Abstract
Molecular identification of neuronal types and genetic and imaging approaches to characterize their properties reveal morphological, physiological and dynamic aspects of sensory circuit development. Here we focus on the mouse tactile sensory circuitry, with particular emphasis on the main trigeminal pathway that connects the whiskers, the major tactile organ in rodents, to the neocortex. At each level of this pathway, neurogenesis, axonal elongation, pathfinding, target recognition and circuit reorganization including dendritic refinement of cortical layer 4 neurons occur contemporaneously and a multitude of molecular signals are used in differing combinations. We highlight recent advances in development of tactile circuitry and note gaps in our understanding.
Introduction
In mammals, tactile sensation develops very early and plays a major role in vital functions such as suckling, moving in space, grasping objects, discrimination of textures and objects, and ultimately in social behaviors and cognition. The tactile sensory periphery is wired to the cerebral cortex through a number of intermediary circuits. Complex molecular signaling events, morphological and physiological differentiations take place in developing these circuitries. Combined genetic, molecular, electrophysiological and imaging tools are revealing the detailed development and adult organization of the somatosensory system of the mouse as a mammalian model.
From the tactile periphery to the central nervous system (CNS)
The tactile sensory system engages with the activation of low threshold mechanoreceptors (LTMRs). These are specialized cells such as Meissner’s, Pacini corpuscles, Krause end bulbs, Merkel cells, Ruffini endings or free nerve endings. The receptor cells are connected to the CNS through the axonal processes of primary sensory neurons that reside in the dorsal root ganglia (DRG) and the trigeminal ganglion (TG) (for the orofacial regions). Tactile sensory axons and their receptors are collectively called the LTMRs. Development and molecular characterization of LTMRs have been detailed in recent reviews [1,2].
Primary sensory neurons arise from the neural crest with some placodal contributions to the TG. In the mouse embryo, neural crest cells migrate and a subpopulation of these cells coalesces into sensory ganglia (E9.5–E13.5) along the sides of the neural tube [3]. Neurogenesis and differentiation into sensory neuron types depend on temporal regulation of neurotrophic signaling and sequential waves of neurogenin 2 (Neurog2) and neurogenin 1 (Neurog1)[ 4].
Spatiotemporal regulation of numerous molecular signals acts in concert in attracting the newborn TG axons to both the peripheral and central targets [5,6]. Peripheral sensory axons that travel through a nonneuronal milieu establish intriguing interactions on the way to and within their final targets. Several target-derived molecules guide these axons, provide trophic support and induce differential gene expression patterns in the parent cell bodies. For example, brain-derived neurotrophic factor (BDNF) and the bone morphogenetic protein 4 (BMP4) expressed in the peripheral trigeminal targets retrogradely control differential gene expression along the dorsoventral axis of the embryonic TG through phosphorylation of Smad family transcription factors [7,8 ]. A more recent study also identified multiple epidermal growth factor like domains 8 (Megf8) as a modifier of BMP4 signaling in the TG and documented Megf8 null mice have impaired TG axon growth and target innervation like mice lacking BMP4 signaling [9].
Central axons of the DRGs enter the spinal cord and bifurcate. One branch terminates in dorsal horns while the other ascends in the dorsal columns (gracilis and cuneatus) to terminate in the dorsal column nuclei (DCN) of the same nomenclature. The orofacial somatosensory epithelium is connected to the CNS via the TG. Like their DRG counterparts, central trigeminal axons enter the pons, bifurcate and develop a short ascending and a long descending branch that stops abruptly in the cervical cord. The molecular signals initiating bifurcation of sensory axons at the entry zone appear to share commonalities between the spinal cord and the trigeminal brainstem. cGMP signaling cascade involving C-type natriuretic peptide (CNP), the natriuretic peptide receptor 2/receptor guanylyl cyclase B (Npr2), and the cGMP-dependent kinase I (cGKI) reportedly regulate sensory axon bifurcation upon entry into the spinal cord and at the brainstem entry zones of the cranial sensory nerves [10,11]. Signals that direct the bifurcated axons along different directions, where they stop and start emitting collateral branches remain elusive, although Slit2 has been identified as a branching factor in organotypic explant cultures of the embryonic trigeminal pathway[ 12].
Several molecular signals such as neurotrophins, integrins, semaphorins have been implicated in directing the ascension of sensory axons along the dorsal columns and even the rhombomeric boundaries for the trigeminal tract extension, but we do not have a definitive picture yet. Somatotopic organization of the ascending somatosensory pathways had been inferred from their positions along the neuraxis. Genetic morphological tracing revealed that LTMRs and proprioceptive afferents are segregated in the dorsal columns and DCN in the mouse [13]. If there is a submodality-specific segregation and topography in the dorsal columns, there must be mechanisms of modality-specific fiber sorting along the pathways.
Tactile nuclei of the brainstem
Little information exists on the development of the gracile and cuneate nuclei (i.e., DCN). In the rat, cuneate neurons are born between E13–E15 [14]. Gracilis and cuneate axons cross the midline and travel along the medial lemniscus to the ventroposterolateral (VPL) nucleus of the thalamus. Developmental schedule of pathfinding, molecular guides and any specific topography along the medial lemniscus are not studied, other than a few inferences from clinical anatomical observations.
Brainstem trigeminal nuclei lie in an elongated axis between the pons and the upper cervical cord. The principal sensory nucleus (PrV) is the most rostral and caudally joined by the spinal trigeminal nucleus with three subdivisions. The main tactile pathway neurons reside in the PrV. Their axons cross the midline, navigate along a well-defined route (trigeminal part of the medial lemniscus) to the contralateral thalamus and terminate in the ventroposteromedial (VPM) nucleus of the dorsal thalamus.
The mouse PrV appears as a peanut-shaped nucleus in coronal sections. From the mid-level, narrow “waist” side, the nucleus can be divided into a dorsal (dPrV) and a ventral (vPrV) portion. Embryologically, neurons of the vPrV are derived from rhombomere (r) 2 and those of the dPrV from r3 [15], and several transcription factors (e.g., Drg11, Lmx1b, Phox2b, Ptf1a) play roles in their differentiation and specification [5,6]. A recent genetic fate mapping study found that Ptf1a plays an important role in regulating GABAergic cell fate decision in neurons derived from r2–r7 [16]. In an earlier study, conditional targeted inactivation of Hoxa2 showed pathfinding errors of trigeminal tract axons, impaired arborization and whisker-specific patterning in the PrV, i.e. barrelette formation[ 15].
The dPrV receives mandibular afferents while vPrV receives ophthalmic and maxillary inputs mainly from the whisker pad. The face is represented along the dorsoventral axis of the PrV, and the 5 rows of whiskers are mapped in an inverted fashion in the vPrV with the nasal axis pointing medially. The whisker map is further accentuated by punctate arrangement of afferent terminals and thalamic projection neurons, collectively known as barrelettes. Thus, there is a topographic map of the face sensory epithelium and within it a patterned organization reflecting the distribution of the whisker follicles. The transcription factor Hoxa2 is normally expressed in vPrV, but not in dPrV, TG or the sensory periphery. Ectopic expression of Hoxa2 in the dPrV attracts whisker-related afferents that form discrete patches in the dPrV [17]. Further, in these mice, dPrV cells orient their dendrites towards these patches, similar to the vPrV barrelette neurons. Thus, Hoxa2 expression could be the determinant of the vPrV neuron identity as a barrelette neuron.
To date, a large body of evidence exists favoring a critical role for the patterning of the sensory epithelium as a key to neural patterns in the brain (reviewed in [18]). In the trigeminal system, ablation of whisker follicles in neonates alters neural patterns in a predictable way. Neonatal denervation of the whisker pad prevents formation of barrelettes, barreloids and barrels. Supernumerary whiskers on the snout or ectopic induction of follicles leads to formation of corresponding barrelette, barreloid and barrel structures[19,20]. Activity blockade, in particular NMDA receptor function disruption, prevents barrel formation [21,22]. However, in almost all these cases the topography of central projections and map formation is not disrupted. Intrinsic molecular cues or periphery-derived signals that alter gene expression downstream are the key players in topographic projections. Classical studies demonstrated that even in the absence of sensory periphery central connections can establish topography, such as the geniculocortical projections in unophthalmic mice [23]. Thus, it is important to distinguish between somatotopy and pattern formation. A recent study [24] examined trigeminal projections in endothelin 1 (Edn1) loss-of-function mouse fetuses which show whisker follicle or epidermal rugae type formations along the mandibular skin. These mutants have severe craniofacial and cardiac defects and do not survive. These trigeminal projections were traced at E14.5 and E17.5 and lipophilic tracer labeling revealed lack of mandibular topography in dPrV. We do not know whether these primordial mandibular “follicles” would eventually develop further and receive normal innervation and afferent density similar to the maxillary whisker follicles, so as to have corresponding barrelettes in the brainstem. The initial studies using the Edn1 null mice reported defects in distal ends of the mandibular and facial nerves, along with ectopic fiber growth [25,26]. Thus, the absence of mandibular topography in the dPrV might reflect aberrations in peripheral projections of the mandibular nerve, rather than any relation to rudimentary follicular structures on the mandibular skin. As indicated above, there are no studies claiming that whisker follicles determine topography of TG projections to the brainstem and establishment of somatotopy. However, there is a large body of literature reporting that patterning within the somatotopy is dependent on the whisker follicles on the snout.
Finally, for the vPrV, a recent review identified and discussed activity-dependent and independent developmental features [27]. Several properties of the vPrV neurons develop independent of peripherally evoked activity. These include the membrane conductances, cell type-specificities of ion channel expression, presynaptic release properties, postsynaptic NMDA receptor subunit composition, and inhibitory inputs they receive. However, whisker-specific patterning of neural elements and synaptic plasticity depends on activity and intact whiskers.
Trigeminothalamic projections and thalamic nuclei
Carbocyanine dye labeling in a novel flattened wholemount preparation of the hindbrain and diencephalon from mouse embryos, and also oblique horizontal sections from postnatal mice, revealed the developing trigeminothalamic tract [28]. Newly generated PrV neurons send out axons as early as E11. Pioneers take a sharp rostral turn as soon as they cross the midline. PrV axons reach the midbrain by E15 and the VPM by E17. By P1, PrV axons form diffuse terminal fields in the VPM and whisker-related patterns by P4. This initial trigeminal lemniscal development study was accompanied by another [29] using in situ hybridization assays and knockout mouse embryos that implicated netrins and slits in attracting PrV axons to the midline and their contralateralization. More recently, genetic labeling of trigeminal lemniscal axons confirmed the initial exuberance of trigeminothalamic projections [30]. In the same study, whole-cell recordings from acute thalamic slices showed that large scale synaptic refinement goes on beyond whisker-specific patterningin the VPM, i.e., barreloid formation.
The r3 origin of vPrV is leading to some very interesting findings. A recent study described the whisker-barrel pathway of a conditional mouse mutant in which Robo3 gene is inactivated in r3 [31]. These mice show partial ipsilateral projection of barrelette axons resulting in an ipsilateral whisker map (barreloids) nestled inside the contralateral whisker map, barreloids in the VPM, and subsequently in the whisker representation area of the SI cortex (barrels). Complete segregation of the ipsilateral and contralateral maps within both the VPM and SI suggest that neighboring axons guide one another. Retrograde labeling from the VPM showed that the ipsi- and contralateral projection neurons are mixed in the vPrV. Why some vPrV axons cross the midline and project contralaterally properly while others fail to do so in these mutant mice remains an unanswered question. There are a few possibilities such as the timing of midline crossing of ipsi- versus contralateral axons with respect to Robo3 expression, incomplete Cre-mediated recombination in these conditional knockout mice, and the r3 not being the sole origin of barrelette neurons.
A recent study also documented that midline crossing defects in spinal cord-specific netrin receptor Dcc null mice show disrupted somatotopy of nociceptive anterolateral system [32]. In these mice, increased ipsilateral spinothalamic projections subsequently lead to aberrant localization of the nociceptive cortical responses. Furthermore, examination of human subjects with DCC mutations also revealed bilateral sensations in response to unilateral stimulation [32]. Collectively, the two studies described above reveal that without any aberrations in the peripheral sensory epithelium or intrinsic cortical mapping signals, axon pathfinding defects in the lower CNS structures can have dramatic alterations of the sensory maps and patterning within them.
PrV barrelette neurons convey the whisker somatotopy and patterning to the VPM and the VPM barreloid neurons do the same for the barrel cortex layer 4 (L4), where thalamic axon terminals are embraced by the dendrites of spiny stellate cells forming the barrels [18]. The dorsolateral part of the VPM receives maxillary afferents and has a distinct whisker patterning, the barreloids; the ventromedial portion receives mandibular afferents. Bechara et al., (2015) reported that ectopic Hoxa2 expression in the dPrV led to projections from the dPrV to the whisker representation zone of the VPM instead of the mandibular portion [17]. This observation prompted the authors to conclude that Hoxa2 plays a role in pathfinding and topographic targeting in cooperation with ephrin-Eph signaling. However, it is not apparent that dPrV cells would do that if there were no vPrV projections to the thalamus, which might have guided them as some of their own Hoxa2 expressing cohort. Definitive support for the mapping role of Hoxa2 would be revealed if Hoxa2 gene expression were completely blocked in the vPrV and ectopically expressed only in the dPrV would lead to switching of the dPrV and vPrV zones in the VPM.
The thalamic VPL nucleus also has topographic afferent, efferent projections with the forepaw region exhibiting barreloid-like larger patches corresponding to the palmar pads. Both VPM and VPL are composed of primarily thalamic projection cells which relay the body map and the patterning to the barrel cortex L4. Incoming lemniscal activity plays a role in differentiation and patterning of thalamic neurons. For example, thalamus-specific genetic interference of NMDA receptors prevents barreloid formation [33]. Genetic and surgical manipulation of input activity also alters dendritic maturation of barreloid neurons that can be restored by overexpression of Kv1.1 type potassium channels, which reduces neuronal excitability[ 34].
Thalamocortical projections and cortical maps and patterns
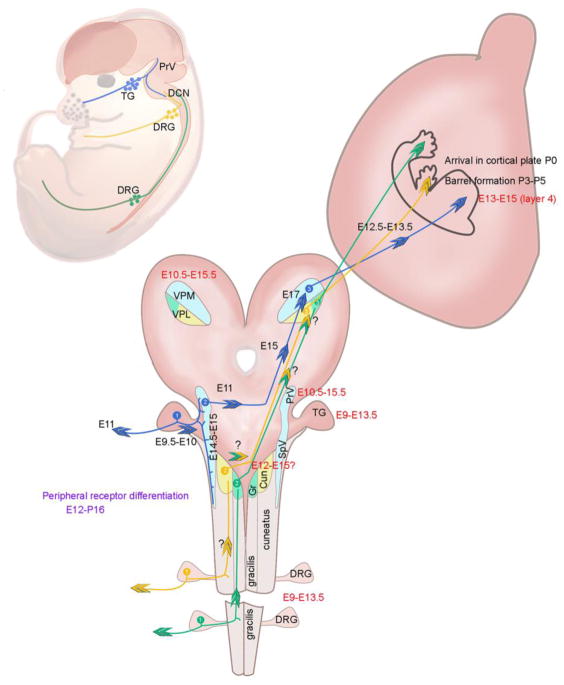
As can be seen from the time periods indicated in Figure 1, neurogenesis, axon extension, and tract formation events along the sensory periphery to the cortex are not temporally linked, rather, several occur independent of one another. Projections from the somatosensory thalamus headed to the cortex start out before lemniscal innervation. Most of the thalamocortical projection developmental studies have been carried out in mice, but it is not yet sorted out whether the VPM and VPL projections start out simultaneously or one follows the other. The initial journey of the thalamocortical axons (TCAs) to specific cortical targets is under the control of multiple thalamic and subpallial molecular guidance cues [35,36 ]. TCAs cross the diencephalon-telencephalon border by E12.5 and a day later they become part of the internal capsule [37,38]. As the neurons destined for specific cortical layers are being generated, TCAs spend some time in the subplate before reaching up to the cortical plate and ultimately L4 of the barrel cortex, their main terminal zone. Several lines of evidence indicate that the TCAs confer the blueprint of the sensory periphery, for example whisker-related patterning, to the topographic projection zone in the primary sensory cortex. During their journey en route to the cortex TCAs are preordered, maintaining near neighbor relationships. Perturbing this topography by genetic means such as conditional inactivation of the Ebf1 (early B cell factor 1) gene, which specifically affects an intermediate target along the TCA, pathway disrupts topographic and functional sensory map in the cortex [39].
Figure 1.
Development of tactile sensory circuitry.
Cortical areas devoted to the sensesare determined during development by both genetic mechanisms intrinsic to the cortex and extrinsic factors delivered by TCAs. Studies employing ectopic expression of intrinsic patterning molecules such as Fgf8, Pax6 [40] [41] showed how cortical areas devoted to the sensory representations can be altered in size and position, independent of sensory inputs. Fgf8 expression from the rostromedial neocortical primordium acts as an organizer for cortical sensory areas; experimental ectopic expression of Fgf8 in the caudal-most neocortical primordium leads to duplication of somatosensory and visual cortical maps [42]. Recent evidence shows that the transcription factor Ctip1, which is highly expressed in the barrel cortex during development, directs acquisition of sensory area identity and represses motor area identity [43]. On the other hand, blocking of visual or auditory inputs during early development results in shrinkage of the corresponding sensory cortex and expansion of the barrel cortex, suggesting cross-modal influences of thalamocortical inputs to the cortical arealization [44]. In thalamocortical slices prepared from mouse embryos, waves of spontaneous activity propagating across thalamic nuclei, corresponding to different sensory modalities, are observed. The authors of this study propose that these prenatal thalamic waves are important for cross-modal communication in the thalamus which may, in turn, modulate cortical area size through gene expression regulation in the thalamus [44]. Another example of the influence of subcortical inputs on cortical area size is observed in conditional Robo3 knockout mice, where a unilateral neonatal infraorbital nerve cut results in shrinkage of the contralateral whisker representation area and the expansion of the ipsilateral whisker area [31]. However, this happens within the face map area without changing overall face representation size. A recent report also suggests that modality of TCA inputs influences the L4 neuron identity [45]. In the mice with genetic ablation of ventrobasal nuclei (VB: VPM and VPL), L4 neurons in SI cortex are innervated by TCAs derived from the posteromedial (Po) nucleus, which projects to the secondary somatosensory (SII) cortex and L1 and L5a of the SI cortex in normal mice. Intriguingly, SI cortex L4 neurons in the VB-ablated mice show gene expression profiles similar to those of SII L4 neuronsin normal mice [45].
TCAs also convey whisker patterning to the barrel cortex. TCA terminals cluster in a whisker-specific manner and cortical L4 neurons surround them with their dendritic arbors embracing the terminals (Figure 2). For thalamocortical circuit refinement within the whisker representation area of the barrel cortex, there is substantial evidence demonstrating important roles for perinatal thalamocortical synaptic inputs. For example, barrel formation and dendritic refinement of L4 neurons are impaired in Rim1/Rim2 double knockout mice and/or VgluT1/VgluT2 double knockout mice; in both double mutants glutamate release from the TCAs is greatly reduced [46,47]. Recently, the presynaptic role of adenylyl cyclase 1, which could be involved in neurotransmitter release, was also revealed by thalamus-specific gene knockout [48]. Postsynaptically, the important role of glutamate receptors and candidates of their downstream signaling molecules are demonstrated. Recent progress on this issue involves the single cell knockout of NMDA receptor NR1 [49] and NR2B [50] subunits, both of which demonstrate impaired dendritic refinement of L4 neurons, and reveal cell-autonomous functions of postsynaptic NMDA receptors. Comparison of subunit-specific knockout mice for NMDA receptors demonstrates distinct functions for NR2B and NR2D subunits in barrel formation [51]. Cortex-specific and single cell knockout mice of metabotropic glutamate receptor 5 (mGluR5) are also reported to demonstrate postsynaptic role for mGluR5 in dendritic refinement and to identify NGF/TrkA signaling as an mGluR5 downstream candidate [52,53]. BTBD3 [54], FGF/FGFR [53], Lhx2 [55] and RORα [56] are also recently identified molecules that play important roles for dendritic refinement of L4 neurons at the postsynaptic side. However, our understanding of the molecular mechanisms of thalamocortical circuit refinement is far from complete. Further identification of molecular players and their cooperative functions in specific circuit sites is needed.
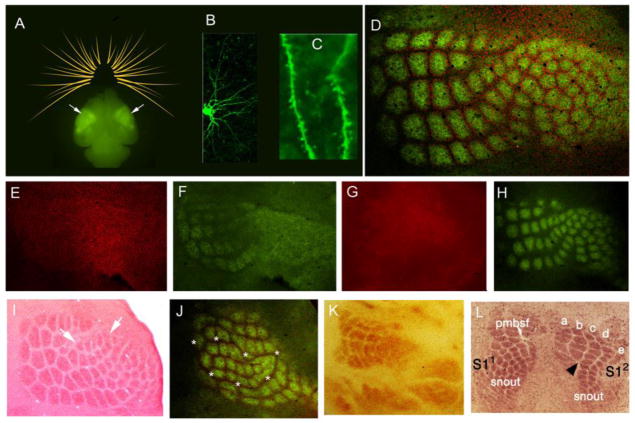
Figure 2.
The barrel cortex of a postnatal mouse brain and examples of barrel cortex defects resulting from peripheral and central alterations.
Activity-independent mechanisms may also be involved in cortical patterning. L4 neurons could be primed to form clusters. A recent study implicates the nuclear orphan receptor RORβ (expressed by L4 neurons) as a potential cluster priming factor; overexpression of RORβ induces periodic barrel-like clustering in L4 independent of TCA inputs, and such clusters indeed attract TCA terminals [57]. However, it is important to note that gain-of-function overexpression and ectopic expression studies show what the neurons can do but not necessarily what they actually do in normal development.
To date, numerous mutant mouse lines with barrel cortex pattern defects or map defects have been identified. Figure 2 illustrates a few examples of such defects arising from region-specific disruption of NMDA receptor function (activity-dependent), peripheral alterations in whisker follicle arrangement, axon pathfinding defects and alterations in the expression of cortical positioning signals.
Spontaneous activity in neonatal barrel cortex
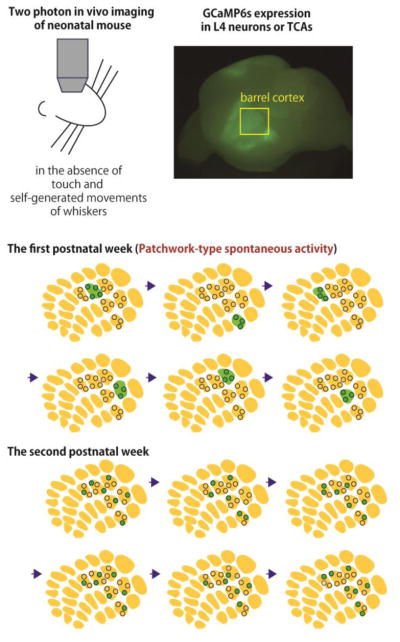
In neonatal rodents, whisker deflection evokes various patterns of cortical activity including gamma oscillations synchronized with the thalamic oscillations [58,59 ]. Because until the second postnatal week rodents do not show whisking behavior, whisker deflection in the first postnatal week occurs mostly by passive stimulation through contact with the dam, littermates, cage and bedding; such mechanical contacts can drive cortical activity [60]. In addition, similar cortical and thalamic oscillations are detected in the neonatal rodent even in the absence of whisker deflection [58]. In the neonatal rodent, self-generated whisker movements are detected, and sensory feedback arising from these whisker movements could drive thalamic and cortical activities without anything touching the whiskers [61–63]. Although this possibility is not excluded, the most recent work reveals a new type of spontaneous activity in the neonatal mouse barrel cortex L4, that arises from the periphery, transmitted via TCAs but mostly independent of whisker movements [64]. This cortical activity shows a “patchwork”-type pattern that corresponds to the barrel map, and neurons in the same barrel tend to be activated together (Figure 3). In this study, the barrel map is clearly visualized in vivo in a newly generated transgenic mouse line that expresses RFP in the TCAs. The synchronization of L4 neuron activity in the same barrel is largely diminished by the second postnatal week. Between the first and second postnatal weeks, however, neuronal circuits in the barrel cortex change drastically. For example, functional excitatory connections between cortical layers [65] and the dendritic spine numbers [66] massively increase. In the second postnatal week, inhibitory and subplate circuits are largely rearranged [67,68]. Further, a developmental switch from electrical synapses mediated by gap junction to chemical synapses between L4 neurons has been reported [69]. These aspects of functional maturation in the barrel cortex could result in changes of activity patterns of L4 neurons. They are also consistent with observations of activity in adjacent neurons in the superficial cortical layers, which also tends to synchronize in the first postnatal week, becoming desynchronized by the second postnatal week [70]. This patchwork activity has features that are suitable for refinement of thalamocortical circuitsin the neonatal barrelcortex.
Figure 3. Patchwork-type spontaneous activity in the neonatal barrel cortex L4.
In the neonatal barrel cortex, L4 excitatory neurons in the same barrel tend to fire together, even when whiskers are not touched or moved. This spontaneous activity is delivered to the cortex by TCAs. In the second postnatal week, the patchwork pattern of activity diminishes and L4 neurons even in the same barrel fire asynchronously.
Dynamics of thalamocortical circuit refinement
As described above, thalamocortical circuits are refined by activity delivered via TCAs. However, the dynamic mechanism of thalamocortical circuit refinement, by which cortical L4 neurons extend their dendrites toward specific sets of presynaptic TCAs, has long remained unexplored. Recently, 18 hour-long time-lapse imaging of L4 spiny stellate neurons in the mouse barrel cortex starting at postnatal day 5 (P5) was reported [49]. It was observed that dendritic morphologies of individual L4 neurons are clearly visualized in vivo using in utero electroporation-based “Supernova” labeling [49,71]. Barrel arrangement is visualized by generating a transgenic mouse line which expresses EGFP in TCAs [49]. This study provides the first evidence that dendritic branches are dynamically moving in the neonatal mammalian brain. Furthermore, by taking advantage of Supernova-mediated single cell knockout, cell-autonomous NMDA receptor function in the regulation of dendrite branch dynamics is also revealed. In NMDAR-deficient barrel cells, dendritic motility was enhanced, and the orientation bias towards TCA terminals did not develop.
Recently, even longer (3 day-long) imaging starting at earlier neonatal stages such as P3 has been achieved [72]. Long-term time-lapse imaging in the neonatal cortex will be a powerful approach to reveal new insights into the dynamic mechanisms that regulate morphological aspects of circuit refinement such as dendritic refinement of barrel cortex L4 neurons. In addition, long-term in vivo time-lapse imaging is useful to characterize neuronal properties in neonatal stages, during which different types of neurons are often difficult to distinguish by histological methods. For example, in early neonatal stages, both spiny stellate and star pyramid neurons in L4 possess apical dendrites and similar morphologies [73]. Furthermore, combined with calcium imaging, these chronic imaging techniques should also reveal developmental changes in physiological properties of individual cortical neurons.
Highlights.
Development and organization of the tactile circuitry in the mouse
Long-term in vivo imaging of developing neural circuits
Mechanisms of barrel cortex patterning
Development of any neural circuitry is a highly orchestrated event involving differentiation of neuronal elements from progenitor cells, acquisition of specific identities under the guidance of various transcription factors, differentiation of dendritic and axonal processes, navigation of axons to the proper targets, establishment of appropriate pre- and postsynaptic contacts, and patterning of neural connections. For the tactile circuitry, from the periphery to the SI cortex, timing of these events does not follow a temporal sequence, rather, many overlaps in time. Cartoon illustrating the timing of neurogenesis (red font) and axon extension and arrival at the targets (black font). The dorsal columns fasciculus gracilis and cuneatus are labeled and the dorsal column nuclei are abbreviated. Gr.: nucleus gracilis; Cun: nucleus cuneatus. Other abbreviations as in the text. Timing of axonal projections along the dorsal columns and the medial lemniscus pathway are not yet defined. Whisker -specific pattern formation begins to emerge in the PrV by P0–P1, in VPM by P2–P3 in SI cortex by P3–P5 [19].
-
A
Body maps in the barrel cortex (arrows) visualized by GFP-tagged thalamocortical axons in a whole brain of neonatal TCA-GFP mouse. Schematic drawing of whiskers is also shown.
-
B
A layer 4 spiny stellate neuron (barrel cell) with oriented basal dendrites intracellularly labeled.
-
C
Higher magnification view of the basal dendrites and dendritic spines.
-
D
In a tangential plane of the somatosensory cortex layer 4, neurons preferentially distribute along the barrel walls and TCA terminals fill inside the barrel hollows, visualized with NeuN (red) and VgluT2 (green)immunohistochemistry, respectively.
-
E,F
Absence of barrels (NeuN, red) and impaired TCA patterning (green) in sensory thalamus-restricted NMDAR NR1 mutant mice.
-
G, H
Absence of barrels (NeuN, red) and impaired TCA patterning (green) in cortex-restricted NMDAR NR1 mutant mice. TCAs form small and less clear clusters.
-
I
Ectopic sonic hedgehog expression in the whisker pad leads to formation of supernumerary whiskers and barrels corresponding to them (arrows) (from ref 19, with permission from Brain Research, Elsevier)
-
J
Bilateral whisker-barrels in conditional Robo3 ko mouse with trigeminothalamic axon midline crossing defects (red: NeuN, green: glutamate transporter immunohistochemistry). Asterisks denote the boundaries of the ipsilateral whisker barrels that are nestled inside the contralateral barrelfield.
-
K
Impaired barrel patterning following TCA pathway defects (ref 39, courtesy of L. Lokmane)
-
L
Duplicate barrel fields following Fgf8 manipulation in neocortex at embryonic ages (adapted from ref. 42)
Acknowledgments
Research supported by NIH grants R01NS084818 and R01NS092216 (R.S.E.) and KAKENHI JP16K14559, JP15H01454, JP15H04263 and Grant-in Scientific Research on Innovation Areas “Dynamic regulation of Brain Function by Scrap & Build System” (JP16H06459) from MEXT( T.I.). We thank A. Kolodkin for comments.
Footnotes
Conflict of interest statement
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommending reading
Papers of particular interest are highlighted as:
• of special interest
•• of outstanding interest
- 1.Olson W, Dong P, Fleming M, Luo W. The specification and wiring of mammalian cutaneous low-threshold mechanoreceptors. Wiley Interdiscip Rev Dev Biol. 2016;5(3):389–404. doi: 10.1002/wdev.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins BA, Lumpkin EA. Developing a sense of touch. Development. 2017;144(22):4078–4090. doi: 10.1242/dev.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serbedzija GN, Fraser SE, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108(4):605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- 4.Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13(13):1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erzurumlu RS, Murakami Y, Rijli FM. Mapping the face in the somatosensory brainstem. Nat Rev Neurosci. 2010;11(4):252–263. doi: 10.1038/nrn2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erzurumlu RS, Chen ZF, Jacquin MF. Molecular determinants of the face map development in the trigeminal brainstem. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(2):121–134. doi: 10.1002/ar.a.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodge LK, Klassen MP, Han BX, Yiu G, Hurrell J, Howell A, Rousseau G, Lemaigre F, Tessier-Lavigne M, Wang F. Retrograde BMP signaling regulates trigeminal sensory neuron identities and the formation of precise face maps. Neuron. 2007;55(4):572–586. doi: 10.1016/j.neuron.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Ji SJ, Jaffrey SR. Intra-axonal translation of SMAD1/5/8 mediates retrograde regulation of trigeminal ganglia subtype specification. Neuron. 2012;74(1):95–107. doi: 10.1016/j.neuron.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelhard C, Sarsfield S, Merte J, Wang Q, Li P, Beppu H, Kolodkin AL, Sucov HM, Ginty DD. MEGF8 is a modifier of BMP signaling in trigeminal sensory neurons. Elife. 2013;2:e01160. doi: 10.7554/eLife.01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt H, Rathjen FG. Signalling mechanisms regulating axonal branching in vivo. Bioessays. 2010;32(11):977–985. doi: 10.1002/bies.201000054. [DOI] [PubMed] [Google Scholar]
- 11.Ter-Avetisyan G, Rathjen FG, Schmidt H. Bifurcation of axons from cranial sensory neurons is disabled in the absence of Npr2-induced cGMP signaling. J Neurosci. 2014;34(3):737–747. doi: 10.1523/JNEUROSCI.4183-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozdinler PH, Erzurumlu RS. Slit2, a branching-arborization factor for sensory axons in the Mammalian CNS. J Neurosci. 2002;22(11):4540–4549. doi: 10.1523/JNEUROSCI.22-11-04540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu J, Ding L, Li JJ, Kim H, Liu J, Li H, Moberly A, Badea TC, Duncan ID, Son YJ, Scherer SS, et al. Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. J Neurosci. 2013;33(45):17691–17709. doi: 10.1523/JNEUROSCI.3429-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TJ, Lue JH, Wu CH, Shieh JY, Wen CY. Neurogenesis of cuneothalamic neurons and NO-containing neurons in the cuneate nucleus of the rat. Exp Brain Res. 2002;142(3):327–334. doi: 10.1007/s00221-001-0950-3. [DOI] [PubMed] [Google Scholar]
- 15.Oury F, Murakami Y, Renaud JS, Pasqualetti M, Charnay P, Ren SY, Rijli FM. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science. 2006;313(5792):1408–1413. doi: 10.1126/science.1130042. [DOI] [PubMed] [Google Scholar]
- 16.Iskusnykh IY, Steshina EY, Chizhikov VV. Loss of Ptf1a Leads to a Widespread Cell-Fate Misspecification in the Brainstem, Affecting the Development of Somatosensory and Viscerosensory Nuclei. J Neurosci. 2016;36(9):2691–2710. doi: 10.1523/JNEUROSCI.2526-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bechara A, Laumonnerie C, Vilain N, Kratochwil CF, Cankovic V, Maiorano NA, Kirschmann MA, Ducret S, Rijli FM. Hoxa2 Selects Barrelette Neuron Identity and Connectivity in the Mouse Somatosensory Brainstem. Cell Rep. 2015;13(4):783–797. doi: 10.1016/j.celrep.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 2012;35(10):1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohsaki K, Osumi N, Nakamura S. Altered whisker patterns induced by ectopic expression of Shh are topographically represented by barrels. Brain Res Dev Brain Res. 2002;137(2):159–170. doi: 10.1016/s0165-3806(02)00462-5. [DOI] [PubMed] [Google Scholar]
- 20.Ohsaki K, Nakamura S. Instructive role of a peripheral pattern for the central patterning of the trigeminal projection at the brainstem and thalamus revealed by an artificially altered whisker pattern. Neuroscience. 2006;141(4):1899–1908. doi: 10.1016/j.neuroscience.2006.04.082. [DOI] [PubMed] [Google Scholar]
- 21.Iwasato T, Erzurumlu RS, Huerta PT, Chen DF, Sasaoka T, Ulupinar E, Tonegawa S. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19(6):1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76(3):427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 23.Godement P, Saillour P, Imbert M. Thalamic afferents to the visual cortex in congenitally anophthalmic mice. Neurosci Lett. 1979;13(3):271–278. doi: 10.1016/0304-3940(79)91506-4. [DOI] [PubMed] [Google Scholar]
- 24.Laumonnerie C, Bechara A, Vilain N, Kurihara Y, Kurihara H, Rijli FM. Facial whisker pattern is not sufficient to instruct a whisker-related topographic map in the mouse somatosensory brainstem. Development. 2015;142(21):3704–3712. doi: 10.1242/dev.128736. [DOI] [PubMed] [Google Scholar]
- 25.Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao WH, Kamada N, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368(6473):703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 26.Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125(5):813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- 27.Lo FS, Erzurumlu RS. Sensory Activity-Dependent and Sensory Activity-Independent Properties of the Developing Rodent Trigeminal Principal Nucleus. Dev Neurosci. 2016;38(3):163–170. doi: 10.1159/000446395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kivrak BG, Erzurumlu RS. Development of the principal nucleus trigeminal lemniscal projections in the mouse. J Comp Neurol. 2013;521(2):299–311. doi: 10.1002/cne.23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirza R, Kivrak BG, Erzurumlu RS. Cooperative slit and netrin signaling in contralateralization of the mouse trigeminothalamic pathway. J Comp Neurol. 2013;521(2):312–325. doi: 10.1002/cne.23188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi Y, Asano H, Katayama Y, Muragaki Y, Imoto K, Miyata M. Large-scale somatotopic refinement via functional synapse elimination in the sensory thalamus of developing mice. J Neurosci. 2014;34(4):1258–1270. doi: 10.1523/JNEUROSCI.3865-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Renier N, Dominici C, Erzurumlu RS, Kratochwil CF, Rijli FM, Gaspar P, Chedotal A. A mutant with bilateral whisker to barrel inputs unveils somatosensory mapping rules in the cerebral cortex. Elife. 2017;6:e23494. doi: 10.7554/eLife.23494. The authors describe the whisker-barrel system in a mouse mutant in which Robo3, a gene that encodes a receptor involved in midline crossing of commissural axons, is deleted in rhombomere 3 neurons. The resulting conditional knockout mouse develops bilateral trigeminothalamic projections from the whisker representation zone of the PrV. Consequently, both ipsi- and contralateral whiskers are mapped to the thalamus and cortex in a non-overlapping fashion. This model mouse should be important in studying trigeminothalamic and thalamocortical wiring rules and competition between sets of afferent projections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva RV, Johannssen HC, Wyss MT, Roome RB, Bourojeni FB, Stifani N, Marsh APL, Ryan MM, Lockhart PJ, Leventer RJ, Richards LJ, et al. DCC Is Required for the Development of Nociceptive Topognosis in Mice and Humans. Cell Rep. 2018;22(5):1105–1114. doi: 10.1016/j.celrep.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Arakawa H, Suzuki A, Zhao S, Tsytsarev V, Lo FS, Hayashi Y, Itohara S, Iwasato T, Erzurumlu RS. Thalamic NMDA receptor function is necessary for patterning of the thalamocortical somatosensory map and for sensorimotor behaviors. J Neurosci. 2014;34(36):12001–12014. doi: 10.1523/JNEUROSCI.1663-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Frangeul L, Kehayas V, Sanchez-Mut JV, Fievre S, Krishna KK, Pouchelon G, Telley L, Bellone C, Holtmaat A, Graff J, Macklis JD, et al. Input-dependent regulation of excitability controls dendritic maturation in somatosensory thalamocortical neurons. Nat Commun. 2017;8(1):2015. doi: 10.1038/s41467-017-02172-1. In this study, the authors focused on thalamic neurons projecting to the SI cortex and found that dendritic maturation of these neurons are affected by membrane excitability, which is regulated by NMDA receptor-induced expression of Kv1.1-type potassium channels. Intriguingly, impairments in aspects of dendritic maturation induced by disruption of NMDA receptors or neonatal infraorbital nerve cut was rescued by Kv1.1 overexpression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garel S, Lopez-Bendito G. Inputs from the thalamocortical system on axon pathfinding mechanisms. Curr Opin Neurobiol. 2014;27:143–150. doi: 10.1016/j.conb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Martini FJ, Moreno-Juan V, Filipchuk A, Valdeolmillos M, Lopez-Bendito G. Impact of thalamocortical input on barrel cortex development. Neuroscience. 2018;368:246–255. doi: 10.1016/j.neuroscience.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125(1):127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uemura M, Nakao S, Suzuki ST, Takeichi M, Hirano S. OL-Protocadherin is essential for growth of striatal axons and thalamocortical projections. Nat Neurosci. 2007;10(9):1151–1159. doi: 10.1038/nn1960. [DOI] [PubMed] [Google Scholar]
- 39.Lokmane L, Proville R, Narboux-Neme N, Gyory I, Keita M, Mailhes C, Lena C, Gaspar P, Grosschedl R, Garel S. Sensory map transfer to the neocortex relies on pretarget ordering of thalamic axons. Curr Biol. 2013;23(9):810–816. doi: 10.1016/j.cub.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 40.Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294(5544):1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- 41.Zembrzycki A, Chou SJ, Ashery-Padan R, Stoykova A, O’Leary DD. Sensory cortex limits cortical maps and drives top-down plasticity in thalamocortical circuits. Nat Neurosci. 2013;16(8):1060–1067. doi: 10.1038/nn.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assimacopoulos S, Kao T, Issa NP, Grove EA. Fibroblast growth factor 8 organizes the neocortical area map and regulates sensory map topography. J Neurosci. 2012;32(21):7191–7201. doi: 10.1523/JNEUROSCI.0071-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greig LC, Woodworth MB, Greppi C, Macklis JD. Ctip1 Controls Acquisition of Sensory Area Identity and Establishment of Sensory Input Fields in the Developing Neocortex. Neuron. 2016;90(2):261–277. doi: 10.1016/j.neuron.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Moreno-Juan V, Filipchuk A, Anton-Bolanos N, Mezzera C, Gezelius H, Andres B, Rodriguez-Malmierca L, Susin R, Schaad O, Iwasato T, Schule R, et al. Prenatal thalamic waves regulate cortical area size prior to sensory processing. Nat Commun. 2017;8:14172. doi: 10.1038/ncomms14172. The authors have discovered that there are spontaneous thalamic calcium waves that propagate among sensory thalamic nuclei to the cortex and that provide communication between different sensory thalamocortical circuits, a mechanism setting up the cortical fields for sensory processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pouchelon G, Gambino F, Bellone C, Telley L, Vitali I, Luscher C, Holtmaat A, Jabaudon D. Modality-specific thalamocortical inputs instruct the identity of postsynaptic L4 neurons. Nature. 2014;511(7510):471–474. doi: 10.1038/nature13390. [DOI] [PubMed] [Google Scholar]
- 46.Narboux-Neme N, Evrard A, Ferezou I, Erzurumlu RS, Kaeser PS, Laine J, Rossier J, Ropert N, Sudhof TC, Gaspar P. Neurotransmitter release at the thalamocortical synapse instructs barrel formation but not axon patterning in the somatosensory cortex. J Neurosci. 2012;32(18):6183–6196. doi: 10.1523/JNEUROSCI.0343-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Fertuzinhos S, Mohns E, Hnasko TS, Verhage M, Edwards R, Sestan N, Crair MC. Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron. 2013;79(5):970–986. doi: 10.1016/j.neuron.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki A, Lee LJ, Hayashi Y, Muglia L, Itohara S, Erzurumlu RS, Iwasato T. Thalamic adenylyl cyclase 1 is required for barrel formation in the somatosensory cortex. Neuroscience. 2015;290:518–529. doi: 10.1016/j.neuroscience.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizuno H, Luo W, Tarusawa E, Saito YM, Sato T, Yoshimura Y, Itohara S, Iwasato T. NMDAR-regulated dynamics of layer 4 neuronal dendrites during thalamocortical reorganization in neonates. Neuron. 2014;82(2):365–379. doi: 10.1016/j.neuron.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62(2):205–217. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamasaki M, Okada R, Takasaki C, Toki S, Fukaya M, Natsume R, Sakimura K, Mishina M, Shirakawa T, Watanabe M. Opposing role of NMDA receptor GluN2B and GluN2D in somatosensory development and maturation. J Neurosci. 2014;34(35):11534–11548. doi: 10.1523/JNEUROSCI.1811-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ballester-Rosado CJ, Sun H, Huang JY, Lu HC. mGluR5 Exerts Cell-Autonomous Influences on the Functional and Anatomical Development of Layer IV Cortical Neurons in the Mouse Primary Somatosensory Cortex. J Neurosci. 2016;36(34):8802–8814. doi: 10.1523/JNEUROSCI.1224-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang JY, Lu HC. mGluR5 Tunes NGF/TrkA Signaling to Orient Spiny Stellate Neuron Dendrites Toward Thalamocortical Axons During Whisker-Barrel Map Formation. Cereb Cortex. 2017:1–16. doi: 10.1093/cercor/bhx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsui A, Tran M, Yoshida AC, Kikuchi SS, UM, Ogawa M, Shimogori T. BTBD3 controls dendrite orientation toward active axons in mammalian neocortex. Science. 2013;342(6162):1114–1118. doi: 10.1126/science.1244505. [DOI] [PubMed] [Google Scholar]
- 55.Wang CF, Hsing HW, Zhuang ZH, Wen MH, Chang WJ, Briz CG, Nieto M, Shyu BC, Chou SJ. Lhx2 Expression in Postmitotic Cortical Neurons Initiates Assembly of the Thalamocortical Somatosensory Circuit. Cell Rep. 2017;18(4):849–856. doi: 10.1016/j.celrep.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 56•.Vitalis T, Dauphinot L, Gressens P, Potier MC, Mariani J, Gaspar P. RORalpha Coordinates Thalamic and Cortical Maturation to Instruct Barrel Cortex Development. Cereb Cortex. 2017:1–14. doi: 10.1093/cercor/bhx262. The role of retinoic acid-related orphan receptor alpha (RORα) in thalamocortical circuit refinement is studied in the staggerer mice and following thalamus or cortical conditional gene deletion. The authors reveal cell-autonomous requirement for RORα in the thalamus for the patterned organization of TCAs in the barrel cortex and for the dendritic maturation of layer 4 neurons. [DOI] [PubMed] [Google Scholar]
- 57.Jabaudon D, Shnider SJ, Tischfield DJ, Galazo MJ, Macklis JD. RORbeta induces barrel-like neuronal clusters in the developing neocortex. Cereb Cortex. 2012;22(5):996–1006. doi: 10.1093/cercor/bhr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minlebaev M, Colonnese M, Tsintsadze T, Sirota A, Khazipov R. Early gamma oscillations synchronize developing thalamus and cortex. Science. 2011;334(6053):226–229. doi: 10.1126/science.1210574. [DOI] [PubMed] [Google Scholar]
- 59.Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29(28):9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Akhmetshina D, Nasretdinov A, Zakharov A, Valeeva G, Khazipov R. The Nature of the Sensory Input to the Neonatal Rat Barrel Cortex. J Neurosci. 2016;36(38):9922–9932. doi: 10.1523/JNEUROSCI.1781-16.2016. Unlike the visual system, the somatosensory system has sensory (tactile) inputs even in neonatal stages. In this paper, the authors classified sensory inputs into endogenous (self-generated movements) and exogenous (stimulation by the littermates) ones and analyzed their relative contributions to the cortical activity. Most likely, both sensory and spontaneous activities contribute to the circuit refinement in the somatosensory system in neonates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang JW, An S, Sun JJ, Reyes-Puerta V, Kindler J, Berger T, Kilb W, Luhmann HJ. Thalamic network oscillations synchronize ontogenetic columns in the newborn rat barrel cortex. Cereb Cortex. 2013;23(6):1299–1316. doi: 10.1093/cercor/bhs103. [DOI] [PubMed] [Google Scholar]
- 62.Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Curr Biol. 2012;22(21):2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432(7018):758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 64••.Mizuno H, Ikezoe K, Nakazawa S, Sato T, Kitamura K, Iwasato T. Patchwork-Type Spontaneous Activity in Neonatal Barrel Cortex Layer 4 Transmitted via Thalamocortical Projections. Cell Rep. 2018;22(1):123–135. doi: 10.1016/j.celrep.2017.12.012. Using two-photon calcium imaging of layer 4 neurons and thalamocortical axon terminals labeled by GCaMP6s, the authors found a novel type of spontaneous activity in the neonatal mouse SI cortex. This activity shows a “patchwork-type” pattern confined to the barrel map, which could be important for thalamocortical circuit maturation. [DOI] [PubMed] [Google Scholar]
- 65.Bureau I, von Saint Paul F, Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 2006;4(12):e382. doi: 10.1371/journal.pbio.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J Comp Neurol. 1996;373(3):340–354. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 67.Marques-Smith A, Lyngholm D, Kaufmann AK, Stacey JA, Hoerder-Suabedissen A, Becker EB, Wilson MC, Molnar Z, Butt SJ. A Transient Translaminar GABAergic Interneuron Circuit Connects Thalamocortical Recipient Layers in Neonatal Somatosensory Cortex. Neuron. 2016;89(3):536–549. doi: 10.1016/j.neuron.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinon MC, Jethwa A, Jacobs E, Campagnoni A, Molnar Z. Dynamic integration of subplate neurons into the cortical barrel field circuitry during postnatal development in the Golli-tau-eGFP (GTE) mouse. J Physiol. 2009;587(Pt 9):1903–1915. doi: 10.1113/jphysiol.2008.167767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valiullina F, Akhmetshina D, Nasretdinov A, Mukhtarov M, Valeeva G, Khazipov R, Rozov A. Developmental Changes in Electrophysiological Properties and a Transition from Electrical to Chemical Coupling between Excitatory Layer 4 Neurons in the Rat Barrel Cortex. Front Neural Circuits. 2016;10:1. doi: 10.3389/fncir.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golshani P, Goncalves JT, Khoshkhoo S, Mostany R, Smirnakis S, Portera-Cailliau C. Internally mediated developmental desynchronization of neocortical network activity. J Neurosci. 2009;29(35):10890–10899. doi: 10.1523/JNEUROSCI.2012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo W, Mizuno H, Iwata R, Nakazawa S, Yasuda K, Itohara S, Iwasato T, Supernova A. Versatile Vector System for Single-Cell Labeling and Gene Function Studies in vivo. Sci Rep. 2016;6:35747. doi: 10.1038/srep35747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakazawa S, Mizuno H, Iwasato T. Selection dynamics of cortical neuron dendrites revealed by long-term in vivo imaging in neonates. FENS Forum 2018 Abstract. 2018 doi: 10.1038/s41467-018-05563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Callaway EM, Borrell V. Developmental sculpting of dendritic morphology of layer 4 neurons in visual cortex: influence of retinal input. J Neurosci. 2011;31(20):7456–7470. doi: 10.1523/JNEUROSCI.5222-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]