Abstract
Dendrites are the conduits for receiving (and in some cases transmitting) neural signals; their ability to do these jobs is a direct result of their morphology. Developmental patterning mechanisms are critical to ensuring concordance between dendritic form and function. This article reviews recent studies in vertebrate retina and brain that elucidate key strategies for dendrite functional maturation. Specific cellular and molecular signals control the initiation and elaboration of dendritic arbors, and facilitate integration of young neurons into particular circuits. In some cells, dendrite growth and remodeling continues into adulthood. Once formed, dendrites subdivide into compartments with distinct physiological properties that enable dendritic computations. Understanding these key stages of dendrite patterning will help reveal how circuit functional properties arise during development.
Overview
Ask a neuroscientist to imagine their favorite cell type (most of us have one). Probably, the first feature that comes fondly to mind is the shape of the cell’s dendrites. This is for good reason: Each of the brain’s many cell types has a distinctive dendritic pattern because each has a distinct function, and the dendrites are where functional differences arise. Through their anatomy, connectivity, and electrical properties, dendrites help make each cell type unique.
During development, dendrite growth and patterning must be tailored to the ultimate function of each cell. Several broad developmental strategies facilitate matching of form to function: First, dendrite growth is coupled to contact and synapse formation with presynaptic afferents [1]. Second, sampling from potential afferents is regulated by extracellular molecular cues, located in the environment or on neighboring arbors, that dictate arbor shape. Third, dendrites within the same cell can become molecularly distinct, such that they receive different types of synapses or filter inputs differently. Together these strategies ensure that the identity, number, and functional impact of inputs are appropriate. Current research seeks to understand mechanistically how these broad strategies are implemented. To this end, a wide variety of experimental model systems have been used; here we focus on insights derived from mouse retina, while touching on other mammalian experimental systems. The retina has been a particularly useful model, especially with the emergence of the retinal direction-selective circuit (Fig. 1) as a unique tool for linking development, anatomy, and circuit computations. We emphasize four select topics that have seen exciting recent advances: 1) How newborn neurons know when and where to initiate dendrite formation; 2) cell-cell recognition cues in dendrite patterning; 3) factors that influence dendrite growth in adulthood; 4) how dendrites subdivide into functionally distinct compartments.
Fig. 1: Organization of cells and synapses in mammalian retina.
Cellular layers (shaded) include outer and inner nuclear layers (ONL, INL); ganglion cell layer (GCL). Outer and inner plexiform layers (OPL, IPL) contain synapses. In outer retina, horizontal cells (purple) receive synapses from cone photoreceptors (red) on their dendrites, and from rods (dark blue) on their axons (a postsynaptic arbor with many dendrite-like properties). Rods and cones also connect to dendrites of specific bipolar cell types; cone bipolar cells (light blue) are illustrated here. In IPL, the direction-selective circuit is illustrated as an example of how inner retinal circuits are organized. Circuit partners converge upon particular IPL sublayers, where direction-selective retinal ganglion cells (RGCs, pink) receive glutamatergic excitation from bipolar cells (light blue) and inhibition from starburst amacrine cells (green). Direction-selective circuit occupies two sublayers (OFF, ON); starburst and bipolar cells project to one or the other, depending on the polarity of their light response. ON-OFF direction-selective RGCs project to both sublayers.
Initiating dendrite formation
Most newborn neurons migrate to their final destination. For example, in neocortex and retina, neurons migrate radially from the ventricular germinal zone to settle in a particular layer of the mature tissue. Almost immediately on arrival they initiate dendrite morphogenesis, beginning the process of integrating into local circuitry. Radial migration and dendrite morphogenesis appear to be mutually exclusive processes: If neurons are forced to grow dendrites early, they stop migrating [2], presumably because the cytoskeletal requirements for each task are incompatible. Therefore, one of the earliest stages in dendrite formation is the decision to switch from one growth mode to the other. This switch must be tightly regulated, so that neurons begin reaching out for their synaptic partners only when they are in the right place to encounter them.
Several cell-intrinsic mechanisms comprising the dendrogenic switch have been identified. These include cytoskeletal polarity regulators [3–5] as well as transcription factors: In mouse cortex, expression of the transcription factor Sox11 suppresses dendritic morphogenesis during radial migration. Moreover, downregulation of Sox11 upon arrival at the cortical plate is required to permit dendrite elaboration, suggesting that Sox11 regulation is a key part of the switching mechanism [2].
To activate dendrite genesis at the right place and time, migrating neurons must be able to detect local cues characteristic of both the migratory route and the final destination. Cues along the migratory route suppress dendrite growth so that migration may continue. In the neocortex, one such cue is N-cadherin, which is expressed by radial glia and mediates adhesion with the neurons migrating along them [6]. This adhesion prevents dendrite growth: In both N-cadherin [7] and Sox11 mutants [2], migrating neurons fall off glial fibers and grow dendrites in ectopic locations. Similarly, Sema3E is expressed on the migratory route of radially migrating olfactory bulb interneurons. Through its receptor PlexinD1, which is expressed on migratory interneurons, Sema3E prevents migration arrest, allowing neurons to traverse deep layers and occupy more superficial ones. In PlexinD1 mutant mice, neurons initiate dendrite formation too soon, causing them to integrate into inappropriate circuits within deeper layers [5].
What about local cues that signal arrival at the migratory destination? Retinal amacrine cells migrate to a final position adjacent to the synaptic neuropil called the inner plexiform layer (IPL; Fig. 2) [8,9]. As they approach the IPL, migrating amacrine cells project transient tangential processes, outside the IPL, that contact neighboring amacrine cell bodies [9,10]. In a recent study of starburst amacrine cells – retinal interneurons that participate in the directionselective circuit (Fig. 1) – these transient contacts were implicated in dendrite initiation: Contact serves as a cue that the neuron has arrived in position to begin innervating the IPL. A starburstspecific cell surface protein, MEGF10, is expressed on the transient arbors and triggers IPL innervation upon homotypic contact. In the absence of homotypic neighbors, or MEGF10, starburst neurons retain a multipolar migratory morphology and fail to innervate the IPL on time (Fig. 2). As a result, formation of direction-selective circuitry is impaired [10]. Using homotypic contact as the cue for dendrite initiation has a clear advantage: The presence of other cells that have successfully completed migration is a reliable signal that the migratory cell has come to the right place. Early-arriving cells could wait for a quorum before differentiating – this is plausible based on the phenotype of experimentally isolated starbursts, which wait in a multipolar state at the edge of the IPL [10] while later-arriving cells would be expected to receive their dendrite initiation signal right away. Such a strategy has the additional advantage that it helps coordinate timing of neuropil innervation across a population of cells born at different times. It will be interesting to see if a similar strategy is used by other cell types.
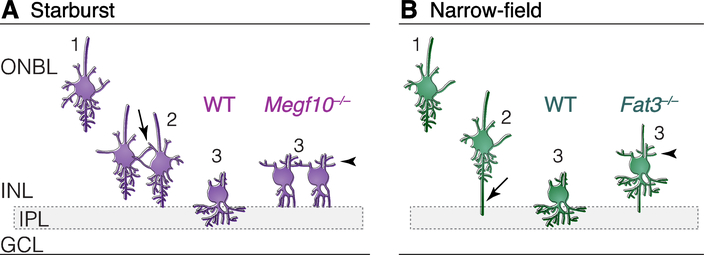
Fig. 2: Initiation of dendrite formation in migrating amacrine cells.
Two types of amacrine cells, starburst (A) and late-born narrow-field cells (B), highlight distinct dendrite initiation strategies. Each cell type is illustrated at three time points: 1) Early radial migration through the outer neuroblast layer (ONBL); 2) nearing their final position adjacent to the IPL; and 3) initiating dendrite projections into the IPL. Starburst cells use homotypic contacts (A, arrow) to detect that they have arrived in the right place for IPL innervation. Narrow-field cells send out long basal processes (B, arrow) to obtain IPL-derived polarity signals that control timing and location of dendrite initiation. Dendrite initiation is impaired when recognition receptors MEGF10 and Fat3 are eliminated. Megf10–/– starburst cells (A) do not receive contact-mediated signals initiating IPL innervation, and therefore remain multipolar (A, time 3, right). Fat3–/– narrow-field cells (B) lose cytoskeletal polarity, which slows migration and permits dendrite initiation on the wrong side of the cell (B, time 3, right). WT, wild-type cells. Arrowheads, mislocalized dendrites in mutant cells. Layer abbreviations as in Fig. 1.
While this homotypic mechanism does not require IPL contact, the IPL does contain cues that regulate dendrite initiation. During radial migration, a population of narrow-field amacrine cells send long processes into the still-distant IPL. These arbors express the cell surface receptor Fat3, which interacts with an unknown IPL ligand to polarize the actin cytoskeleton via Ena/VASP signaling. In the absence of Fat3, cytoskeletal polarization is lost, leading to a slowing of radial migration and a concomitant increase in dendrite sprouting (Fig. 2). Many dendrites emerge on the wrong side of the cell – i.e. away from the IPL – giving rise to ectopic neuropil [11]. These findings suggest that loss of polarization drives neurons away from migratory behavior and towards dendrite initiation. An analogous situation likely exists for cortical neurons, with Reelin serving as the polarization cue located at the migratory terminus. Loss of Reelin causes cortical migration and dendrite polarization phenotypes similar to Fat3–/– amacrine cells [12]. Dendrite mislocalization errors in these two mutants might be a direct result of polarization errors; however, they also might arise because dendrites began growing too soon, before the slower migration could be completed. Thus, premature [11] or tardy [10] dendrite outgrowth both lead to targeting errors (Fig. 2), highlighting the importance of precise control over the timing of dendrite initiation.
Cell-cell recognition in dendrite patterning
Once growth is initiated, dendrites begin to sample their local environment. Molecular cues encountered during exploration determine which branches will survive into maturity. Key cues derive from extracellular matrix; from sister dendrites of the same cell [13,14]; from neighboring neurons and glia [15]; and from circuit partners [1,16]. Depending on the cellular and molecular context, these cues may promote either branch stabilization or retraction. In this way, they influence many anatomical parameters including dendrite shape, laminar targeting, and synaptic partner matching.
The laminar structure of the retinal IPL provides a key advantage to study this exploratory phase of dendrite development: Neurons comprising an individual circuit project to stereotyped IPL sublayers where they encounter their circuit partners (Fig. 1). By knowing where a developing neuron is ultimately going to grow dendrites, it becomes easier to identify factors that regulate arbor survival during the exploratory phase. The mouse direction-selective circuit is particularly convenient for studying this problem because reagents are available to mark and manipulate each of its cell types. These advantages have led to a series of recent insights into how cell-cell recognition establishes dendrite morphologies underlying retinal circuit function.
A key principle emerging from the direction-selective circuit work is that “pioneer dendrites” can nucleate a developing circuit by guiding projections from their later-developing circuit partners. In the direction-selective circuit (Fig. 1), the pioneers are the starburst cells: They stratify their dendrites into two IPL sublayers at early developmental stages, when their direction-selective ganglion cell (DSGC) circuit partners still send dendrites diffusely throughout the IPL [10,17,18]. Subsequently, the stratified starburst dendrites serve as a scaffold that recruits DSGC dendrite projections. How does the starburst scaffold guide DSGC dendrites? One appealing model is that branches contacting the scaffold are stabilized while those that fail to do so are eliminated. This model is consistent with the phenotype of MEGF10 mutants, in which the starburst scaffold has errors including large gaps and ectopic projections. In this setting, DSGC dendrites strictly follow starburst mistakes, suggesting that survival of DSGC arbors is dictated by starburst contact [10]. Further supporting this model, Peng et al. found a molecular mechanism that stabilizes DSGC dendrites within the ON portion of the scaffold: Bistratified ON-OFF DSGCs (Fig. 1) express a Contactin5/Caspr4 complex on their ON arbors, which binds homophillically to the same complex on the ON starburst dendrites [18]. Other molecular mediators of starburst scaffolding include type II classical cadherins, which are expressed by all members of the circuit and function to stabilize bipolar cell-starburst interactions [19,20]. Whether cadherins do the same for DSGC dendrites remains to be determined.
The scaffolding strategy ensures that dendrites have plenty of access to presynaptic partners. What happens to dendrites that cannot find enough partners? Recent studies have addressed this question using genetically targeted cell ablation strategies [21,22]. It turns out that several retinal neuron types, including color-selective horizontal cells and ON-alpha retinal ganglion cells (RGCs), can target alternate partners in an attempt to preserve circuit function. When the preferred bipolar cell partners of ON-alpha RGCs are partially removed, they make more connections with locally available, second-choice bipolar cell types [21]. In a more complete ablation setting, ON-alpha RGC dendrites connect with new ON [22] and even some OFF [21] bipolar cell types. These second-choice connections remain selective: only certain bipolar types are chosen. Second-choice partners likely have similar temporal tuning to the preferred options, because ON-alpha RGCs remain selective for low-frequency stimuli in the absence of preferred partners [22]. These results suggest that a hierarchy of partner choices is one factor contributing robustness to the developmental mechanisms that establish circuit function.
If retinal dendrites really have a hard-wired “back-up” plan in case their preferred partners are missing, this raises a new problem: What normally prevents wiring with the secondchoice partners? One possibility is that repulsion could evict exploratory dendrites from neighboring sublayers before they stabilize second-choice contacts. Whether this actually occurs in vivo is unclear: Repulsive semaphorins and plexins have important roles in setting up the overall laminar structure of the IPL, but whether their removal promotes second-choice connections is untested [23–25]. Few other repulsive molecules have been studied in the IPL. Recently, the mutually repulsive cell surface molecules FLRT2 and Unc5C were found in adjacent IPL sublayers: FLRT2 marks the direction-selective circuit (Fig. 1), while Unc5C marks surrounding strata [26]. Therefore, these molecules are positioned to prevent straying of direction-selective circuit dendrites. Whether they do so in vivo remains to be seen.
Late plasticity of dendrites
When do developing dendrites become fixed in their final form? In the outer plexiform layer (OPL) of mouse retina (Fig. 1), it turns out that signals shaping dendrites are active not only during development, but also into adulthood. This implies that adult dendrites retain intrinsic plasticity that allows them to remodel. Since OPL dendrites receive synapses from photoreceptors, the cells most often lost in retinal degenerative disease, such plasticity could have important therapeutic implications.
In the OPL, photoreceptors synapse onto bipolar and horizontal cell targets with a high degree of specificity: Rods and cones connect to different bipolar cells, and to distinct postsynaptic arbors of horizontal cells (Fig. 1). Further, there are different types of cone bipolar cells with different dendritic shapes and synaptic properties, in line with their distinct spatiotemporal response properties [27–31]. A common feature of bipolar cell dendrites is that they tile visual space – that is, they collectively fill available OPL territory with minimal overlap [27]. This structure serves as the anatomical basis for their spatial receptive fields, so one might assume that it would be static in the mature retina. Surprisingly, however, mouse rod bipolar cells expand their territories, increasing photoreceptor contacts, until about one month of age [32]; cone bipolars continue to expand until 3 months [33], well into mouse adulthood.
Unregulated, such growth would ultimately degrade spatial maps in the bipolar populations and their downstream targets, potentially also degrading visual acuity. A recent study demonstrates that DSCAM, a homophilic adhesion molecule, is part of the system limiting this growth. In Dscam mutant mice, type 4 cone bipolar cells have larger dendritic territories and larger receptive fields due to tiling defects. The phenotype arises in development but worsens into adulthood: Mutant bipolar dendrites continue expanding, exploring their environment, and establishing new cone synapses. Tiling errors can even be induced by adult Dscam gene ablation [33]. Therefore, one role of DSCAM is to suppress young adult dendritic plasticity mechanisms in mouse middle age. Other adhesion molecules perform similar functions in horizontal cell “axons” – i.e. the arbor postsynaptic to the rods (Fig. 1). When the transsynaptic adhesion protein NGL-2 is deleted from adult horizontal cells, their axons are liberated to expand [34]. Thus, manipulation of homotypic or transsynaptic cell-cell interactions can unlock intrinsic dendrite plasticity in adults.
Before this work, there were other hints that bipolar cell dendrites might be malleable into adulthood. Loss of photoreceptor synapses, or impairment of their function in aging, causes rod bipolar cell dendrites to sprout as if searching for their lost partners [35–37]. To test whether sprouting is really a search strategy, Beier et al. [38] made focal laser lesions in rabbit retina that selectively ablated photoreceptors but not bipolar cells. They then asked if deafferented bipolar cells would connect with spared photoreceptors surrounding the lesion. Rod bipolars succeeded in doing so, partially restoring visual sensitivity. By contrast, cone bipolars could not, suggesting that there is cell-type specificity to the adult dendrite plasticity mechanisms [38]. As shown by the DSCAM work, however, cone bipolar dendrites have the potential to become plastic. It will be exciting to see whether intrinsic adult plasticity can be harnessed to mediate repair of synapses lost to photoreceptor degeneration.
Compartmentalization establishes dendrite functional properties
Dendritic computations depend on compartmentalization – the precise arrangement of inputs and ion channels within the arbor [39]. But outside of certain specific cases [40], little is known about how such patterns are established during development. The radially symmetric dendritic arbor of the starburst amacrine cell has recently emerged as a useful model system to address this problem (Fig. 3). Starburst dendrites compute the direction of image motion: Moving bars traveling along the dendrite in the soma-to-tip (i.e. centrifugal) direction, but not the opposite direction, drive calcium transients in starburst presynaptic terminals [41,42] (Fig. 3). This causes the terminals to release GABA onto DSGCs, supplying the null-direction inhibition that makes DSGC responses direction-selective.
Fig. 3: Functional compartmentalization of starburst amacrine cell dendrites.
A: Starburst dendrites are radially symmetric. GABAergic presynaptic boutons (magenta) are restricted to the distal third of their dendrites. B: Detail of part of a starburst dendritic arbor. Circles illustrate restricted distribution of inhibitory (orange) and excitatory (blue) synaptic inputs. C: Individual wild-type starburst boutons have preferred directions (PDs, magenta arrows) aligned to the cell’s centrifugal axis (gray arrow). Local dendrite angle determines bouton PD, as shown by Sema6a–/– dendrites (illustrated at right). Dendrite mispatterning causes uncoupling of bouton PD and centrifugal axis (arrows, right panel).
The preference of starburst dendrites for centrifugal motion necessitates two types of dendritic compartmentalization. First, molecular specializations along each dendrite likely generate centrifugal direction selectivity. Calcium imaging of starburst presynaptic terminals demonstrated that a terminal’s preferred direction is dictated by its local dendrite angle. This is true both in normal dendrites and in Sema6a mutant dendrites, which have patterning errors that decouple local dendrite angle from the centrifugal axis [43,44] (Fig. 3). The strong influence of local dendrite structure strongly suggests that centrifugal motion preference is driven by dendrite-intrinsic properties. Several centrifugal dendritic asymmetries have recently been identified, affecting the sub-dendritic localization of particular synaptic inputs and outputs (Fig. 3) [31,41,45–47]. Little is known of how this sub-dendritic synaptic specificity arises in development; undoubtedly, this will be an area of intense interest going forward.
A second kind of compartmentalization, distinguishing starburst dendritic branches from one another, is needed to ensure that directional information is delivered to appropriate postsynaptic DSGC partners. A starburst cell has dendrites encoding many different directions (Fig. 3), whereas DSGCs prefer only a single direction. Thus, DSGCs do not sample broadly from the starburst dendrite population; instead, they receive selective input from the subset of dendrites with the complementary preferred direction. To achieve this wiring specificity, starburst dendrites must acquire distinct molecular identities matching their preferred direction. In the absence of such identity cues, DSGCs would be expected to wire with starburst dendrites encoding a broader range of directions, impairing their directional tuning. Such a phenotype was recently reported in mice lacking the starburst-specific gene Frmd7: Nasally- and temporallyaligned starburst dendrites lost their respective specificity for temporal- and nasal-preferring DSGCs, suggesting that dendritic molecular identity was compromised. How FRMD7 accomplishes this compartmentalization task remains unclear, given that 1) it is an intracellular protein of unknown function; and 2) it does not preferentially localize to any particular dendrite(s). Perhaps it is involved in trafficking of dendrite-specific molecular signatures. Its mysterious functions are likely similar in humans because FRMD7 is implicated in diseases of horizontal motion vision [48].
Conclusions and Perspective
Here we have highlighted provocative results from specific model systems that shed light on critical yet underappreciated aspects of dendrite formation. It will be important to find out which aspects of this work generalize to other cell types. We have inevitably neglected exciting work that did not fit our four topics; for example, the critical role of neural activity in elaboration and stabilization of dendrite branches [49]. Integrating activity-dependent events with the molecular events that we highlighted will be an important future direction.
HIGHLIGHTS.
A switch from radial migration to dendrite initiation is regulated by local cues
Dendrites can select backup synaptic partners when their first choice is missing
Some dendrites retain intrinsic plasticity into adulthood
Starburst amacrine cells are a useful model of dendrite compartmentalization
ACKNOWLEDGEMENTS
For financial support we thank the National Eye Institute (F32EY027998 to CLP; R01EY024694 to JNK; core facilities grant EY5722 to Duke University Eye Center), Pew Charitable Trusts, E. Matilda Ziegler Foundation, McKnight Endowment Fund for Neuroscience, Alfred P. Sloan foundation (JNK), and Research to Prevent Blindness (Unrestricted Grant to Duke University). The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cline H, Haas K: The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol 2008, 586:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoshiba Y, Toda T, Ebisu H, Wakimoto M, Yanagi S, Kawasaki H: Sox11 Balances Dendritic Morphogenesis with Neuronal Migration in the Developing Cerebral Cortex. J Neurosci 2016, 36:5775–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerrier S, Coutinho-Budd J, Sassa T, Gresset A, Jordan NV, Chen K, Jin WL, Frost A,Polleux F: The F-BAR domain of srGAP2 induces membrane protrusions required for neuronal migration and morphogenesis. Cell 2009, 138:990–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Icha J, Kunath C, Rocha-Martins M, Norden C: Independent modes of ganglion cell translocation ensure correct lamination of the zebrafish retina. J Cell Biol 2016, 215:259–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawada M, Ohno N, Kawaguchi M, Huang SH, Hikita T, Sakurai Y, Bang Nguyen H, Quynh Thai T, Ishido Y, Yoshida Y, et al. : PlexinD1 signaling controls morphological changes and migration termination in newborn neurons. EMBO J 2018, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper JA: Molecules and mechanisms that regulate multipolar migration in the intermediate zone. Front Cell Neurosci 2014, 8:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shikanai M, Nakajima K, Kawauchi T: N-cadherin regulates radial glial fiber-dependent migration of cortical locomoting neurons. Commun Integr Biol 2011, 4:326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong RO: Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development 2005, 132:5069–5079. [DOI] [PubMed] [Google Scholar]
- 9.Chow RW, Almeida AD, Randlett O, Norden C, Harris WA: Inhibitory neuron migration and IPL formation in the developing zebrafish retina. Development 2015, 142:2665–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.*.Ray TA, Roy S, Kozlowski C, Wang J, Cafaro J, Hulbert SW, Wright CVE, Field GD, Kay JN: Formation of retinal direction-selective circuitry initiated by starburst amacrine cell homotypic contact. Elife 2018, 7.The authors show that, for starburst amacrine cells, the switch from migration to dendrite initiation occurs via homotypic interactions involving MEGF10. Homotypic recognition confirms that the cells are in the right place to begin seeking circuit partners. Failure of this recognition system disrupts direction-selective circuit assembly and function.
- 11.Krol A, Henle SJ, Goodrich LV: Fat3 and Ena/VASP proteins influence the emergence of asymmetric cell morphology in the developing retina. Development 2016, 143:2172–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Dell RS, Cameron DA, Zipfel WR, Olson EC: Reelin Prevents Apical Neurite Retraction during Terminal Translocation and Dendrite Initiation. J Neurosci 2015, 35:10659–10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ing-Esteves S, Kostadinov D, Marocha J, Sing AD, Joseph KS, Laboulaye MA, Sanes JR,Lefebvre JL: Combinatorial Effects of Alpha- and Gamma-Protocadherins on Neuronal Survival and Dendritic Self-Avoidance. J Neurosci 2018, 38:2713–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Sanes JR: Cellular and Molecular Analysis of Dendritic Morphogenesis in a Retinal Cell Type That Senses Color Contrast and Ventral Motion. J Neurosci 2017, 37:12247–12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molumby MJ, Keeler AB, Weiner JA: Homophilic Protocadherin Cell-Cell Interactions Promote Dendrite Complexity. Cell Rep 2016, 15:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao YT, Zhu JX, Hanamura K, Iurilli G, Datta SR, Dalva MB: Filopodia Conduct Target Selection in Cortical Neurons Using Differences in Signal Kinetics of a Single Kinase. Neuron 2018, 98:767–782 e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stacy RC, Wong RO: Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J Comp Neurol 2003, 456:154–166. [DOI] [PubMed] [Google Scholar]
- 18.**.Peng YR, Tran NM, Krishnaswamy A, Kostadinov D, Martersteck EM, Sanes JR: Satb1 Regulates Contactin 5 to Pattern Dendrites of a Mammalian Retinal Ganglion Cell. Neuron 2017, 95:869–883 e866.This work demonstrates that the bistratification of ON-OFF DSGCs is controlled by the transcription factor Satb1 and its downstream target Contactin5. This homophilic adhesion molecule anchors the ON arbor of ON-OFF DSGCs to the ON starburst scaffold, preventing their elimination during the exploratory phase of dendrite development.
- 19.Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR: Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci 2011, 31:7753–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan X, Krishnaswamy A, De la Huerta I, Sanes JR: Type II cadherins guide assembly of a direction-selective retinal circuit. Cell 2014, 158:793–807. [DOI] [PubMed] [Google Scholar]
- 21.Okawa H, Della Santina L, Schwartz GW, Rieke F, Wong RO: Interplay of cellautonomous and nonautonomous mechanisms tailors synaptic connectivity of converging axons in vivo. Neuron 2014, 82:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.*.Tien NW, Soto F, Kerschensteiner D: Homeostatic Plasticity Shapes Cell-Type-Specific Wiring in the Retina. Neuron 2017, 94:656–665 e654.Here the authors acutely ablate the preferred presynaptic bipolar cell partners of ON-alpha RGCs and show that, in turn, the RGCs make highly selective new connections with second-choice partners. The combination of new synaptic partners effectively restores alpha cell function.
- 23.Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR,Suto F, Chedotal A, Peachey NS, et al. : Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron 2011, 71:460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL: Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature 2011, 470:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun LO, Jiang Z, Rivlin-Etzion M, Hand R, Brady CM, Matsuoka RL, Yau KW, Feller MB,Kolodkin AL: On and off retinal circuit assembly by divergent molecular mechanisms. Science 2013, 342:1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visser JJ, Cheng Y, Perry SC, Chastain AB, Parsa B, Masri SS, Ray TA, Kay JN, Wojtowicz WM: An extracellular biochemical screen reveals that FLRTs and Unc5s mediate neuronal subtype recognition in the retina. Elife 2015, 4:e08149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wassle H, Puller C, Muller F, Haverkamp S: Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci 2009, 29:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baden T, Berens P, Bethge M, Euler T: Spikes in mammalian bipolar cells support temporal layering of the inner retina. Curr Biol 2013, 23:48–52. [DOI] [PubMed] [Google Scholar]
- 29.Ichinose T, Hellmer CB: Differential signalling and glutamate receptor compositions in the OFF bipolar cell types in the mouse retina. J Physiol 2016, 594:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franke K, Berens P, Schubert T, Bethge M, Euler T, Baden T: Inhibition decorrelates visual feature representations in the inner retina. Nature 2017, 542:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fransen JW, Borghuis BG: Temporally Diverse Excitation Generates Direction-Selective Responses in ON- and OFF-Type Retinal Starburst Amacrine Cells. Cell Rep 2017, 18:1356–1365. [DOI] [PubMed] [Google Scholar]
- 32.Anastassov IA, Wang W, Dunn FA: Synaptogenesis and synaptic protein localization in the postnatal development of rod bipolar cell dendrites in mouse retina. J Comp Neurol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons AB, Bloomsburg SJ, Sukeena JM, Miller CJ, Ortega-Burgos Y, Borghuis BG,Fuerst PG: DSCAM-mediated control of dendritic and axonal arbor outgrowth enforces tiling and inhibits synaptic plasticity. Proc Natl Acad Sci U S A 2017, 114:E10224–E10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soto F, Zhao L, Kerschensteiner D: Synapse maintenance and restoration in the retina by NGL2. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuel MA, Voinescu PE, Lilley BN, de Cabo R, Foretz M, Viollet B, Pawlyk B, Sandberg MA, Vavvas DG, Sanes JR: LKB1 and AMPK regulate synaptic remodeling in old age. Nat Neurosci 2014, 17:1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haverkamp S, Michalakis S, Claes E, Seeliger MW, Humphries P, Biel M, Feigenspan A: Synaptic plasticity in CNGA3(−/−) mice: cone bipolar cells react on the missing cone input and form ectopic synapses with rods. J Neurosci 2006, 26:5248–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarin S, Zuniga-Sanchez E, Kurmangaliyev YZ, Cousins H, Patel M, Hernandez J, Zhang KX, Samuel MA, Morey M, Sanes JR, et al. : Role for Wnt Signaling in Retinal Neuropil Development: Analysis via RNA-Seq and In Vivo Somatic CRISPR Mutagenesis. Neuron 2018, 98:109–126 e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beier C, Hovhannisyan A, Weiser S, Kung J, Lee S, Lee DY, Huie P, Dalal R, Palanker D, Sher A: Deafferented Adult Rod Bipolar Cells Create New Synapses with Photoreceptors to Restore Vision. J Neurosci 2017, 37:4635–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Branco T, Hausser M: The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol 2010, 20:494–502. [DOI] [PubMed] [Google Scholar]
- 40.Kupferman JV, Basu J, Russo MJ, Guevarra J, Cheung SK, Siegelbaum SA: Reelin signaling specifies the molecular identity of the pyramidal neuron distal dendritic compartment. Cell 2014, 158:1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlasits AL, Morrie RD, Tran-Van-Minh A, Bleckert A, Gainer CF, DiGregorio DA, Feller MB: A Role for Synaptic Input Distribution in a Dendritic Computation of Motion Direction in the Retina. Neuron 2016, 89:1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Euler T, Detwiler PB, Denk W: Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 2002, 418:845–852. [DOI] [PubMed] [Google Scholar]
- 43.Poleg-Polsky A, Ding H, Diamond JS: Functional Compartmentalization within Starburst Amacrine Cell Dendrites in the Retina. Cell Rep 2018, 22:2898–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrie RD, Feller MB: A Dense Starburst Plexus Is Critical for Generating Direction Selectivity. Curr Biol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.**.Ding H, Smith RG, Poleg-Polsky A, Diamond JS, Briggman KL: Species-specific wiring for direction selectivity in the mammalian retina. Nature 2016, 535:105–110.This study used serial block-face electron microscopy to map starburst amacrine cell synaptic inputs. In mice, inhibitory synapses are located largely within the proximal onethird of the starburst arbor, whereas excitatory bipolar cell input occurs along the inner two-thirds of the arbor (see Fig. 3 for schematic). In rabbit retina, by contrast, both input types are more uniform across the arbors.
- 46.Kim JS, Greene MJ, Zlateski A, Lee K, Richardson M, Turaga SC, Purcaro M, Balkam M,Robinson A, Behabadi BF, et al. : Space-time wiring specificity supports direction selectivity in the retina. Nature 2014, 509:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greene MJ, Kim JS, Seung HS, EyeWirers: Analogous Convergence of Sustained and Transient Inputs in Parallel On and Off Pathways for Retinal Motion Computation. Cell Rep 2016, 14:1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.**.Yonehara K, Fiscella M, Drinnenberg A, Esposti F, Trenholm S, Krol J, Franke F, Scherf BG, Kusnyerik A, Muller J, et al. : Congenital Nystagmus Gene FRMD7 Is Necessary for Establishing a Neuronal Circuit Asymmetry for Direction Selectivity. Neuron 2016, 89:177–193.This study demonstrates that starburst-specific gene Frmd7 is required for correct asymmetrical wiring of mouse starbursts with horizontal motion-preferring DSGCs. Humans with FRMD7 mutations present with a horizontal motion disorder known as congenital nystagmus; thus, this work is the first example of a genetic mutation in the DS circuit that is implicated in a human disease.
- 49.Frangeul L, Kehayas V, Sanchez-Mut JV, Fievre S, Krishna KK, Pouchelon G, Telley L,Bellone C, Holtmaat A, Graff J, et al. : Input-dependent regulation of excitability controls dendritic maturation in somatosensory thalamocortical neurons. Nat Commun 2017, 8:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]