carotid (red), facial nerve (yellow).
C# scripts allowed the MR headset to recognize different voice, gesture, and gaze commands. Gaze commands allowed for placement of the object in 2 dimensions. Gesture commands were used to allow holographic images to move and rotate in all three xyz coordinate directions. Voice commands e.g. “lock”, “unlock” allowed for stability of object placement in relation to its surrounding, and also disabled simultaneous manipulation by gesture or gaze commands. Our C# scripts also created a pseudo-state for each object depending on the command. For example, an image can initially start off in its movable state, where manipulation of the image and overlay onto a physical object can take place. However, when the command for “lock” is performed, the image then shifts to its locked state. This pseudo-state allows for more refined control of the holographic image as the user has specific commands for each state.
These results were reproducible in both the cadaveric head and temporal bone models as well as the 3D-printed skull model.
Accuracy measurements
3 different observers performed triplicate measurements of 7 pre-specified landmarks on the 3D holographic skull and 3D-printed skull. The left earlobe, left lateral canthus, midpoint of the maxillary central incisors, right lateral canthus, and right earlobe were chosen as external landmarks, and the right and left internal auditory canal (IAC) for internal landmarks. We first used surface registration as described above to align the hologram and 3D skull model. We were then able to use voice commands to remove the external surface of our hologram revealing just the internal landmarks to perform TRE of the right and left IAC.
For the 3 observers, the average TRE (±sem) was 6.46 mm ± 0.37, 4.69 mm ± 0.10, and 6.14 mm ± 0.14, respectively (Table 1). The overall average TRE between the 3 observers was 5.76 mm ± 0.54.
Table 1.
Average Target Localization Error (TLE) of external and internal landmarks as measured by 3 different observers.
| Measurement Points | Average TLE (mm) ± SEM |
||
|---|---|---|---|
| Observer 1 | Observer 2 | Observer 3 | |
| Left earlobe | 4.32 ± 0.25 | 5.69 ± 0.10 | 4.89 ± 0.11 |
| Left lateral canthus | 6.18 ± 0.36 | 1.65 ± 0.015 | 4.70 ± 0.17 |
| Midpoint maxillary central incisors | 9.23 ± 0.53 | 2.31 ± 0.05 | 5.98 ± 0.04 |
| Midpoint maxillary central incisors | 1.72 ± 0.10 | 2.04 ± 0.03 | 4.19 ± 0.17 |
| Right earlobe | 8.22 ± 0.47 | 4.13 ± 0.11 | 3.89 ± 0.19 |
| Left IAC | 7.65 ± 0.44 | 7.50 ± 0.06 | 7.04 ± 0.13 |
| Right IAC | 7.87 ± 0.45 | 9.50 ± 34 | 12.3 ± 0.15 |
| Total Average | 6.46 ± 0.37 | 4.69 ± 0.10 | 6.14 ± 0.14 |
Discussion
One of the key surgical principles taught to surgical trainees is to obtain adequate exposure. Safe surgical dissection requires knowledge of relevant anatomy. With experience, surgeons build a mental 3D map of the surgical anatomy and are able to navigate more efficiently with less exposure. An accurate surgical navigation system, using ExR, can potentially enhance a surgeon’s operative experience, by enabling more efficient and safe dissection.
Image guided surgery (IGS) utilizing ExR, follows several basic principles: 1) target anatomy 3D model generation, 2) visual display, 3) registration and tracking, and 4) accuracy measurement.
Target anatomy 3D models are typically derived from either CT or MRI images. Modern CT scanners have accuracy to less than 0.234 mm10. The DICOM data is segmented using one of several available software programs to generate 3D reconstructions. In our study, we used semiautomatic segmentation for skin and bone surfaces and manual segmentation for individual anatomic structures of interest. Lateral skull base anatomy is well suited for image guidance application as the relevant structures are fixed in bone. The manual segmentation process can be time-consuming11 and efforts have been made by others to automate this process for temporal bone structures12. ExR IGS has been described in multiple fields including neurosurgery, maxillofacial, orthopedic, cardiac and laparoscopic surgery13. In otolaryngology, AR has been applied in sentinel lymph node biopsy14, endoscopic sinus and skull base surgery15,16, and cochlear implantation17.
Visual display of digital models in ExR has been achieved in multiple ways for IGS. A computer generated (CG) image can be superimposed on real-world imagery and displayed on a monitor, which has most often been described in laparoscopic and endoscopic surgery18. Several groups have described using a surgical microscope with digital images projected into the eyepieces stereoscopically to allow for depth perception19,20. The MR headset projects digital images onto a transparent lens on a HMD. The headset is wireless and allows the user freedom of motion. We were able to visualize and maneuver our digital 3D anatomic images in the headset to overlay the physical specimens (Fig. 3).
Registration and tracking in IGS are essential for reliable navigation. Registration is the process of alignment of the image space with the physical space.7 Current commercial IGS systems use either fiducial marker points21 or surface anatomy maps22 for registration. Fiducials can be affixed to the skin or bone of the patient before obtaining CT or MRI. In the operating room, the physical location of the fiducials is then defined with a tracking probe. Tracking is accomplished with either optical (infrared) or electromagnetic field systems. The IGS system then aligns the image space points with the physical space points as closely as possible. In our project, we visually registered a holographic image reconstruction to its physical model using the HMD. The conceptual shift when comparing the MR headset to current IGS systems is that the registration and tracking components are contained within the headset. Our next objective was to measure the accuracy of the HMD after registration.
Accuracy in IGS is most commonly measured using TRE, which refers to the distance between a physical space point and the corresponding point as located by an IGS system. Our study found TRE to be an average of 5.76 mm using the MR headset to locate points on a 3D-printed model. Prior studies comparing accuracy using different registration methods have found bone-anchored fiducials to be the most accurate. Metzger23 reported TREs of 1.13 mm with bone-anchored fiducials, 2.03 mm with skin fiducials, 3.17 mm with bony landmarks and 3.79 mm with a splint during maxillofacial surgery. Mascott24 found TREs of 1.7 mm using bone-anchored fiducials and 4.4 mm when using surface map registration.
There are various limitations to our research and the MR HMD. When measuring the TRE, we used a 3D-printed model in place of the original cadaveric head. In addition, to view our models in the MR headset, the number of triangular meshes in our 3D reconstructions was reduced to permit image viewing at a smooth and stable frame rate. This reduction smooths the edges of the reconstruction and could affect model accuracy in the sub-millimeter range. We found that around 30,000 triangles frame rate and spatial mapping accuracy significantly decreased, causing the model to shift as the observer moves and increasing TRE. Higher resolution reconstructions can have >200,000 surfaces, and thus are not tractable for this type of display without some refinement. As computational power is continually improving with lower power draws, newer MR headset models will have increased computing power that should help with this issue. However, given that the improvement needed is more than an order of magnitude, we will likely need to produce models that have more surfaces in areas/structures of interest while decreasing resolution elsewhere. We perceive the majority of the inaccuracy is due to image distortion and difficulty with accurate depth perception. The MR HMD creates the perception of depth by presenting images stereoscopically- a separate image to each eye on the lens. Visualizing the location of a probe in 3 planes is difficult without moving to different perspectives. The user can experience discomfort trying to localize points in space for extended periods. Inattentional blindness- missing abnormal events when the user’s attention is focused elsewhere- is a concern with AR surgery25. It is difficult to describe the user experience in static format as in the figures and we would encourage the reader to view this video (https://wustl.box.com/s/38o05xoygr50hmbf3ou4nj7w6lk1yhel). We attempted to capture the utility of this platform with objective measurements(TRE), but the potential application is more readily apparent with headset demonstration.
Despite the limitations identified with the MR HMD as an IGS tool, we believe that this technology is promising and warrants further development. We plan to improve our surface registration method by improving the user interface to make fine adjustments of the hologram easier. Autoregistration will require defining the region of physical space where our image model will be anchored to be better mapped by the headset.
Conclusion
The MR HMD is capable of providing ‘x-ray’ vision of human anatomy. The logistics of registration, navigation and accuracy warrant improvement before it is ready to be used for image guided surgery. We are currently working to improve each of these areas. The device has several key features that make it ideal for IGS. The technology has significant promise to improve surgical navigation by helping the surgical trainee more efficiently develop a mental 3D anatomical map. Future iterations of the MR headsets may well be capable of reliable registration and better accuracy for intraoperative surgical guidance to improve surgical outcomes and improve physician training.
Acknowledgements:
none
Funding: Washington University in St. Louis School of Engineering and Applied Science Collaboration Initiation Grant
Research reported in this publication was supported by the National Institute of Deafness and Other Communication Disorders within the National Institutes of Health, through the “Development of Clinician/Researchers in Academic ENT” training grant, award number T32DC000022. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest:
None
References
- 1.Milgram P, Kishino F. A Taxonomy of Mixed Reality Visual-Displays. Ieice T Inf Syst 1994;E77d:1321–1329. [Google Scholar]
- 2.Silva Jennifer NA, et al. “Emerging Applications of Virtual Reality in Cardiovascular Medicine.” JACC: Basic to Translational Science 3.3 (2018): 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae Hyojoon, Mani Golparvar-Fard, and Jules White. “High-precision vision-based mobile augmented reality system for context-aware architectural, engineering, construction and facility management (AEC/FM) applications”. Visualization in Engineering 1.1 (2013), p. 3. [Google Scholar]
- 4.Carrino Francesco et al. “Augmented reality treatment for phantom limb pain”. International Conference on Virtual, Augmented and Mixed Reality. Springer; 2014, pp. 248–257. [Google Scholar]
- 5.Fang Te-Yung et al. “Evaluation of a haptics-based virtual reality temporal bone simulator for anatomy and surgery training”. Computer methods and programs in biomedicine 113.2 (2014), pp. 674–681. [DOI] [PubMed] [Google Scholar]
- 6.Liarokapis Fotis et al. “Web3D and augmented reality to support engineering education”. World Transactions on Engineering and Technology Education 3.1 (2004), pp. 11–14. [Google Scholar]
- 7.Labadie RF, Fitzpatrick JM (2016). Image-Guided Surgery. San Diego, CA: Plural Publishing. [Google Scholar]
- 8.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, & Gerig G (2006). User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage, 31(3), 1116–1128. www.itksnap.org [DOI] [PubMed] [Google Scholar]
- 9.Cignoni P, Callieri M, Corsini M, Dellepiane M, Ganovelli F, Ranzuglia G. MeshLab: an Open-Source Mesh Processing Tool Sixth Eurographics Italian Chapter Conference, page 129–136, 2008. www.meshlab.net [Google Scholar]
- 10.Dillon NP, Siebold MA, Mitchell JE, Blachon GS, Balachandran R, Fitzpatrick JM, & Webster RJ (2016). Increasing Safety of a Robotic System for Inner Ear Surgery Using Probabilistic Error Modeling Near Vital Anatomy. Proceedings of SPIE--the International Society for Optical Engineering, 9786, 97861G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakashima S Sando I , Takahashi H and Fujita S (1993), Computer‐aided 3‐D reconstruction and measurement of the facial canal and facial nerve. I. cross‐sectional area and diameter: Preliminary report. The Laryngoscope, 103: 1150–1156. [DOI] [PubMed] [Google Scholar]
- 12.Noble JH, Dawant BM, Warren FM, Labadie RF (2009). Automatic identification and 3D rendering of temporal bone anatomy. Otology &Neurotology, 30(4), 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kersten-Oertel M, Jannin P, & Collins DL (2013). The state of the art of visualization in mixed reality image guided surgery. Computerized Medical Imaging and Graphics : the Official Journal of the Computerized Medical Imaging Society, 37(2), 98–112. [DOI] [PubMed] [Google Scholar]
- 14.Profeta AC, Schilling C, & McGurk M (2016). Augmented reality visualization in head and neck surgery: an overview of recent findings in sentinel node biopsy and future perspectives. The British Journal of Oral & Maxillofacial Surgery, 54(6), 694–696. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Yang J, Chu Y, Wu W, Xue J, Liang P, & Chen L (2016). A Novel Augmented Reality Navigation System for Endoscopic Sinus and Skull Base Surgery: A Feasibility Study. PloS One, 11(1), e0146996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Citardi MJ, Agbetoba A, Bigcas J-L, & Luong A (2016). Augmented reality for endoscopic sinus surgery with surgical navigation: a cadaver study. International Forum of Allergy & Rhinology, 6(5), 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu WP, Azizian M, Sorger J, Taylor RH, Reilly BK, Cleary K, & Preciado D (2014). Cadaveric feasibility study of da Vinci Si-assisted cochlear implant with augmented visual navigation for otologic surgery. JAMA Otolaryngology-- Head & Neck Surgery, 140(3), 208–214. [DOI] [PubMed] [Google Scholar]
- 18.Vávra P, Roman J, Zonča P, Ihnát P, Němec M, Kumar J, et al. (2017). Recent Development of Augmented Reality in Surgery: A Review. Journal of Healthcare Engineering, 2017, 4574172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birkfellner W, Figl M, Matula C, Hummel J, Hanel R, Imhof H, et al. (2003). Computer-enhanced stereoscopic vision in a head-mounted operating binocular. Physics in Medicine and Biology, 48(3), N49–57. [DOI] [PubMed] [Google Scholar]
- 20.Edwards PJ, Hawkes DJ, Hill DL, Jewell D, Spink R, Strong A, & Gleeson M (1995). Augmentation of reality using an operating microscope for otolaryngology and neurosurgical guidance. Journal of Image Guided Surgery, 1(3), 172–178. [DOI] [PubMed] [Google Scholar]
- 21.Grunert P, Müller-Forell W, Darabi K, Reisch R, Busert C, Hopf N, & Perneczky A (1998). Basic principles and clinical applications of neuronavigation and intraoperative computed tomography. Computer Aided Surgery : Official Journal of the International Society for Computer Aided Surgery, 3(4), 166–173. [DOI] [PubMed] [Google Scholar]
- 22.Pelizzari CA, Chen GT, Spelbring DR, Weichselbaum RR, & Chen CT (1989). Accurate three-dimensional registration of CT, PET, and/or MR images of the brain. Journal of Computer Assisted Tomography, 13(1), 20–26. [DOI] [PubMed] [Google Scholar]
- 23.Metzger MC, Rafii A, Holhweg-Majert B, Pham AM, & Strong B (2007). Comparison of 4 registration strategies for computer-aided maxillofacial surgery. Otolaryngology--Head and Neck Surgery : Official Journal of American Academy of Otolaryngology-Head and Neck Surgery, 137(1), 93–99. [DOI] [PubMed] [Google Scholar]
- 24.Mascott CR, Sol J-C, Bousquet P, Lagarrigue J, Lazorthes Y, & Lauwers-Cances V (2006). Quantification of true in vivo (application) accuracy in cranial image-guided surgery: influence of mode of patient registration. Neurosurgery, 59(1 Suppl 1), ONS146–56– discussion ONS146–56. [DOI] [PubMed] [Google Scholar]
- 25.Dixon BJ, Daly MJ, Chan HHL, Vescan A, Witterick IJ, & Irish JC (2014). Inattentional blindness increased with augmented reality surgical navigation. American Journal of Rhinology & Allergy, 28(5), 433–437. [DOI] [PubMed] [Google Scholar]


