Abstract
Background:
Cystic echinococcosis is a zoonotic infection and considered as a major economic and public health concern worldwide. This research was conducted to determine genotypic characteristics of livestock and human hydatid cyst isolates from Hamadan area, western Iran.
Methods:
Sampling was conducted in Hamadan industrial slaughterhouse and Beast Hospital of Hamadan City, western Iran, from 2015 to 2016. Overall, 74 livestock isolates including 69 sheep, 3 cattle and 2 goats and 9 human hydatid cysts were genotyped by PCR amplification of the rDNA ITS1 region and followed restriction fragment length polymorphism (RFLP) analysis with four restriction endonuclease enzymes, RsaI, HpaII, AluI, and TaqI, and sequencing.
Results:
The PCR amplicon size of each isolate was approximately 1 kb which was the same with that of sheep strain. According to the RFLP patterns, the isolates belonged to a single species, E. granulosus sensu stricto (G1–G3 complex). Furthermore, sequencing of representative amplicons confirmed that the RFLP-genotyped isolates corresponded to E. granulosus sensu stricto.
Conclusion:
E. granulosus sensu stricto is the prevailing species of E. granulosus sensu lato in the region and pointed out the importance of sheep/dog cycle in human transmission.
Keywords: Echinococcus granulosus, Genotype, Iran, ITS1, Humans, Livestock, PCR-RFLP
Introduction
Cystic echinococcosis (CE) is a cosmopolitan zoonotic infection caused by the larval stage of a taeniid tape-worm, Echinococcus granulosus sensu lato. The parasite life cycle engages two hosts including carnivorous, as a definitive host, and herbivorous, as an intermediate host. In the domestic life cycle, dog and livestock have the main role in survival and distribution of the infection. Hydatid cyst, the encysted larval stages of the parasite, develops from ingested eggs in inner organs of the intermediate host animals and gradually grows (1). Hydatid cyst establishes generally in liver (more than 65% of the cases) and lungs (25% of the cases) in intermediate hosts, however, other organs can be involved, including heart, kidneys, spleen, bone, eyes, muscles and central nervous system (2). A human can be infected by the metacestode stage of the parasite as an accidental intermediate host (1).
CE is considered a public health problem and economic concern in many countries worldwide (3). This zoonotic infection has extensive distribution in the world with highly endemic regions including Australia, China, the Middle East, Western and Central Asia, Northern and Eastern Africa and Southern America (2). Echinococcosis/hydatidosis should be considered as a prevalent infection in Iran with a prevalence rate of 5%–49% in stray dogs and with an average of 6.73% among livestock (4). Phylogenetic analyses and molecular epidemiological studies based on mitochondrial genes (COI, NDI and ATP6) and nuclear rRNA genes (ITS1) revealed that E. granulosus s.l. is a set of species/genotypes including E. granulosus sensu stricto (G1–G3 complex), E. equinus (G4), E. ortleppi (G5) and E. canadensis (G6–G10) (5–7). Situation of E. canadensis genotypes is controversial and it suggested to be divided into two distinct species consist of E. canadensis (G8 and G10), cervid genotypes, and E. intermedius, (G6/G7), camel/pig genotypes (3, 8).
Variation in species/genotypes of E. granulosus s.l. are reflected in morphological and biological characteristics of the parasite and it can influence the life cycle pattern, host specificity, development rates, pathogenicity, treatment, transmission dynamics, epidemiology and finally control of CE (9).
Morphological and molecular epidemiological studies have been conducted in Iran showed existence of E. granulosus sensu stricto and camel genotype in livestock, dog and human (10–14). Hamadan Province is one of the endemic regions of echinococcosis/hydatidosis in Iran (15, 16). However, there is little information about the genetic characterization of E. granulosus s.l. in this area.
This study was conducted to investigate the species/genotypes of the parasite in the region by using PCR-RFLP and nucleotide sequences analysis of the ITS1-rDNA locus.
Materials and Methods
Parasites
During Mar 2015 to Apr 2016, livestock hydatid cysts including sheep, cattle, goats and human hydatid cysts were collected from Hamadan industrial slaughterhouse and Beast Hospital of Hamadan city, western Iran, respectively. Fertile hydatid cysts with colorless transparent fluid and whitish germinal layer were selected for molecular analysis. Aspirated protoscoleces from individual hydatid cyst washed three times with sterile normal saline solution and preserved in 70% (v/v) ethanol at −20 °C, until use for molecular processes.
DNA extraction
Total genomic DNA (gDNA) extraction was performed on 50 mg protoscolices pellet of each individual hydatid cyst by using High Pure PCR Template Preparation Kit (Roche Diagnostics, Mannheim, Germany) following the manufacturer’s recommended protocol. The extracted gDNA was evaluated by 0.8% agarose gel electrophoresis and spectrophotometer (NanoDrop-ND1000) and the DNA stored at −20 °C until molecular analysis.
PCR amplification and RFLP analysis
PCR amplification was performed on DNA samples to amplify the ITS1 rDNA region of E. granulosus s.l. using forward (EgF: 5′ GTC GTA ACA AGG TTT CCG TAG G 3′) and reverse (EgR: 5′ TAG ATG CGT TCG AAG TGT CG 3′) primers (17). The PCR reaction was done in a final volume of 50 μl, including 7 μl gDNA, 25 pmol of each primer, 5 μl buffer 10X, 2 μl dNTP, 2 μl MgCl2, 2 unit Taq DNA polymerase (Sinagene Company) and 29 μl distilled water. Negative (no added DNA) and positive controls were included in PCR experiment. The PCR conditions were as follows: one cycle of primary denaturation (2 min at 95 °C), followed by 35 cycles of denaturation (30 sec at 94 °C), annealing (45 sec at 55 °C), extension (1 min at 72 °C), and followed by a final extension (5 min at 72 °C). Then, the PCR product was evaluated by electrophoresis in a 1% (w/v) Tris-borate/EDTA (TBE) agarose gels stained with SYBR Safe DNA gel stain (Invitrogen).
RFLP analysis of the PCR product was performed by four restriction enzymes including RsaI, HpaII, AluI, and TaqI which identify nucleotide sequences of GT/AC, C/CGG, AG/CT, and T/CGA, respectively. The digestion reaction was carried out on 10 μl of the PCR amplicon in a final 20 μl volume and incubation at 37 °C for 4 h following the manufacturer’s instructions (fermentas, Germany), with the exception of TaqI, incubated at 65 °C. The restriction fragments were conducted to 2% agarose gel electrophoresis and visualized under UV light after SYBR Safe DNA gel staining.
Sequence analysis
Representative PCR amplicons of different intermediate host including sheep, cattle, goat, and human were subjected to sequencing using Applied Biosystems Automated 3730xl DNA Analyzer (Bioneer Inc., Korea) and the same primers utilized in the primary amplification. Sequence editing and alignment were done by Chromas software, ver. 2.6, and MultAlin program (18). Homology survey of the consensus sequences was carried out by using the BLASTn program (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and comparison with other reference sequences in GenBank.
Results
The PCR amplification of the ITS1 region of rDNA was successfully performed on 74 animal DNA (69 sheep, 3 cattle, and 2 goats) and 9 human DNA samples and produced amplicons of approximately 1 kb in length which are similar to the sheep strain (Fig. 1).
Fig. 1:
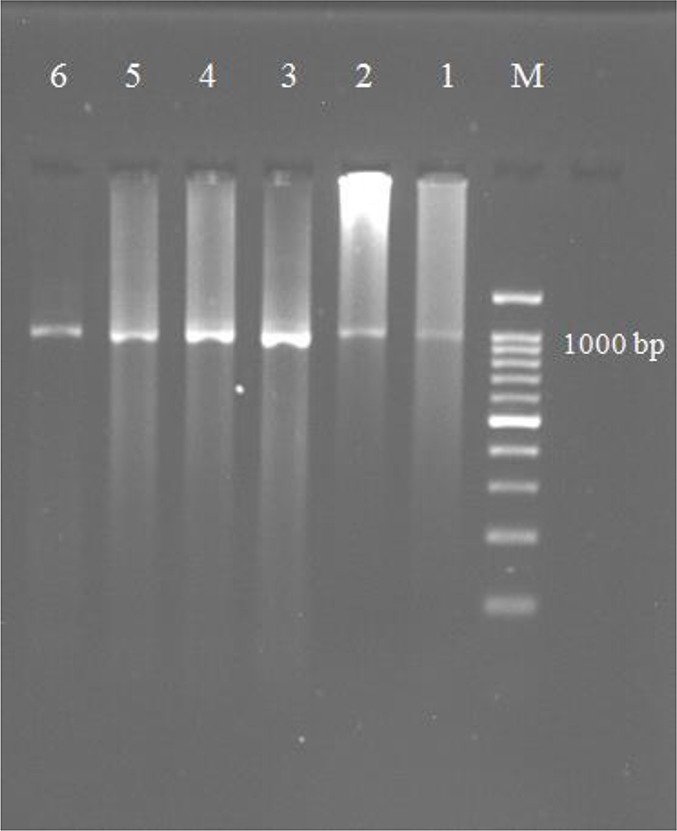
Agarose gel electrophoresis of PCR-ITS1 amplification of the 1000 bp fragment from E. granulosus isolates. Lane M: DNA marker (100 bp); Lane 1–6: some of the E. granulosus isolates
The samples used for PCR tests were 36 liver and 47 lung hydatid cysts. No amplification was observed in negative control in each PCR run. RFLP analysis of the ITS1 rDNA region was done by using the four restriction endonucleases and one identical pattern was obtained from the animal and human hydatid cyst isolates by each of the restriction enzymes. The size of fragments produced by the restriction enzymes was as follows: AluI: 200 and 800 bp; RsaI: 345 and 655; HpaII: 300 and 700 bp; TaqI: without any digestion (Fig. 2). The four RFLP patterns indicate that the isolates belong to E. granulosus s.s. (19).
Fig. 2:
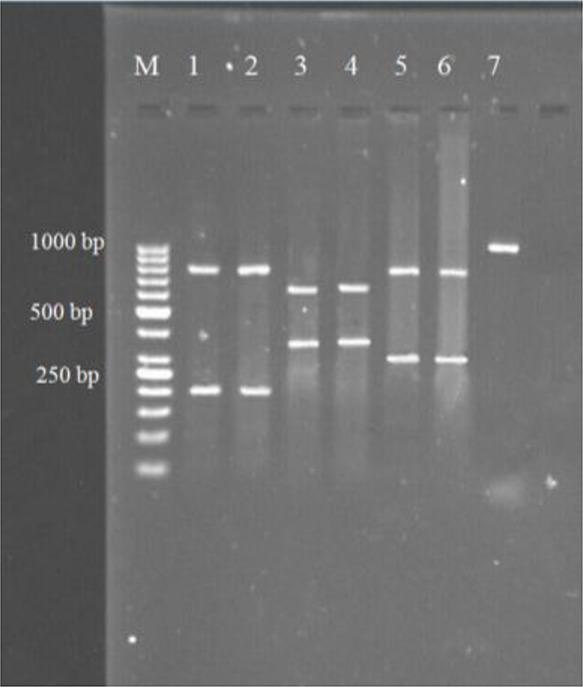
Representative RFLP analysis patterns of the amplified ITS1 rDNA region of E. granulosus isolates were digested by AluI (lane 1 & 2), RsaI (lane 3 & 4), HpaII (lane 5 & 6) and TaqI (lane 7)
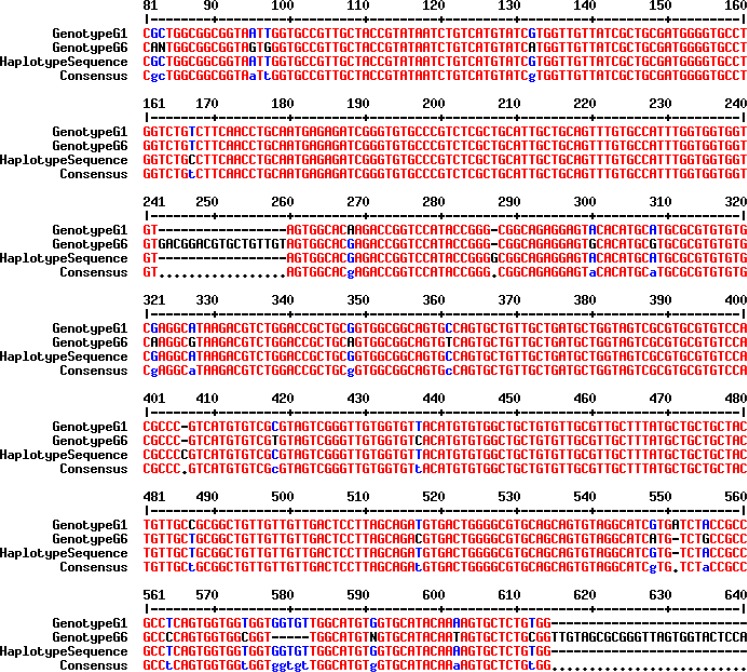
Fourteen representative amplicons of ITS1-PCR, including animal and human isolates, were subjected to sequencing. Partial consensus sequences of E. granulosus ITS1 rDNA region of the isolates were achieved and compared with the reference sequence genotype G1 (Accession No. AJ237777) (20) and other sequences deposited in GenBank. Alignment of the obtained sequences with the reference sequence revealed 99% homology and indicated that the isolates corresponded to E. granulosus genotype G1 (Fig. 3). Finally, a consensus sequence was deposited at GenBank database under accession numbers MF004421.
Fig. 3:
Partial sequence (MF004421) alignment of the ITS1 rDNA region of E. granulosus isolates was compared with reference strains (G1 genotype: GenBank Accession No. AJ237777 & G6 genotype: Accession No. AJ237776)
Discussion
As for any infectious disease, control of hydatid disease requires a clear species-level classification and genetic identification of the species. Comprehensive molecular genetic analysis of E. granulosus, based on mitochondrial and nuclear rRNA genes, revealed some of the strains of E. granulosus can be considered as distinct species (3). PCR–RFLP analysis of ITS1 region of Echinococcus can distinguish E. granulosus s.s., E. equinus, E. ortleppi, G6/G7 genotypes, E. multilocularis, E. vogeli and E. oligarthrus (10, 21). Human can be infected by all E. granulosus genotypes except G4 (3, 22). Thus, various animals can play a role in the epidemiology of human echinococcosis/hydatidosis and design an appropriate strategy to control the infection requires a good understanding of transmission cycle in each region.
Until now, two main genotypes including G1–G3 complex and G6 have been reported from Iran based on mitochondrial genes (cox1 and nad1) sequencing and PCR-RFLP analysis of rDNA-ITS1 (10–14, 19, 23). In this study, E. granulosus s.s. was detected in the examined livestock and human hydatid cysts and it indicates the genotypic cluster G1–G3 is the dominant genotype of E. granulosus in the area. This finding is consistent with the previous studies from other parts of Iran that they have reported E. granulosus s.s. as the prevailing type of the parasite in animals and human isolates (10–14, 19). Moreover, some of the workers have reported genotype G1 (common sheep strain) as the only genotype in animal and human hydatid cysts (19, 23).
In the present study, genotype G6 was not detected among examined animal and human isolates which may be due to the absence of camel, the natural intermediate host of the camel strain, in the region. Some species/strains of E. granulosus, especially sheep strain (G1) has a higher potential for adaptation to a wide range of host species under various environmental conditions. Thus, this strain is likely the most widespread E. granulosus strain worldwide and the common etiologic agent of human CE (22).
Various molecular studies were conducted for genetic identification of E. granulosus in the endemic areas in the world. Villalobos and colleagues reported genotypes G1 and G7 in pigs from Mexico, known as a hypoendemic area of echinococcosis, using ITS1-PCRRFLP analysis and mitochondrial genes sequencing (24). In Argentina, a hyperendemic in South America, 76 hydatid cysts from cattle and sheep were genetically identified and E. granulosus s.s. genotype G1 was dominant genotype and two isolates from sheep and cattle were detected as genotype G2 and G5, respectively (25). Human CE was investigated in South Africa. In this study, 32 human isolates were subjected to genetic identification using PCR-RFLP and sequencing of 12s rRNA gene. The most of the isolates [26/32] belonged to E. granulosus s.s., and G6/G7 and G5 genotypes constituted five and one of the isolates, respectively (26). However, in some areas of Africa such as Mauritania and Sudan, G6 is the prevailing genotype of the parasite in sheep, cattle, camel and human (27, 28). In Russia, an endemic area for both multilocular and unilocular hydatid cyst, genetic identification of Echinococcus spp. was conducted. Seventy-five Echinococcus isolates obtained from 14 host species were identified using mitochondrial DNA sequencing. In this study, in addition to E. granulosus s.s., genotype G6, G8 and G10 and also E. multilocularis were detected in the different regions of Russia (29). In another study on human and dog isolates in northwest of China, the Xinjiang Uygur Autonomous Region, one of the important foci of human CE in the world, the most of the human [45/47] and dog [42/45] isolates was detected as G1 genotype and the others were G6 genotype (30). In Turkey, the neighbor of Iran, 112 livestock isolates including sheep [100] and cattle [12] hydatid cysts were genetically categorized based on the mitochondrial cox1 gene. Genotype G1 was found the major genotype [107/112] and five isolates (two sheep and three cattle) corresponded to G3 genotype (31).
In the present study, the results of representative sequencing of the rDNA-ITS1 region of E. granulosus isolates were consistent with those of genotyping of the isolates by RFLP analysis and the obtained G1 sequences had high similarity (99%) with Bowles isolate (G1 reference sequence, AJ237777) (20) and other G1 sequences.
Conclusion
This research as the first genetic characterization of E. granulosus in the Hamadan area showed that E. granulosus s.s (genotype G1–G3 complex) is the common species/genotypes of E. granulosus s.l. in the area and the sheep/dog cycle is the most probable parasite life cycle in this area. Thus, further genetic studies are needed to exactly determine the genotypes of E. granulosus s.s in the region. Eventually, this information may be considered when implementing hydatidosis control programs, because antigenic variation and differences in other biological characteristics of Echinococcus species were reported (9).
Acknowledgements
This article (derived from a master’s thesis) was supported financially by Vice-chancellor of Research and Technology, Hamadan University of Medical Sciences (Project No. 9312126502). The authors thank the staffs of Research Center for Molecular Medicine and Parasitology Research Laboratory of Hamadan University of Medical Sciences for their cooperation and help.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004; 17(1):107–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardona GA, Carmena D. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Vet Parasitol. 2013;192(1–3):10–32. [DOI] [PubMed] [Google Scholar]
- 3.Thompson RC. The taxonomy, phylogeny and transmission of Echinococcus. Exp Parasitol. 2008; 119(4):439–46. [DOI] [PubMed] [Google Scholar]
- 4.Rokni MB. Echinococcosis/hydatidosis in Iran. Iran J Parasitol. 2009; 4(2):1–16. [Google Scholar]
- 5.Nakao M, McManus DP, Schantz PM, Craig PS, Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology. 2007; 134(Pt 5):713–22. [DOI] [PubMed] [Google Scholar]
- 6.Moks E, Jõgisalu I, Valdmann H, Saarma U. First report of Echinococcus granulosus G8 in Eurasia and a reappraisal of the phylogenetic relationships of ‘genotypes’ G5–G10. Parasitology. 2008; 135(5):647–54. [DOI] [PubMed] [Google Scholar]
- 7.Knapp J, Nakao M, Yanagida T, et al. Phylogenetic relationships within Echinococcus and Taenia tapeworms (Cestoda: Taeniidae): An inference from nuclear protein-coding genes. Mol Phylogenet Evol. 2011; 61(3):628–38. [DOI] [PubMed] [Google Scholar]
- 8.Saarma U, Jõgisalu I, Moks E, et al. A novel phylogeny for the genus Echinococcus, based on nuclear data, challenges relationships based on mitochondrial evidence. Parasitology. 2009; 136(3):317–28. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RCA, McManus DP. Aetiology: parasites and life-cycles. In: Eckert J, Gemmell M, Meslin F-X, Pawlowski Z, eds. WHOI/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. Paris: World Organisation for Animal Health, 2001: 1–19. [Google Scholar]
- 10.Harandi MF, Hobbs RP, Adams PJ, et al. Molecular and Morphological Characterization of Echinococcus granulosus of Human and Animal Origin in Iran. Parasitology. 2002; 125(Pt 4):367–73. [DOI] [PubMed] [Google Scholar]
- 11.Sharbatkhori M, Fasihi Harandi M, Mirhendi H, Hajialilo E, Kia EB. Sequence Analysis of cox1 and nad1 Genes in Echinococcus granulosus G3 Genotype in Camels (Camelus Dromedarius) from Central Iran. Parasitol Res. 2011; 108(3):521–7. [DOI] [PubMed] [Google Scholar]
- 12.Sharbatkhori M, Tanzifi A, Rostami S, Rostami M, Fasihi Harandi M. Echinococcus granulosus sensu lato Genopypes in Domestic Livestock and Humans in Golestan Province, Iran. Rev Inst Med Trop Sao Paulo. 2016; 58:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsa F, Fasihi Harandi M, Rostami S, Sharbatkhori M. Genotyping Echinococcus granulosus from dogs from Western Iran. Exp Parasitol. 2012; 132(2):308–12. [DOI] [PubMed] [Google Scholar]
- 14.Shahnazi M, Hejazi H, Salehi M, Andalib AR. Molecular characterization of human and animal Echinococcus granulosus isolates in Isfahan, Iran. Acta Trop. 2011; 117(1):47–50. [DOI] [PubMed] [Google Scholar]
- 15.Fallah M, Taherkhani H, Sajadi M. Echinococcosis in stray dogs in Hamedan, west of Iran. Iran J Med Sci. 1995; 29:170–2. [Google Scholar]
- 16.Fallah M, Matini M, Beygomkia E, Mobedi I. Study of Zoonotic Tissue Parasites (Hydatid Cyst, Fasciola, Dicrocoelium and Sarcocystis) in Hamadan Abattoir. Sci J Hamadan Univ Med Sci. 2010; 17(3):5–12. [Google Scholar]
- 17.Rahimi H, Kia E, Mirhendi S, et al. A new Primer Pair in ITS1 Region for Molecular Studies on Echinococcus granulosus. Iran J Public Health. 2007; 36(1):45–9. [Google Scholar]
- 18.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988; 16(22):10881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dousti M, Abdi J, Bakhtiyari S, et al. Genotyping of Hydatid Cyst Isolated from Human and Domestic Animals in Ilam Province, Western Iran Using PCR-RFLP. Iran J Parasitol. 2013; 8(1):47–52. [PMC free article] [PubMed] [Google Scholar]
- 20.Bowles J, Blair D, McManus DP. A molecular phylogeny of the genus Echinococcus. Parasitology. 1995; 110 (Pt 3):317–28. [DOI] [PubMed] [Google Scholar]
- 21.Bowles J, McManus DP. Rapid discrimination of Echinococcus species and strains using a polymerase chain reaction-based RFLP method. Mol Biochem Parasitol. 1993; 57(2):231–9. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez Rojas CA, Romig T, Lightowlers MW. Echinococcus granulosus sensu lato genotypes infecting humans – review of current knowledge. Int J Parasitol. 2014; 44(1):9–18. [DOI] [PubMed] [Google Scholar]
- 23.Kia EB, Rahimi H, Sharbatkhori M, Talebi A, Fasihi Harandi M, Mirhendi H. Genotype identification of human cystic echinococcosis in Isfahan, central Iran. Parasitol Res. 2010; 107(3):757–60. [DOI] [PubMed] [Google Scholar]
- 24.Villalobos N, González LM, Morales J, et al. Molecular identification of Echinococcus granulosus genotypes (G1 and G7) isolated from pigs in Mexico. Vet Parasitol. 2007; 147(1–2):185–9. [DOI] [PubMed] [Google Scholar]
- 25.Andresiuk MV, Gordo FP, Saarma M, et al. Echinococcus granulosus genotype G1 dominated in cattle and sheep during 2003–2006 in Buenos Aires province, an endemic area for cystic echinococcosis in Argentina. Acta Trop. 2013; 127(2):136–42. [DOI] [PubMed] [Google Scholar]
- 26.Mogoye BK, Menezes CN, Wong ML, et al. First insights into species and genotypes of Echinococcus in South Africa. Vet Parasitol. 2013; 196(3–4):427–32. [DOI] [PubMed] [Google Scholar]
- 27.Bardonnet K, Piarroux R, Dia L, et al. Combined Eco-Epidemiological and Molecular Biology Approaches to Assess Echinococcus granulosus Transmission to Humans in Mauritania: Occurrence of the ‘Camel’Strain and Human Cystic Echinococcosis. Trans R Soc Trop Med Hyg. 2002; 96(4):383–6. [DOI] [PubMed] [Google Scholar]
- 28.Omer RA, Dinkel A, Romig T, et al. A Molecular Survey of Cystic Echinococcosis in Sudan. Vet Parasitol. 2010; 169(3–4):340–6. [DOI] [PubMed] [Google Scholar]
- 29.Konyaev SV, Yanagida T, Nakao M, et al. Genetic diversity of Echinococcus spp. in Russia. Parasitology. 2013; 140(13):1637–47. [DOI] [PubMed] [Google Scholar]
- 30.Bart JM, Abdukader M, Zhang YL, et al. Genotyping of human cystic echinococcosis in Xinjiang, PR China. Parasitology. 2006; 133(Pt 5):571–9. [DOI] [PubMed] [Google Scholar]
- 31.Vural G, Baca AU, Gauci CG, et al. Variability in the Echinococcus granulosus Cytochrome C oxidase 1 mitochondrial gene sequence from livestock in Turkey and a re-appraisal of the G1–3 genotype cluster. Vet Parasitol. 2008; 154(3–4):347–50. [DOI] [PubMed] [Google Scholar]