Abstract
Objective:
Cardioplegic arrest (CP) and cardiopulmonary bypass (CPB) are associated with vasomotor dysfunction of coronary arterioles in diabetic patients undergoing cardiac surgery. We hypothesized that diabetes may up-regulate vasopressin receptor expression and alter the contractile response of coronary arterioles to vasopressin in the setting of CP/CPB.
Methods:
Right atrial tissue samples of diabetic (DM) and non-diabetic (ND) patients (n = 8/ each group) undergoing cardiac surgery were harvested before and after CP/CPB. The isolated coronary arterioles (80–150μm) dissected from the harvested right atrial tissue samples, were cannulated and pressurized (40 mmHg) in a no-flow state. The changes in diameter were measured with video microscopy. The protein expression/localization of vasopressin V1A and 1B receptors in the atrial tissue were measured by immune-blotting and immunohistochemistry.
Results:
The pre-CP/CPB contractile responses of the coronary arterioles to vasopressin were significantly increased post-CP/CPB in both ND and DM groups. This effect was more pronounced in the vessels from patients with diabetes than that of vessels from nondiabetic patients (P<0.05). Vasopressin-induced contractile response of the coronary arterioles was inhibited in the presence of the specific V1A antagonist SR 49059 (10−7M) in both ND and DM vessels (P<0.05). The post-CP/CPB protein levels of V1A were significantly increased compared with pre-CP/CPB values in both ND and DM groups (P<0.05), whereas, this increase was higher in DM than that of ND (P<0.05). Immunohistochemistry staining further indicates that V1B receptors were mainly expressed in the myocardium, but not in vascular smooth muscle.
Conclusions:
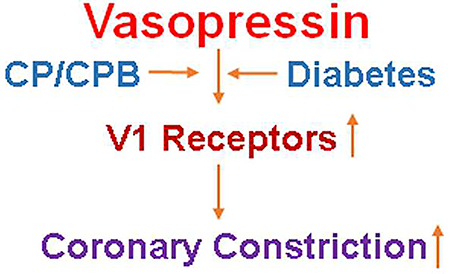
CP/CPB and diabetes are both associated with up-regulation in V1 receptor expression/localization in human myocardium. Vasopressin may induce coronary arteriolar constriction via V1A receptors. This alteration may lead to increased coronary arteriolar spasm in diabetic patients undergoing CP/CPB and cardiac surgery.
Keywords: Cardioplegia, Cardiopulmonary bypass, vasopressin, coronary arterioles, diabetes, microvascular reactivity
Graphical Abstract
Introduction
Diabetes (DM) places patients at an increased risk for cardiovascular disease that often progresses more rapidly and is more extensive than in non-diabetic (ND) patients.1 In addition, patients with DM tend to suffer worse outcomes following cardiac surgery,2 which may in part be related to vasomotor and endothelial dysfunction in arteries and arterioles.3–8 The mechanism resulting in this microvascular dysfunction is not completely understood, but may be related to an altered response to vasopressin.9–12
Prior work has demonstrated that myocardial ischemia is associated with a marked increase in the contractile response of coronary microvasculature to vasopressin in the left ventricular myocardium of dogs.11 Interestingly, large epicardial coronary arterial rings responded minimally to vasopressin and this was not significantly altered by ischemia or reperfusion.11 Circulating levels of vasopressin are elevated after myocardial ischemia and other stresses,13 exemplifying the important role of vasopressin on vascular tone. Indeed, vasopressin is still used clinically as a first line drug to increase vascular tone for maintaining systemic blood pressure after cardiac surgery,14 and to decrease mesenteric blood flow in the setting of gastrointestinal hemorrhage.15 Few studies have examined the altered effects of vasopressin or the expression/localization of the vasopressin receptors in the human coronary microvasculature. In fact, to our knowledge, no such studies in patients have been conducted in response to cardioplegia (CP) and cardiopulmonary bypass (CPB) in the setting of diabetes. Our hypothesis is that diabetes up-regulates the vasopressin receptor expression and alters the contractile response of coronary arterioles to vasopressin in the setting of CP/CPB. We first examined whether diabetes alters the human coronary arteriolar response to vasopressin using an in-vitro microvessel preparation. We then measured the effect of diabetes and CP/CPB on vasopressin receptor expression/localization in coronary arterioles and myocardium using western blotting and immunohistochemistry.
Methods
Human Subjects and Tissue Harvesting
Two sequential tissue samples were harvested from the right atrial appendage of patients undergoing coronary artery bypass grafting (CABG) procedure. The surgeons used a double-purse-string technique with 3–0 polypropylene sutures on the right atrial appendage. The first specimen (pre-CP/CPB tissue sample) was taken during initial atrial cannulation and before tightening the superior suture to secure the cannula. Then, the inferior suture remained loose throughout the procedure, enough to allow the respective atrial section to be exposed to the CP solution during cardioplegic arrest and subsequently reperfused once the aortic cross-clamp was removed. The second specimen (post-CP/CPB) was harvested after removal of cross-clamp and atrial cannula. Thus, the atrium was exposed to the effects of CP/CPB, and a brief period of reperfusion (approximately 5 to 10 minutes). The harvesting procedure has been described in detail. 4–6, 10, 16 Harvested tissue was either frozen in liquid nitrogen (immunoblotting), fixed in 10% formalin for 24 hours followed by paraffinization and sectioning into 5μm slices (immunofluorescent staining), or stored in cold Krebs buffer (in-vitro analysis).
Using Hemoglobin A1C (HbA1C) and their medical history, qualified patients were divided into two groups: 1) patients with a normal HbA1C (<6.5) and no documented history of diabetes were placed in the non-diabetic (ND) group; 2) patients with a HbA1C equal to or greater than 8.5 were placed in the diabetic (DM) group. If a patient had a HbA1C between 6.5 to 8.5, they were excluded. Patients who experienced an aortic cross-clamp time longer than 120 minutes or a CPB time longer than 180 minutes were also excluded from the study. Each group contained 8 patients. All procedures were approved by the Institutional Review Board of Rhode Island Hospital and Brown Medical School, and informed consent was obtained from all enrolled patients as required by the institutional review board.
Cold Blood Cardioplegic Solution
Cold blood CP solution (4º to 8ºC) consisted of a 4:1 mixture of oxygenated blood and hyperkalemic crystalloid CP solution (CAPS, Lanham, MD). An initial 600 to 1,000 ml (introduction) of cold-blood (4º to 8ºC) hyperkalemic (K+ 25 mmol/L) CP solution was delivered antegrade into the aortic root, followed by 200 to 500 mL (K+ 8mmol/L, maintenance) of cold blood CP solution every 15 to 20 minutes.
In-Vitro Analysis
Coronary arterioles (90–150 μm internal diameters) were dissected from the atrial myocardium using a 10 to 60x dissecting microscope. 4–6, 17 Microvessels were placed in the microvessel chamber and cannulated using dual glass micropipettes (40 to 80 μm in diameter), and then secured with 10–0 nylon monofilament sutures. Oxygenated (95% oxygen and 5% carbon dioxide) Krebs buffer solution warmed to 37°C was continuously circulated through the microvessel chamber and reservoir. The microvessels were pressurized to 40 mm Hg in a no-flow state with a burette manometer filled with a Krebs buffer solution. An inverted microscope (40 to 200×, Olympus CK2, Olympus Optical) connected to a video camera was used to project the vessel image onto a monitor. The internal luminal diameter was observed using an electronic dimension analyzer. Vessels were bathed in the organ chamber for at least 30 minutes before being subjected to a pharmacologic intervention.4–6 Vasopressin (10−8-10−5M)9, 11, 18 with or without SR 49059, a selective V1A receptor antagonist (10−7M) was applied to the vessel and the response was measured.
Immunoblotting
Right atrial tissue samples harvested from six patients per group were dissected and cleaned of connective tissues, then placed in SDS-PAGE buffer. Total protein (40 μg) was fractionated on an 8–16% SDS-PAGE gel, and then transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, MA). The membranes were incubated for 1 hour in 1:1000 dilutions of individual primary antibodies to Anti-V1A and V1B receptors (ABCAM) at room temperature. The detailed methods have been described previously.4–6
Immunoperoxidase Staining of V1A Receptors
Atrial myocardial tissue sections (n = 5/group) from ND and DM groups were de-paraffinized in xylene, and rehydrated in graded ethanol and phosphate buffered saline (PBS) solution. The sections were incubated in 3% H2O2 for 20 minutes. The section was then incubated with anti-V1A antibody (1mg/mL), for 60 minutes at room temperature and washed with PBS. The sections were then incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). The chromogen 3,30-diaminobenzidine was then used. Atrial tissue was visualized using a Nikon E800 microscope system (Nikon, Melville, NY). Six image photos per slide were taken at the same magnification resolution (x20) for optical density (intensity) analysis (Spot RT3; Diagnostic Instruments, Sterling Heights, MI).
Immunofluorescence
Atrial tissue sections from five patients per group were first de-paraffinized in xylene then rehydrated using graded ethanol and phosphate-buffered saline solutions (PBS). The antigen was unmasked using sodium citrate (10mmol/L, pH = 6.0), followed by a PBS wash and blocking with 2% bovine serum albumin in PBS at room temperature for 2 hrs.4–6 After PBS wash, sections were incubated overnight with anti-V1A and V1B antibodies (each used at 1:200) (ABCAM, Cambridge, MA) and smooth muscle actin antibody at a temperature of 4°C. Sect ions were then washed in PBS and incubated with the appropriate Alexa-fluor secondary antibodies and mounted using fluorescent mounting medium (Vector Labs, Burlingame, Calif.). Tissue was visualized using a Zeiss LSM510 confocal microscope system (Carl Zeiss MicroImaging, Inc. Thornwood, NY). Tissue labeled with only the actin antibody and secondary antibody served as a negative control.
Chemicals
Vasopressin and SR49059 were obtained from Sigma-Aldrich and dissolved in ultrapure distilled water on the day of the study.
Data Analysis
Data are presented as the mean and standard deviation (SD). Categorical data of patient characteristics were analyzed with Fisher’s exact test and the other clinical data were analyzed with Paired student t-test. Microvascular responses are expressed as percent contraction of the baseline diameter. Microvascular data of vasopressin dose--responses were analyzed using two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparisons test. The data of immuno-histochemistry and Western blot were analyzed using one-way ANOVA followed by Bonferroni’s multiple comparisons test or Newman-Keuls multiple comparisons test. P values < 0.05 were considered significant (GraphPad Software, Inc, San Diego, CA).
Results
Patient Characteristics
Patient characteristics are listed in Table 1. All patients (8/group) were on aspirin and β-blockers and patients with preoperative hypertension were on antihypertensive medication. The pre-operative hemoglobin A1C level averaged 8.9 ± 0.45 in DM 304 patients and 5.3 ± 0.3 in ND patients.
Table 1:
Patient Characteristics
| Patient Characteristics | ND | DM | P values |
|---|---|---|---|
| Age (y)* | 65 ± 7 | 67 ± 6 | 0.64 |
| Male/Female (n) | 5/3 | 6/2 | 0.96 |
| HbA1c (%)* | 5.3 ± 0.3 | 8.9 ± 0.45 | 0.0001 |
| Patient Blood Glucose (mg/dL, pre-CP/CPB)* | 106 ± 14 | 210 ± 21 | 0.0001 |
| Patient Blood Glucose (mg/dL,During-CP/CPB)* | 140 ± 20 | 184± 16 # | 0.01 |
| Pre-operative Insulin (n) | 0 | 8 | 0.001 |
| Intra-operative Insulin (n) | 2 | 8 | 0.002 |
| Hypercholestesterolemia (n) | 6 | 7 | 0.52 |
| Oral hypoglycemic drugs | 0 | 8 | 0.005 |
| Obesity (BMI>30) | 4 | 6 | 0.59 |
| Aspirin | 8 | 8 | 0.78 |
| Hypertension (n) | 7 | 8 | 0.86 |
| Atrial fibrillation (n) | 0 | 0 | 1.0 |
| Duration of CPB (min)* | 112 ± 20 | 116 ± 23 | 0.79 |
| Cross-clamp time (min)* | 90 ± 8 | 98 ± 19 | 0.70 |
| CABG | 8 | 8 | 1.0 |
mean ± SD, BMI = body mass index, CABG = coronary artery bypass.
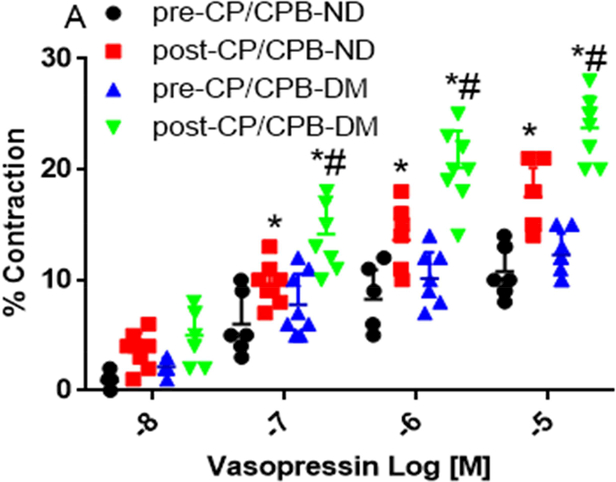
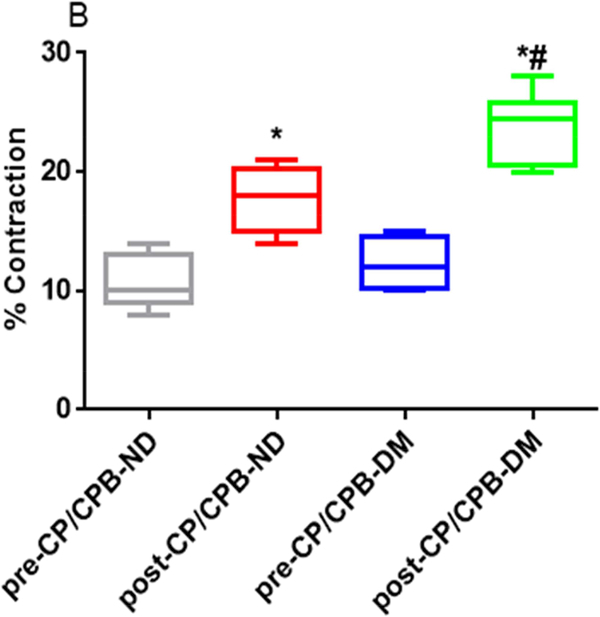
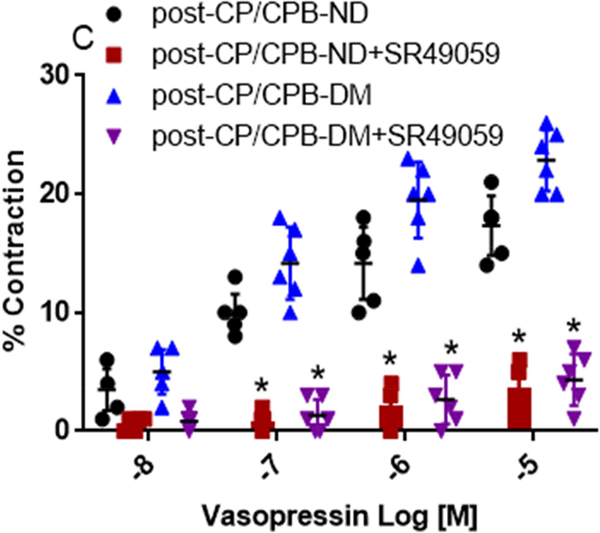
Microvascular Contraction to Vasopressin
There were no significant differences in microvessel diameters among subgroups at baseline before vasopressin treatment (Table 2). Vasopressin induced dose-dependent contractile responses in the microvessels of all studied groups (Figure 1A, Figure 2A and 2C). There were no significant differences in the dose-dependent contractile response to vasopressin between pre-CP/CPB-ND and pre-CP/CPB-DM groups (P=0.45, Figure 1A). In contrast, the dose-dependent responses to vasopressin were significantly increased post-CP/CPB-ND and post-CP/CPB-DM compared with its 314 pre-CP/CPB-ND or pre-CP/CPB-DM at tested concentrations of 10−7M, 10−6M and 10−5M, respectively (*P<0.05, vs. pre-CP/CPB-ND, or pre-CP/CPB-DM, Figure 1A). This increased dose-dependent response was more pronounced in the post-CP/CPB-DM vessels than that of post-CP/CPB-ND vessels at 10−7M, 10−6M and 10−5M, respectively (#P < 0.05, vs. post-CP/CPB-ND, Figure 1A). In particular, the contractile responses of the pre-CP/CPB-ND coronary arterioles to vasopressin (10−5M) were significantly 320 increased post-CP/CPB-ND (*P = 0.0001, vs. pre-CP/CPB-ND, Figure 1B) and post321 CP/CPB-DM (*P = 0.0001 vs. pre-CP/CPB-DM, Figure 1B) with significant, more pronounced effects in the DM vessels than in the ND group (#P = 0.0001 vs. post-CP/CPB-ND, Figure 1B).
Table 2.
Baseline Microvessel Diameters (in micrometer)
| Micovessels | P values | |
|---|---|---|
| pre-CP/CPB-ND (114 ± 24 ) | pre-CP/CPB-DM (122 ± 22) | 0.4984 |
| post-CP/CPB-ND (117 ± 20) | post-CP/CPB-DM (115 ± 21) | 0.5256 |
| pre-CP/CPB-ND (114 ± 24 ) | post-CP/CPB-ND (117 ± 20) | 0.7899 |
| pre-CP/CPB-ND (114 ± 24 ) | post-CP/CPB-DM (115 ± 21) | 0.9306 |
| pre-CP/CPB-DM (122 ± 22) | post-CP/CPB-DM (115 ± 21) | 0.8481 |
Mean ± SD, n = 8/group.
Figure 1.
A, Dose-dependent contractile response of coronary arterioles to vasopressin (10−8-10−5M) from non-diabetic (ND) patients before cardoplegic ischemia and cardiopulmonary bypass (pre-CP/CPB-ND), post-CP/CPB-ND; and from diabetic patients (DM), pre-CP/CPB-DM and post-CP/CPB-DM. B, the bar graph showing the coronary arteriolar contractile response to vasopressin at 10−5M. *P<0.05 vs. pre-CP/CPB-ND, #P<0.05 vs. post-CP/CPB-ND; n = 8/group, mean ± SD.
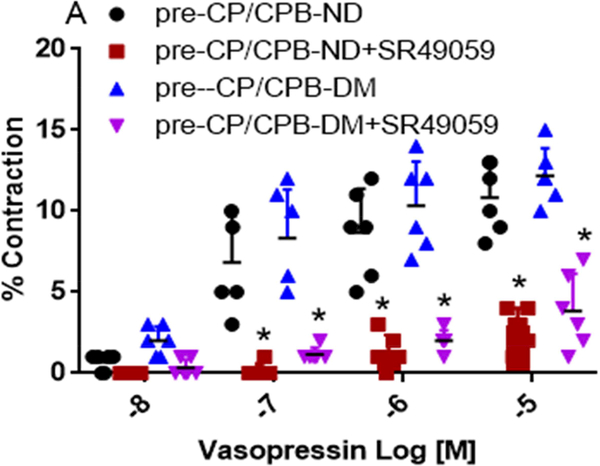
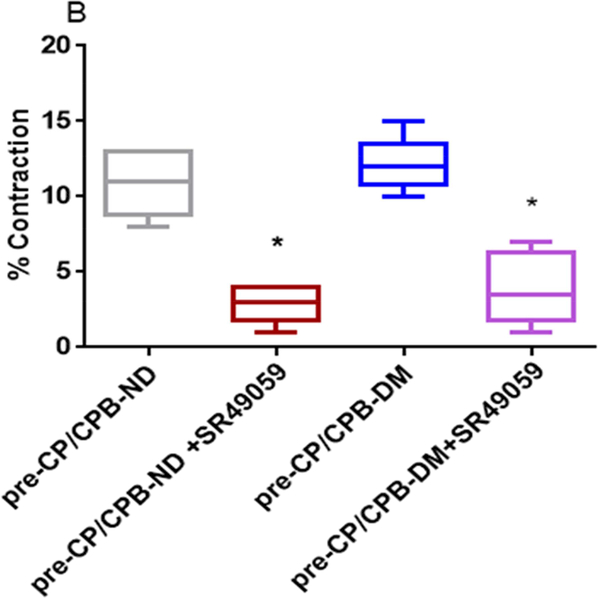
Figure 2.
A, Dose-dependent contractile response of atrial coronary arterioles to vasopressin in the presence or absence of V1 receptor blocker SR490099 (10−7M) in the pre-CP/CPB-ND and the pre-CP/CPB-DM subgroups; B, the coronary arteriolar contractile response to vasopressin at 10−5M in the presence or absence of SR490099 (10−7M) in the pre-CP/CPB-ND and the pre-CP/CPB-DM subgroups; C, Dose-dependent contractile response of atrial coronary arterioles to vasopressin in the presence or absence of V1 receptor blocker SR490099 (10−7M) in the post-CP/CPB-ND and the post-CP/CPB-DM subgroups; D, the coronary arteriolar contractile response to vasopressin at 10−5M in the presence or absence of SR490099 in the post-CP/CPB-ND and the post-CP/CPB-DM subgroups;*P<0.05 vs. pre-CP/CPB-ND alone or #P <0.05 vs. pre-CP/CPB-DM alone, n = 6/group, mean ± SD.
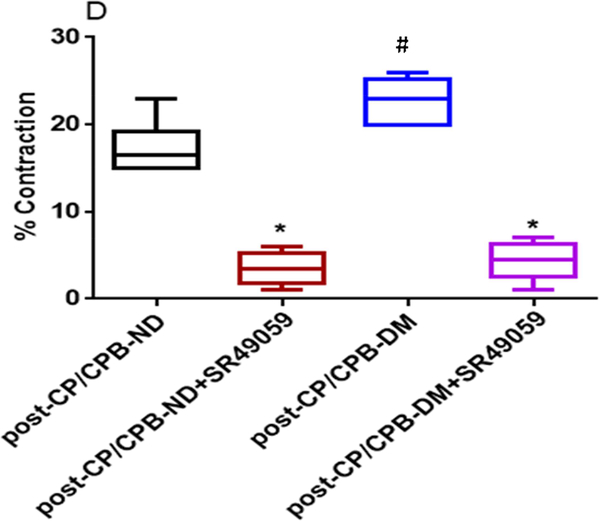
Effect of V1 Receptor antagonist
Vasopressin-induced dose-dependent (at 10−7M, 10−6M, and 10−5M) contractile responses of the coronary arterioles were significantly inhibited in the presence of the specific V1A antagonist SR 49059 (10−7M) in both ND and DM vessels (*P < 0.0001 vs. pre-CP/CPB-ND alone, or pre-CP/CPB-DM alone, Figure 2A; *P <0.0001, vs. post-CP/CPB-ND alone or post-CP/CPB-DM alone, Figure 2C). In particular, the contractile responses of vasopressin at 10−5M were significantly diminished in the presence of SR49059 (10−7M) in all studied vessels of ND and DM (*P = 0.0002 vs. pre-CP/CPB-ND alone; *P < 0.0001 vs. pre-CP/CPB-DM alone, Figure 2B; *P < 0.0001 vs. post-CP/CPB-ND alone;, *P < 0.0001 vs. post-CP/CPB-DM alone, Figure 2D).
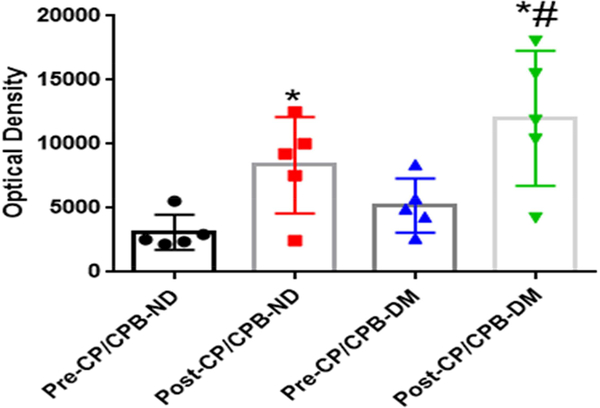
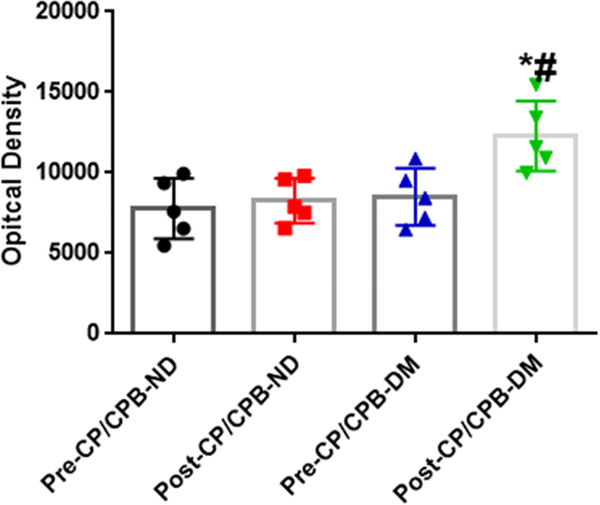
Vasopressin Receptor Expression
There were no significant differences in the protein expression of the V1A receptors before CP/CPB between ND and DM patients (P = 0.65 vs. pre-CP/CPB-ND, Figure 3A and Figure 3C). The post-CP/CPB protein levels of V1A receptors were significantly increased compared with pre-CP/CPB values in both ND and DM groups (*P = 0.04 vs. pre-CP/CPB-ND or *P = 0.001 vs. pre-CP/CPB-DM, Figure 3A and Figure 3C). This increase was significantly higher in post-CP/CPB-DM than in the post-CP/CPB-ND (P = 0.03, Figure 3C). There were no significant differences in the V1B receptor protein expression between pre-CP/CPB-ND and pre-CP/CPB-DM groups (P = 0.9, pre-CP/CPB-DM, Figure 3B and Figure 3D) or between pre- and post-CP/CPB-ND (P = 0.9 vs. pre-CP/CPB-ND, Figure 3B and Figure 3D). In contrast, the post-CP/CPBDM protein levels of V1B receptors in the DM myocardium were significantly higher than those of pre-CP/CPB-DM (*P =0.02, Figure 3B and Figure 3D) or post-CP/CPB-ND (# P = 0.01, Figure 3D).
Figure 3.
A and B, Representative immunoblots of atrial tissue lysates for V1A and V1B receptors, harvested from the diabetic (DM) and non-diabetic (ND) patients pre- and post-CP/CPB. C, Immunoblot quantification shows significantly increased V1A receptor expression post-CP/CPB-ND and post-CPB-DM; * P <0.05 vs. pre-CP/CPB-ND or preCP/CPB-DM, #P <0.05 vs. post-CP/CPB-ND; D, Immunoblot quantification shows significantly increased V1B receptor expression post-CP/CPB-DM, *P <0.05 vs. preCP/CPB-ND or pre-CP/CPB-DM, #P <0.05 vs. post-CP/CPB-ND, n = 6/group, mean ± SD.
Immuno-localization of V1 Receptors
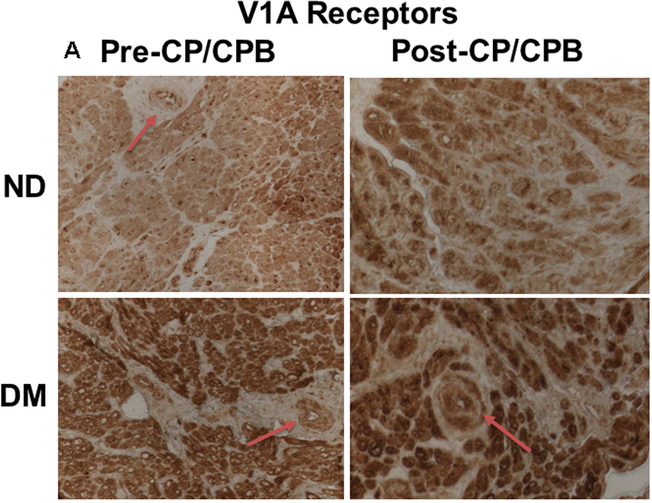
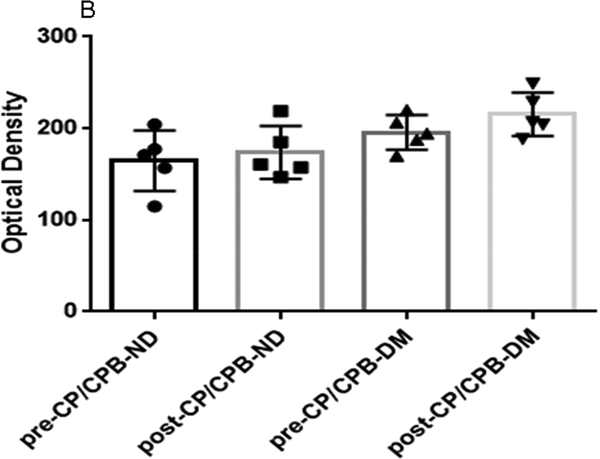
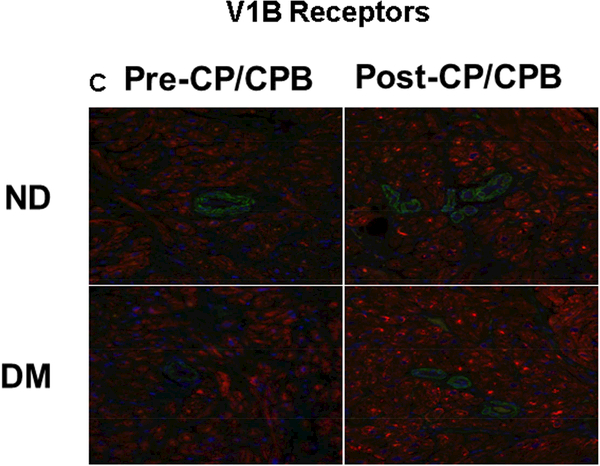
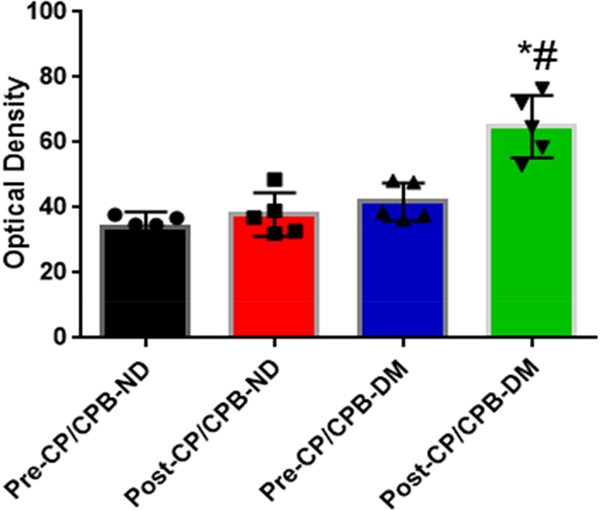
Immunohistochemistry staining indicates that the V1A receptors were expressed in atrial microvessels (red, arrow, Figure 4A) and myocardium (brown, Figure 4A). The intensities of V1A tended to be increased in the pre-CP/CPB-DM myocardium compared to pre-CP/CPB-ND (Figure 4A), but failed to reach statistical significance (P = 0.06, Figure 4B). The pre-CP/CPB V1A levels also tended to be increased post-CP/CPB in both ND (P = 0.18 vs. pre-CP/CPB-ND) and DM groups (P = 0.34, Figure 4B). V1B receptors were mainly expressed in the myocardium (red, Figure 4C), but not in vascular smooth muscle (green, smooth muscle alpha actin staining, Figure 4C). The intensities of V1B pre-CP/CPB-DM (Figure 4D) tended to be increased in the DM myocardium, but once again failed to reach statistical significance compared to pre-CP/CPB-ND (P = 0.12, Figure 4D). There were no significant differences in V1B distribution between pre- and post-CP/CPB-ND in the ND group (P = 0.3, Figure 4C and Figure 4D). In contrast, the pre-CP/CPB-DM levels of V1B were significantly increased post-CP/CPB-DM in the atrial myocardium of DM patients (*P = 0.001 vs. pre-CP/CPB-DM; #P = 0.0001 vs. post-CP/CPC-ND, Figure 4C and Figure 4D).
Figure 4.
A, Immunohistochemical image of V1A receptors in paraffin-embedded human atrial tissue slides from non-diabetic (ND) and diabetes (DM) patients. B, Densitometric analysis of signal intensities, C, Immunofluorescence staining of V1B receptors in the embedded human atrial tissue slides from the ND and DM patients before and after CP/CPB. D, Densitometric analysis of signal intensities. *P<0.05 vs. pre-CP/CP-DM, #P < 0.05 vs. post-CP/CPB-ND; n = 5/group, mean ± SD.
Discussion
In our study of the coronary arteriolar reactivity in response to vasopressin, the main findings are as follows: (1) the contractile response to vasopressin after CP/CPB in patients with the poorly controlled DM was significantly more intense than in the ND patients; (2) there is a minimal contractile response to vasopressin after the addition of the V1A receptor antagonist, SR49059; (3) expression of V1A receptor protein was increased after CP/CPB for both ND and DM patients, and was significantly increased in post-CP/CPB in DM patients as compared to ND patients; (4) V1B expression was increased in DM post-CP/CPB; (5) V1B receptor distribution was increased in atrial myocardium in DM patients post-CP/CPB but not in vascular smooth muscle, which expresses little if any V1B receptors.
Vasopressin is produced in the posterior pituitary gland. Circulating levels of vasopressin are known to be increased at the beginning of septic shock and reduced in the later stages.19 After cardiac surgery, circulating levels of vasopressin are either diminished or increased.20, 21 Vasopressin may be elevated during hemorrhagic shock, hypotension, acidosis and hypoxia. 22, 23 While the administration of vasopressin has been advocated as a first line drug for the treatment of post-cardiac surgical vasoplegia,21, 23 the use of vasopressin in the setting of ischemic heart disease is associated with increased mortality.24, 25 Thus, the use of vasopressin to support vasomotor tone in the setting of cardiac surgery has potential implications on the perfusion of the myocardium. The concentration of vasopressin we used in the current study was based on the previous in-vitro vessel studies in dogs,11 pigs12 and humans.18 In a preliminary study, in the setting of CP/CPB, we observed that the change in the contractile response of human skeletal muscle arterioles to vasopressin was opposite from that observed in coronary microvasculature, but occurred at similar concentrations, suggesting that the concentrations we examined have clinical relevance.
Studies in animals and humans have shown that diabetes results in higher levels of plasma vasopressin 26 and decreased levels of V1 receptors in the liver and kidney of type-1 diabetic rats 27 In this study, we hypothesized that the coronary microvascular response to vasopressin would be increased after CP/CPB, and that the up-regulation of vasopressin receptors is greater in patients with diabetes, and this may in part be responsible for the altered coronary arteriolar contractile response to vasopressin in the setting of CP/CPB. This study suggests that the increase in coronary arteriolar contractile response in patients’ post-CP/CPB could be the result of increased expression of V1A receptor proteins and increased distribution of the receptors in the atrial tissue. In the patients with DM, this mechanism is augmented significantly. In the atrial myocardium from patients with DM, we also observed a significant increase in V1B receptors after CP/CPB, but this increase was not seen in the vascular smooth muscle.
Our group and others have extensively studied the effect of CP/CPB on microvascular reactivity, permeability, neurocognitive decline and other effects. 4–6, 7, 28–30 Most of the vascular effects result in a diminished response to vasoconstrictor agents such as phenylephrine, endothelin-1, and thromboxane-A2 or endothelium-dependent vasodilator agonists such as substance P.4–6, 8, 30 Two exceptions to this are the increased coronary contractile response observed to serotonin10, 16 and now the response to vasopressin. The diminished contractile responses to many vasoconstrictors are due in large part to damage to the downstream contractile mechanism including mitogen activated protein kinase and protein kinase C31, altered relaxation mechanisms relate to increased oxidative stress,32 oxygen derived free radicals,33 neutrophil related mechanisms34 and receptor desensitization.35 The increase in vasopressin receptor expression and responsiveness likely has to do with tissue ischemia/reperfusion and increased oxidative stress. Cell cultured experiments studying the cellular response to oxidative stress, and hypoxia, may be conducted in the future to determine the specifics mechanisms responsible for the observed increased contraction to vasopressin.
Limitations
Our study does have several possible limitations. First, we did not include patients with well controlled diabetes. However, previous studies6, 32 have shown that vascular responses in patients with controlled diabetes have less endothelial dysfunction than that observed in vessels from patients with poorly controlled diabetes. Indeed, most vascular responses in patients with well controlled diabetes tend to be very similar to that observed in vessels from ND patients. Second, we did not examine mechanisms causing the observed effects. We did not examine mechanisms causing the observed effects. However, even though sample size is small, the groups were fairly well matched. Thus, any difference in response to vasopressin must have been due to diabetes or some factors related to it. These factors could include chronic hyperglycemia, increased oxidative stress, advanced glycosylation end products, or diabetes-induced dyslipidemia. It is likely that all of these factors could have contributed in some degrees to the change in vascular reactivity. While the diabetic patients in this study had received perioperative oral hypoglycemic agents and/or insulin, previous study in dogs found a similar increased contractile response to vasopressin. In that study, the animals did not receive either insulin or hypoglycemic medications.11 Third, we did not measure blood vasopressin concentrations during cardiac surgery. This would have possibly increased the clinical impact of the study. Fourth, this study focused on the response of atrial microcirculation. These effects on the ventricular myocardium could be clinically more significant. Unfortunately, due to the nature of the study, it would be difficult to sample ventricular myocardium of our patients undergoing coronary artery bypass surgery. However, we feel comfortable generalizing our study to the ventricular myocardium based on previous studies in animals. In canine studies in which arterial microvessels were harvested from the ventricular myocardium, the investigators observed a similar increase in reactivity of the ventricular microvasculature to vasopressin after ischemia as was observed in the atrial microvasculature in the current study.11
In summary, we observed an increased coronary microvascular contractile response to vasopressin after cardiac surgery, associated with an increased expression of V1 receptors. These changes were more evident in patients with poorly controlled diabetes. Thus, practitioners should be aware of the increased contractile response of coronary arterioles patients may have to vasopressin after cardiac surgery, when 461 considering administration of this drug.23, 36–39
Supplementary Material
Central Message:
Cardioplegic ischemia and cardiopulmonary bypass enhanced coronary arteriolar contractile response to vasopressin and increased V1 receptor expression and distribution in patients with diabetes.
Perspective Statement:
Vasopressin may induce coronary arteriolar constriction via V1A receptors. CP/CPB and diabetes are both associated with up-regulation in V1 receptor expression/localization in human myocardium. This alteration may lead to increased coronary arteriolar contraction in diabetic patients undergoing CP/CPB and cardiac surgery.
Acknowledgment
We would like to thank all the nurses, physician assistants and perfusionists working in cardiac surgery in Rhode Island Hospital for collecting the tissue samples and recording patient characteristics. We would also like to thank the nurses and physician assistants at Division of Cardiac Surgery of Rhode Island Hospital for collecting patient consent forms.
Funding: This study was supported by the National Heart, Lung, and Blood Institute HL-46716 (F.W.S.), HL128831 (F.W.S.) and IDeA-CTR grant U54GM115677 (F.W.S.). This study was also supported in part by 1RO1HL127072 (J.F.), 1R01 HL136347–01 (J.F.), the Institutional Development Award (IDeA) from the National Institute of General Medical Science (NIGMS) of the NIH [5P20-GM103652(Pilot Project)] (J.F.), AHAGrant-in-Aid-15GRNT25710105 (J.F.), Summer Assistantship Award through Basic and Translational Research (BTR) Program (NIH T35HL094308) to N.S. and A.K, and Summer Research-Early Identification Program (SR-EIP) of Leadership Alliance at Brown University (NIH-R25HL088992) (V.C.).
Abbreviations:
- ANOVA
analysis of variance
- BMI
body mass index
- CABG
coronary artery bypass grafting
- CP
cardioplegia
- CPB
cardiopulmonary bypass
- ND
non-diabetes
- DM
diabetes
- pre-CP/CPB
before cardioplegia and cardiopulmonary bypass
- post-CP/CPB
after cardioplegia and cardiopulmonary bypass
- pre-CP/CPB-ND
before cardioplegia and cardiopulmonary bypass in non-diabetics
- post-CP/CPB-ND
after cardioplegia and cardiopulmonary bypass in non-diabetics
- pre-CP/CPB-DM
before cardioplegia and cardiopulmonary bypass in diabetics
- post-CP/CPB-DM
after cardioplegia and cardiopulmonary bypass in diabetics
- V1A
vasopressin 1A receptors
- V1B
vasopressin 1B receptors
Footnotes
Disclosure: No potential conflicts of interest exist in the preparation of this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New England Journal of Medicine. 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 2.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, Guyton RA. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. The Annals of thoracic surgery. 1999;67:1045–1052 [DOI] [PubMed] [Google Scholar]
- 3.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Liu Y, Chu LM, Clements RT, Khabbaz KR, Robich MP, Bianchi C, Sellke FW. Thromboxane-induced contractile response of human coronary arterioles is diminished after cardioplegic arrest. The Annals of thoracic surgery. 2011;92:829–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng J, Chu LM, Dobrilovic N, Liu Y, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. Surgery. 2012;152:262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng J, Liu Y, Sabe AA, Sadek AA, Singh AK, Sodha NR, Sellke FW. Differential impairment of adherens-junction expression/phosphorylation after cardioplegia in diabetic versus non-diabetic patients. European Journal of Cardio-Thoracic Surgery. 2015;49:937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma S, Maitland A, Weisel RD, Fedak PW, Li SH, Mickle DA, Li RK, Ko L, Rao V. Increased endothelin-1 production in diabetic patients after cardioplegic arrest and reperfusion impairs coronary vascular reactivity: Reversal by means of endothelin antagonism. The Journal of thoracic and cardiovascular surgery. 2002;123:1114–1119 [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Sellke F. Microvascular dysfunction in patients with diabetes after cardioplegic arrest and cardiopulmonary bypass. Current opinion in cardiology. 2016;31:618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan TA, Bianchi C, Ruel M, Feng J, Sellke FW. Differential effects on the mesenteric microcirculatory response to vasopressin and phenylephrine after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2007;133:682–688 [DOI] [PubMed] [Google Scholar]
- 10.Robich MP, Araujo EG, Feng J, Osipov RM, Clements RT, Bianchi C, Sellke FW. Altered coronary microvascular serotonin receptor expression after coronary artery bypass grafting with cardiopulmonary bypass. The Journal of thoracic and cardiovascular surgery. 2010;139:1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellke F, Quillen J. Altered effects of vasopressin on the coronary circulation after ischemia. The Journal of thoracic and cardiovascular surgery. 1992;104:357–363 [PubMed] [Google Scholar]
- 12.Khan TA, Bianchi C, Ruel M, Feng J, Sellke FW. Differential effects on the mesenteric microcirculatory response to vasopressin and phenylephrine after cardiopulmonary bypass. The Journal of thoracic and cardiovascular surgery. 2007;133:682–688 [DOI] [PubMed] [Google Scholar]
- 13.Novella S, Martínez AC, Pagán RM, Hernández M, García-Sacristán A, González-Pinto A, González-Santos JM, Benedito S. Plasma levels and vascular effects of vasopressin in patients undergoing coronary artery bypass grafting. European Journal of Cardio-Thoracic Surgery. 2007;32:69–76 [DOI] [PubMed] [Google Scholar]
- 14.Masetti P, Murphy SF, Kouchoukos NT. Vasopressin therapy for vasoplegic syndrome following cardiopulmonary bypass. Journal of cardiac surgery. 2002;17:485–489 [DOI] [PubMed] [Google Scholar]
- 15.Darcy M Treatment of lower gastrointestinal bleeding: Vasopressin infusion versus embolization. Journal of vascular and interventional radiology. 2003;14:535–543 [DOI] [PubMed] [Google Scholar]
- 16.Métais C, Li J, Li J, Simons M, Sellke FW. Serotonin-induced coronary contraction increases after blood cardioplegia-reperfusion. Circulation. 1999;100:II-328-Ii-334. [DOI] [PubMed] [Google Scholar]
- 17.Sabe AA, Potz BA, Elmadhun NY, Liu Y, Feng J, Abid MR, Abbott JD, Senger DR, Sellke FW. Calpain inhibition improves collateral-dependent perfusion in a hypercholesterolemic swine model of chronic myocardial ischemia. The Journal of thoracic and cardiovascular surgery. 2016;151:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bax WA, Van der Graaf PH, Stam WB, Bos E, Nisato D, Saxena PR. [arg8] vasopressin-induced responses of the human isolated coronary artery: Effects of non-peptide receptor antagonists. European journal of pharmacology. 1995;285:199–202 [DOI] [PubMed] [Google Scholar]
- 19.Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D. Circulating vasopressin levels in septic shock. Critical care medicine. 2003;31:1752–1758 [DOI] [PubMed] [Google Scholar]
- 20.Bomberg H, Bierbach B, Flache S, Wagner I, Glaser L, Groesdonk HV, Menger MD, Schafers HJ. Endothelin and vasopressin influence splanchnic blood flow distribution during and after cardiopulmonary bypass. The Journal of thoracic and cardiovascular surgery. 2013;145:539–547 [DOI] [PubMed] [Google Scholar]
- 21.Hajjar LA, Vincent JL, Galas FRBG, Rhodes A, Landoni G, Osawa EA, Melo RR, Sundin MR, Grande SM, Gaiotto FA . Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgerythe vancs randomized controlled trial. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2017;126:85–93 [DOI] [PubMed] [Google Scholar]
- 22.Rurak D Plasma vasopressin levels during hypoxaemia and the cardiovascular effects of exogenous vasopressin in foetal and adult sheep. The Journal of physiology. 1978;277:341–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talbot MP, Tremblay I, Denault AY, Bélisle S. Vasopressin for refractory hypotension during cardiopulmonary bypass. The Journal of thoracic and cardiovascular surgery. 2000;120:401–402 [DOI] [PubMed] [Google Scholar]
- 24.Indrambarya T, Boyd JH, Wang Y, McConechy M, Walley KR. Low-dose vasopressin infusion results in increased mortality and cardiac dysfunction following ischemiareperfusion injury in mice. Critical Care. 2009;13:R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bomberg H, Bierbach B, Flache S, Scheuer C, Novak M, Schafers HJ, Menger MD. Vasopressin induces rectosigmoidal mucosal ischemia during cardiopulmonary bypass. Journal of cardiac surgery. 2014;29:108–115 [DOI] [PubMed] [Google Scholar]
- 26.Bankir L, Bardoux P, Ahloulay M. Vasopressin and diabetes mellitus. Nephron. 2001;87:8–18 [DOI] [PubMed] [Google Scholar]
- 27.Trinder D, Phillips PA, Stephenson JM, Risvanis J, Aminian A, Adam W, Cooper M, Johnston CI. Vasopressin v1 and v2 receptors in diabetes mellitus. Am J Physiol. 1994;266:E217–223 [DOI] [PubMed] [Google Scholar]
- 28.Feng J, Anderson K, Singh AK, Ehsan A, Mitchell H, Liu Y, Sellke FW. Diabetes upregulation of cyclooxygenase 2 contributes to altered coronary reactivity after cardiac surgery. The Annals of thoracic surgery. 2017;104:568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabe AA, Dalal RS, Chu LM, Elmadhun NY, Ramlawi B, Bianchi C, Sellke FW. Preoperative gene expression may be associated with neurocognitive decline after cardiopulmonary bypass. The Journal of thoracic and cardiovascular surgery. 2015;149:613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sodha NR, Feng J, Clements RT, Bianchi C, Boodhwani M, Ramlawi B, Mieno S, Khabbaz KR, Sellke FW. Protein kinase c alpha modulates microvascular reactivity in the human coronary and skeletal microcirculation. Surgery. 2007;142:243–252 [DOI] [PubMed] [Google Scholar]
- 31.Khan TA, Bianchi C, Araujo EG, Ruel M, Voisine P, Li J, Liddicoat JR, Sellke FW. Cardiopulmonary bypass reduces peripheral microvascular contractile function by inhibition of mitogen-activated protein kinase activity. Surgery. 2003;134:247–254 [DOI] [PubMed] [Google Scholar]
- 32.Feng J, Chu LM, Nikola DN, Liu Y, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with poorly controlled diabetes. Surgery. 2012;152:262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sellke FW, Shafique T, Ely DL, Weintraub RM. Coronary endothelial injury after cardiopulmonary bypass and ischemic cardioplegia is mediated by oxygen-derived free radicals. Circulation. 1993;88:II395–400 [PubMed] [Google Scholar]
- 34.Friedman M, Wang SY, Sellke FW, Cohn WE, Weintraub RM, Johnson RG. Neutrophil adhesion blockade with npc 15669 decreases pulmonary injury after total cardiopulmonary bypass. The Journal of thoracic and cardiovascular surgery. 1996;111:460–468 [DOI] [PubMed] [Google Scholar]
- 35.Wang SY, Friedman M, Johnson RG, Weintraub RM, Sellke FW. Adrenergic regulation of coronary microcirculation after extracorporeal circulation and crystalloid cardioplegia. American Journal of Physiology-Heart and Circulatory Physiology. 1994;267:H2462–H2470 [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulos G, Sintou E, Siminelakis S, Koletsis E, Baikoussis NG, Apostolakis E. Perioperative infusion of low-dose of vasopressin for prevention and management of vasodilatory vasoplegic syndrome in patients undergoing coronary artery bypass grafting-a double-blind randomized study. Journal of cardiothoracic surgery. 2010;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Argenziano M, Chen JM, Choudhri AF, Cullinane S, Garfein E, Weinberg AD, Smith CR, Rose EA, Landry DW, Oz MC. Management of vasodilatory shock after cardiac surgery: Identification of predisposing factors and use of a novel pressor agent. The Journal of thoracic and cardiovascular surgery. 1998;116:973–980 [DOI] [PubMed] [Google Scholar]
- 38.Noto A, Lentini S, Versaci A, Giardina M, Risitano DC, Messina R, David A. A retrospective analysis of terlipressin in bolus for the management of refractory vasoplegic hypotension after cardiac surgery. Interactive cardiovascular and thoracic surgery. 2009;9:588–592 [DOI] [PubMed] [Google Scholar]
- 39.Colson PH, Bernard C, Struck J, Morgenthaler NG, Albat B, Guillon G. Post cardiac surgery vasoplegia is associated with high preoperative copeptin plasma concentration. Critical Care. 2011;15:R255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.