Abstract
Background:
Dyspnea on exertion is common to both heart failure (HF) and chronic obstructive pulmonary disease (COPD), and it is important to discriminate whether symptoms are caused by HF or COPD in clinical practice. The ventilatory equivalent for carbon dioxide (V̇E/V̇CO2) slope and V̇E intercept (a reflection of pulmonary dead space) are two candidate non-invasive indices that could be used for this purpose. Thus, we compared non-invasive indexes of ventilatory efficiency in patients with HF and preserved or reduced ejection fraction (HFpEF and HFrEF, respectively) or COPD.
Methods:
Patients with HFpEF (n=21), HFrEF (n=20), and COPD (n=22) patients performed cardiopulmonary exercise testing to volitional fatigue. V̇E and gas exchange were measured via breath-by-breath open circuit spirometry. All data from rest to peak exercise were used to calculate V̇E/V̇CO2 slope and V̇E intercept using linear regression. Receiver operating characteristic (ROC) curves were constructed to determine optimized cutoffs for V̇E/V̇CO2 slope and V̇E intercept to discriminate HFpEF and HFrEF from COPD.
Results:
HFrEF patients had a greater V̇E/V̇CO2 slope than HFpEF and COPD patients (HFrEF: 40±9; HFpEF: 32±7; COPD: 32±7) (p<0.01). COPD patients had a greater V̇E intercept than HFpEF and HFrEF patients (COPD: 3.32±1.66; HFpEF: 0.77±1.23; HFrEF: 1.28±1.19 L/min) (p<0.01). A V̇E intercept of 2.64 L/min discriminated COPD from HF patients (AUC: 0.88, p<0.01), while V̇E/V̇CO2 slope did not (p=0.11).
Conclusion:
These findings demonstrate that V̇E intercept, not V̇E/V̇CO2 slope, may discriminate COPD from both HFpEF and HFrEF patients.
Keywords: ventilatory intercept, VE/VCO2 slope, diastolic heart failure, systolic heart failure, breathing strategy
1. Introduction
Patients with heart failure with preserved (HFpEF) or reduced ejection fraction (HFrEF) and chronic obstructive pulmonary disease (COPD) present with overlapping symptoms such as dyspnea on exertion, exercise intolerance, muscle weakness, and fatigue (1). Furthermore, HF and COPD patients can exhibit multiple co-morbidities necessitating differentiating indices to align the most appropriate treatment strategy. The ventilatory equivalent for carbon dioxide (V̇E/V̇CO2) slope, determined from cardiopulmonary exercise testing (CPET), has been used as a prognostic tool in HF (2–4) as well as to assess disease progression and identify possible comorbidities including COPD (5–7).
Higher V̇E/V̇CO2 slope is indicative of greater disease severity and worse outcomes in HF (4), which is in contrast to COPD, where decreases in V̇E/V̇CO2 slope are associated with worsening COPD severity due to pulmonary abnormalities and mechanical constraints (7). Thus, the V̇E/V̇CO2 slope would theoretically be useful to differentiate HF from COPD. However, patients with HF also exhibit pulmonary abnormalities similar to COPD (e.g., impaired lung diffusion capacity and mechanical constraints) (8–11), which may mask the differentiating impact of the V̇E/V̇CO2 slope. To this point, previous studies investigating the ability of V̇E/V̇CO2 slope to discriminate HFrEF and COPD have been inconclusive (12, 13).
The ventilatory intercept (V̇ E intercept) is a novel parameter derived from the V̇E to V̇CO2 relationship during exercise, which theoretically equates to dead space and is not influenced by pulmonary mechanical constraints (7, 14). Unlike V̇E/V̇CO2 slope, V̇E intercept increases with greater disease severity in COPD (7), and COPD patients have a greater V̇E intercept than HFrEF patients (12, 13). To date, V̇E/V̇CO2 slope and V̇E intercept have been exclusively investigated in HFrEF and COPD patients. Because HFpEF comprises ~50% of the HF population, it is important to understand these ventilatory inefficiency indices in HFpEF. In fact, our lab and others have found that ventilatory efficiency (and the components of the alveolar air equation) can differ between HFrEF and HFpEF where HFrEF patients exhibit worse ventilatory efficiency (i.e. greater V̇E/V̇CO2 slope) (2, 15).
The purpose of this study was to compare the V̇E/V̇CO2 slope and V̇E intercept in patients with COPD, HFpEF, or HFrEF. We hypothesized that 1) COPD patients will have a greater V̇E intercept compared to HF patients and 2) HFrEF patients will exhibit a greater V̇E/V̇CO2 slope compared to COPD and HFpEF patients.
2. Methods
2.1. Participants
Patients with HFpEF (n=21), HFrEF (n=20), or COPD (n=22) were referred to our study by their primary cardiologist or pulmonologist. HFrEF was defined by left ventricular ejection fraction (LVEF) ≤40% and HFpEF was defined by a LVEF ≥50%, clinical symptoms (e.g., exertional dyspnea) and elevated left heart filling pressures at rest and/or with exercise in accordance with established guidelines (16). COPD patients with GOLD stages 1–4 were recruited.
Participants were excluded using the following criteria: primary pulmonary hypertension, diagnosed pulmonary disease (in HF patients), diagnosed heart failure (in COPD patients), significant coronary artery disease (stenosis ≥50%), cor pulmonale, primary renal or hepatic disease, valvular heart disease (any stenosis, >mild regurgitation, etc.), hypertrophic or infiltrative cardiomyopathy, constrictive pericarditis, or deep vein thrombosis. All participants provided written informed consent after being provided a written and verbal description of the study requirements. All aspects of this study were approved by the Mayo Clinic Institutional Review Board and conformed to principles outlined in the Declaration of Helsinki.
2.2. Echocardiography
Resting two-dimensional and tissue Doppler echocardiography according to guidelines of the American Society of Echocardiography were used to assess LVEF, morphology, and function (i.e. early transmitral flow velocity (E), late transmitral flow velocity (A), E/A ratio, early diastolic mitral annular velocity (e’), and peak E to e’ ratio) in HF patients (16).
2.3. Pulmonary function tests
Patients performed standard pulmonary function tests according to the ATS/ERS guidelines. All HFrEF and COPD patients as well as a subset (n=12) of the HFpEF patients performed the pulmonary function tests. Forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC are reported. FEV1 and FVC are reported as percent (%) predicted values.
2.4. CPET protocol
Patients performed the CPET while remaining on standard pharmacologic therapy. Patients performed cycling exercise at an initial workload of 15–20 watts with an increasing workload of 15–20 watts every 3 minutes until volitional fatigue. Heart rate and rhythm were continuously monitored using a 12-lead electrocardiogram.
2.5. Ventilation and gas exchange
Breath-by-breath open circuit spirometry (MedGraphics, St. Paul, MN) was used to continuously measure ventilation and gas exchange throughout the CPET. The final 30 seconds of rest and of the final completed exercise stage (i.e., peak oxygen uptake (V̇O2peak)) were used for data analyses. Criteria for achievement of V̇O2peak included one of the following: heart rate <10 beats/min of the age-predicted maximum, plateau in V̇O2 (<150 mL/min) with increases in workload, respiratory exchange ratio >1.1, or rating of perceived exertion >17. Percent (%) predicted V̇O2peak was calculated from Hansen et al. (Predicted V̇O2peak = weight (kg) * (50.75–0.372*age)) (17). Data acquired included V̇O2, V̇CO2, respiratory exchange ratio (RER), breathing frequency (Fb), tidal volume (VT), ventilatory equivalents for V̇O2 and V̇CO2 (V̇E/V̇O2 and V̇E/V̇CO2, respectively), inspiratory time (Ti), expiratory time (Te), and VT/Ti (an index of ventilatory drive). The ventilatory efficiency response was expressed as a linear regression by plotting V̇E (ordinate) and V̇CO2 (abscissa) using data at rest and V̇O2peak and the slope (i.e. V̇E/V̇CO2 slope) and y-intercept (i.e. V̇E intercept) were determined for each patient as reviously done (2–4, 15, 18, 19). Using all exercise data to derive these ventilatory efficiency parameters is clinically relevant and prognostically superior to determining ventilatory efficiency parameters with exercise data prior to the respiratory compensation threshold in HF patients (15, 19).
2.6. Statistical analyses
Values are reported as mean±standard deviation (SD). Statistical analyses were performed by using SigmaStat 2.0 (Jandel Scientific, San Rafael, CA). All data were checked for normal distribution using the Shapiro-Wilk test and, if normality was not met, data were reciprocally transformed. Participant characteristics and peak exercise data were compared using a one-way analysis of variance, unpaired t-tests, and Kruskal-Wallis one-way analysis of variance (for categorical data) when appropriate. Tukey post-hoc tests were used when significant F-tests were found. Linear regression was used to determine V̇E/V̇CO2 slope and V̇E intercept. The receiver operating characteristic (ROC) curve model performed with JMP (JMP, Cary, NC) was used to determine area under the curve (AUC) and different cutoffs for V̇E/V̇CO2 slope and V̇E intercept to discriminate patients with HFpEF and HFrEF from COPD patients. Statistical significance was set at p<0.05.
3. Results
3.1. Participant characteristics
Age was similar in the groups but HFpEF had a greater BMI than HFrEF (Table 1). COPD patients had a lower FEV1 (% predicted) and FEV1/FVC than the HFpEF and HFrEF patients. Resting echocardiography measurements indicate there were increased filling pressures in HFpEF and HFrEF patients. All participants completed all aspects of this study in the absence of adverse events.
Table 1:
Patient characteristics
| COPD | HFpEF | HFrEF | p-value | |
|---|---|---|---|---|
| n | 22 | 21 | 20 | |
| Age (years) | 58 ± 8 | 63 ± 9 | 58 ± 7 | 0.07 |
| Sex (men/women) | 16/6† | 7/14 | 17/3† | <0.01 |
| Height (cm) | 170 ± 8 | 168 ± 10 | 173 ± 8 | 0.13 |
| Weight (kg) | 91 ± 25 | 101 ± 20 | 86 ± 14 | 0.07 |
| Body mass index (kg/m2) | 31 ± 8 | 36 ± 7‡ | 29 ± 4 | <0.01 |
| Body surface area (m2) | 2.1 ± 0.3 | 2.2 ± 0.3 | 2.0 ± 0.2 | 0.25 |
| FEV1 (% predicted) | 52 ± 14 | 75 ± 18* | 80 ± 17* | <0.01 |
| FVC (% predicted) | 79 ± 14 | 80 ± 15 | 81 ± 16 | 0.89 |
| FEV1/FVC (%) | 53 ± 14 | 74 ± 7* | 77 ± 5* | <0.01 |
| LV ejection fraction (%) | 63 ± 6‡ | 22 ± 7 | <0.01 | |
| GOLD stage, n (%) | ||||
| 1 | 1 (5) | |||
| 2 | 12 (55) | |||
| 3 | 8 (36) | |||
| 4 | 1 (5) | |||
| NYHA class, n (%) | ||||
| II | 6 (29) | 11 (55) | 0.09 | |
| III | 14 (67) | 9 (45) | 0.17 | |
| IV | 1 (5) | 0 (0) | 0.34 | |
| Weber-Janicki class [peak VO2], n (%) | ||||
| C, 10–16 mL/kg/min | 3 (14) | 6 (30) | 0.24 | |
| D, <10 mL/kg/min | 18 (86)* | 14 (70)* | 0.24 | |
| Hemoglobin (g/dL) | 12.2 ± 1.5‡ | 13.7 ± 1.7 | <0.01 | |
| Creatinine (mg/dL) | 1.24 ± 0.29 | 1.46 ± 0.44 | 0.07 | |
| eGFR (mL/min per 1.73 m2) | 48 ± 17 | 52 ± 14 | 0.58 | |
| Drug Therapy, n (%) | ||||
| Steroid | 13 (59) | 0 (0)* | 0 (0)* | <0.01 |
| Bronchodilator | 15 (68) | 0 (0)* | 0 (0)* | <0.01 |
| ACE I or ARB | 6 (27) | 14 (67)* | 14 (70)* | <0.01 |
| Antiarrhythmic | 0 (0) | 4 (19) | 9 (45)* | <0.01 |
| β-blocker | 4 (18) | 16 (76)* | 17 (85)* | <0.01 |
| Ca2+ channel blocker | 1 (5) | 6 (29) | 0 (0) | <0.01 |
| Digoxin | 0 (0) | 2 (10)‡ | 12 (60)* | <0.01 |
| Nitrate (oral, SL, or topical) | 0 (0) | 7 (33) | 6 (30) | 0.01 |
| Aspirin | 5 (23) | 15 (71)* | 15 (75)* | <0.01 |
| Diuretics | 5 (23) | 9 (43)‡ | 18 (90)* | <0.01 |
| Echocardiography | ||||
| LA volume (mL) | 76 ± 27‡ | 114 ± 42 | <0.01 | |
| LA volume index (mL/m2) | 37 ± 13‡ | 56 ± 21 | <0.01 | |
| Mitral E-wave VEL (cm/s) | 95.4 ± 24.6 | 88.9 ± 37.7 | 0.53 | |
| Mitral A-wave VEL (cm/s) | 74.7 ± 19.7 | 71.3 ± 37.6 | 0.75 | |
| Mitral E/A ratio | 1.4 ± 0.7 | 1.5 ± 1.0 | 0.67 | |
| Mitral septal tissue Doppler VEL (e’) (cm/s) | 6.7 ± 2.1‡ | 4.3 ± 1.1 | <0.01 | |
| Mitral E/e’ ratio | 15.9 ± 8.0‡ | 22.5 ± 10.8 | 0.04 | |
| IV septum thickness (mm) | 10.3 ± 1.2 | 9.5 ± 1.3 | 0.06 | |
| Posterior wall thickness (mm) | 9.9 ± 1.7 | 9.9 ± 1.3 | 0.99 |
Mean±SD.
, significantly different from COPD
, significantly different from HFpEF
, significantly different from HFrEF. FEV1, FVC, and FEV1/FVC are from 12 of 21 HFpEF patients.
3.2. Peak Exercise
COPD patients exhibited a greater workload, relative and absolute V̇O2, V̇CO2, HR, V̇E, VT, Fb, Ti, and VT/Ti compared to HFpEF and HFrEF patients during peak exercise (Table 2). At peak exercise, HFrEF patients had a greater V̇E/V̇CO2 ratio, V̇E/V̇O2 ratio, and Ti/Ttot compared to COPD and HFpEF patients.
Table 2:
Peak Exercise Data
| COPD | HFpEF | HFrEF | p-value | |
|---|---|---|---|---|
| Workload (watts) | 84 ± 35 | 40 ± 13* | 40 ± 12* | <0.01 |
| V̇O2 (mL/kg/min) | 17 ± 4 | 8 ± 2* | 9 ± 3* | <0.01 |
| V̇O2 (% predicted) | 59 ± 13 | 31 ± 9* | 30 ± 8* | <0.01 |
| V̇O2 (mL/min) | 1516 ± 431 | 825 ± 220* | 770 ± 203* | <0.01 |
| V̇CO2 (mL/min) | 1562 ± 551 | 860 ± 247* | 756 ± 217* | <0.01 |
| RER | 1.01 ± 0.10 | 1.04 ± 0.13 | 1.07 ± 0.09 | 0.33 |
| HR (beats/min) | 130 ± 22 | 102 ± 14* | 103 ± 26* | <0.01 |
| V̇E (L/min) | 53 ± 17 | 28 ± 9* | 31 ± 9* | <0.01 |
| Fb (breaths/min) | 34 ± 5 | 28 ± 9* | 28 ± 7* | <0.01 |
| VT (L) | 1.6 ± 0.5 | 1.0 ± 0.3* | 1.1 ± 0.4* | <0.01 |
| V̇E/V̇ O2 | 35 ± 6‡ | 34 ± 9‡ | 44 ± 12 | <0.01 |
| V̇E/V̇ CO2 | 35 ± 7‡ | 33 ± 6‡ | 41 ± 9 | <0.01 |
| Ti (s) | 0.58 ± 0.10 | 0.75 ± 0.22* | 0.85 ± 0.24* | <0.01 |
| Te (s) | 1.21 ± 0.22 | 1.62 ± 0.54 | 1.46 ± 0.80 | 0.07 |
| Ti/Ttot | 0.32 ± 0.05‡ | 0.32 ± 0.06‡ | 0.38 ± 0.07 | <0.01 |
| VT/Ti | 2683 ± 614 | 1443 ± 409* | 1354 ± 309* | <0.01 |
| O2 saturation (%) | 96 ± 3 | 94 ± 3 | 95 ± 4 | 0.15 |
Mean ± SD.
significantly different from COPD
significantly different from HFpEF
significantly different from HFrEF
3.3. Ventilatory inefficiency parameters
HFrEF patients had a greater V̇E/V̇CO2 slope than HFpEF and COPD patients (HFrEF: 40±9; HFpEF: 32±7; COPD: 32±7) (p<0.01) (Figure 1A). COPD patients had a greater V̇E intercept compared to HFpEF and HFrEF patients (COPD: 3.32±1.66; HFpEF: 0.77±1.23; HFrEF: 1.28±1.19 L/min) (p<0.01) (Figure 1B). Importantly, these group differences in V̇E/V̇CO2 slope and V̇E intercept were still present after adjustment for sex and BMI (all, p<0.01). Significant inverse relationships were observed between V̇E/V̇CO2 slope and V̇E intercept in HFpEF (r2=0.40, p<0.01), but not COPD (r2=0.11, p=0.17) or HFrEF (r2=0.00, p=0.78).
Figure 1: V̇E/V̇CO2 slope and V̇E intercept in COPD, HFpEF, and HFrEF patients.
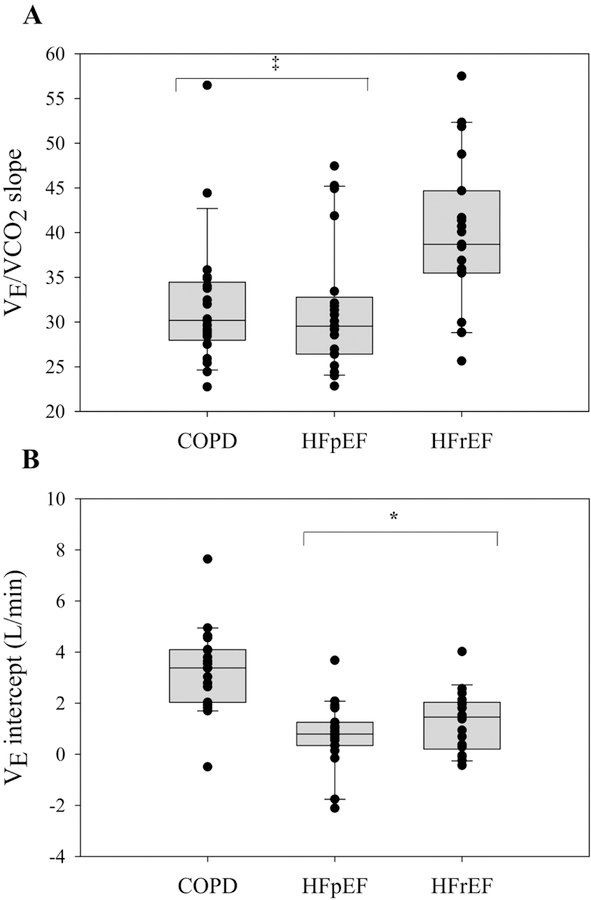
HFrEF patients had a greater V̇E/V̇CO2 slope compared to COPD and HFpEF patients (p<0.01). COPD patients had a greater V̇E intercept compared to HFpEF and HFrEF patients (p<0.01). ‡, significantly different from HFrEF patients. *, significantly different from COPD patients. Data are reported as median and 25–75 interquartile range.
3.4. ROC curves
A ROC curve analysis considering COPD and all HF patients identified a cutoff for V̇E intercept of ≥2.64 L/min to indicate patients with a high probability of having COPD (AUC: 0.88; p<0.01) (Figure 2) (Table 3), with 74% of COPD and 5% of HF patients exceeding this value. When considering COPD and HFpEF patients, ROC curve analysis identified a cutoff for V̇E intercept of ≥1.82 L/min to indicate patients with a high probability of having COPD (AUC: 0.90; p<0.01). Using this cutoff, 89% of COPD and 16% of HFpEF patients had a V̇E intercept ≥1.36 L/min. For COPD and HFrEF patients, a ROC curve analysis identified a cutoff for V̇E intercept of ≥2.64 L/min to indicate patients with a high probability of having COPD (AUC: 0.85; p<0.01). Using this cutoff, 74% of COPD and 6% of HFrEF patients had a V̇E intercept of ≥2.64 L/min. A ROC curve analysis considering COPD and HFrEF patients identified cutoff for V̇E/V̇CO2 slope of ≤35 to indicate patients with a high probability of having COPD (AUC: 0.79; p<0.01). Using this cutoff, 86% of COPD and 21% of HFrEF patients had a V̇E/V̇CO2 slope ≤35.
Figure 2: ROC curve analysis considering all HF and COPD patients.
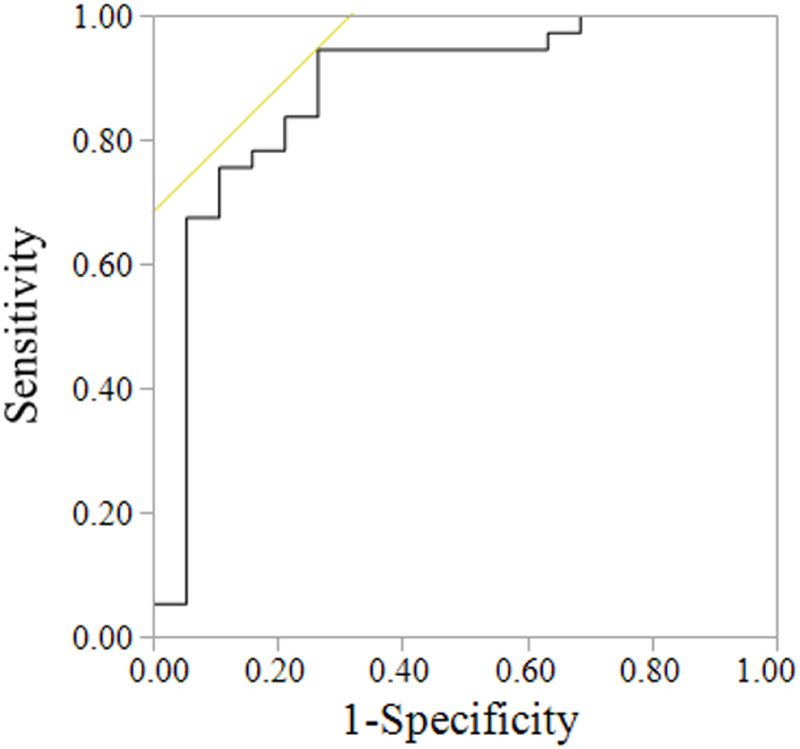
The ROC curve identified a cutoff for V̇E intercept of ≥2.64 L/min to indicate patients with a high probability of having COPD (AUC: 0.88; p<0.01).
Table 3:
ROC analysis
| COPD and All HF | COPD and HFpEF | COPD and HFrEF | ||||
|---|---|---|---|---|---|---|
| VE/VCO2 slope |
VE intercept |
VE/VCO2 slope |
VE intercept |
VE/VCO2 slope |
VE intercept |
|
| AUC | 0.62 | 0.88 | 0.54 | 0.90 | 0.79 | 0.85 |
| p-value | 0.11 | <0.01 | 0.85 | <0.01 | <0.01 | <0.01 |
| Cutoff | 2.64 | 1.82 | 35 | 2.64 | ||
| Sensitivity | 0.74 | 0.89 | 0.86 | 0.74 | ||
| Specificity | 0.95 | 0.84 | 0.79 | 0.94 | ||
4. Discussion
This is the first study comparing non-invasive indices of ventilatory efficiency during incremental exercise (i.e., V̇E/V̇CO2 slope and V̇E intercept) across COPD, HFpEF and HFrEF patients. Our major findings confirm the study hypothesis that V̇E intercept is greater in COPD patients compared to both HF groups with no differences between HFpEF and HFrEF. Furthermore, we found that the V̇E/V̇CO2 slope was greater in HFrEF compared to COPD and HFpEF patients (while no differences were present between the latter). Lastly, we found that COPD patients have a greater likelihood of having a V̇E intercept ≥2.64 L/min than both HF groups. Taken together, these findings are the first to demonstrate that V̇E intercept, not V̇E/V̇CO2 slope, can discriminate COPD from both HFpEF and HFrEF patients. This is clinically important given the high volume of patients presenting with unexplained dyspnea and/or co- morbid disease states including HF and COPD. Furthermore, because HF patients often demonstrate pulmonary abnormalities and COPD patients often demonstrate cardiac output impairment, these data allows us to begin to understand different patterns of data presented by patients with these diseases which influence both aspects of the cardiopulmonary system. Subsequently, this will allow clinicians the ability to differentiate the clinical treatment priority and align treatment strategies which will have the greatest impact.
The ability to non-invasively distinguish HF and COPD patients has important clinical utility as HF and COPD have overlapping symptoms (e.g., dyspnea on exertion, exercise intolerance, muscle weakness, fatigue) (1). Therefore, alternative indices are required to differentiate COPD from HFpEF and HFrEF. The V̇E intercept, a measure of ventilatory efficiency quantified from CPET, has been found to be an important and useful parameter in COPD as it is not confounded by mechanical abnormalities and constraints (7). An important novel finding of the present study was that the V̇E intercept was effective in differentiating COPD from HFpEF and HFrEF patients. This is clinically relevant given no currently available tools can non-invasively discriminate between HFpEF and COPD. These findings are consistent with and extend previous studies that have reported a greater V̇E intercept in COPD compared to HFrEF patients (12, 13). The elevated V̇E intercept in COPD patients theoretically equates to increased dead space (i.e. increased V̇E when metabolic demand is null) (20) and likely results from an altered breathing strategy (increased breathing frequency to compensate for reduced VT secondary to greater mechanical constraints) and/or a progressive ventilation-perfusion mismatch in COPD patients (21).
Importantly, the ROC curve analysis identified a significant cutoff for V̇E intercept (i.e. 2.64 L/min), but not V̇E/V̇CO2 slope when all HF patients were included in the analysis. Moreover, V̇E intercept AUC was significant for HFpEF patients, while V̇E intercept and V̇E/V̇CO2 slope AUCs were significant for HFrEF. These findings suggest that the V̇E/V̇CO2 relationship is important for both HF groups; however, the V̇E intercept component provides clinical value for HFpEF, while both V̇E intercept and V̇E/V̇CO2 provide clinical value for HFrEF. In the present study, the V̇E intercept cutoff value for the HFrEF patients (i.e. 2.64 L/min) is lower than previously reported (2.72–4.07 L/min) (12, 13) likely due to the methodology used to derive the V̇E/V̇CO2 slope and V̇E intercept as well as the severity of disease in the HF and COPD patients (7, 12, 22, 23).
An elevated V̇E/V̇CO2 slope has been found in several clinical populations including HFpEF, HFrEF, and COPD. However, disease severity in HF and COPD has opposing effects on V̇E/V̇CO2 slope. Because of these divergent responses, the V̇E/V̇CO2 slope has been found to be greater in HFrEF or not different compared to COPD patients (12, 13). In the present study, we found that the V̇E/V̇CO2 slope was greater in HFrEF compared to HFpEF and COPD, while not different between the latter. Our finding of a greater ventilatory inefficiency as indicated by a higher V̇E/V̇CO2 slope in HFrEF than HFpEF patients is consistent with previous studies (2, 15). Furthermore, the V̇E/V̇CO2 slope in HFpEF patients in the present study (~32) is consistent with the V̇E/V̇CO2 slope (~33) reported in HFpEF patients of a similar age (18). The heightened ventilatory response in HFrEF likely arises from increased physiological dead space, neural ventilatory drive, and/or activation of group III/IV locomotor muscle afferents during exercise (24, 25). Furthermore, the exaggerated ventilatory response in HFrEF has important implications in blood flow redistribution during exercise (26, 27) as well as possibly influencing the ventilatory efficiency in patients with HFrEF and co-existing COPD. Specifically, HFrEF patients with co-existing COPD have a higher V̇E/V̇CO2 slope than COPD alone (23).
There are several methodological factors to consider when interpreting our findings. First, we acknowledge the relatively small sample size in the present investigation. Studies with larger sample sizes may be necessary to confirm our findings. Second, echocardiography measurements were not performed in the COPD patients. Third, the physiologic mechanisms responsible for the ventilatory inefficiency in HF and COPD patients were outside the scope of the present non-invasive study. Future studies are warranted using invasive techniques (e.g., arterial blood gas measurements, locomotor muscle neural feedback inhibition, etc.) in combination with interventions such as altering central-peripheral hemodynamics (e.g. via inorganic nitrite supplementation (28, 29)) and modifying dead space (20) to better understand the underlying pathophysiology of ventilatory inefficiency in these populations.
In summary, we have demonstrated that V̇E intercept, determined non-invasively from a CPET, can discriminate HFpEF and HFrEF from COPD patients. These results demonstrate the importance of quantifying CPET metrics in HF and COPD patients while providing additional support for the use of the V̇E intercept as a ventilatory efficiency parameter. In contrast, V̇E/V̇CO2 slope was only sufficient in distinguishing HFrEF from COPD patients. Future studies are warranted to determine if V̇E intercept is capable of distinguishing HFpEF with concurrent COPD diagnoses from HFpEF patients.
Acknowledgements:
The authors would like to acknowledge and thank all the participants who volunteered for this study.
Funding: This work was supported by the National Institutes of Health [HL126638 to T.P.O, HL071478 to B.D.J., and HL128526 to B.A.B] and American Heart Association [16POST30260021 to E.H.V. and 18POST3990251 to J.R.S.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflicts of interest are reported.
References
- 1.Hawkins NM, Virani S, Ceconi C. Heart failure and chronic obstructive pulmonary disease: the challenges facing physicians and health services. Eur Heart J 2013. September;34(36):2795–2803. [DOI] [PubMed] [Google Scholar]
- 2.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol 2005. November 15;46(10):1883–1890. [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Humphrey R. Comparison of ventilatory expired gas parameters used to predict hospitalization in patients with heart failure. Am Heart J 2002. March;143(3):427–432. [DOI] [PubMed] [Google Scholar]
- 4.Guazzi M, Reina G, Tumminello G, Guazzi MD. Exercise ventilation inefficiency and cardiovascular mortality in heart failure: the critical independent prognostic value of the arterial CO2 partial pressure. Eur Heart J 2005. March;26(5):472–480. [DOI] [PubMed] [Google Scholar]
- 5.Thirapatarapong W, Armstrong HF, Thomashow BM, Bartels MN. Differences in gas exchange between severities of chronic obstructive pulmonary disease. Respir Physiol Neurobiol 2013. March 01;186(1):81–86. [DOI] [PubMed] [Google Scholar]
- 6.Thirapatarapong W, Armstrong HF, Bartels MN. Comparison of cardiopulmonary exercise testing variables in COPD patients with and without coronary artery disease. Heart Lung 2014. Mar-Apr;43(2):146–151. [DOI] [PubMed] [Google Scholar]
- 7.Neder JA, Arbex FF, Alencar MC, O’Donnell CD, Cory J, Webb KA, O’Donnell DE. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur Respir J 2015. February;45(2):377–387. [DOI] [PubMed] [Google Scholar]
- 8.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010. September;3(5):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson BD, Beck KC, Olson LJ, O’Malley KA, Allison TG, Squires RW, Gau GT. Ventilatory constraints during exercise in patients with chronic heart failure. Chest 2000. February;117(2):321–332. [DOI] [PubMed] [Google Scholar]
- 10.Olson TP, Johnson BD, Borlaug BA. Impaired Pulmonary Diffusion in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2016. June;4(6):490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agostoni P, Bussotti M, Cattadori G, Margutti E, Contini M, Muratori M, Marenzi G, Fiorentini C. Gas diffusion and alveolar-capillary unit in chronic heart failure. Eur Heart J 2006. November;27(21):2538– 2543. [DOI] [PubMed] [Google Scholar]
- 12.Apostolo A, Laveneziana P, Palange P, Agalbato C, Molle R, Popovic D, Bussotti M, Internullo M, Sciomer S, Bonini M, Alencar MC, Godinas L, Arbex F, Garcia G, Neder JA, Agostoni P. Impact of chronic obstructive pulmonary disease on exercise ventilatory efficiency in heart failure. Int J Cardiol 2015;189:134–140. [DOI] [PubMed] [Google Scholar]
- 13.Teopompi E, Tzani P, Aiello M, Ramponi S, Visca D, Gioia MR, Marangio E, Serra W, Chetta A. Ventilatory response to carbon dioxide output in subjects with congestive heart failure and in patients with COPD with comparable exercise capacity. Respir Care 2014. July;59(7):1034–1041. [DOI] [PubMed] [Google Scholar]
- 14.Agostoni P, Apostolo A, Sciomer S. Evolution of the concept of ventilatory limitation during exercise. Combining the pneumologist and cardiologist point of view. Respir Physiol Neurobiol 2011. December 15;179(2–3):127–128. [DOI] [PubMed] [Google Scholar]
- 15.Van Iterson EH, Johnson BD, Borlaug BA, Olson TP. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail 2017. October 08. [DOI] [PMC free article] [PubMed]
- 16.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task Force for the D, Treatment of A, Chronic Heart Failure of the European Society of C, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, Guidelines ESCCfP. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012. August;14(8):803–869. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis 1984. February;129(2 Pt 2):S49–55. [DOI] [PubMed] [Google Scholar]
- 18.Guazzi M, Labate V, Cahalin LP, Arena R. Cardiopulmonary exercise testing reflects similar pathophysiology and disease severity in heart failure patients with reduced and preserved ejection fraction. Eur J Prev Cardiol 2014. July;21(7):847–854. [DOI] [PubMed] [Google Scholar]
- 19.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest 2003. August;124(2):720–727. [DOI] [PubMed] [Google Scholar]
- 20.Gargiulo P, Apostolo A, Perrone-Filardi P, Sciomer S, Palange P, Agostoni P. A non invasive estimate of dead space ventilation from exercise measurements. PLoS One 2014;9(1):e87395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell DE, Laveneziana P, Webb K, Neder JA. Chronic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med 2014. March;35(1):51–69. [DOI] [PubMed] [Google Scholar]
- 22.Ward SA. Commentary on “Mechanism of augmented exercise hyperpnea in chronic heart failure and dead space loading” by Poon and Tin. Respir Physiol Neurobiol 2013. October 01;189(1):203–210. [DOI] [PubMed] [Google Scholar]
- 23.Arbex FF, Alencar MC, Souza A, Mazzuco A, Sperandio PA, Rocha A, Hirai DM, Mancuso F, Berton DC, Borghi-Silva A, Almeida DR, O’Donnell DE, Neder JA. Exercise Ventilation in COPD: Influence of Systolic Heart Failure. COPD 2016. December;13(6):693–699. [DOI] [PubMed] [Google Scholar]
- 24.Olson TP, Snyder EM, Johnson BD. Exercise-disordered breathing in chronic heart failure. Exerc Sport Sci Rev 2006. October;34(4):194–201. [DOI] [PubMed] [Google Scholar]
- 25.Olson TP, Joyner MJ, Eisenach JH, Curry TB, Johnson BD. Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp Physiol 2014. February;99(2):414–426. [DOI] [PubMed] [Google Scholar]
- 26.Smith JR, Hageman KS, Harms CA, Poole DC, Musch TI. Effect of chronic heart failure in older rats on respiratory muscle and hindlimb blood flow during submaximal exercise. Respir Physiol Neurobiol 2017. September;243:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 2010. July 01;588(Pt 13):2487– 2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borlaug BA, Melenovsky V, Koepp KE. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circ Res 2016. September 16;119(7):880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borlaug BA, Koepp KE, Melenovsky V. Sodium Nitrite Improves Exercise Hemodynamics and Ventricular Performance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2015. October 13;66(15):1672–1682. [DOI] [PubMed] [Google Scholar]


