Abstract
Known giant virus diversity is currently skewed towards viruses isolated from aquatic environments and cultivated in the laboratory. Here, we employ cultivation-independent metagenomics and mini-metagenomics on soils from the Harvard Forest, leading to the discovery of 16 novel giant viruses, chiefly recovered by mini-metagenomics. The candidate viruses greatly expand phylogenetic diversity of known giant viruses and either represented novel lineages or are affiliated with klosneuviruses, Cafeteria roenbergensis virus or tupanviruses. One assembled genome with a size of 2.4 Mb represents the largest currently known viral genome in the Mimiviridae, and others encode up to 80% orphan genes. In addition, we find more than 240 major capsid proteins encoded on unbinned metagenome fragments, further indicating that giant viruses are underexplored in soil ecosystems. The fact that most of these novel viruses evaded detection in bulk metagenomes suggests that mini-metagenomics could be a valuable approach to unearth viral giants.
Current knowledge of giant virus diversity is largely based on samples from aquatic environments. Here the authors employ cultivation-independent metagenomics and mini-metagenomics on forest soil and identify 16 novel giant virus genomes, some of which representing novel lineages, including the so far largest genome in Mimiviridae.
Introduction
Viruses larger than some cellular organisms and with genomes up to several megabases in size have been discovered in diverse environments across the globe, primarily from aquatic systems, such as freshwater, seawater and wastewater1,2, but also from terrestrial environments3–5 including permafrost6,7. These viruses are nucleocytoplasmic large DNA viruses (NCDLV), and they infect a wide range of eukaryotes, in particular protists and algae8–11. Only a few protist-infecting NCDLV have been recovered with their native hosts, such as Cafeteria roenbergensis virus (CroV) in the marine flagellate Cafeteria roenbergensis12 and the Bodo saltans virus (BsV)13. Many of the NCDLV are referred to as giant viruses based on their large physical size and a genome size of at least 300 kb14, although the term has also been applied to members of the NCLDV with genomes of at least 200 kb regardless of their particle size15. Importantly, for many of these NCDLV genome size and particle diameter do no correlate8.
Most of our current understanding of giant viruses comes from isolates retrieved in co-cultivation with laboratory strains of Acanthamoeba1,3. Only recently have the genomes of giant viruses been recovered by approaches, such as bulk shotgun metagenomics16–20, flow-cytometric sorting21–23, and after successful isolation using a wider range of protist hosts23–25. Recent large-scale marker gene-based environmental surveys26–28 hinted at an immense phylogenetic breadth of giant viruses of which, however, only a small fraction has been isolated to date. Possible reasons include challenges in providing a suitable host during co-cultivation and the inability to recover the viruses together with their native hosts29. In addition, a systematic recovery of giant virus genomes from metagenomic datasets is lacking and thus, the genetic diversity of giant viruses remains underexplored.
Here we describe 16 giant virus genomes from a forest soil ecosystem that were recovered using a cultivation-independent approach. We shed light on their coding potential and expand the phylogenetic framework of the NCLDV. Importantly, the novel genomes represent only the tip of the iceberg as revealed by a survey of the major capsid protein (MCP) encoded on unbinned metagenome fragments, which indicates a much higher untapped diversity of giant virus genetic material in soil.
Results
Mini-metagenomics facilitated the discovery of giant virus genomes
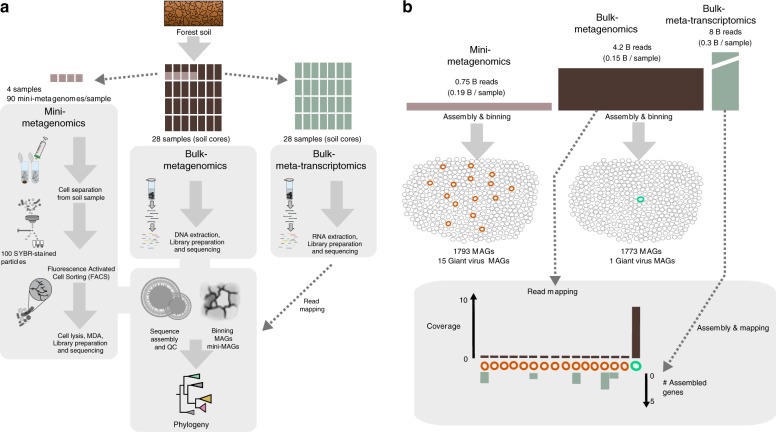
Soil samples from the Harvard Forest were subjected to standard shotgun sequencing of microbial communities. Four of the 28 samples were also analyzed using a ‘mini-metagenomics’30–32 approach, where multiple sets of 100 DNA-stained particles were flow sorted and subjected to whole genome amplification and sequencing (Fig. 1a). Metagenomic binning of assembled contigs produced 15 metagenome assembled genomes (MAGs) from the mini-metagenomes and 1 MAG from the bulk metagenomes (Supplementary Tables 1–4) that displayed features typically found in most NCLDV genomes33,34, such as hallmark genes encoding for MCP(s), factors for maturation of the viral capsid, and packaging ATPases (Supplementary Table 1, Supplementary Fig. 1). Furthermore, we observed on most contigs a uniform distribution of genes of viral, bacterial, or eukaryotic origin and many without matches in public databases (Supplementary Figs. 2, 3). In addition, these new viruses encoded numerous paralogous genes, a feature common to many NCLDV35,36 (Supplementary Fig. 2). Many of the duplicated genes were located on different contigs and often unique to the respective genomes, providing additional evidence that these contigs belong to a single viral MAG (Supplementary Fig. 1). Moreover, presence, absence, and copy number of nucleocytoplasmic virus orthologous genes (NCVOGs)34 were comparable to previously described giant viruses, suggesting that the MAGs are made up by single viral genomes and several of them being nearly complete (Supplementary Table 1, Supplementary Figs. 1, 4). An independently conducted benchmarking experiment of the mini-metagenomics approach revealed that no chimeric contigs are being created during this workflow which further supports the quality of the genomes derived here (Supplementary Figs. 5, 6).
Fig. 1.
Discovery pipeline for soil giant viruses. a Overall workflow. Fourteen forest soil cores from Barre Woods long-term experimental warming site were sub-sampled into organic horizon and mineral zone resulting in 28 total samples. Total DNA and RNA were extracted from 28 soil samples for bulk metagenomics and metatranscriptomics. Of these samples, a subset of four encompassing two organic and two mineral layers were selected for flow-sorted mini-metagenomics. Cells and presumably viral particles, were separated from soil, stained with SYBR green nucleic acid stain and sorted using fluorescence activated cell sorting (FACS). Ninety sorted pools of 100 SYBR+ particles underwent lysis, whole genome amplification, library preparation, and sequencing on the Illumina NextSeq platform. Phylogenomic analysis of metagenome assembled genomes (MAGs) facilitated the identification of novel giant viruses. There was no correlation of presence or absence of giant viruses and sample treatment (Supplementary Table 3). b Data analysis summary. Fifteen giant virus MAGs (orange circles) were recovered from sorted samples, while only one giant virus MAG (turquoise circle) was recovered from the bulk metagenomes. The other 1778 MAGs from the mini-metagenomes (gray circles) and 1772 MAGs from the bulk metagenomes (gray circles) were of bacterial or archaeal origin and not analyzed further in this study. Mapping of bulk metagenome reads to MAGs revealed ~9× coverage of the bulk-metagenome derived MAG and <1× coverage of MAGs derived from mini-metagenomes, confirming the inability to recover these novel giant virus genomes using bulk metagenomics despite deep sequencing efforts. Assembly and mapping of metatranscriptome data indicated expression of only few of the novel giant virus genes of MAGs derived from mini-metagenomes
Despite the bulk metagenome approach generating five-fold more reads, it only yielded in a single giant virus genome, whereas mini-metagenomics lead to the recovery of 15 additional bins attributable to NCLDV (Fig. 1b). Bulk metagenome reads only mapped to the MAG recovered from bulk metagenomes (at ~9× coverage) and not to any mini-metagenome MAGs, suggesting most of the discovered viruses were of low abundance in the sampled forest soil (Fig. 1b). This was also reflected in the soil metatranscriptomes in which no or only low transcriptional activity of the giant viruses could be detected (Fig. 1b, Supplementary Table 5).
Sorted viral particles expand known diversity of NCLDV
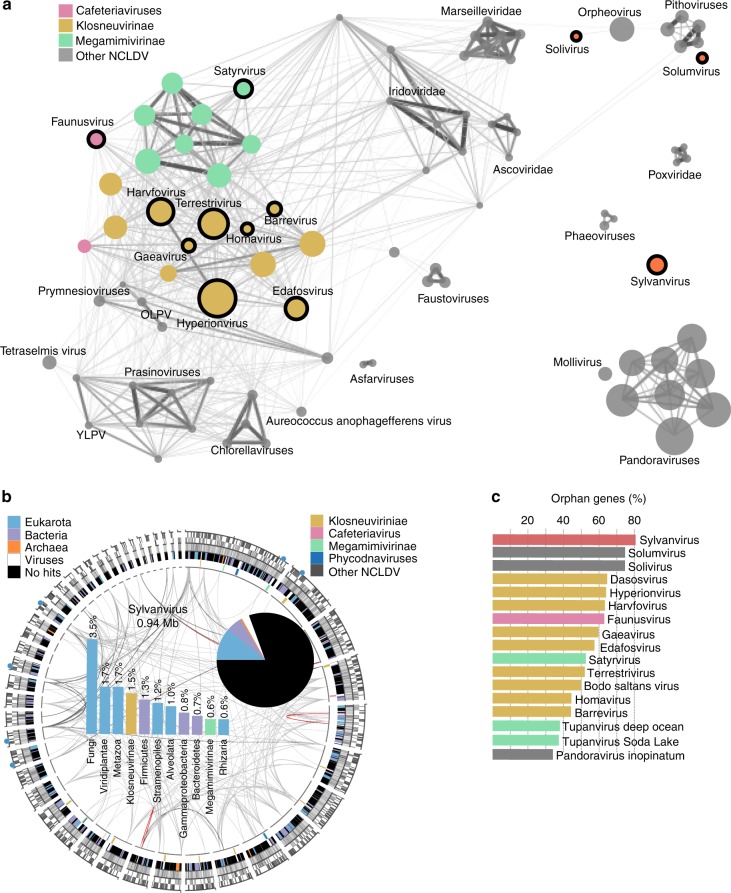
The phylogenetic relationships inferred from the tree built from a concatenated alignment of five core NCVOGs34,37 (Fig. 2a; Supplementary Fig. 1) and the consensus of single protein phylogenies (Supplementary Figs. 7, 8) showed that newly discovered viruses from forest soil were affiliated with diverse lineages in the NCLDV. Two of the new viruses, solivirus, and solumvirus, were in sister-position to the pithoviruses, cedratviruses and the recently isolated orpheovirus38. Sylvanvirus represented a long branch on its own. Most novel soil NCLDV were positioned within the family Mimiviridae, which comprises the proposed subfamilies Megamimivirinae, the Klosneuvirinae, the algae-infecting Mesomimivirinae and the genus Cafeteriavirus17,39 (Fig. 2b). One of the new viruses, faunusvirus, grouped with CroV and represents the second viral genome sampled in this clade (Fig. 2b). Another novel virus, satyrvirus, branched as sister lineage to the two recently isolated tupanviruses, which were derived from deep sea and a soda lake samples9, together forming a monophyletic clade in the Megamimivirinae (Fig. 2b). Thus, satyrvirus can be considered as a third member of the proposed genus Tupanvirus40. Notably, none of the new lineages were directly affiliated with any of the three other subgroups of well-studied Megamimivirinae41,42. Eight of the new viruses branched within the proposed Klosneuvirinae, currently the largest subfamily in the Mimiviridae based on phylogenetic diversity (PD)43 (Fig. 2c).
Fig. 2.
Expansion of NCLDV diversity by novel soil giant viruses. a Phylogenetic tree (IQ-tree LG+F+R6) of NCLDV inferred from a concatenated protein alignment of five core nucleocytoplasmic virus orthologous genes (NCVOGs)34. The tree was built from a representative set of NCDLV genomes after de-replication by ANI clustering (95% id). Novel soil NCLDV lineages and existing major NCLDV lineages grouping together with soil NCLDV are highlighted in black. The scale bar represents substitutions per site. Branch support values are shown in data S1. Branches are collapsed if support was low (<50), filled circles indicate moderate support (50–80, white) or high support (80–97, black), branches without circles are fully supported (>97). b Detailed phylogenetic tree of the Mimiviridae. Diameter of filled circles correlates with assembly size and shades of gray with GC% ranging from 20% (light gray) to 60% (dark gray). Bar plots summarize total number of encoded aminoacyl-tRNA synthetases (aaRS) and tRNAs. In addition, completeness was estimated based on number of identified marker genes out of 20 ancestral NCVOGs (more details are shown in Supplementary Fig. 1). c Increase of phylogenetic diversity (PD) after adding the soil NCLDV MAGs (black) to representative sets of NCLDV reference genomes (gray). Naming considerations are shown in Supplementary Table 2
Strikingly, the addition of the novel giant viruses to the NCLDV tree lead to a 21% increase of the total PD in the NCLDV (Fig. 2c), expanded the diversity of the Mimiviridae by 77% and nearly tripled the PD of the Klosneuvirinae (Fig. 2c). It is important to note that this expansion of PD was from a single study using cultivation-independent techniques, thereby building upon decades of previous giant virus discovery work1,8,10,41. The fact that all these newly discovered viruses represent distinct lineages in the NCLDV hints that additional sampling is expected to lead to a further substantial increase in giant virus PD.
Genomic features of soil giant viruses
The assembled viral genomes assigned to the klosneuviruses were among the largest ever found (Fig. 2b; Supplementary Fig. 1, Supplementary Table 1). With a genome size of up to 2.4 Mb the hyperionvirus would become the new record for genome size in the Mimiviridae, dwarfing klosneuvirus and tupanvirus with their ~ 1.5 Mb genomes9,17. Considering that several of the forest soil MAGs are potentially only partially complete, the true genome size of the new viruses might be even larger. Similar to recently discovered klosneuviruses and tupanviruses9,17, several of the new viruses affiliated with the Klosneuvirinae encode for expanded sets of aminoacyl tRNA synthetases (aaRS), e.g. terrestrivirus with up to 19 different aaRS and up to 50 tRNAs with specificity for all 20 different amino acids, a feature only very recently described in the tupanviruses9. In concert with other viral components of the eukaryotic translation system, such viruses likely override host protein biosynthesis using their own enzymes to ensure efficient production of viral proteins. Being less dependent on the host cell machinery might allow these viruses to infect multiple hosts, i.e. fewer proteins are necessary to target and interact with alternative hosts. A broader host range has been experimentally verified for tupanviruses9 which were able to infect different protists, however, viral titer did not increase in all the cases9.
Genome novelty of soil giant viruses
Complementary to the phylogenetic analysis (Fig. 2a), we inferred a gene sharing network to provide further insights into the relationship of the novel viral genomes to known NCLDV lineages based on shared gene content. In agreement with the species tree, viral lineages such as the Mimiviridae, the Marseilleviridae, the pithoviruses and cedratviruses, the faustoviruses and the molliviruses and pandoraviruses remained well connected (Fig. 3a). Among the novel viruses with the lowest percentage of genes shared with other NCLDV were solumvirus and solivirus, with solivirus being only connected to orpheovirus and Marseilleviridae and solumvirus to the cedratviruses. In contrast to the phylogenetic tree in which solivirus and solumvirus were affiliated to each other, there was no particular linkage between them in the network. This suggests limited taxon sampling and we expect that with discovery of additional giant virus genomes, the phylogenetic position of these viruses will be better resolved.
Fig. 3.
Genome novelty of soil giant viruses. a Nucleocytoplasmic large DNA virus (NCLDV) gene sharing network, with nodes representing genomes, node diameter correlating with genome size, edge diameter and color intensity with normalized percentage of genes in shared gene families between node pairs above a threshold of 18%. b Circular representation of the sylvanvirus genome. From outside to inside: Blue filled circles depict location of encoded tRNAs. The second ring displays positions of genes (gray) either on the minus or the plus strand. The next track illustrates GC content in shades of gray ranging from 20% (white) to 60% (dark gray). The fourth track shows color-coded origin of proteins with best blastp hits (e-value 1e−5) to cellular homologs. Best hits against viral proteins are indicated in white and if possible, further broken down based on their taxonomic origin color-coded on the most inner track. Finally, lines in the middle of the plot connect paralogs (gray) and nearly identical repeats (orange). The pi chart in the center of the plot summarizes the percentage of genes with and without cellular homologs, which are further broken down based on best blastp hits (e-value 1e−5) hits in the adjacent bar plot. c Percentage of genes in NCLDV genomes with bacterial or eukaryotic homologs and with no blastp hits (e-value 1e−5) in the NCBI nr database, highlighting the unique position of sylvanvirus
Another of the soil giant viruses denoted as sylvanvirus featured a genome completely disconnected from all other NCLDV (Fig. 3a). With a size of almost 1 Mb it represents one of the largest viral genomes outside pandoraviruses and the Mimiviridae (Fig. 3a; Supplementary Fig. 1)8,44. With the presence of 10 ancestral NCLDV genes, a number similar to several other NCLDV, the sylvanvirus genome can be considered near complete (Supplementary Fig. 1). Intriguingly, the vast majority (~80%) of its proteins had neither matches in the NCBI non-redundant (nr) database (Fig. 3b). From the proteins with database hits, 57% had matches to eukaryotes and 27% to bacteria but only 13% to other viruses (Fig. 3c). Importantly, there was no trend in taxonomic affiliation of the hits (Fig. 3c), again emphasizing the lack of any affiliation to known viruses and organisms. Among the identifiable genes were 18 potential kinases, five ubiquitin ligases, and a histone, all potentially playing important roles in interaction with a currently unknown host.
True diversity of giant viruses in forest soil
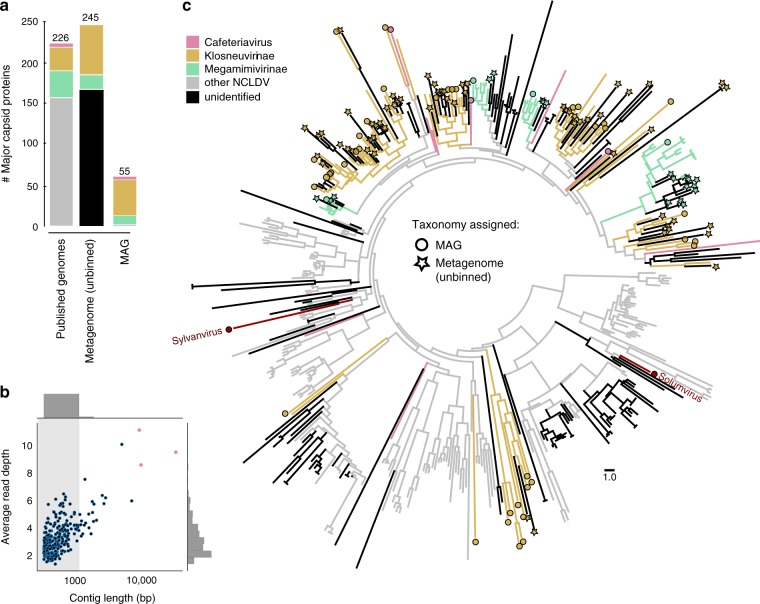
The MCPs in the bulk metagenomes revealed that the 16 novel viral genomes represent just a small fraction of giant virus diversity in the soil samples (Fig. 4a). In total, 245 different MCP genes were detected, of which 99% were part of the unbinned metagenome fraction. Most of these MCPs were located on short contigs with a read coverage of below 2, indicating an extremely low abundance of corresponding NCLDV in the respective samples (Fig. 4b). Importantly, none of the bulk-metagenome MCPs matched MCPs from the mini-metagenome-derived MAGs, further underlining the much greater diversity of giant viruses in these samples. MCPs can be heavily duplicated but usually branch together in lineage-specific clades enabling taxonomic classification based on their nearest neighbors in the tree45. Based on identified phylogenetic relationships it was possible to assign taxonomy to several of the bulk metagenome MCPs, of which most could be attributed to the klosneuviruses (Fig. 4a, c). A hint of the true dimension of the NCLDV diversity is revealed when considering that the total number of nearly 300 MCPs discovered in this study, which includes MCPs from all the MAGs, exceeds the 226 MCPs identified in previously published NCLDV genomes.
Fig. 4.
Hidden diversity of giant viruses in bulk metagenomes. a Total number of major capsid proteins (MCPs) found in reference nucleocytoplasmic large DNA virus (NCLDV) genomes, metagenome assembled genomes (MAGs), or recovered from bulk metagenomes on contigs >1 kb and contigs <1 kb (dark gray), colored by taxonomy. b Size and cover of bulk metagenome contigs containing MCP genes, either from the unbinned fraction (filled blue circles) or the MAGs (filled pink circles). c Phylogenetic tree of the MCPs of NCLDV. Branches are color-coded based on taxonomic origin of MCPs inferred by relationship in the tree to MCPs of known reference NCLDV. MCPs of novel giant viruses from this study which are not members of the Mimiviridae are indicated in red. Branches labeled with a circle represent novel MCP from MAGs generated in this study while stars indicate MCPs recovered from the unassembled fraction (contigs >1 kb) of bulk-metagenomes. Circles and stars are filled in color if taxonomy could be assigned based on the tree and in black if it was not possible to assign taxonomy
Discussion
Our results illustrate that employing cultivation independent methods on a minute sample from forest soil, a habitat in which giant viruses have rarely been found previously3,45, can lead to key discoveries. Recovery of solumvirus, solivirus, and sylvanvirus, three potentially genus, subfamily, or even family level NCLDV lineages together with 13 other novel giant virus genomes vastly expands the PD of the NCLDV and provides new insights into their genetic makeup.
The fact that only a single giant virus MAG was recovered in the bulk metagenomes suggests extremely low abundance of these viruses compared to bacterial and archaeal community members in forest soil. However, mini-metagenomics has proven most effective in recovering these viruses, yet without any detectable traces of host sequences (Supplementary Tables 6, 7). It is noteworthy that oftentimes the average read coverage of the giant virus MAGs was the highest or among the highest compared to non-viral MAGs derived from the same mini-metagenomes pool of 100 DNA-stained particles (Supplementary Fig. 9). The high coverage and completeness of giant virus genomes is consistent with having several copies of the same viral genome in the same mini-metagenome pool, but the overall low abundance of giant viruses in the system makes it unlikely that several identical viral particles were sorted by chance (Supplementary Figs. 1, 9). A plausible scenario could be that host vacuoles already filled with giant viruses may have been recovered during sorting, thereby delivering several clonal copies of a giant virus genome into a single mini-metagenome pool. This would enable genome assembly of higher quality and completeness, as previously shown for polyploid bacterial symbionts46.
Of the few available studies that have used this mini-metagenomes method, one describes the discovery of a novel intracellular bacterium30 and another a new group of giant viruses17, suggesting mini-metagenomics is a compelling method for elucidating the hidden diversity of intracellular entities such as giant viruses. As shown by the MCP diversity in the unbinned metagenome fraction many novel giant viruses are readily awaiting discovery. Importantly, the mini-metagenomics approach has not been exhaustively performed in soil or any other ecosystem and thus represents a promising addition to the toolkit for exploring the untapped diversity in the giant virus universe.
Methods
Sampling and sample preparation
Fourteen forest soil cores from the Barre Woods warming experiment located at the Harvard Forest Long-Term Ecological Research site (Petersham, MA) were collected and sub-sampled into organic horizon and mineral zone, resulting in 28 total samples. Mineral zone samples were flash-frozen while organic horizons were incubated with deuterium oxide for 2 weeks prior to freezing to label the active bacterial and archaeal communities. This incubation was carried out as part of a different experiment that will be addressed in a later manuscript. Total DNA and RNA were extracted from 28 soil samples for bulk metagenomics and metatranscriptomics using the MoBio PowerSoil DNA and RNA kits, respectively. Bacterial and Plant rRNA depletion was performed on the RNA samples prior to sequencing. Of these 28 soil samples, a subset of four encompassing two organic and two mineral layers were selected for mini-metagenomics. Cells, and presumably viral particles and/or eukaryote vacuoles containing them, were separated from soil particles using a mild detergent, followed by vortexing, centrifugation, and filtration through a 5 μm syringe filter. The filtrates were stained with SYBR Green nucleic acid stain. For each of the four samples, 90 pools containing 100 SYBR+ particles were sorted into microwell plates using fluorescence activated cell sorting (FACS). Sorted pools underwent lysis and whole genome amplification through multiple displacement amplification (MDA) following methods outlined previously47. A total of 360 sequencing libraries were generated with the Nextera XT v2 kit (Illumina) with 9 rounds of PCR amplification.
Mini-metagenomes
The 360 libraries derived from sorted particles were sequenced at the DOE Joint Genome Institute (JGI, Walnut Creek, CA) using the Illumina NextSeq platform. Pools of 90 libraries were processed in four sequencing runs that generated 2 × 150 bp read lengths. Raw Illumina reads were quality filtered to remove contamination and low-quality reads using BBTools (http://bbtools.jgi.doe.gov, version 37.38). Read normalization was performed using BBNorm (http://bbtools.jgi.doe.gov) and error correction with Tadpole (http://bbtools.jgi.doe.gov). Assembly of filtered, normalized Illumina reads was performed using SPAdes (v3.10.1)48 with the following options:–phred-offset 33 -t 16 -m 115–sc -k 25,55,95. All contig ends were then trimmed of 200 bp and contigs were discarded if the length was <2 kb or read coverage <2 using BBMap (http://bbtools.jgi.doe.gov) with the following options: nodisk ambig, filterbycoverage.sh: mincov.
Bulk metagenomes
Unamplified TruSeq libraries were prepared for the 28 DNA samples for metagenomic sequencing on the Illumina HiSeq-2000 platform at the DOE JGI. Raw Illumina reads were trimmed, quality filtered, and corrected using bfc (version r181)49 with the following options: -1 -s 10g -k 21 -t 10. Following quality filtering, reads were assembled using SPAdes (v3.11.1)48,50 with the following options:-m 2000–only-assembler -k 33,55,77,99,127–meta -t 32. The entire filtered read set was mapped to the final assembly and coverage information generated using bbmap (http://bbtools.jgi.doe.gov, version 37.62) with default parameters except ambiguous = random. The version of the processing pipeline was jgi_mga_meta_rqc.py, 2.1.0.
Metatranscriptomes
Libraries were prepared and sequenced on the Illumina NextSeq platform at the DOE JGI. Following sequencing, metatranscriptome reads were quality cleaned and a combined assembly was generated using the MEGAHIT assembler (v1.1.2)51 using the following options: -m 0.2—k-list 23,43,63,83,103,123—continue -o out.megahit—12. These cleaned reads were aligned to metagenome reference sequences using BBMap (http://bbtools.jgi.doe.gov, version 37.38) with the following options: nodisk = true interleaved = true ambiguous = random.
Metagenome binning
Contigs were organized into genome bins based on tetranucleotide sequence composition with MetaBat252. Genome bins were generated for mini-metagenomes without contig coverage patterns due to MDA bias53. Coverage was determined for the bulk metagenomes by mapping reads to the completed assemblies using the Burrows–Wheeler aligner54. Taxonomy of bins was determined with the genome taxonomy database classifier (https://github.com/Ecogenomics/GTDBTk).
Screening for giant viruses
Metagenomic bins were screened for presence of the 20 ancestral NCVOGs34 with hmmsearch (version 3.1b2, hmmer.org). Bins with more than five different hits and/or that contained the NCLDV MCP gene (NCVOG0022) were selected and further evaluated (see below).
Annotation and quality control of viral genome bins
Gene calling was performed with GeneMarkS using the virus model55. For functional annotation proteins were blasted against previously established NCVOGs34 and the NCBI non-redundant database (nr) using Diamond blastp56 with an e-value cutoff of 1.0e−5. In addition, protein domains were identified by hmmsearch (version 3.1b2, hmmer.org) against Pfam-A (version 29.0)57, and tRNAs and introns were identified using tRNAscan-SE58 and cmsearch from the Infernal package59 against the Rfam database (version 13.0)60. Nearly identical sequences within genome bins (>100 bp, identity >94%) were detected using the MUMmer repeat-match algorithm61 and visualized with Circos62 together with the respective genome bins. For all MAGs, paralogs and best diamond blastp vs. NCBI nr hits were visualized with Circos62. Furthermore, distribution of read depth across contigs was evaluated and regions with low average coverage were identified (Supplementary Table 4).
Experimental benchmarking of the mini-metagenomics approach
Benchmarking of the mini-metagenomics approach to assess potential chimera formation during MDA was performed by randomly sorting 10 cells from a bacterial mock community consisting of five different bacterial isolates; Escherichia coli K12, Echinicola vietnamensis DSM 17526, Shewanella oneidensis MR-1, Pseudomonas putida F1, and Meiothermus ruber. In total 59 of these 10-cell sorts were subject to MDA and sequencing. Resulting reads were filtered, assembled and analyzed with the same bioinformatics pipeline used for the mini-metagenomes generated in this study. Assembly statistics of recovered MAGs were generated with MetaQUAST63.
Computational benchmarking of giant virus metagenomic binning
In addition, benchmarking of the binning workflow was performed to assess its applicability to giant virus data. First, binning of a simulated mock community consisting of 12 giant viruses was tested, each a representative of a subfamily or family in the NCLDV. In addition, the herein newly discovered giant viruses were used as template for a second simulated mock community. In brief, MDA was simulated on the genomes of the mock communities with MDAsim64 (https://github.com/hzi-bifo/mdasim/releases/v2.1.1). In the following, Illumina reads were generated with ART65 and the same bioinformatics pipeline used for the mini-metagenomes in this study employed for read error-correction, normalization, assembly, and binning.
Phylogenomics
To remove redundancy, the set of 186 published NCLDV genomes and 16 novel soil giant viruses were clustered at an average nucleotide identity (ANI) of 95% with at least 100 kb-aligned fraction using fastANI66 resulting in 132 clusters and singletons. None of the newly discovered viruses clustered with any other virus. The three most incomplete novel giant virus genomes were removed from the data set (Supplementary Table 1, Supplementary Fig. 2). To infer the positions of novel soil giant viruses in the NCLDV, five core NCLDV proteins34 were selected: DNA polymerase elongation subunit family B (NCVOG0038), D5-like helicase-primase (NCVOG0023), packaging ATPase (NCVOG0249), and DNA or RNA helicases of superfamily II (NCVOG0076) and Poxvirus Late Transcription Factor VLTF3-like (NCVOG0262), and identified with hmmsearch (version 3.1b2, hmmer.org). Three of the MAGs derived from mini-metagenomes were excluded from the analysis as they had less than three conserved NCLDV proteins (Supplementary Table 1). Protein sequences were aligned using mafft67. Gapped columns in alignments (<10% sequence information) and columns with low information content were removed from the alignment with trimal68. Phylogenetic trees for each protein and for a concatenated alignment of all five proteins were constructed using IQ-tree with LG+F+R6 as suggested by model test as best-fit substitution model69. The percentage increase in PD41 was calculated based on the difference of the sum of branch lengths of phylogenetic species of the NCLDV trees with and without the metagenomic soil giant viruses.
MCP analysis
Bulk metagenome assemblies and 186 published NCLDV genomes and 16 soil MAGs were screened for presence of the NCLDV MCP gene (NCVOG0022)17,34 with hmmsearch (version 3.1b2, hmmer.org) and applying a cutoff of 1e−6. This cutoff has been evaluated against ~60,000 available bacterial, archaeal, eukaryotic, and other non-NCLDV genomes in the Integrated Microbial Genomes database70 yielding in only few false positives. Resulting protein hits were extracted from the metagenome and to reduce redundancy clustered with cd-hit at a sequence similarity of 95%71. Cluster representatives were then subject to diamond blastp56 against nr database (June 2018) and proteins which had hits but no NCLDV MCP in the top 10 were excluded from further analysis as potentially false positives. For tree construction, MCPs were extracted and aligned with mafft-ginsi (–unalignlevel 0.8,–allowshift)67. Gapped columns in the alignment (<10% sequence information) were removed with trimal68 and proteins with <50 aligned amino acids were removed. A phylogenetic tree was constructed with IQ-tree and the LG+F+R8 as suggested by model test as the best-fit substitution model69.
Gene sharing network
Protein families were inferred with OrthoFinder 1.0372 on a representative dataset of 93 NCLDV genomes for comparative analysis (after de-replication using 95% ANI clustering66, details described above, and removal of 36 poxviruses). For each pair of NCLDV genomes (ANI 95% cluster representatives) the average percentage of proteins in shared orthogroups in relation to the total number of proteins in the respective genome was calculated and used as edge weight in the network.The network was created in Gephi73 using a force layout and filtered at an edge weight of 18%.
Electronic supplementary material
Acknowledgements
The work conducted by the U.S. Department of Energy Joint Genome Institute, a DOE Office of Science User Facility, is supported under Contract no. DE-AC02-05CH11231. The Harvard Forest experimental warming plots are supported by NSF grants DEB 1237491 (Long-Term Ecological Research) and DEB 1456528 (Long-Term Research in Environmental Biology). Lauren Alteio was supported by a DOE Office of Science Graduate Student Research Program award. We thank William Warner and Michael Bernard for assistance with sample collection and processing. We would like to thank Simon Roux, David Paez, and Natalia Ivanova for helpful discussions.
Author contributions
L.A. collected and processed soil samples. D.G. and E.M.R. conducted cell sorting, whole genome amplification, and library creation. F.B.Y. prepared performed experimental work for benchmarking of the mini-metagenomics approach. The JGI production team performed sequencing and processed resulting sequence data and metagenomic binning. F.S. performed benchmarking of mini-metagenomics data, screening, and identification of the viral bins, analyzed the genome data and prepared phylogenetic trees. L.A. and F.S. visualized the data. J.B., R.R.M., and T.W. supervised research. F.S. and T.W. prepared the manuscript, with contributions from all authors. All authors read and approved the final manuscript.
Data availability
The giant virus genomes were deposited at NCBI Genbank (MK071979–MK072551) and at https://bitbucket.org/berkeleylab/forestsoil-gv, together with sequence alignments and phylogenetic trees underlying this study. Metagenomes and corresponding metadata are available at https://img.jgi.doe.gov/m, accession numbers indicated in Supplementary Table 3.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Frederik Schulz, Lauren Alteio.
Contributor Information
Frederik Schulz, Email: fschulz@lbl.gov.
Jeffrey Blanchard, Email: jeffb@bio.umass.edu.
Tanja Woyke, Email: twoyke@lbl.gov.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-07335-2.
References
- 1.Aherfi S, Colson P, La Scola B, Raoult D. Giant viruses of amoebas: an update. Front. Microbiol. 2016;7:349. doi: 10.3389/fmicb.2016.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade ACDSP, et al. Ubiquitous giants: a plethora of giant viruses found in Brazil and Antarctica. Virol. J. 2018;15:22. doi: 10.1186/s12985-018-0930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagnier I, et al. A decade of improvements in Mimiviridae and Marseilleviridae isolation from amoeba. Intervirology. 2013;56:354–363. doi: 10.1159/000354556. [DOI] [PubMed] [Google Scholar]
- 4.Yoosuf N, et al. Draft genome sequences of Terra1 and Terra2 viruses, new members of the family Mimiviridae isolated from soil. Virology. 2014;452-453:125–132. doi: 10.1016/j.virol.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Boughalmi M, et al. High-throughput isolation of giant viruses of the Mimiviridae and Marseilleviridae families in the Tunisian environment. Environ. Microbiol. 2013;15:2000–2007. doi: 10.1111/1462-2920.12068. [DOI] [PubMed] [Google Scholar]
- 6.Legendre M, et al. In-depth study of Mollivirus sibericum, a new 30,000-y-old giant virus infecting Acanthamoeba. Proc. Natl Acad. Sci. USA. 2015;112:E5327–E5335. doi: 10.1073/pnas.1510795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legendre M, et al. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proc. Natl Acad. Sci. USA. 2014;111:4274–4279. doi: 10.1073/pnas.1320670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abergel C, Legendre M, Claverie JM. The rapidly expanding universe of giant viruses: Mimivirus, Pandoravirus, Pithovirus and Mollivirus. FEMS Microbiol. Rev. 2015;39:779–796. doi: 10.1093/femsre/fuv037. [DOI] [PubMed] [Google Scholar]
- 9.Abrahão J, et al. Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere. Nat. Commun. 2018;9:749. doi: 10.1038/s41467-018-03168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer MG. Giant viruses come of age. Curr. Opin. Microbiol. 2016;31:50–57. doi: 10.1016/j.mib.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Wilson WH, Van Etten JL, Allen MJ. The Phycodnaviridae: the story of how tiny giants rule the world. Curr. Top. Microbiol. Immunol. 2009;328:1–42. doi: 10.1007/978-3-540-68618-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer MG, Allen MJ, Wilson WH, Suttle CA. Giant virus with a remarkable complement of genes infects marine zooplankton. Proc. Natl Acad. Sci. USA. 2010;107:19508–19513. doi: 10.1073/pnas.1007615107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeg, C. M., Chow, C.-E. T. & Suttle, C. A. The kinetoplastid-infecting Bodo saltans virus (BsV), a window into the most abundant giant viruses in the sea. eLife7, e33014 (2018). [DOI] [PMC free article] [PubMed]
- 14.Claverie JM, Abergel C. Giant viruses: the difficult breaking of multiple epistemological barriers. Stud. Hist. Philos. Biol. Biomed. Sci. 2016;59:89–99. doi: 10.1016/j.shpsc.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm Steven, Bird Jordan, Bonifer Kyle, Calfee Benjamin, Chen Tian, Coy Samantha, Gainer P., Gann Eric, Heatherly Huston, Lee Jasper, Liang Xiaolong, Liu Jiang, Armes April, Moniruzzaman Mohammad, Rice J., Stough Joshua, Tams Robert, Williams Evan, LeCleir Gary. A Student’s Guide to Giant Viruses Infecting Small Eukaryotes: From Acanthamoeba to Zooxanthellae. Viruses. 2017;9(3):46. doi: 10.3390/v9030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verneau J, Levasseur A, Raoult D, La Scola B, Colson P. MG-Digger: an automated pipeline to search for giant virus-related sequences in metagenomes. Front. Microbiol. 2016;7:428. doi: 10.3389/fmicb.2016.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz F, et al. Giant viruses with an expanded complement of translation system components. Science. 2017;356:82–85. doi: 10.1126/science.aal4657. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, et al. Four novel algal virus genomes discovered from Yellowstone Lake metagenomes. Sci. Rep. 2015;5:15131. doi: 10.1038/srep15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreani J, Verneau J, Raoult D, Levasseur A, La Scola B. Deciphering viral presences: two novel partial giant viruses detected in marine metagenome and in a mine drainage metagenome. Virol. J. 2018;15:66. doi: 10.1186/s12985-018-0976-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roux S, et al. Ecogenomics of virophages and their giant virus hosts assessed through time series metagenomics. Nat. Commun. 2017;8:858. doi: 10.1038/s41467-017-01086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson WH, et al. Genomic exploration of individual giant ocean viruses. ISME J. 2017;11:1736–1745. doi: 10.1038/ismej.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez Martínez J, Swan BK, Wilson WH. Marine viruses, a genetic reservoir revealed by targeted viromics. ISME J. 2014;8:1079–1088. doi: 10.1038/ismej.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil JYB, et al. High-throughput isolation of giant viruses in liquid medium using automated flow cytometry and fluorescence staining. Front. Microbiol. 2016;7:26. doi: 10.3389/fmicb.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajrai LH, et al. Kaumoebavirus, a new virus that clusters with faustoviruses and Asfarviridae. Viruses. 2016;8:278. doi: 10.3390/v8110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reteno DG, et al. Faustovirus, an asfarvirus-related new lineage of giant viruses infecting amoebae. J. Virol. 2015;89:6585–6594. doi: 10.1128/JVI.00115-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mihara Tomoko, Koyano Hitoshi, Hingamp Pascal, Grimsley Nigel, Goto Susumu, Ogata Hiroyuki. Taxon Richness of “Megaviridae” Exceeds those of Bacteria and Archaea in the Ocean. Microbes and Environments. 2018;33(2):162–171. doi: 10.1264/jsme2.ME17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colson P, Aherfi S, La Scola B. Evidence of giant viruses of amoebae in the human gut. Hum. Microbiome J. 2017;5-6:14–19. doi: 10.1016/j.humic.2017.11.001. [DOI] [Google Scholar]
- 28.Hingamp P, et al. Exploring nucleo-cytoplasmic large DNA viruses in Tara Oceans microbial metagenomes. ISME J. 2013;7:1678–1695. doi: 10.1038/ismej.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halary S, Temmam S, Raoult D, Desnues C. Viral metagenomics: are we missing the giants? Curr. Opin. Microbiol. 2016;31:34–43. doi: 10.1016/j.mib.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 30.McLean JS, et al. Candidate phylum TM6 genome recovered from a hospital sink biofilm provides genomic insights into this uncultivated phylum. Proc. Natl Acad. Sci. USA. 2013;110:E2390–E2399. doi: 10.1073/pnas.1219809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu, F. B. et al. Microfluidic-based mini-metagenomics enables discovery of novel microbial lineages from complex environmental samples. eLife6, e26580 (2017). [DOI] [PMC free article] [PubMed]
- 32.Berghuis, B. A. et al. Hydrogenotrophic methanogenesis in archaeal phylum Verstraetearchaeota reveals the shared ancestry of all methanogens. Preprint at 10.1101/391417 (2018). [DOI] [PMC free article] [PubMed]
- 33.Iyer LM, Aravind L, Koonin EV. Common origin of four diverse families of large eukaryotic DNA viruses. J. Virol. 2001;75:11720–11734. doi: 10.1128/JVI.75.23.11720-11734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yutin N, Wolf YI, Raoult D, Koonin EV. Eukaryotic large nucleo-cytoplasmic DNA viruses: clusters of orthologous genes and reconstruction of viral genome evolution. Virol. J. 2009;6:223. doi: 10.1186/1743-422X-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filée J. Route of NCLDV evolution: the genomic accordion. Curr. Opin. Virol. 2013;3:595–599. doi: 10.1016/j.coviro.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Suhre K. Gene and genome duplication in Acanthamoeba polyphaga Mimivirus. J. Virol. 2005;79:14095–14101. doi: 10.1128/JVI.79.22.14095-14101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yutin N, Wolf YI, Koonin EV. Origin of giant viruses from smaller DNA viruses not from a fourth domain of cellular life. Virology. 2014;466-467:38–52. doi: 10.1016/j.virol.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreani J, et al. Orpheovirus IHUMI-LCC2: a new virus among the iant viruses. Front. Microbiol. 2017;8:2643. doi: 10.3389/fmicb.2017.02643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallot-Lavallée, L., Blanc, G. & Claverie, J.-M. Comparative genomics of Chrysochromulina ericinavirus and other microalga-infecting large DNA viruses highlights their intricate evolutionary relationship with the established Mimiviridae family. J. Virol. 91, e00230-17 (2017). [DOI] [PMC free article] [PubMed]
- 40.Rodrigues, R. A. L., Mougari, S., Colson, P., La Scola, B. & Abrahão, J. S. ‘Tupanvirus’, a new genus in the family Mimiviridae. Arch. Virol. doi: 10.1007/s00705-018-4067-4 (2018). [DOI] [PubMed]
- 41.Colson P, La Scola B, Levasseur A, Caetano-Anollés G, Raoult D. Mimivirus: leading the way in the discovery of giant viruses of amoebae. Nat. Rev. Microbiol. 2017;15:243–254. doi: 10.1038/nrmicro.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Scola B, et al. A giant virus in amoebae. Science. 2003;299:2033. doi: 10.1126/science.1081867. [DOI] [PubMed] [Google Scholar]
- 43.Wu D, et al. A phylogeny-driven genomic encyclopaedia of Bacteria and Archaea. Nature. 2009;462:1056–1060. doi: 10.1038/nature08656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Legendre M, et al. Diversity and evolution of the emerging Pandoraviridae family. Nat. Commun. 2018;9:2285. doi: 10.1038/s41467-018-04698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilhelm SW, Coy SR, Gann ER, Moniruzzaman M, Stough JMA. Standing on the shoulders of giant viruses: five lessons learned about large viruses infecting small eukaryotes and the opportunities they create. PLoS Pathog. 2016;12:e1005752. doi: 10.1371/journal.ppat.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woyke T, et al. One bacterial cell, one complete genome. PLoS One. 2010;5:e10314. doi: 10.1371/journal.pone.0010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinke C, et al. Obtaining genomes from uncultivated environmental microorganisms using FACS-based single-cell genomics. Nat. Protoc. 2014;9:1038–1048. doi: 10.1038/nprot.2014.067. [DOI] [PubMed] [Google Scholar]
- 48.Bankevich A, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H. BFC: correcting Illumina sequencing errors. Bioinformatics. 2015;31:2885–2887. doi: 10.1093/bioinformatics/btv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nurk S, Meleshko D, Korobeynikov A, Pevzner P. A. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 2017;27:824–834. doi: 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li D, et al. MEGAHIT v1.0: a fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods. 2016;102:3–11. doi: 10.1016/j.ymeth.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 52.Kang DD, Froula J, Egan R, Wang Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ. 2015;3:e1165. doi: 10.7717/peerj.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woyke T, et al. Assembling the marine metagenome, one cell at a time. PLoS One. 2009;4:e5299. doi: 10.1371/journal.pone.0005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borodovsky, M. & Lomsadze, A. Gene identification in prokaryotic genomes, phages, metagenomes, and EST sequences with GeneMarkS suite. Curr. Protoc. Bioinformatics 27, 3911 (2002). [DOI] [PubMed]
- 56.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 57.Finn RD, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowe TM, Chan PP. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016;44:W54–W57. doi: 10.1093/nar/gkw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nawrocki EP, Eddy SR. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics. 2013;29:2933–2935. doi: 10.1093/bioinformatics/btt509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalvari I, et al. Rfam 13.0: shifting to a genome-centric resource for non-coding RNA families. Nucleic Acids Res. 2018;46:D335–D342. doi: 10.1093/nar/gkx1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delcher AL, Salzberg SL, Phillippy AM. Using MUMmer to identify similar regions in large sequence sets. Curr. Protoc. Bioinforma. 2003;Chapter 10:Unit 10.3. doi: 10.1002/0471250953.bi1003s00. [DOI] [PubMed] [Google Scholar]
- 62.Krzywinski M, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mikheenko A, Saveliev V, Gurevich A. MetaQUAST: evaluation of metagenome assemblies. Bioinformatics. 2016;32:1088–1090. doi: 10.1093/bioinformatics/btv697. [DOI] [PubMed] [Google Scholar]
- 64.Tagliavi, Z. & Draghici, S. MDAsim: a multiple displacement amplification simulator. In 2012 IEEE International Conference on Bioinformatics and Biomedicine (BIBM) 1–4 (IEEE, 2012).
- 65.Huang W, Li L, Myers JR, Marth GT. ART: a next-generation sequencing read simulator. Bioinformatics. 2012;28:593–594. doi: 10.1093/bioinformatics/btr708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jain, C., Rodriguez-R. L. M., Phillippy, A. M., Konstantinidis, K. T. & Aluru, S. High-throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Preprint at https://www.biorxiv.org/content/early/2017/11/27/225342 (2017). [DOI] [PMC free article] [PubMed]
- 67.Katoh K, Standley DM. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics. 2016;32:1933–1942. doi: 10.1093/bioinformatics/btw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen IMA, et al. IMG/M: integrated genome and metagenome comparative data analysis system. Nucleic Acids Res. 2017;45:D507–D516. doi: 10.1093/nar/gkw929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 72.Emms DM, Kelly S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015;16:157. doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bastian M, Heymann S, Jacomy M, et al. Gephi: an open source software for exploring and manipulating networks. Icwsm. 2009;8:361–362. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The giant virus genomes were deposited at NCBI Genbank (MK071979–MK072551) and at https://bitbucket.org/berkeleylab/forestsoil-gv, together with sequence alignments and phylogenetic trees underlying this study. Metagenomes and corresponding metadata are available at https://img.jgi.doe.gov/m, accession numbers indicated in Supplementary Table 3.