Abstract
Epilepsy produces chronic chemical changes induced by altered cellular structures, and acute ones produced by conditions leading into individual seizures. Here, we aim to quantify 24 molecules simultaneously at baseline and during periods of lowered seizure threshold in rats. Using serial hippocampal microdialysis collections starting two weeks after the pilocarpine-induced status epilepticus, we evaluated how this chronic epilepsy model affects molecule levels and their interactions. Then, we quantified the changes occurring when the brain moves into a pro-seizure state using a novel model of physiological ictogenesis. Compared with controls, pilocarpine animals had significantly decreased baseline levels of adenosine, homovanillic acid, and serotonin, but significantly increased levels of choline, glutamate, phenylalanine, and tyrosine. Step-wise linear regression identified that choline, homovanillic acid, adenosine, and serotonin are the most important features to characterize the difference in the extracellular milieu between pilocarpine and control animals. When increasing the hippocampal seizure risk, the concentrations of normetanephrine, serine, aspartate, and 5-hydroxyindoleacetic acid were the most prominent; however, there were no specific, consistent changes prior to individual seizures.
Keywords: Ictogenesis, Nucleus reuniens, Epilepsy, Pilocarpine, Hippocampus, Microdialysis
1. Introduction
Understanding the mechanisms underlying epileptogenesis and ictogenesis are a primary goal of epilepsy research. Biochemical changes are integral to these processes yet have received limited attention. Investigations of focal biochemical changes are challenging since they require frequent measurements of a large number of molecules over prolonged periods. In this work, we evaluate 24 molecules simultaneously at baseline and during periods of lowered seizure threshold.
Researchers have developed techniques to measure neurotransmitter activity in vivo such as positron emission tomography, microsensors, magnetic resonance spectroscopy, and cerebral microdialysis sampling (Anderzhanova and Wotjak, 2013; Chowdhury et al., 2015; Dupont et al., 2017; van der Zeyden et al., 2008). Cerebral microdialysis is a well-established technique used to sample the immediate vicinity of the catheter tip in order to monitor basal concentrations over a specified sampling time. In epilepsy, past clinical and experimental microdialysis studies support the notion that an imbalance between excitatory and inhibitory neurotransmission is crucial to the pathophysiology of temporal lobe epilepsy (TLE) (Cavus et al., 2016; During and Spencer, 1993; Luna-Munguia et al., 2011; Thomas et al., 2005; Wilson et al., 1996). Past studies associated glutamate elevation with excitotoxicity (Wang and Qin, 2010), increased hippocampal cellular excitability (Pan et al., 2008), decreased hippocampal volume, and impaired glucose metabolism (Cavus et al., 2005, 2008). Later technical improvements such as high-performance liquid chromatography allowed more detailed evaluation of neurotransmitters and neurometabolites. Clinckers et al. (2005) reported that glutamate alone is not enough to induce seizures within the hippocampus in the pilocarpine rat model. Others focused on monoamines as additional seizure triggers, given the role of the cholinergic and histaminergic systems in the pathogenesis of epilepsy (Hillert et al., 2014; Valle-Dorado, 2015). However, studies of the biochemical changes during epilepsy are quite limited. Not only are past studies limited to a small number of molecules, it has not been feasible to measure time-dependent changes. A major limitation is that there were previously no methods to control seizure threshold experimentally in a spontaneous seizure model. Prior work has utilized the kindling model, which provides excellent control of seizure timing, but is not a model of spontaneous epilepsy (Bertram, 2007), limiting its ability to inform of the chemical changes of epileptogenesis and ictogenesis (Luna-Munguia et al., 2017).
This study focuses on the rat pilocarpine model of TLE, which is the most common form of partial epilepsy, characterized by spontaneous, recurrent seizures that often originate in the limbic system (Löscher and Schmidt, 2011; Sloviter, 2005). We used this model to simulate the initial injury and reproduce the main pathologic characteristics observed in human TLE, as well as the development of spontaneous recurrent seizures (Curia et al., 2008; Turski et al., 1983). However, as with all models of epilepsy, the timing of seizures is inherently random. Therefore, it is difficult to evaluate the changes preceding seizures, especially with labor-intensive microdialysis. We recently developed a novel model of ictogenesis, which allows control of seizure threshold through physiological pathways (Luna-Munguia et al., 2017). This model utilizes rats that have developed spontaneous seizures several weeks after pilocarpine-induced status epilepticus, and increases the risk of those seizures by increasing the random afferent synaptic activity to the hippocampus. This model, combined with state-of-the-art microdialysis techniques, allowed us to monitor 24 molecules simultaneously during ictogenesis.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (230-260 g; Charles River, U.S.A.) were individually housed in a facility under controlled illumination (12-h light/dark cycle; light on 09:00 am) and environmental conditions (22°C, 40-45% humidity). Rat chow and tap water were available ad libitum. All animals were acclimatized to the animal room conditions for at least 5 days before any experimental manipulation. Procedures involving animals and their care were carried out in accordance with protocols approved by the University Committee on Use and Care of Animals of the University of Michigan. The number of animals was kept as small as possible.
2.2. Pilocarpine-induced status epilepticus
These experiments utilized the method described in Luna-Munguia et al. (2017). Briefly, 56-days-old rats were randomly assigned to a group that received an injection of pilocarpine hydrochloride (340 mg/kg ip; Sigma-Aldrich, St. Louis, MO) 20 min after a single intraperitoneal injection of methylatropine bromide (5 mg/kg; Sigma-Aldrich, St. Louis, MO). Five to ten minutes after the pilocarpine administration, the animals started with head nodding, evolving into recurrent generalized convulsions (status epilepticus) within 40 min. Rats that did not develop status epilepticus within this period of time, received an additional dose of pilocarpine hydrochloride (170 mg/kg; ip). Animals were behaviorally monitored by experienced researchers and after 90 min of status epilepticus, seizures were interrupted with diazepam (10 mg/kg ip; Hospira, Lake Forest, IL). Control animals received a single injection of methylatropine bromide (5 mg/kg; ip) 20 min prior to vehicle (0.9% saline solution; ip), and treated with diazepam 2 h after the saline injection. Monitoring of the spontaneous seizures began immediately after the pilocarpine injection and was continued for 14 days using a video recording system. Rats with epilepsy that did not achieve the initial body weight within the first 12 days after pilocarpine-induced status epilepticus were excluded from the study.
2.3. Surgery
Procedures for affixing the subdural and depth electrodes were performed as previously described (Luna-Munguia et al., 2017), with the sole exception being the introduction of the microdialysis probe. Briefly, Pilocarpine (n = 15) and Control (n = 15) rats were anesthetized with a ketamine/xylazine mix (70/10 mg/kg ip, respectively) and placed in a stereotactic frame (David Kopf Instrument, Tujunga, CA) 14 days after the pilocarpine injection. Electrodes were positioned and fastened using mounting screws (E363/20; PlasticsOne, Roanoke, VA) on four different places (left and right frontal, one cerebellar, and one reference over the sinus cavity). Then, two single channel depth electrodes (E363-1-SPC; PlasticsOne, Roanoke, VA) were stereotactically implanted, one in the left hippocampus (AP −4.8, ML 5.2, DV −7.5) and one in the right hippocampus (AP −4.8, ML −5.2, DV −7.5). In all cases, a 19-gauge guide cannula (C310GA/SPC; PlasticsOne, Roanoke, VA) was coupled to the depth electrode and stereotactically implanted in the left hippocampus (AP −4.8, ML 5.2, DV −4.5) to guide a dialysis probe constructed by ourselves as previously described (Luna-Munguia et al., 2011) and designed to protrude 3 mm beyond the guide cannula tip in order to stay at hippocampus. The sockets were fitted into a 6-pin electrode pedestal (MS363; PlasticsOne, Roanoke, VA) and the entire apparatus was secured with dental cement. For local drug injection into the nucleus reuniens, we followed our previously described procedure (Luna-Munguia et al., 2017). Here, a 26-gauge guiding cannula (C315GA/SPC; PlasticsOne, Roanoke, VA) was stereotactically implanted (AP −1.8, ML −1, DV −7.2, with a 15° arm angle from vertical axis) to guide a 33-gauge injecting cannula (C315IA/SPC; PlasticsOne, Roanoke, VA). Once in place, the assembly was cemented to the skull. All animals were given a subcutaneous post-operatory analgesic treatment (buprenorphine hydrochloride 0.05 mg/kg; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) every 12 h for two days, and allowed to recover for at least 12 days.
2.4. Experimental groups
Starting on day 13 post-surgery, Pilocarpine (i.e. rats with epilepsy) and Control animals were monitored for up to 48 h by continuous video/EEG recording (Ceegraph Vision; Bio-logic System Corporation or Natus Neuroworks).
2.4.1. Pilocarpine group (n = 15)
Microdialysis experiments began 2 days after starting the continuous video/EEG recording. A continuously perfused dialysis probe, constructed in the lab (Luna-Munguia et al., 2011), was inserted into the guide cannula and fixed to the socket with dental acrylic. The inlet of the dialysis probe was connected through a dual-channel swivel to a syringe mounted on a microperfusion pump.
The dialysis system was continuously perfused during all microdialysis experiments at a rate of 2 μl/min with fresh filtered artificial cerebro-spinal fluid (145 mM NaCl, 2.68 mM KCl, 1.4 mM CaCl2 · 2H2O, 1.01 mM MgSO4 · 7H2O, 1.55 mM Na2HPO4, 0.45 mM NaH2PO4 · H2O, 250 mM ascorbic acid; pH 7.4). All compounds purchased from Sigma-Aldrich (St. Louis, MO). A 2-h recovery period was allowed after probe implantation. Then, the dialysates were collected at 20 min intervals for 2 h under basal conditions in transparent/coned/capped vials (Macherey-Nagel, Düren, Germany).
The 15 animals were split into 3 groups: ictogenesis (n = 6), phosphate buffered saline (PBS)-injection (n = 6), and sham-injection (n = 3). For the 6 animals in the ictogenesis group, KCl (120 mM) was injected (0.1 μl/min over 5 min) into the nucleusreuniens of freely moving rats via the injecting cannula connected with polyethylene tubing to a micro-syringe (Hamilton Co., Reno, NV). There were a total of 9 injections, occurring every 20 min, which includes 15 min recovery time between injections (Figure 1). Once the ninth administration was completed, the injection cannula was removed from the guide cannula but the animal remained connected to the video/EEG recording. Hippocampal microdialysate collections were done at 3, 8, and 15 min after each of the 9 KCl injections started. After the ictogenesis cycles were completed, 4 additional collections were done over the next 2 hours, each 30 min apart. Thus, in total each animal had 6 baseline, 27 ictogenesis, and 4 post-ictogenesis samples. Immediately after collection, perfusates were processed as described below. The 6 PBS-injection animals had precisely the same procedure, except that instead of KCl they had injections with PBS 1X solution (Fisher Scientific, Fair Lawn, NJ). The 3 sham-injection had the same manipulation and instrumentation as the other two groups, except that no compound was placed into the nucleus reuniens injecting cannula.
Figure 1. Experimental protocol.
Top: EEG was monitored for 48 hrs prior to the experiment, 2 hours of which occurred after implantation of the microdialysis catheter. The injections lasted 3 hrs, followed by 2 hrs of additional EEG. Hazard rate determined the risk of seizures over time, as described previously, showing that KCl injections increased risk from about 0.2/hour to 0.6/hour (Luna-Munguia et al., 2017). Although sham and PBS animals had seizures, the risk was not increased during the injection time. Dashed lines: Poisson uncertainty. Bottom: Microdialysis collection schedule included 6 samples before the experiment, 3 samples during each of 9 injections (27 total), and 4 samples after the injections. All samples were 4 μL collected over 2 min.
All experiments began at 09:00 am and used the same protocol, and there was always only a single experiment per 24-hour period. EEG recordings were sampled at 256 Hz and concurrent time-synched video was analyzed offline. Seizures were identified manually by an observer blinded to the groups assisted by EEG viewing software (Insight 12, Persyst Corp., San Diego, CA). There were no seizures during the pre-experiment baselines, and four seizures in Pilocarpine animals during the post-experiment period; the collection after each seizure was not included in the analysis.
2.4.2. Control group (n = 15)
The Control group was never exposed to pilocarpine and did not develop epilepsy. This group was treated exactly the same as the Pilocarpine group, with 6 animals receiving the ictogenesis injection, 6 receiving PBS injections, and 3 having sham injections. The microdialysis procedure, EEG monitoring, and all analyses were identical to the Pilocarpine group.
2.5. Sample derivatization and analysis
Each dialysate was derivatized with benzoyl chloride, mixed with 100 mM sodium carbonate and internal standard, and analyzed by liquid chromatography-tandem mass spectrometry (LC- MS/MS) as previously described (Song et al., 2012; Zestos et al., 2016). All samples were analyzed for the concentrations of: acetylcholine, adenosine, aspartate, choline, dihydroxyphenylalanine (DOPA), dihydroxyphenylacetic acid (DOPAC), dopamine, GABA, glucose, glutamate, glutamine, glutathione, glycine, histamine, homovanillic acid (HVA), 3-methoxytyramine (3-MT), normetanephrine (NM), norepinephrine, phenylalanine, serine, serotonin, 5-hydroxyindoleacetic acid (5-HIAA), taurine, and tyrosine. The peak areas of each analyte were divided by the area of the internal standard for quantification.
2.6. Histology
At the completion of the experiments, rats were deeply anesthetized using isoflurane (VetOne, Boise, ID) inhalation and overdosed using an intraperitoneal injection of pentobarbital (Vortech Pharmaceuticals, Dearborn, MI). Animals were transcardially perfused with 0.9% saline solution followed by a 4% paraformaldehyde solution, then stored at 4°C in 30% sucrose followed by freezing, cutting with a cryostat, and cresyl violet staining. The targeting for both the nucleus reuniens (with the injection cannula) and the hippocampus (with the microdialysis probe) were verified as described previously (Luna-Munguia et al., 2011, 2017).
2.7. Statistical analysis
Our analysis involved three aspects: quantifying changes in individual molecules, correlation between different molecules, and a multidimensional approach to assess overall differences between various situations and cohorts. We evaluated each of these aspects for two conditions: the baseline difference between Pilocarpine and Control groups, and the effect of the induced pro-ictal state. For baseline comparisons, the groups were Control and pilocarpine-treated animals, and the analysis used data acquired during the two-hour baseline prior to the induced ictogenesis (Figure 1). For determining the effect of the pro-ictal state, the two groups were animals receiving sham and KCl injections (with Control and Pilocarpine groups being analyzed separately), comparing data during the three-hour injection experiment with baseline. Measured levels at baseline were reported using mean and S.E.M. (Table 1). Next, to control for interanimal variability in the statistical testing, molecular concentrations for each animal were converted to z-scores using the mean and standard deviation over the two-hour baseline window (difference of each measurement from the mean, divided by the standard deviation). Any molecule with mean less than 0.01 nM was omitted from further analysis due to lack of sufficient precision (3-MT, dopamine, norepinephrine). Because of this, all subsequent analysis was limited to 21 molecules.
Table 1.
Molecule levels at baseline
| Compound | Control (nM) |
Pilocarpine model (nM) |
Compound | Control (nM) |
Pilocarpine model (nM) |
|---|---|---|---|---|---|
| Acetylcholine | 27.37 ± 5.91 | 34.61 ± 5.93 | Adenosine*** | 34.58 ± 4.88 | 7.4 ± 1.22 *** |
| Aspartate | 269.3 ± 27.57 | 291 ± 24.3 | Choline*** | 124.2 ± 26.22 | 479.2 ± 42.56 *** |
| DOPA | 3.73 ± 0.4 | 2.65 ± 0.37 | DOPAC | 9.03 ± 1.6 | 5.64 ± 0.82 |
| Dopamine | 0.091 ± 0.013 | 0.062 ± 0.01 | GABA | 105.7 ± 24.5 | 44.48 ± 5.28 |
| Glucose | 89.12 ± 7.66 | 77.37 ± 7.64 | Glutamate*** | 972.3 ± 87.95 | 1615 ± 129.5 *** |
| Glutamine | 37168 ± 3626 | 34616 ± 3037 | Glutathione | 91.79 ± 7.46 | 93.32 ± 7.29 |
| Glycine | 3462 ± 354 | 2763 ± 225.2 | Histamine | 2.75 ± 0.3 | 2.81 ± 0.22 |
| HVA** | 16.52 ± 1.94 | 7.46 ± 0.9 *** | 3-MT | 0.096 ± 0.02 | 0.046 ± 0.007 |
| NM | 0.33 ± 0.04 | 0.23 ± 0.029 | Norepinephrine | 0.18 ± 0.041 | 0.14 ± 0.021 |
| Phenylalanine*** | 975.8 ± 77.59 | 1627 ± 105.6 *** | Serine | 3696 ± 127 | 3519 ± 140.6 |
| Serotonin*** | 5.56 ± 1.17 | 0.76 ± 0.18*** | 5-HIAA | 27.18 ± 3.41 | 26.66 ± 3.01 |
| Taurine | 5792 ± 495.7 | 6525 ± 410.2 | Tyrosine*** | 919.4 ± 76.26 | 1387 ± 76.9 *** |
Data shown as mean ± S.E.M. Statistical significance computed using Wilcoxon Rank Sum on z-scored data and the Bonferroni correction for multiple comparisons (n = 24).
p < 0.01/24
p < 0.001/24.
DOPA = dihydroxyphenylalanine; DOPAC = dihydroxyphenylacetic acid; HVA = homovanillic acid; 3-MT = 3-methoxytyramine; NM = normetanephrine; 5-HIAA = 5-hydroxyindolacetic acid; S.E.M. = standard error of the mean
Non-parametric statistics were used for further analyses since the z-score distributions were not always normal (Kolmogorov-Smirnov test). Individual molecules were compared between groups using the Wilcoxon Rank-Sum test with Bonferroni correction. A corrected p-value of < 0.05 was used as criterion for significance. Pearson's correlation coefficients were also computed. Correlations were additionally visualized using hierarchical clustering and unweighted, average (group-wise) Euclidean distance. Lastly, step-wise logistic regression was used to identify a minimal subset of molecules descriptive of the difference between each of the two groups under comparison, selecting from the molecules found to be statistically significant in the Wilcoxon Rank-Sums. Hold-out-one-animal cross validation was used to ensure generalizability of logistic regression results.
3. Results
3.1. KCl microinjection to the nucleus reuniens induces hippocampal seizures
During the focal microinjections of KCl in the Pilocarpine group, 4 out of 6 subjects had seizures. In total, 11 seizures were observed in these four animals during the KCl-nucleus reuniens injections.
No Control animals had seizures during the injections or at any other time. Seizures only occurred in one Pilocarpine animal during phosphate buffered saline (PBS) injection, though that seizure was expected given that animal's high baseline seizure rate (~ 1 per hour, and the injections are 3 hours long). The other Pilocarpine animals with PBS injections did indeed have spontaneous seizures, but there were none in the 3 hours prior to or during the injection. As is expected for the pilocarpine model, seizure rates on different days were quite variable in many animals, which did not allow the identification of a stable baseline over long periods. We calculated the risk of seizures over time for each group, and found that the KCl injections increased seizure risk more than 3-fold, while there was no increase in the sham or PBS injections (Figure 1, risk calculated as previously described (Luna-Munguia et al., 2017)).
3.2. Pilocarpine model alters baseline extracellular molecule levels
Microdialysis experiments revealed increased interictal extracellular levels of choline (+286%, p 0.001), glutamate (+66%, p < 0.001), phenylalanine (+67%, p < 0.001), and tyrosine (+51%, p < 0.001) in hippocampi of rats with epilepsy, when compared to Controls. On the other hand, in rats with epilepsy, hippocampal extracellular levels of adenosine (−78%,p < 0.001), homovanillic acid (HVA; −55%,p < 0.01), and serotonin (−86%,p < 0.001) were significantly lower when compared to the Control group (Table 1).
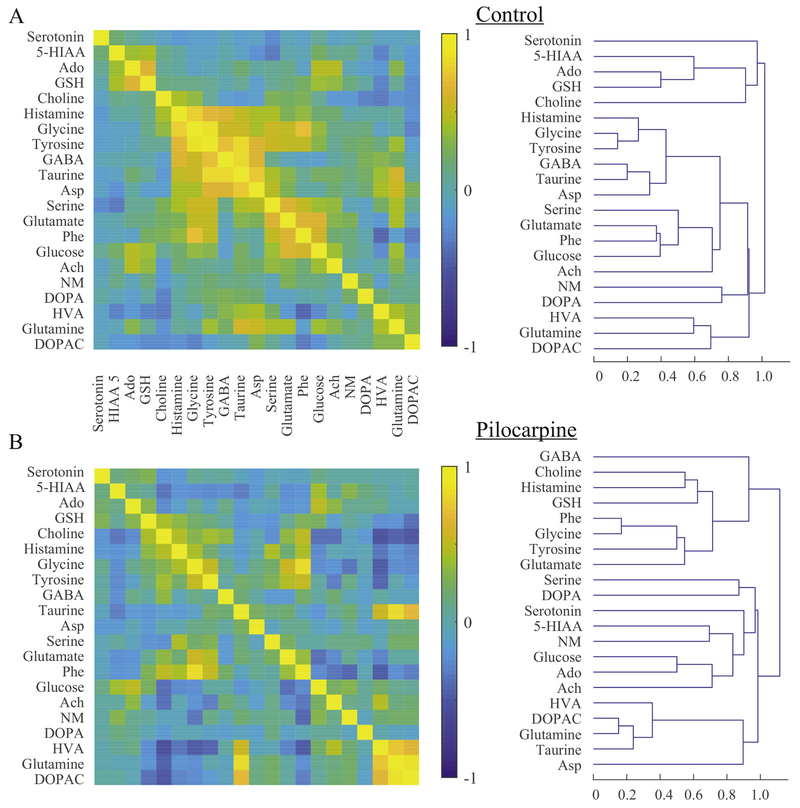
With simultaneous measurements, it is possible to determine interactions between each molecule as well as which molecules are the most important in determining the difference between Control and Pilocarpine groups. Pearson correlations between each pair of molecules and hierarchical clustering dendrograms are shown in Figure 2. In normal animals without epilepsy, there is a high correlation within four groups of molecules: 1) serotonin, 5-hydroxyindolacetic acid (5- HIAA), adenosine, glutathione, and choline; 2) GABA, taurine, aspartate, tyrosine, glycine, and histamine; 3) glutamate, phenylalanine, glucose, and acetylcholine; 4) HVA, glutamine, and dihydroxyphenylacetic acid (DOPAC). In pilocarpine-treated animals, these relationships are drastically changed (Figure 2B), including the finding that aspartate, glutamate, and GABA are correlated with different molecules. Both GABA and aspartate, which had low hierarchies in normal animals, had the highest hierarchies in pilocarpine-treated rats, indicating that they behave independent of the others. In other words, even though the GABA level did not change significantly from baseline (Table 1), its correlation with other molecules was very different. These results illustrate the complex effects of pilocarpine-induced epilepsy on the extracellular milieu.
Figure 2. Multivariate correlations.
Multivariate correlations in Control (A) and Pilocarpine (B) rats. Left: correlations are significantly altered in pilocarpine-treated animals. Right: hierarchical dendrograms show that connectivity between each molecule also changes in the Pilocarpine group. All scales are normalized to local peak values.
Multivariate methods are helpful to determine which molecules are most descriptive of the differences between Pilocarpine and Control animals. One method to accomplish this is step-wise logistic regression, which determines the subset of all the molecules that is responsible for the largest contribution to the difference. Another feature of this tool is that it provides an odds ratio, in which one standard deviation change in a specific molecule accounts for a ten-fold change in odds that a given animal was from the Pilocarpine group. We performed this analysis on the 21 molecules and found that four were primarily responsible for the difference between Control and Pilocarpine animals: adenosine, serotonin, HVA, and choline, with adenosine being the most significant and serotonin being the most variable (Figure 3). What is perhaps the most unexpected is that glutamate and GABA were not on the list: i.e., they were statistically not as important in distinguishing the two groups of animals as the other four molecules. Overall, there were many molecules in which the baseline concentrations and interactions with other molecules were altered by the pilocarpine model.
Figure 3. Multivariate analysis of pilocarpine effect.
Although 7 molecules were significantly changed in pilocarpine-treated animals (Table 1), stepwise linear regression found that, due to correlations, changes in these four molecules are sufficient to characterize the difference between the Pilocarpine and Control groups. Error bars: 1 stdev.
3.3. Pro-ictal states alter extracellular molecule levels
We then quantified the changes during a procedure that increases seizure risk, creating a pro-ictal state without directly affecting the hippocampus (Luna-Munguia et al., 2017). Note that the KCl injection is far from where the microdialysis probe is placed in the hippocampus and is very slow (0.1 μl/min), so the changes are unlikely to be due to local injection effects. Because both the baseline and the changes during ictogenesis were highly varied across animals, it was not possible to show results similar to Table 1. In addition, we found that there were changes in many molecules during both the sham and PBS injections, which is not surprising since being connected to the microdialysis pump is an unusual environment for the animals. We thus determined that the best way to show the biochemical effect of the pro-ictal injections was to compare the effect of Sham + PBS injections with that of the KCl injection, normalized to its own baseline (z-score). This comparison allows identification of how the change from baseline due to KCl injections is different from change due to sham and PBS injections. Because Control animals are already significantly different from pilocarpine-treated animals at baseline, we report the results only for Pilocarpine group. There are several changes during Sham + PBS (Figure 4A), but different effects during KCl (Figure 4B). Several molecules have similar effects in both conditions (e.g. the cluster of phenylalanine, tyrosine, taurine, and serine). There are also many differences; in particular, several molecules became less correlated (5-HIAA, HVA, and glutamate) with other molecules, while others developed new correlations (serine, aspartate, histamine, glutamine, and normetanephrine (NM)). These complex relationships are also visible in the dendrograms, showing that many molecules cluster with different groups during KCl injections. 5-HIAA, serotonin, and HVA become more independent of the other molecules, and the hierarchical pairings of many other molecules are altered.
Figure 4. Correlations in Sham + PBS and KCl injections.
(A) Sham+PBS produced several biochemical changes from baseline, likely due to the stress of the experiment. (B) The KCl injection, which is pro-ictal, produces many different effects.
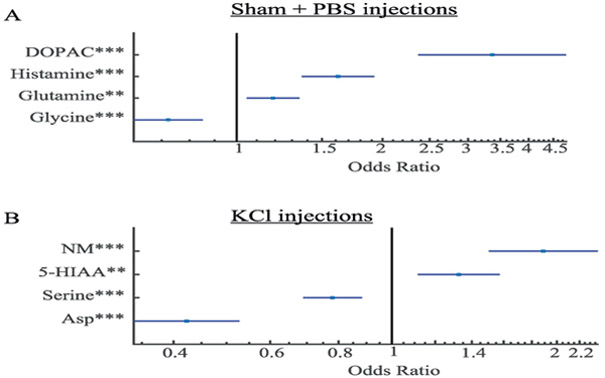
Stepwise logistic regression quantifies these complex interactions. Results show that four molecules are most characteristic of the changes that occur during the Sham + PBS injections: DOPAC, histamine, glutamine, and glycine (Figure 5A). In contrast, KCl injections are characterized by changes in NM, 5-HIAA, serine, and aspartate (Figure 5B). Again, it is notable that in neither case is glutamate or GABA the most prominent change. However, is clear from Figure 4 that glutamate is tightly correlated with aspartate and GABA with NM, suggesting that the changes in aspartate and NM are associated with glutamate and GABA levels.
Figure 5. Multivariate analysis of injections.
(A) Stepwise linear regression finds that the effect of Sham + PBS can be best described by changes in four molecules. (B) The KCl injections produce alterations in four different molecules. Error bars: 1 stdev.
3.4. Biochemical changes prior to seizures
One of the primary goals of the ictogenesis model is to record the biochemical changes that produce seizures. During the KCl injections, there were 11 seizures, 5 of which occurred less than 60 seconds after microdialysis sampling in three different animals. We compared the results of these five seizures with the other samples during the KCl injection to determine if there were any acute changes immediately prior to a seizure. We first analyzed all 21 molecules with rank sum statistics. None of the molecules were significantly changed after accounting for multiple comparisons. Unfortunately, multivariate methods were not possible in this case, as there are 21 molecules but only 5 seizure events. We analyzed each of the cases individually and found that certain molecules were different in individual seizures, but changes were not consistent and were not the most extreme values. To illustrate this, we plot the normalized values of several molecules (Figure 6), including the four primary candidates in Figure 5 (aspartate, 5-HIAA, NM, and serine), as well as glutamate, GABA, and glutamine. These plots show the normalized values of every measurement taken during KCl injections, with the pre-seizure events marked in red. Although the sample size is small, the pre-seizure measurements are widespread and variable. There were individual seizures with extreme values, such as one seizure with low aspartate and serine, and a different one with high NM, but these were not consistent even in the two animals with multiple seizures nor were they the most extreme values. Note that all these measurements were normalized to the samples during KCl injections; a similar analysis with values normalized to the pre-injection baseline yielded similar results (not shown). Thus, although the previous section identified several changes that were induced by KCl injections (pro-ictal state), it was not possible to identify a stereotyped biochemical effect that heralded the imminent approach of a seizure.
Figure 6. Distribution of concentrations.
The four graphs show all measurements of the selected molecules in the KCl injections from all animals. The samples that occurred < 60 s prior to a seizure are shown in red. There was no consistent change in any molecule. Measurements from each animal were individually normalized (z-score) using the mean and standard deviation of the values during the KCl injections.
4. Discussion
Identifying biomarkers is a key benchmark for epilepsy research (Dlugos et al., 2016). This work provides two important innovations to this research. The first is the simultaneous measurements of 24 molecules, of which 21 were sufficiently high to analyze. Microdialysis is capable of measuring extracellular levels of neurotransmitters (Schultz and Kennedy, 2008), peptides (Zhou et al., 2013), proteins (Clough, 2005), but such work was previously limited to selected molecules. Using a sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) technique allowed us to measure down to picomolar concentrations (Song et al., 2012). Derivatizing with benzoyl chloride increased sensitivity and improved the quantification via stable isotope-labeled internal standards. This helped us detect and evaluate for the first time the basal extracellular levels of 24 compounds in a series of samples taken from the hippocampus of live rats. The second innovation was the ability to control seizure threshold, allowing the first investigation of the effects of seizure threshold on these molecules.
Several studies have analyzed the biochemistry of epilepsy, focusing on the extracellular imbalance between glutamatergic and GABAergic neurotransmission. This imbalance produces neuronal hyperexcitability, a logical trigger for seizures (Bernard et al., 2000). Past results from patients demonstrated that glutamate levels were higher in epileptic tissue both interictally and during seizures (Cavus et al., 2008; Thomas et al., 2005). In contrast, GABA was shown either to increase (During and Spencer, 1993; Thomas et al., 2005; Wilson et al., 1996) or decrease (Pan et al., 2008) before seizures. Further research is necessary to determine how these molecules affect the pathogenesis of epilepsy. Animal studies support the human results, showing increased hippocampal glutamate and GABA levels after microperfusion of chemoconvulsants (Meurs et al., 2008; Wilson et al., 1996) or other epilepsy models (Ueda and Tsuru, 1995). Only one prior study focused on the interictal and ictal levels of glutamate and GABA in kindled rats, reporting alterations that resemble those found in hippocampi of refractory TLE patients (Luna-Munguia et al., 2011). In our study we found changes in these molecules, but there were several others that were changed more significantly in both epileptogenesis and ictogenesis. This finding agrees with prior work suggesting that increased glutamate levels alone are not enough to induce seizures (Clinckers et al., 2005).
Another novel contribution of this work is the ability to identify interactions between these different molecules. We tested the biochemical changes present in three conditions: due to the pilocarpine model (interictal or baseline changes) (Figures 2, 3), due to the ictogenesis model (pro-ictal changes) (Figures 4, 5), and immediately before a seizure (pre-ictal changes) (Figure 6). Each of these conditions had different interactions that merit some discussion.
The effect of the pilocarpine model on underlying biochemistry has been evaluated previously, typically limited to a few chosen molecules. Our simultaneous analysis reveals previously-unrecognized interactions. Three molecules existed in such low concentrations that it was unreliable to compare them with the other molecules (3-methoxytyramine (3-MT), dopamine, and norepinephrine). Seven had significant changes in mean concentration in the Pilocarpine group (increased choline, glutamate, phenylalanine, tyrosine; decreased serotonin, adenosine, HVA). While glutamate is expected based upon past literature, the other compounds are less studied.
The increased level of tyrosine is a novel finding, possibly specific to the pilocarpine model. This result is unexpected in human epilepsy based on previous studies reporting no changes in its activity (Pintor et al., 1990), enzymatic expression (Rocha et al., 2012) or extracellular concentration (Ronne-Engstrom et al., 1992) in patients. Although acetylcholine is the second most prevalent brain excitatory neurotransmitter, few epilepsy microdialysis studies have focused on it or its metabolites. We found an increase in choline levels, which likely reflects an increase in the activity of the septo-hippocampal cholinergic pathway. Evidence shows that changes in cholinergic neurotransmission can be involved in hippocampal epileptogenesis (Leite et al., 2002). Jope et al. (1987) and Hillert et al. (2014) found increased acetylcholine during seizures, but only the former also tested choline. In this sense, it is unclear why choline levels were significantly higher, despite insignificant changes in acetylcholine in our experiment. Although more work is needed to understand this effect, one possibility is reduced activity of the hippocampal choline acetyltransferase (Vezzani et al., 1991).
Decreased serotonin is related to an increased seizure risk (Bagdy et al., 2007; Vermoesen et al., 2012), while augmenting its level can be protective (Clinckers et al., 2004). Serotonin is also known to decrease in the presence of elevated phenylalanine in mice and humans (Pascucci et al., 2002; Weglage et al., 1995) due to enzymatic and transporter inhibition (Ogawa and Ichinose, 2006; Pietz et al., 1999). While dopamine was too low to measure, its metabolite HVA was decreased. This agrees with prior work showing reduced tissue content of dopamine, DOPAC, and HVA in the temporal neocortex of TLE patients (Mori et al., 1987; Pacia et al., 2001; Rocha et al., 2012). Our finding of decreased adenosine is novel; prior works had identified increased levels during seizures (During and Spencer, 1992), while here we show for the first time a decreased interictal extracellular level of adenosine in animals with epilepsy from the pilocarpine model, which is possibly crucial in epilepsy development. Adenosine is an endogenous inhibitor of excitatory synaptic transmission with potent anticonvulsant properties, and also has neuroprotective effects as well in ischemia and seizures (Boison, 2008), and thus is likely an essential player for natural seizure termination. Seizure control is also improved when adenosine is administered directly into the epileptic focus in rats (Anschel et al., 2004).
The interactions between molecules also changed in the pilocarpine model. In general, this model disrupts the normal correlations between several different molecules. Although GABA levels did not change, it behaves more independently in pilocarpine-treated animals (as judged by the hierarchical trees), as did aspartate. Conversely, serotonin is less independent. When evaluated together, the molecules most characteristic of the differences were the decreased adenosine, serotonin, HVA, and increased choline. The full extent and consequences of these findings will require additional research, but it is clear that such complex interactions would be missed without analyzing several of these molecules together.
Researchers have been looking for evidence of a pro-ictal state, a condition in which seizures are more likely (Jirsa et al., 2014). Our ictogenesis model provides a novel, controlled environment to test for pro-ictal changes. We found four molecules were most characteristic of the KCl injection: NM, 5-HIAA, serine, and aspartate. Each of these represents an interesting aspect of ictogenesis. The most extreme changes were increased NM and decreased aspartate. Our analysis demonstrated that NM is tightly correlated with GABA, and aspartate with glutamate. Thus, these changes can be understood by thinking that the pro-ictal state produces low levels of glutamate/aspartate, and high levels of GABA/NM. This is somewhat counterintuitive, and may represent a systemic compensatory reaction to increased ictogenicity, explaining why the hippocampus was not having a seizure despite increased seizure risk. A similar effect occurs with 5-HIAA. Like its metabolite serotonin, it increases during pro-ictal states and behaves quite independently during KCl injection. As described above, increased serotonin protects against seizures. The decrease in serine may be important because of how the logistic regression works, rather than an independent physiological effect. Stepwise linear regression determines the most efficient way to describe the difference caused by KCl injections. In Figure 4B, serine has extensive correlation with a large number of molecules (phenylalanine, taurine, glycine, glutamine, and histamine). In effect, combining Figure 4 and 5 suggests that measuring serine levels is an efficient method to monitor the concentration of 5 other important molecules, an efficient probe of several biochemical effects. This suggests an intriguing alternative approach to biomarker research: identification of a small group of molecules that are correlated with the effects of many others.
We were unable to find reliable changes leading to the 5 seizures induced by the ictogenesis model. The number of seizures was small, but even so the results did not appear to be converging: even with only 5 samples there was great disparity in the different responses. This may indicate that the changes induced by the pro-ictal state were already sufficient to produce seizures; however, even when comparing against baseline there was no clear answer. One limitation is that each microdialysis collection lasted 2 min and possibly the acute changes leading to seizures are too fast to be detected in that time scale. Alternative, these results could suggest that there are multiple pathways into a seizure, i.e. there is not a single biochemical condition that causes the brain to start a seizure. Instead, as has been predicted by mathematical modeling of seizure dynamics, the transition into seizure may occur as a random process once the brain is already close to seizure threshold (Jirsa et al., 2014).
Identifying biochemical signatures of epileptogenesis and ictogenesis will have tremendous implications in epilepsy research. Our findings will help guide future work by identifying the complex interactions that exist between various molecules, isolating the best candidates for future research using higher temporal and spatial resolution. We propose that biochemical assays should monitor several molecules together, and analyze both the levels and interactions of these molecules to provide important candidates for future research.
Highlights.
Microdialysis measured 24 molecules simultaneously in pilocarpine rats over hours
Multivariate techniques analyzed the effects and interactions of the 24 molecules
Several molecules have significant changes in rat hippocampi due to epileptogenesis
An ictogenesis model alters several molecules other than GABA and glutamate
These findings show potential biochemical biomarkers of icto- and epilepto-genesis
Acknowledgements
Funding for this work was provided by NIH 1K08NS069783, 1R01NS094399, T32DA007268, R37 EB003320, R01 EB003320, and the Michigan Brain Initiative Working Group.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Abbreviations
- DOPA
dihydroxyphenylalanine
- DOPAC
dihydroxyphenylacetic acid
- GABA
gamma-aminobutyric acid
- HVA
homovanillic acid
- 3-MT
3-methoxytyramine
- NM
normetanephrine
- 5-HIAA
5-hydroxyindoleacetic acid
Footnotes
Competing interests No competing interests declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderzhanova E and Wotjak CT (2013). Brain microdialysis and its applications in experimental neurochemistry. Cell Tissue Res 354(1),27–39. DOI:10.1007/s00441-013-1709-4 [DOI] [PubMed] [Google Scholar]
- 2.Anschel DJ, Ortega EL, Kraus AC, and Fisher RS (2004). Focally injected adenosine prevents seizures in the rat. Exp Neurol 190, 544–547. DOI:10.1016/j.expneurol.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 3.Bagdy G, Kecskemeti V, Riba P, and Jakus R (2007). Serotonin and epilepsy. J. Neurochem 100(4), 857–873. DOI:10.1111/j.1471-4159.2006.04277.x [DOI] [PubMed] [Google Scholar]
- 4.Bernard C, Costar R, Hirsch JC, Esclapez M, and Ben-Ari Y (2000). What is GABAergic inhibition? How is it modified in epilepsy? Epilepsia. 41, S90–S95. [DOI] [PubMed] [Google Scholar]
- 5.Bertram E (2007). The relevance of kindling for human epilepsy. Epilepsia 48 Suppl 2, 65–74 [DOI] [PubMed] [Google Scholar]
- 6.Boison D (2008). Adenosine as a neuromodulator in neurological diseases. Curr. Opin. Pharmacol 8, 2–7. DOI: 10.1016/j.coph.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, and Abi-Saab WM (2005). Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann. Neurol 57(2), 226–235. DOI: 10.1002/ana.20380 [DOI] [PubMed] [Google Scholar]
- 8.Cavus I, Pan JW, Hetherington HP, Abi-Saab W, Zaveri HP, Vives KP, Krystal JH, Spencer SS, and Spencer DD (2008). Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia. 49(8), 1358–1366. DOI: 10.1111/j.1528-1167.2008.01603.x [DOI] [PubMed] [Google Scholar]
- 9.Cavus I, Romanyshyn JC, Kennard JT, Farooque P, Williamson A, Eid T, Spencer SS, Duckrow R, Dziura J, and Spencer DD (2016). Elevated basal glutamate and unchanged glutamine and GABA in refractory epilepsy: Microdialysis study of 79 patients at the yale epilepsy surgery program. Ann. Neurol 80, 35–45. DOI: 10.1002/ana.24673 [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury FA, O'Gorman RL, Nashef L, Elwes RD, Edden RA, Murdoch JB, Barker GJ, and Richardson MP (2015). Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J. Magn. Reson. Imaging 41(3), 694–699. DOI: 10.1002/jmri.24611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinckers R, Smolders I, Meurs A, Ebinger G, and Michotte Y (2004). Anticonvulsant action of hippocampal dopamine and serotonin is independently mediated by D and 5-HT receptors. J. Neurochem 89(4), 834–843. DOI: 10.1111/j.1471-4159.2004.02355.x [DOI] [PubMed] [Google Scholar]
- 12.Clinckers R, Gheuens S, Smolders I, Meurs A, Ebinger G, and Michotte Y (2005). In vivo modulatory action of extracellular glutamate on the anticonvulsant effects of hippocampal dopamine and serotonin. Epilepsia. 46, 828–836. DOI: 10.1111/j.1528-1167.2005.57004.x [DOI] [PubMed] [Google Scholar]
- 13.Clough GF (2005). Microdialysis of large molecules. AAPS J. 7(3), E686–692. DOI: 10.1208/aapsj070369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curia G, Longo D, Biagini G, Jones RS, and Avoli M (2008). The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 172, 143–157. DOI: 10.1016/j.jneumeth.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dlugos D, Worrell G, Davis K, Stacey W, Szaflarski J, Kanner A, Sunderam S, Rogawski M, Jackson-Ayotunde P, Loddenkemper T, Diehl B, Fureman B, and Dingledine R (2016). 2014 Epilepsy benchmarks area III: improve treatment options for controlling seizures and epilepsy-related conditions without side effects. Epilepsy Curr 16(3), 192–197. DOI: 10.5698/1535-7511-16.3.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont AC, Largeau B, and Santiago Ribeiro MJ (2017). Translocator protein-18 kDa (TSPO) Positron Emission Tomography (PET) imaging and its clinical impact in neurodegenerative diseases. Int. J. Mol. Sci 18(4), pii:E785. DOI: 10.3390/ijms18040785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.During MJ and Spencer DD (1992). Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann. Neurol 32(5), 618–624. DOI: 10.1002/ana.410320504 [DOI] [PubMed] [Google Scholar]
- 18.During MJ and Spencer DD (1993) Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 341, 1607–1610. [DOI] [PubMed] [Google Scholar]
- 19.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr., Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshe SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, and Wiebe S (2014). ILAE Official report: a practical clinical definition of epilepsy. Epilepsia. 55, 475–482. DOI: 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 20.Hillert MH, Imran I, Zimmermann M, Lau H, Weinfurter S, and Klein J (2014). Dynamics of hippocampal acetylcholine release during lithium-pilocarpine-induced status epilepticus in rats. J. Neurochem 131, 42–52. DOI: 10.1111/jnc.12787 [DOI] [PubMed] [Google Scholar]
- 21.Jirsa VK, Stacey WC, Quilichini PP, Ivanov AI, and Bernard C (2014). On the nature of seizure dynamics. Brain. 137(Pt 8), 2210–2230. DOI: 10.1093/brain/awu133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jope RS, Simonato M, and Lally K (1987). Acetylcholine content in rat brain is elevated by status epilepticus induced by lithium and pilocarpine. J. Neurochem 49, 944–951. [DOI] [PubMed] [Google Scholar]
- 23.Leite JP, Garcia-Cairasco N, and Cavalheiro EA (2002). New insights from the use of pilocarpine and kainate models. Epilepsy Res 50, 93–103. [DOI] [PubMed] [Google Scholar]
- 24.Löscher W and Schmidt D (2011). Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia. 52, 657–678. DOI: 10.1111/j.1528-1167.2011.03024.x [DOI] [PubMed] [Google Scholar]
- 25.Luna-Munguia H, Orozco-Suarez S, and Rocha L (2011). Effects of high frequency electrical stimulation and R-verapamil on seizure susceptibility and glutamate and GABA release in a model of phenytoin-resistant seizures. Neuropharmacology. 61, 807–814. DOI: 10.1016/j.neuropharm.2011.05.027 [DOI] [PubMed] [Google Scholar]
- 26.Luna-Munguia H, Starski P, Chen W, Gliske S, and Stacey WC (2017). Control of in vivo ictogenesis via endogenous synaptic pathways. Sci. Rep 7(1), 1311 DOI: 10.1038/s41598-017-01450-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meurs A, Clinckers R, Ebinger G, Michotte Y, and Smolders I (2008). Seizure activity and changes in hippocampal extracellular glutamate, GABA, dopamine and serotonin. Epilepsy Res 78(1), 50–59. DOI: 10.1016/j.eplepsyres.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 28.Mori A, Hiramatsu M, Namba S, Nishimoto A, Ohmoto T, Mayanagi Y, and Asakura T (1987). Decreased dopamine level in the epileptic focus. Res. Commun. Chem. Pathol. Pharmacol 56, 157–164. [PubMed] [Google Scholar]
- 29.Ogawa S and Ichinose H (2006). Effect of metals and phenylalanine on the activity of human tryptophan hydroxylase-2: comparison with that on tyrosine hydroxylase activity. Neurosci. Lett 401(3), 261–265. DOI: 10.1016/j.neulet.2006.03.031 [DOI] [PubMed] [Google Scholar]
- 30.Pacia SV, Doyle WK, and Broderick PA (2001). Biogenic amines in the human neocortex in patients with neocortical and mesial temporal lobe epilepsy: identification with in situ microvoltammetry. Brain Res 899, 106–111. [DOI] [PubMed] [Google Scholar]
- 31.Pan JW, Williamson A, Cavus I, Hetherington HP, Zaveri H, Petroff OA, and Spencer DD (2008). Neurometabolism in human epilepsy. Epilepsia. 49 (Suppl 3), 31–41. DOI: 10.1111/j.1528-1167.2008.01508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pascucci T, Ventura R, Puglisi-Allegra S, and Cabib S (2002). Deficits in brain serotonin synthesis in a genetic mouse model of phenylketonuria. Neuroreport. 13, 2561–2564. DOI: 10.1097/01.wnr.0000047690.08940.39 [DOI] [PubMed] [Google Scholar]
- 33.Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, and Bremer HJ (1999). Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J. Clin. Invest 103, 1169–1178. DOI: 10.1172/JCI5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pintor M, Mefford IN, Hutter I, Pocotte SL, Wyler AR, and Nadi NS (1990). Levels of biogenic amines, their metabolites, and tyrosine hydroxylase activity in the human epileptic temporal cortex. Synapse. 5, 152–156. DOI: 10.1002/syn.890050210 [DOI] [PubMed] [Google Scholar]
- 35.Rocha L, Alonso-Vanegas M, Villeda-Hernandez J, Mujica M, Cisneros-Franco JM, Lopez-Gomez M, Zavala-Tecuapetla C, Frias-Soria CL, Segovia-Vila J, and Borsodi A (2012). Dopamine abnormalities in the neocortex of patients with temporal lobe epilepsy. Neurobiol. Dis 45(1), 499–507. DOI: 10.1016/j.nbd.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 36.Ronne-Engstrom E, Hillered L, Flink R, Spannare B, Ungerstedt U, and Carlson H (1992). Intracerebral microdialysis of extracellular amino acids in the human epileptic focus. J. Cereb. Blood Flow Metab 12, 873–876. DOI: 10.1038/jcbfm.1992.119 [DOI] [PubMed] [Google Scholar]
- 37.Schultz KN and Kennedy RT (2008). Time-resolved microdialysis for in vivo neurochemical measurements and other applications. Annu. Rev. Anal. Chem 1, 627–661. DOI: 10.1146/annurev.anchem.1.031207.113047 [DOI] [PubMed] [Google Scholar]
- 38.Sloviter RS (2005). The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. C. R. Biol 328(2), 143–153. [DOI] [PubMed] [Google Scholar]
- 39.Song P, Mabrouk OS, Hershey ND, and Kennedy RT (2012). In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography-mass spectrometry. Anal. Chem 84(1), 412–419. DOI: 10.1021/ac202794q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas PM, Phillips JP, and O'Connor WT (2005). Microdialysis of the lateral and medial temporal lobe during temporal lobe epilepsy surgery. Surg. Neurol 63, 70–79. DOI: 10.1016/j.surneu.2004.02.031 [DOI] [PubMed] [Google Scholar]
- 41.Turski WA, Czuczwar SJ, Kleinrok Z, and Turski L (1983). Cholinomimetics produce seizures and brain damage in rats. Experientia. 39(12), 1408–1411. [DOI] [PubMed] [Google Scholar]
- 42.Ueda Y and Tsuru N (1995). Simultaneous monitoring of the seizure-related changes in extracellular glutamate and gamma-aminobutyric acid concentration in bilateral hippocampi following development of amygdaloid kindling. Epilepsy Res 20(3), 213–219. [DOI] [PubMed] [Google Scholar]
- 43.Valle-Dorado MG, Santana-Gomez CE, Orozco-Suarez SA, and Rocha L (2015). The mast cell stabilizer sodium cromoglycate reduces histamine release and status epilepticus-induced neuronal damage in the rat hippocampus. Neuropharmacology. 92, 49–55. DOI: 10.1016/j.neuropharm.2014.12.032 [DOI] [PubMed] [Google Scholar]
- 44.van der Zeyden M, Oldenziel WH, Rea K, Cremers TI, and Westerink BH (2008). Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol. Biochem. Behav 90(2), 135–147. DOI: 10.1016/j.pbb.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 45.Vermoesen K, Massie A, Smolders I, and Clinckers R (2012). The antidepresants citalopram and reboxetine reduce seizure frequency in rats with chronic epilepsy. Epilepsia. 53(5), 870–878. DOI: 10.1111/j.1528-1167.2012.03436.x [DOI] [PubMed] [Google Scholar]
- 46.Vezzani A, Serafini R, Stasi MA, Vigano G, Rizzi M, and Samanin R (1991). A peptidase-resistant cyclic octapeptide analogue of somatostatin (SMS 201-995) modulates seizures induced by quinolinic and kainic acids differently in the rat hippocampus. Neuropharmacology. 30(4), 345–352. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y and Qin ZH (2010). Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 15, 1382–1402. DOI: 10.1007/s10495-010-0481-0 [DOI] [PubMed] [Google Scholar]
- 48.Weglage J, Pietsch M, Funders B, Koch HG, and Ullrich K (1995). Neurological findings in early treated phenylketonuria. Acta Paediatrica. 84, 411–415. [DOI] [PubMed] [Google Scholar]
- 49.Wilson CL, Maidment NT, Shomer MH, Behnke EJ, Ackerson L, Fried I, and Engel J Jr. (1996). Comparison of seizure related amino acid release in human epileptic hippocampus versus a chronic, kainate, rat model of hippocampal epilepsy. Epilepsy Res 26, 245–254. [DOI] [PubMed] [Google Scholar]
- 50.Zestos AG, Mikelman SR, Kennedy RT, and Gnegy ME (2016). PKCβ inhibitors attenuate amphetamine-stimulated dopamine efflux. ACS Chem. Neurosci 7(6), 757–766. DOI: 10.1021/acschemneuro.6b00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Mabrouk OS, and Kennedy RT (2013). Rapid preconcentration for liquid chromatography-mass spectrometry assay of trace level neuropeptides. J. Am. Soc. Mass. Spectrom 24(11), 1700–1709. DOI: 10.1007/s13361-013-0605-1 [DOI] [PMC free article] [PubMed] [Google Scholar]