Abstract
The use of personal continuous glucose monitoring (CGM) has expanded dramatically among individuals with diabetes. CGM systems provide retrospective data, as well as the current glucose value and trend arrow data, which indicate the direction and velocity of changing glucose. In 2017, Aleppo and colleagues developed a simplified approach for adults with diabetes to safely adjust rapid-acting insulin doses using trend arrow information in the Dexcom G5 CGM system. Since then, the FreeStyle Libre and FreeStyle Libre 14-day CGM systems have become available in the United States; however, guidance on using trend arrow data that take the unique features of these systems into consideration is lacking. Specifically, the FreeStyle Libre systems do not have automatic alarms, which impact how the system and trend arrow data are used. The Endocrine Society convened an expert panel to address this gap and develop an approach to adjusting rapid-acting insulin doses for adults using trend arrows in the FreeStyle Libre systems. We based our approach on previous work and expanded upon engagement and scanning recommendations, and we incorporated pre-exercise planning specific to these systems. Our approach provides insulin dose adjustments as discrete insulin units based on an individual’s insulin sensitivity and directionality of the trend arrow. We focus on the needs of patients treated with multiple daily injections because these individuals currently make up a greater proportion of individuals on intensive insulin therapy. Our recommendations are intended to provide a safe, practical approach to using trend arrows in the FreeStyle Libre systems.
Keywords: continuous glucose monitoring, flash glucose monitoring, trend arrows, scanning, diabetes, diabetes technology
Use of personal continuous glucose monitoring (CGM) has expanded dramatically over the past decade and is recommended as the gold standard of care for individuals with diabetes treated with intensive insulin therapy [1–3].
CGM provides information that can be critical for safe and effective diabetes management. Unlike fingerstick monitoring, which measures glucose at a single point in time, CGM displays the immediate glucose value within the context of both prior glucose data and, importantly, the direction and velocity of changing glucose using trend arrows. Real-time information allows patients to react immediately to mitigate or prevent acute glycemic events. Retrospective review of CGM data in combination with standardized data management tools such as the ambulatory glucose profile [4, 5] help clinicians and patients fine-tune management strategies.
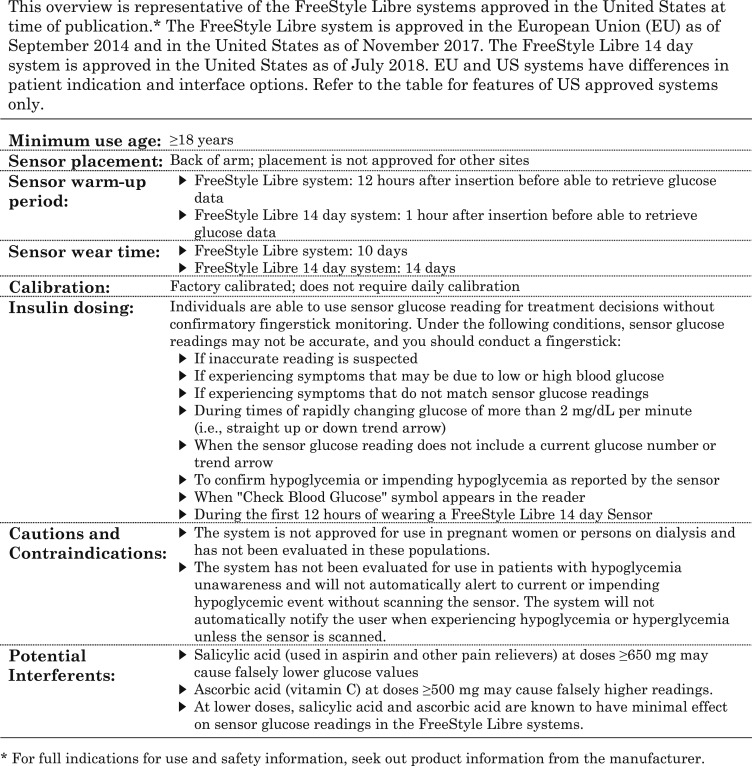
One of the newest CGM systems available in the United States is the FreeStyle Libre flash glucose monitoring system (FreeStyle Libre system; Abbott Diabetes Care, Alameda, CA), often referred to as flash CGM or intermittently scanned CGM. The system received US Food and Drug Administration clearance in September 2017 for nonadjunctive use in adults (age 18 years and older) with diabetes. The FreeStyle Libre 14-day flash glucose monitoring system (FreeStyle Libre 14-day system; Abbott Diabetes Care) was more recently approved by the US Food and Drug Administration with the same indications but with a longer wear time and shorter warm-up period that is comparable to systems available in Europe (Table 1). The nonadjunctive indication allows patients to calculate insulin doses based on current sensor value using individual insulin dosing parameters, target glucose concentration, and trend arrow information.
Table 1.
Overview of Features in the FreeStyle Libre Systems Approved in the United States
|
Large randomized controlled trials demonstrated that use of the FreeStyle Libre system resulted in significant reductions in hypoglycemia, increased time in target range, reduced glycemic variability, and greater patient satisfaction compared with fingerstick monitoring [6, 7]. These benefits were seen in well-controlled type 1 diabetes (T1D) [6] as well as suboptimally controlled type 2 diabetes (T2D) treated with intensive insulin therapy [7]. Studies also showed high device utilization, suggesting that the FreeStyle Libre system may enhance patient engagement [6, 7]. Smaller observational and prospective studies have also shown improvements in both A1c and a reduction in hypoglycemic events [8–10].
Although research provides compelling evidence of the benefits of the FreeStyle Libre system, there is sparse guidance for what actions individuals should take based on the trend arrow data. The purpose of this article is to provide safe, practical guidance on the use of trend arrow data in the FreeStyle Libre and FreeStyle Libre 14-day systems—collectively called the FreeStyle Libre systems unless denoted otherwise—in the United States for clinicians and their patients on intensive, rapid-acting insulin therapy. Current sensor warm-up and wear time in US-approved systems has been an iterative process that has resulted in closer alignment between systems approved in the United States and beyond. Our hope is that this better alignment of systems will improve access and utility, but we note differences outside the United States in patient indications (systems are available to children as young as 4 years of age and to women during pregnancy) as well as interface options (systems may use the mobile LibreLink application on a compatible smart device to scan the sensor). We focus on the US-approved systems, recognizing the differences in patient indications and interface options in the FreeStyle Libre systems available outside the United States.
Our approach is based on the recently published guidance by Aleppo et al. [11], which focused on a different CGM system (Dexcom, San Diego, CA). We have modified our approach to account for these differences. Specifically, we address that the FreeStyle Libre systems do not have automatic alarms or double up and double down trend arrows, which are present in the Dexcom systems. We developed our approach to be applicable to any patient with diabetes treated by intensive insulin therapy who is using continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDIs). However, we focus on the needs of patients treated with MDIs because these individuals currently make up a greater proportion of T1D and T2D populations on intensive insulin therapy. Additionally, our approach is based on the anticipated changes in individuals using rapid-acting insulin analogs for prandial and correction insulin doses. Our approach is not intended for individuals using more recently available ultra–rapid-acting insulins, which have reduced insulin action times and may affect rate of glucose change differently. We also incorporate our clinical experiences, our personal experiences as people living with diabetes and using CGM, and guidance from other diabetes specialists.
1. FreeStyle Libre Flash Glucose Monitoring System
A. System Overview
The FreeStyle Libre systems use two components: a disposable sensor that is inserted into the user’s upper arm and a separate handheld touchscreen reader device used to scan and retrieve CGM glucose readings. Following sensor insertion, the FreeStyle Libre 10-day system has a 12-hour warm-up period until the reader is able to retrieve sensor glucose readings. The system is factory calibrated, which eliminates the need for daily calibration during the 10-day wear time (Table 1). Similarly, the FreeStyle Libre 14-day system is factory calibrated, but it has a 1-hour warm-up period and 14-day wear time.
When the reader is swiped close to the sensor in either system, the sensor glucose data are transmitted to the reader. The reader displays the current glucose concentration and the most recent 8 hours of sensor glucose readings, as well as trend arrow data when present. When >8 hours occur between scans, only the last 8 hours of data are reported. Importantly, the system lacks automatic alarms. Therefore, patients must be actively engaged in scanning because they will not receive automatic alarms in the event of hypoglycemia or hyperglycemia, as is the case with other CGM systems.
B. Trend Arrows
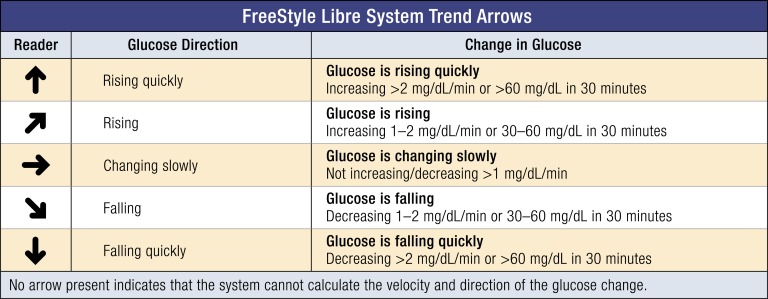
The system measures glucose concentrations every minute and, when scanned, transmits the current glucose reading and historical glucose readings in 15-minute increments to the reader. The trend arrows are calculated from glucose readings with an emphasis on the most recent 15 minutes. The directionality of trend arrows allows individuals to anticipate future glucose concentrations. This additional information can be used proactively to adjust therapy and prevent hypoglycemia or hyperglycemia. For individuals on intensive insulin therapy, upward trend arrows indicate rising glucose concentrations and may suggest a need for additional rapid-acting insulin. Downward trend arrows indicate falling glucose concentrations and may suggest a need for less rapid-acting insulin or carbohydrate ingestion to avoid hypoglycemia. Flat arrows indicate that glucose concentrations are changing very slowly and that therapy adjustments are probably not needed if in the target range. Figure 1 provides an example of how these trend arrows appear in the FreeStyle Libre system and the anticipated glucose change they represent.
Figure 1.
Trend arrows in the FreeStyle Libre systems. The FreeStyle Libre systems present trend arrow data as icons on the reader. Trend arrows indicate rates of glucose change (mg/dL per min) and can be described as the anticipated glucose change. Notably, the flat arrow does not indicate no change in sensor glucose readings. The flat arrow indicates steady glucose values with a change of <1 mg/dL per min. For individuals using the FreeStyle Libre systems, more frequent scanning may be warranted to monitor for hypoglycemia when a flat arrow is present and sensor glucose is near the low end of the target range. In general, anticipated glucose may be less accurate when trying to predict changes over extended periods of time (e.g., beyond 20 to 30 min) due to the many factors that may influence glucose concentrations. Conversion: mg/dL × 0.0555 = mmol/L.
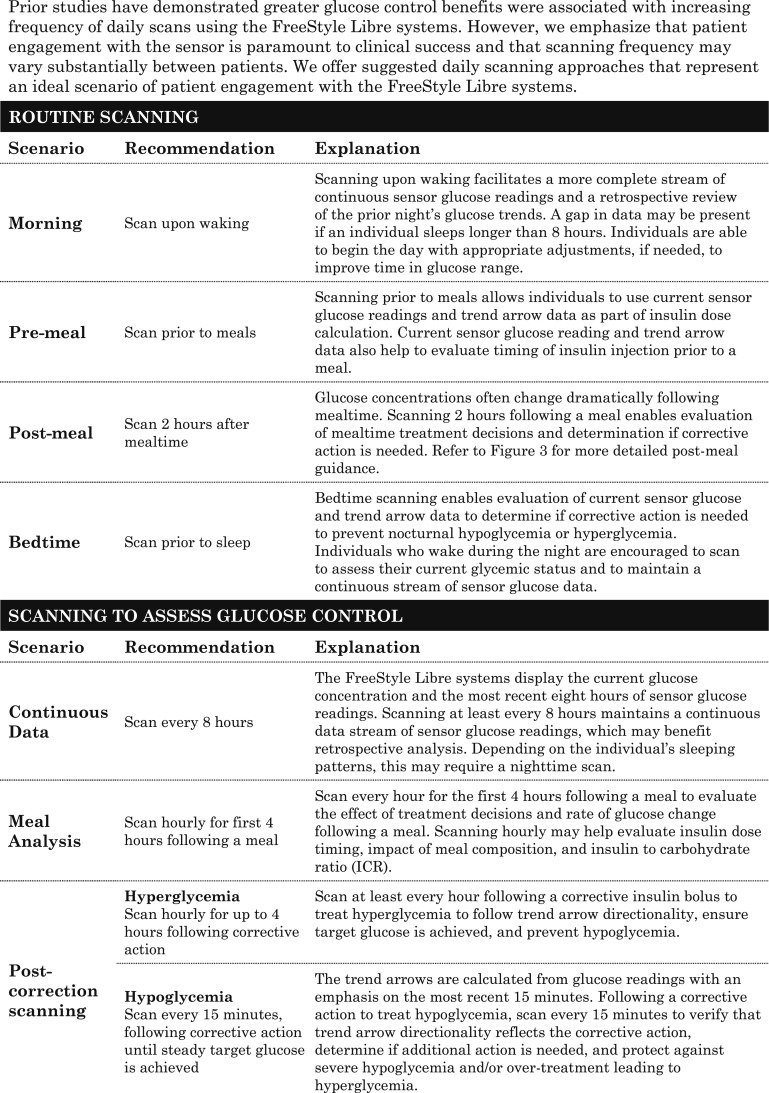
C. Scanning Frequency
Although, there are no “hard and fast” rules for scanning frequency, large clinical studies have shown that the glycemic benefits of the FreeStyle Libre system use were achieved at mean scanning frequencies ranging from 8 times per day in patients with T2D [7] to 15 times per day in patients with T1D [6]. However, scanning at any frequency has been associated with clinical benefit, and an analysis of real-world data from 50,831 users revealed a mean of 16.3 scans per day, suggesting that this scanning frequency is reasonable among most users [12]. The recommendations for scanning frequency presented in Table 2 are based on our clinical judgment and personal experiences using the FreeStyle Libre system in a clinical setting.
Table 2.
Scanning Recommendations for Optimal Diabetes Management Using the FreeStyle Libre Systems
|

|
2. Approach to Using Trend Arrows in the FreeStyle Libre Systems to Adjust Insulin Doses
In 2017, Aleppo et al. [11] published recommendations for using trend arrows with the Dexcom G5 CGM system in adults. Those recommendations were based on review of four previously published methods to adjust insulin doses using trend arrows: the DirecNet Applied Treatment Algorithm [13], the Scheiner method [14], Pettus and Edelman method [15], and the Klonoff and Kerr equation [16]. We have modified those recommendations to accommodate the features and functionalities of the FreeStyle Libre systems.
Our approach may be applied to any patient with diabetes treated by intensive insulin therapy. Our recommendations are applicable to both CSII and MDI therapy; however, we focus mainly on trend arrow use with patients treated with MDIs, who comprise more than half of the T1D population [17] and a growing number of patients with T2D [18].
Recognizing that many patients treated with MDIs may be unfamiliar with CGM use, we have endeavored to make our method as simple and intuitive as possible. Because adjusting insulin dose using trend arrows adds a layer of complexity, we recommend patients wait until they are comfortable with the general application of CGM data and learn how their body responds to various meals (quantity/composition) and physical activity before adjusting insulin dose using trend arrows. Safe and effective use of the FreeStyle Libre systems requires that patients have a basic understanding of how to use their current glucose value, target glucose value, food intake (if any), and insulin dosing parameters to calculate their insulin doses. Importantly, the approach relies on the accurate determination of insulin-to-carbohydrate ratio and correction factors as insulin dose parameters.
A. Mealtime Insulin Calculation
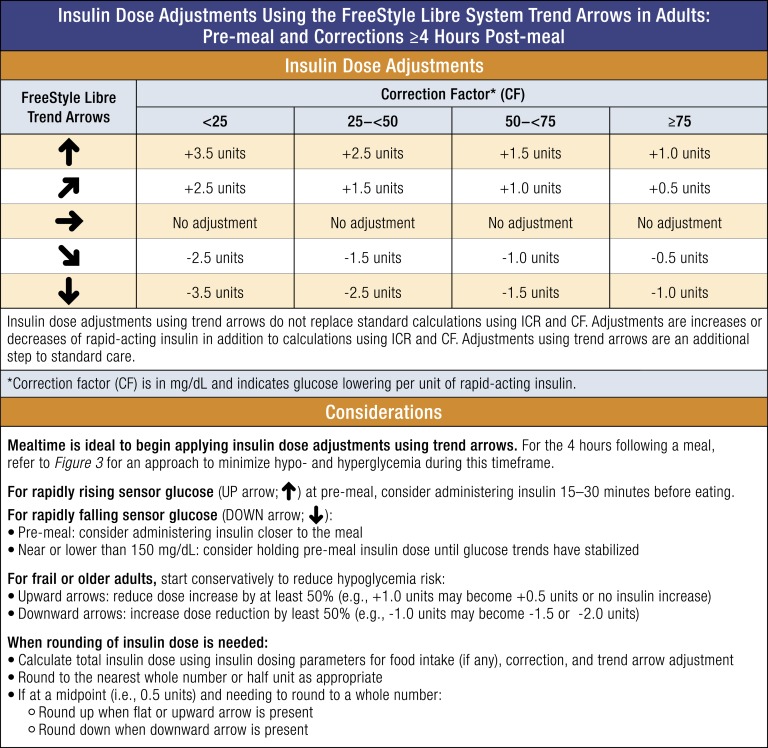
Figure 2 outlines our method for adjusting insulin doses at meal time and at least 4 hours after a meal, using trend arrows. The method utilizes a simple approach to insulin dose calculation: Total premeal insulin dose = food ± correction ± arrow adjustment. Patients would calculate their rapid-acting insulin dose for food and correction, and then add or subtract insulin based on the trend arrow.
Figure 2.
Insulin dose adjustments for adults using trend arrows in the FreeStyle Libre systems. Our recommended approach to adjusting insulin dose using trend arrow data in the FreeStyle Libre systems assumes that the patient has insulin-requiring diabetes, is using rapid-acting insulin for meals and correction, and is using insulin-to-carbohydrate ratio (ICR) and correction factors (CFs) that have been optimized as much as possible. The approach is based on anticipated glucose change and typical insulin sensitivity ranges in adults. The approach utilizes a simple approach to insulin dose calculation: Total insulin dose = food ± correction ± arrow adjustment. It provides adjustments in terms of insulin units over the range of insulin sensitivities to minimize additional calculations. It is generally recommended to start adjusting conservatively and at mealtime to understand how the recommendations impact individual glucose responses. Adjusting the insulin dose using trend arrows does not replace but, rather, adds to standard calculations using ICR and CFs. Importantly, a single arrow up may require additional corrections due to unknown velocity of glucose increase (e.g., >2 mg/dL). The CF (in mg/dL) indicates glucose lowering per unit of rapid-acting insulin. Conversion: mg/dL × 0.0555 = mmol/L.
The insulin dose adjustment recommendations are based on typical insulin sensitivity ranges for adult patients as determined by Aleppo et al. [11]. Similarly, we offer an insulin dose adjustment in insulin units for each insulin sensitivity range. In this manner, insulin adjustments can be simply added to or subtracted from standard calculations. The adjustments also take into consideration the limitations of 0.5-U increment minimums for individuals treated with MDI with significant insulin sensitivity. Although 0.5-U increment insulin pens are generally reserved for use in highly insulin-sensitive patients, it is not uncommon for adults with T1D to also be insulin sensitive, and use of 0.5-U increment insulin pens can be a practical tool for diabetes management [19]. Alternatively, adult patients using 1.0-U increments can round the total insulin dose, including food, correction, and adjustment, to the closest full unit.
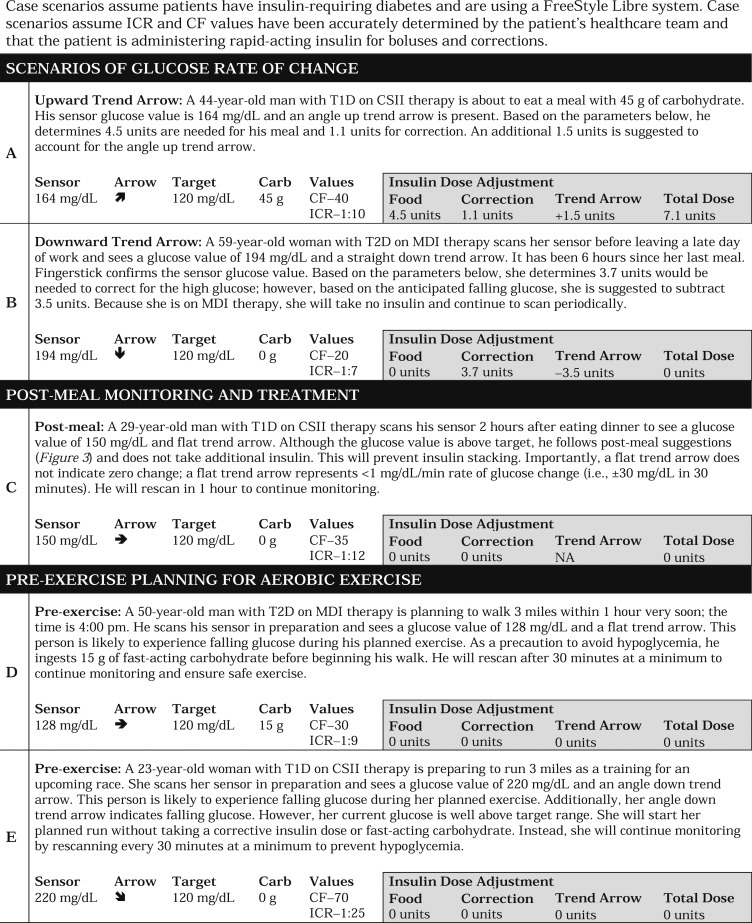
Because the previous approach determined by Aleppo et al. focused on the Dexcom G5 system, which includes double up and double down trend arrows that correspond to >3 mg/dL per minute rate of glucose change, we modified our guidance to accommodate the FreeStyle Libre systems, which do not have double up or double down trend arrows. Specifically, we retained the same adjustment value as Aleppo et al. for the single up and single down trend arrows rather than increase the suggested insulin dose adjustment to account for a lack of double up and double down trend arrows. We think this is a safe approach to using trend arrows in the FreeStyle Libre systems, which lack automatic alarms. Based on clinical experience and a study by Kovatchev et al. [20], we also think that the time spent at >3 mg/dL per minute is relatively infrequent in the absence of food intake or exercise, for which we offer specific guidance. In this manner, we think our suggested approach provides a safe starting point for individuals seeking to use trend arrows in the FreeStyle Libre systems to reduce glycemic variability. It is important to differentiate MDIs from CSII when using trend arrows; for instance, CSII users may need to adjust insulin dose more conservatively when they are using temporary basal rates to address trend arrows following mealtime. Table 3 presents sample scenarios that demonstrate how the approach can be used.
Table 3.
Illustrative Examples of Using Trend Arrows in the FreeStyle Libre Systems
|
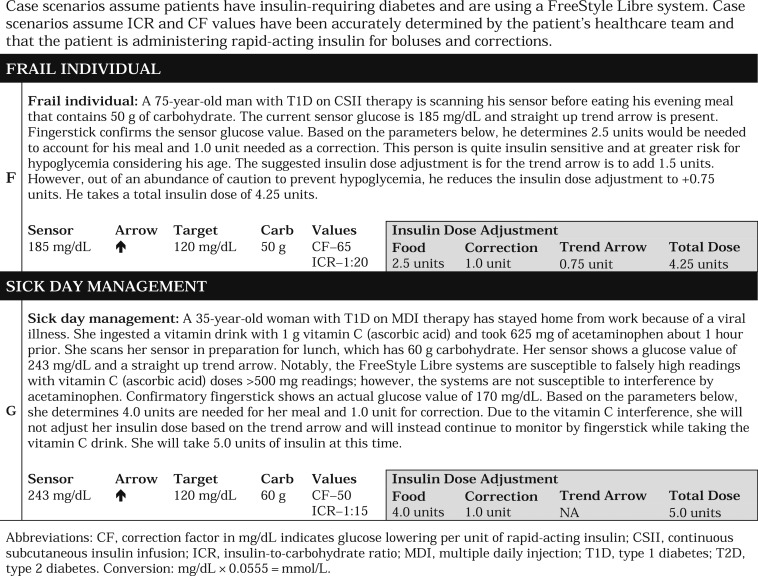
|
Our approach to adjusting insulin dose is not intended to replace standard care in the event of a missed insulin dose for meals. In this case, it is standard practice to calculate the insulin dose based on the carbohydrate ingested, CGM value, and CGM trend arrow at the time of the meal. Adjusting insulin doses using trend arrows should also be avoided in cases of underestimating carbohydrate intake from a previous meal (i.e., miscalculations) and overcorrecting for hypoglycemia with fast-acting carbohydrate. At these times, the trend arrows can play an important role as indications to patients of a missed dose or a miscalculation. For other unplanned situations, more specific strategies for the use of trend arrows should be established between patients and their health care providers.
B. Postmeal Monitoring (2 to 4 Hours After Meal)
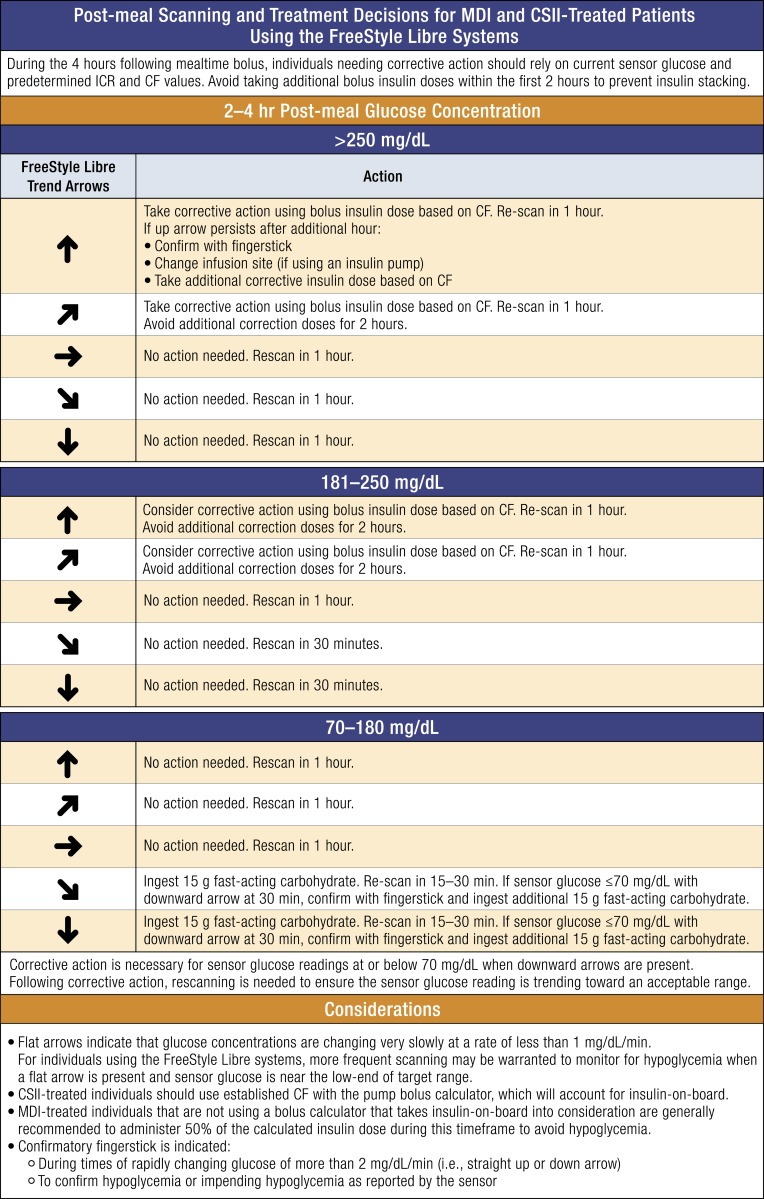
We also include an approach to postmeal monitoring and treatment in adults based on the guidelines used in the REPLACE-BG trial, which suggested a method to minimize hypoglycemia and hyperglycemia during the 4 hours following a meal [21]. Figure 3 outlines the approach that can be used by both patients treated with MDIs and patients treated with insulin pumps.
Figure 3.
Monitoring and treatment decisions using trend arrows in the FreeStyle Libre systems 2 to 4 h following mealtime bolus. Several variables impact glycemia following mealtime bolus. During the 4 h following mealtime bolus, individuals needing corrective active should rely on current sensor glucose and predetermined CF and ICR values. Importantly, the approach relies on the accurate determination and use of insulin-to-carbohydrate ratio (ICR) and correction factors (CFs) as insulin dose parameters. Individuals should not rely on insulin dose adjustments using trend arrow data during this time. The following is based on the protocol used in the REPLACE-BG study [21] and has been modified to account for the unique features of the FreeStyle Libre system and includes observed trend arrows for the FreeStyle Libre systems. In general, individuals should avoid taking corrective action by bolus insulin dose within the first 2 h after eating. Insulin stacking is a primary concern during this time. Beyond 4 h, it is assumed that most, if not all, carbohydrate has entered the system and that there is no active bolus insulin on board. In this case, the authors recommend using the trend arrows for dose adjustment (Fig. 2). The CF (in mg/dL) indicates glucose lowering per unit of rapid-acting insulin. Conversion: mg/dL × 0.0555 = mmol/L.
As a general rule, we recommend caution when adjusting insulin dose using trend arrows during the 4 hours following a meal due to the many variables (e.g., meal composition, total calories, physical activity/exercise, insulin on board) that affect rate of glucose change during this time. Notably, our approach assumes the use of rapid-acting insulin. More recently available ultra–rapid-acting insulins may also affect rate of glucose change during this time. Individuals using ultra–rapid-acting insulin should take caution when considering our postmeal approach. Importantly, patients should be cautioned to wait at least 2 hours after a meal-time bolus before taking any corrective action (e.g., standard corrections based on correction factor or insulin dose adjustments using trend arrows).
3. Engaging Your Patients
Optimal use of the FreeStyle Libre systems requires that patients and clinicians become actively engaged with utilizing CGM data in both real-time decision-making and retrospective analysis. Real-time decision-making refers to patients actively using their CGM data to make insulin dosing decisions to achieve desired glycemic control and prevent or mitigate acute glycemic events. Retrospective analysis refers to review of historical CGM data to identify glucose patterns that may indicate a need for therapy adjustment and/or changes in behaviors.
A. Real-Time Decision-Making
The FreeStyle Libre systems require patients to make a conscious effort to obtain glucose data with periodic scanning. As such, patients need guidance regarding when and how often to scan based on their current glucose and trend arrows (see Table 2). However, monitoring is not managing. Patients also need guidance about what they should do with the information they obtain and, importantly, they need to be empowered to make changes when needed. These measures will enhance patients’ confidence in the device and their ability to avoid acute glycemic events and effectively manage their diabetes.
B. Retrospective Analysis
Use of retrospective CGM data by patients and clinicians is equally important. Patients can use built-in reader reports (e.g., Glucose Pattern Summary or Weekly Summary) and detailed information downloaded via the LibreView software to see the effects of their current activities (e.g., insulin dosing, exercise, meal amounts/composition) and adjust their behaviors accordingly. Importantly, patients should be encouraged to use retrospective analysis to evaluate glucose variability, the reasons behind glycemic excursions, and whether they are spending more time in target range without hypoglycemia. Each clinician and patient will have to individualize the type of data they look at to personalize their therapy and/or behavioral measures.
Reflection is also valuable to evaluate an individual’s use of trend arrow adjustments, scanning frequency, and level of patient engagement. When patients make frequent adjustments for trend arrow data and/or hyperglycemia and hypoglycemia events, it may be an indication that their current treatment plan or insulin parameters are inaccurate and need modification. It may also indicate the need for further education in insulin management and/or diabetes self-management behaviors.
4. Special Considerations When Using the FreeStyle Libre Systems
Insulin dosing decisions during special circumstances are complex and multifactorial and may be impacted by food intake, physical activity, and stressors among other factors. These areas are of great concern and our suggestions should serve as a starting point for future discussion on how our suggested approach may apply. In particular, bedtime insulin dose decisions and potential nocturnal hypoglycemia are common concerns impacted by several factors, particularly when patients engage in intensive physical activity during the day. Our approach to insulin dose adjustments may be considered at bedtime; however, patients should use caution when adding insulin to calculated correction insulin doses at that time. Additional guidance during other special circumstances is provided.
A. Sick Day Management and Medication Considerations
During illness, there is increased risk of glucose instability that can lead to severe hypoglycemia, hyperglycemia, and diabetic ketoacidosis. Use of the FreeStyle Libre systems likely offers some benefit in patients with acute illness, but special precautions must be considered. Based on our clinical experience, we recommend more frequent scanning and suggest that individuals consider insulin correction every 2 to 3 hours with appropriate ketone monitoring during illness. Additionally, patients should consider using fingerstick monitoring for treatment decisions when glucose is >250 mg/dL and ketones (blood or urine) are present and when glucose is <70 mg/dL or symptoms of hypoglycemia are present.
A confirmatory fingerstick may be required when high doses of salicylic acid and/or ascorbic acid are used, which is more likely to occur during sick days. Salicylic acid (used in aspirin and other pain relievers) at doses ≥650 mg may cause falsely lower glucose values, and ascorbic acid (vitamin C) at doses ≥500 mg may cause falsely higher readings. At lower doses, salicylic acid and ascorbic acid are known to have minimal effect on sensor glucose readings in the FreeStyle Libre systems.
B. Young Adults
In the United States, the FreeStyle Libre systems are approved for all adults ≥18 years of age; however, there are distinct differences between younger and older adults that should be considered when initiating CGM in patients within these age groups. As reported by Miller et al. [22], achieving adequate glucose control among younger adults with T1D remains problematic. Moreover, early CGM studies with older CGM devices found that CGM adherence among patients <21 years of age was suboptimal [23].
The FreeStyle Libre systems’ ease of use, scanning reminders, low-profile sensor, and absence of alarms offer distinct advantages for younger adult patients. Nevertheless, finding ways to motivate patients and sustain their engagement with CGM is paramount when initiating CGM use in younger adults. It is important that clinicians have frank discussions with their younger adult patients to identify and resolve barriers to sustained use. It is also important that patients receive adequate training in using their CGM data to make appropriate adjustments, when needed. Guidance about when to scan and how to use trend arrows in treatment decisions is essential. Based on clinical experience, clinicians should de-emphasize “the numbers” and emphasize “staying flat within the lines,” with a focus on minimizing glycemic variability. This will facilitate more positive, supportive discussions with patients that focus on achieving desired glycemic control.
C. Elderly and Frail Adults
Elderly patients with diabetes are at a notably higher risk for severe hypoglycemia and its ramifications due to age, duration of diabetes, and greater prevalence of hypoglycemia unawareness [24–28]. The increased risk is compounded by cognitive and physical impairments and other comorbidities. Hence, it is important to start conservatively when using our approach to adjust insulin doses using trend arrows.
In the older and frail diabetes populations, there is a tendency to relax A1c targets; however, this clinical decision needs to be individualized based on recent epidemiologic data. For example, severe hypoglycemia is common in T1D and to a lesser extent in adults with T2D with both higher and lower A1c levels, although the relationship between A1c goal setting and frequency of severe hypoglycemia in those individuals is less clear [29, 30]. Also, as discussed earlier, use of the FreeStyle Libre system reduces hypoglycemia and hyperglycemia in both well-controlled T1D [6] and poorly controlled insulin-treated patients with T2D [7], whereas frequent fingerstick monitoring does not protect against severe hypoglycemia [28].
In short, rather than relax glycemic goals, we may instead strive to optimize the medications and self-management tools that are now available to create a stable glucose profile. However, we recognize that there is a learning curve challenge with newer technology for older patients and emphasize the value in ongoing patient education.
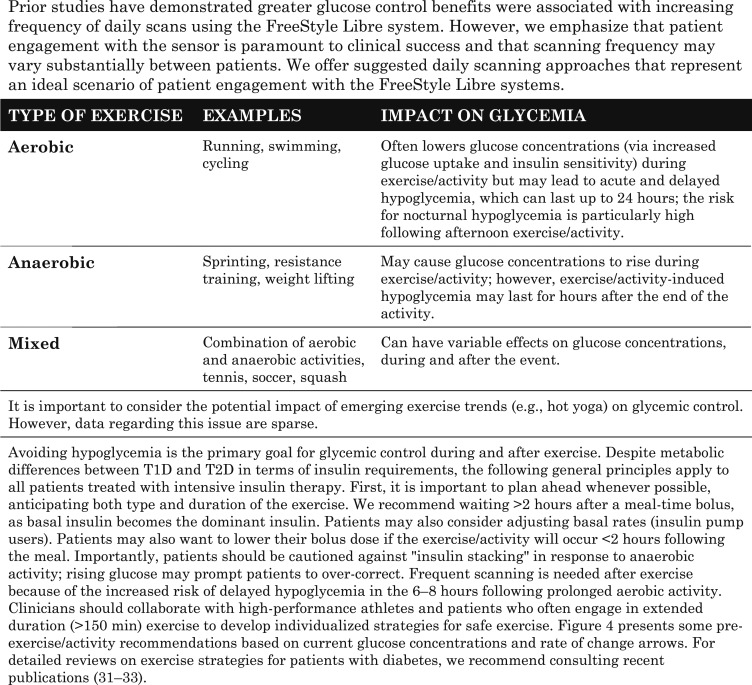
D. Exercise/Physical Activity
Glycemic responses to exercise and physical activity are complex and can be influenced by multiple factors: type of diabetes; fitness level, timing/dose of last bolus; timing/content of last meal; current glucose concentration; rate of changing glucose at start of exercise/activity; rate of insulin infusion (insulin pump basal rate); insulin on board; and type, intensity, and duration of exercise/activity [31]. Information about the impact of various types of exercise on glycemia is presented in Table 4.
Table 4.
Impact of Exercise on Glycemia
|
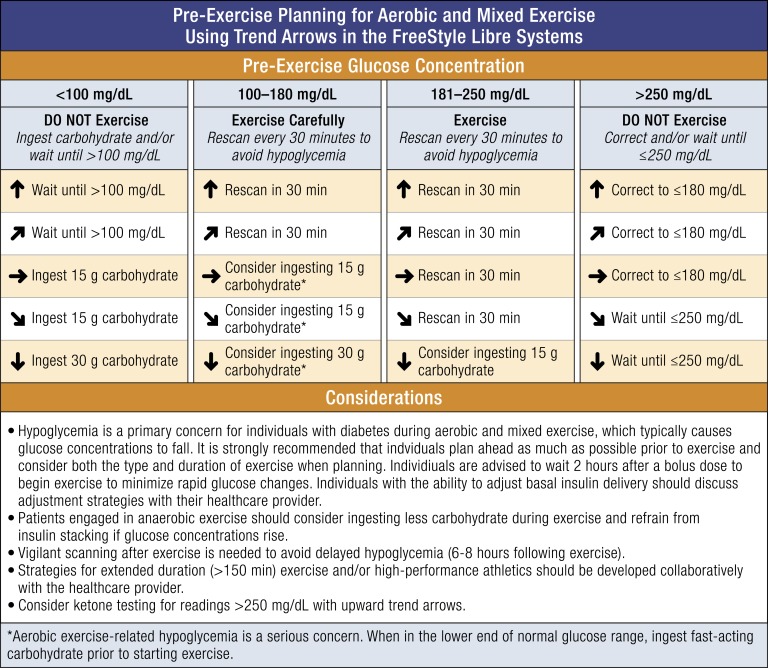
A suggested guide to using the trend arrow data prior to and during exercise in the FreeStyle Libre systems is presented in Fig. 4. Our guide is not intended to be comprehensive, and we recognize that numerous factors can influence glycemia prior to, during, and up to 8 hours or longer following exercise [31]. We strive to highlight the unique features of the FreeStyle Libre systems to provide a starting point to individualize an exercise strategy for individuals performing moderate aerobic exercise.
Figure 4.
Approach for pre-exercise/activity planning based on current glucose concentration and trend arrow direction. The following recommendations are based on the consensus statement by Riddell et al. [31] and our own clinical experience. Our pre-exercise planning approach takes the unique features of the FreeStyle Libre systems approved in the United States into consideration including the scanning requirement and trend arrow information. In addition to the suggested planning steps, individuals should be aware of how they respond to different types and/or durations of exercise and plan accordingly in advance of activities as best as possible. In general, frequent scanning is recommended for individuals using the FreeStyle Libre systems during exercise.
E. Environmental Factors
There are no indications that environmental factors, such as extreme temperature, altitude, or humidity, impact CGM accuracy, but they do affect fingerstick monitoring values [34]. Certain medications may impact reliability of data stream as indicated above in the sick day management section.
5. Discussion
A large proportion of individuals with diabetes treated with intensive insulin therapy are not achieving their glycemic goals [22, 35] and hypoglycemia remains a key obstacle to effective diabetes management [36]. Today’s CGM devices, such as the FreeStyle Libre systems, can help patients overcome obstacles and improve quality of life [6, 7, 12, 37]. However, monitoring glucose is only the first step in optimizing care; patients need to know how to use their glucose data, including trend arrow information, to make informed decisions.
Our recommendations are intended to provide a safe, practical approach to using the FreeStyle Libre systems, in general, and trend arrows, in particular. Our goal is to provide guidance that facilitates individualized recommendations for trend arrow use and data assessment. Our approach focuses on typical insulin sensitivity ranges used in adults and provides a range of adjustments in discrete insulin units. We believe this simplified approach reduces numeracy requirements and the number of calculations, which will help patients improve glucose control and increase glucose time in range without hypoglycemia, while promoting clinical discussion.
CGM has the potential to transform diabetes management. With this approach as a starting point, we hope to address the needs of clinicians and the growing number of patients using CGM seeking an approach to safely reduce glycemic variability using trend arrow data. We also hope to see this approach applied in practice to derive empirically based information for all currently available and emerging glucose monitoring devices.
Acknowledgments
We thank Irl B. Hirsch for his contributions as an editorial advisor, the Endocrine Society for convening and facilitating the writing group, and Christopher G. Parkin for editorial support in developing this manuscript.
Financial Support: This work was supported by an unrestricted educational grant to the Endocrine Society from Abbott Diabetes Care Inc., Alameda, California.
Author Contributions: Y.C.K. led the writing group as chair and facilitated development and group consensus of the methods proposed. All authors contributed equally in manuscript review, discussion of the results and implications, and comments on the manuscript at all stages.
Disclosure Summary: Y.C.K. has received research support from Dexcom, Roche Diabetes Care, and Tandem Diabetes. A.J.A.’s institution has received research support from Dexcom and Medtronic, and he has consulted for Dexcom. R.M.B. has received research support, consulted, and/or has been on the scientific advisory boards for Abbott Diabetes Care, Dexcom, Eli Lilly, Johnson & Johnson, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi, and United Healthcare. R.M.B.’s employer, nonprofit HealthPartners Institute, contracts for his services and no personal income goes to R.M.B. J.R.G. III has served on advisory boards and/or speaker bureaus for Abbott Diabetes Care. D.F.K. has served on advisory boards and speaker bureaus for Abbott Diabetes Care and Dexcom; her institution has received research support from Abbott Diabetes Care and Dexcom; and she owns stock in Dexcom. L.K.M. has served on scientific advisory boards for Abbott Diabetes Care, and his institution has received research support from Abbott Diabetes Care. E.M. has served on advisory boards and/or speaker bureaus for Abbott Diabetes Care. D.R.H. has nothing to disclose.
Glossary
Abbreviations:
- CGM
continuous glucose monitoring
- CSII
continuous subcutaneous insulin infusion
- MDI
multiple daily injection
- T1D
type 1 diabetes
- T2D
type 2 diabetes
References and Notes
- 1. Fonseca VA, Grunberger G, Anhalt H, Bailey TS, Blevins T, Garg SK, Handelsman Y, Hirsch IB, Orzeck EA, Roberts VL, Tamborlane W; Consensus Conference Writing Committee . Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22(8):1008–1021. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S55–S64. [DOI] [PubMed] [Google Scholar]
- 3. Peters AL, Ahmann AJ, Battelino T, Evert A, Hirsch IB, Murad MH, Winter WE, Wolpert H. Diabetes technology—continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3922–3937. [DOI] [PubMed] [Google Scholar]
- 4. Bergenstal RM, Ahmann AJ, Bailey T, Beck RW, Bissen J, Buckingham B, Deeb L, Dolin RH, Garg SK, Goland R, Hirsch IB, Klonoff DC, Kruger DF, Matfin G, Mazze RS, Olson BA, Parkin C, Peters A, Powers MA, Rodriguez H, Southerland P, Strock ES, Tamborlane W, Wesley DM. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther. 2013;15(3):198–211. [DOI] [PubMed] [Google Scholar]
- 5. International Diabetes Center Ambulatory glucose profile: AGP reports. Available at: www.agpreport.org/agp/agpreports. Accessed 22 April 2018.
- 6. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254–2263. [DOI] [PubMed] [Google Scholar]
- 7. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ish-Shalom M, Wainstein J, Raz I, Mosenzon O. Improvement in glucose control in difficult-to-control patients with diabetes using a novel flash glucose monitoring device. J Diabetes Sci Technol. 2016;10(6):1412–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKnight JA, Gibb FW. Flash glucose monitoring is associated with improved glycaemic control but use is largely limited to more affluent people in a UK diabetes centre. Diabet Med. 2017;34(5):732. [DOI] [PubMed] [Google Scholar]
- 10. Dover AR, Stimson RH, Zammitt NN, Gibb FW. Flash glucose monitoring improves outcomes in a type 1 diabetes clinic. J Diabetes Sci Technol. 2017;11(2):442–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aleppo G, Laffel LM, Ahmann AJ, Hirsch IB, Kruger DF, Peters A, Weinstock RS, Harris DR. A practical approach to using trend arrows on the Dexcom G5 CGM system for the management of adults with diabetes. J Endocr Soc. 2017;1(12):1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. 2018;137:37–46. [DOI] [PubMed] [Google Scholar]
- 13. Buckingham B, Xing D, Weinzimer S, Fiallo-Scharer R, Kollman C, Mauras N, Tsalikian E, Tamborlane W, Wysocki T, Ruedy K, Beck R; Diabetes Research In Children Network (DirecNet) Study Group . Use of the DirecNet applied treatment algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the FreeStyle Navigator). Pediatr Diabetes. 2008;9(2):142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scheiner G. Practical CGM: Improving Patient Outcomes through Continuous Glucose Monitoring. 4th ed. Alexandria, VA: American Diabetes Association; 2015.
- 15. Pettus J, Edelman SV. Recommendations for using real-time continuous glucose monitoring (rtCGM) data for insulin adjustments in type 1 diabetes. J Diabetes Sci Technol. 2017;11(1):138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klonoff DC, Kerr D. A simplified approach using rate of change arrows to adjust insulin with real-time continuous glucose monitoring. J Diabetes Sci Technol. 2017;11(6):1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grunberger G, Abelseth JM, Bailey TS, Bode BW, Handelsman Y, Hellman R, Jovanovič L, Lane WS, Raskin P, Tamborlane WV, Rothermel C. Consensus statement by the American Association of Clinical Endocrinologists/American College of Endocrinology insulin pump management task force. Endocr Pract. 2014;20(5):463–489. [DOI] [PubMed] [Google Scholar]
- 18. McGill JB, Ahmann A. Continuous glucose monitoring with multiple daily insulin treatment: outcome studies. Diabetes Technol Ther. 2017;19(S3):S3–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klonoff DC, Nayberg I, Stauder U, Oualali H, Domenger C. Half-unit insulin pens: disease management in patients with diabetes who are sensitive to insulin. J Diabetes Sci Technol. 2017;11(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kovatchev BP, Clarke WL, Breton M, Brayman K, McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther. 2005;7(6):849–862. [DOI] [PubMed] [Google Scholar]
- 21. Aleppo G, Ruedy KJ, Riddlesworth TD, Kruger DF, Peters AL, Hirsch I, Bergenstal RM, Toschi E, Ahmann AJ, Shah VN, Rickels MR, Bode BW, Philis-Tsimikas A, Pop-Busui R, Rodriguez H, Eyth E, Bhargava A, Kollman C, Beck RW; REPLACE-BG Study Group . REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care. 2017;40(4):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM, Tamborlane WV; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–978. [DOI] [PubMed] [Google Scholar]
- 23. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. [DOI] [PubMed] [Google Scholar]
- 24. Weinstock RS, DuBose SN, Bergenstal RM, Chaytor NS, Peterson C, Olson BA, Munshi MN, Perrin AJ, Miller KM, Beck RW, Liljenquist DR, Aleppo G, Buse JB, Kruger D, Bhargava A, Goland RS, Edelen RC, Pratley RE, Peters AL, Rodriguez H, Ahmann AJ, Lock JP, Garg SK, Rickels MR, Hirsch IB; T1D Exchange Severe Hypoglycemia in Older Adults With Type 1 Diabetes Study Group . Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care. 2016;39(4):603–610. [DOI] [PubMed] [Google Scholar]
- 25. Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32(8):1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Punthakee Z, Miller ME, Launer LJ, Williamson JD, Lazar RM, Cukierman-Yaffee T, Seaquist ER, Ismail-Beigi F, Sullivan MD, Lovato LC, Bergenstal RM, Gerstein HC; ACCORD Group of Investigators; ACCORD-MIND Investigators . Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012;35(4):787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giorda CB, Ozzello A, Gentile S, Aglialoro A, Chiambretti A, Baccetti F, Gentile FM, Lucisano G, Nicolucci A, Rossi MC; HYPOS-1 Study Group of AMD . Incidence and risk factors for severe and symptomatic hypoglycemia in type 1 diabetes. Results of the HYPOS-1 study. Acta Diabetol. 2015;52(5):845–853. [DOI] [PubMed] [Google Scholar]
- 28. Cariou B, Fontaine P, Eschwege E, Lièvre M, Gouet D, Huet D, Madani S, Lavigne S, Charbonnel B. Frequency and predictors of confirmed hypoglycaemia in type 1 and insulin-treated type 2 diabetes mellitus patients in a real-life setting: results from the DIALOG study. Diabetes Metab. 2015;41(2):116–125. [DOI] [PubMed] [Google Scholar]
- 29. Weinstock RS, Xing D, Maahs DM, Michels A, Rickels MR, Peters AL, Bergenstal RM, Harris B, Dubose SN, Miller KM, Beck RW; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab. 2013;98(8):3411–3419. [DOI] [PubMed] [Google Scholar]
- 30. Lipska KJ, Warton EM, Huang ES, Moffet HH, Inzucchi SE, Krumholz HM, Karter AJ. HbA1c and risk of severe hypoglycemia in type 2 diabetes: the Diabetes and Aging Study. Diabetes Care. 2013;36(11):3535–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, Kowalski A, Rabasa-Lhoret R, McCrimmon RJ, Hume C, Annan F, Fournier PA, Graham C, Bode B, Galassetti P, Jones TW, Millán IS, Heise T, Peters AL, Petz A, Laffel LM. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5(5):377–390. [DOI] [PubMed] [Google Scholar]
- 32. Bally L, Laimer M, Stettler C. Exercise-associated glucose metabolism in individuals with type 1 diabetes mellitus. Curr Opin Clin Nutr Metab Care. 2015;18(4):428–433. [DOI] [PubMed] [Google Scholar]
- 33. Rabasa-Lhoret R, Bourque J, Ducros F, Chiasson JL. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes Care. 2001;24(4):625–630. [DOI] [PubMed] [Google Scholar]
- 34. Erbach M, Freckmann G, Hinzmann R, Kulzer B, Ziegler R, Heinemann L, Schnell O. Interferences and limitations in blood glucose self-testing: an overview of the current knowledge. J Diabetes Sci Technol. 2016;10(5):1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017;8(4):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cryer PE. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14(6):750–756. [DOI] [PubMed] [Google Scholar]
- 37. Al Hayek AA, Robert AA, Al Dawish MA. Evaluation of FreeStyle Libre flash glucose monitoring system on glycemic control, health-related quality of life, and fear of hypoglycemia in patients with type 1 diabetes. Clin Med Insights Endocrinol Diabetes. 2017;10:1179551417746957. [DOI] [PMC free article] [PubMed] [Google Scholar]