Abstract
Background:
Coccidiosis causes morphologic alteration in intestinal mucosa resulting in reduction of absorptive surface. Anticoccidials used as feed additives may induce changes in the intestinal mucosa. This study was designed to assess intestinal morphometry in broilers infected with Eimeria under different anticoccidial treatments.
Methods:
To evaluate the effect of salinomycin and amprolium+ethopabate on intestinal morphometry in broilers experimental coccidiosis, in Tehran, Iran in May 2015, fifty-four Ross 308 birds were randomly divided into two challenged and unchallenged groups at the age of 12 days. The birds were challenged with Eimeria field isolate at day 14. Different growth and parasitological parameters including weight gain, feed consumption, FCR, macroscopic lesion score and oocyst score were recorded 7 d post-inoculation. Histological sections from four main parts of intestine (anterior, middle, lower intestines and cecum) were prepared and analyzed. Villus width and length and total mucosal thickness were measured microscopically.
Results:
Amprolium+ethopabate and salinomycin significantly reduced coccidiosis gross lesions in infected birds. Microscopically anticoccidial administration in the presence of infection has significantly increased the villus length while the presence of amprolium+ethopabate in the absence of infection has greatly increased the mucosal thickness and villi height in comparison to the control group.
Conclusion:
Anticoccidials may induce some histological changes in the mucosa when there is no parasite to be affected. Some of these effects may be advantageous for the intestinal epithelium integrity and hence the birds’ performance.
Keywords: Anticoccidal drug, Experimental coccidiosis, Histomorphometric analysis, Intestinal macroscopic lesions, Eimeria
Introduction
Eimeria, an apicomplexan protozoan parasite, is the cause of coccidiosis in the poultry industry. Six species of Eimeria are known to have an adverse economic impact on chicken (1) and annually impose nearly 2 billion US$ lose on commercial chicken producers at least 1.5 (2, 3). The integrity of the gastrointestinal (GI) tract is definite in digestion and absorption of nutrients and hence in the gainful poultry production (4). Eimeria proliferates in all parts of the intestinal tract, destructs the epithelial cells and thus disrupts nutrient absorption, weight gain and therefore financial effectiveness (4). The capability of the intestinal tract is adjustable based on different stimuli such as therapeutic or herbal feed additives and even pathogenic microorganisms. Anticoccidial food additives are the major route of coccidiosis prevention in today’s intensive broiler production industry. Amprolium occludes thiamin transporters in Eimeria species and salinomycin is an ionophore anticoccidial drug which alters the parasites’ cation balance (5, 6). Few studies have investigated the effect of anticoccidials on intestinal histomorphometry during a coccidial challenge.
We evaluated the influence of amprolium+ ethopabate and salinomycin on macroscopic intestinal lesions and microscopic intestinal morphology of infected broilers in experimental coccidiosis.
Materials and Methods
Oocyst collection
Litters of a commercial broiler house in west of Iran, Hamadan, was inspected for Eimeria oocysts (7, 8). Isolated oocysts were purified and identified (based on shape index) as E. acervulina, E. maxima, E. tenella, and E. necatrix. The challenge dose was chosen to induce the most pathologic lesions and the least mortality based on the experiment performed on four 14 d old chicks (9).
Animals and experiment design
In Tehran, Iran in May 2015, 41 one-day-old male broilers (Ross 308) were randomly allocated to 6 groups each included three replicates of 3. The experiment was conducted in battery cages under controlled condition. The negative control, drug control, and treatment groups are summarized in Table 1.
Table 1:
Experimental design for evaluation of the effect of salinomycin and amprolium+ethopabate on broilers intestinal morphometry
| Groups | Inoculated infective dose | Anticoccidials |
|---|---|---|
| 1 | 250000 oocyst/bird | Salinomycin @ 500 ppm |
| 2 | 250000 oocyst/bird | Amprolium+ethopabate @ 500 ppm |
| 3 | 250000 oocyst/bird | Non-medicated |
| 4 (salinomycin control) | - | Salinomycin |
| 5 (amprolium+ethopabate control) | - | Amprolium+ethopabate |
| 6 (Negative control) | - | Non-medicated |
The diet was formulated to meet all essential nutrients for the birds, and food and water were provided ad-libitum. No vaccine was administered during the experiment. To enable individual data recording, tags were attached to the birds’ leg.
At day 12th, anticoccidials were introduced in the birds’ diets and the diet was provided up to 7 d post-inoculation. Kimiamycin® 12 (Kimiafaam Group, Iran) and Ethoamprox® (JamedatAfagh Pharmaceutical Company, Iran) were prescribed 500 ppm in the feed. Overall, 14-day-old birds were challenged orally. On the day of experimental challenge and a week later, all of the birds were weighed individually. Weight gain (WG) and feed intake (FI) were recorded. Gross lesion score (GLS), microscopic lesion score (MLS) and oocysts index (OI) were investigated after necropsy and feed conversion ratio (FCR) was calculated consequently (8, 10).
Pathological assay
At the end of the experiment (21st day), all the birds were euthanized by neck severing and necropsied. For the preparation of pathologic samples the whole GI tract was removed, slit open and both the mucosal surface and the unopened serosal surface of the intestine were examined for macroscopic lesions (11). Fragments of about two centimeters from the upper intestine (duodenum and jejunum), mid intestine, lower intestine (ileum) and cecum were cut, pinned and stowed in 10% buffered formalin solution. The segments were processed by routine histology methods, embedded longitudinally in paraffin, sectioned 5 μm thick parallel to the cut edge (Microm, Germany) and stained with hematoxylin and eosin (H&E) for microscopic evaluations (Nikon Eclipse E400, USA). At least 6 histological sections for each bird, totalizing about 300 slides were prepared and analyzed using H&E staining.
Microscopic lesion score was assessed by evaluating villus height, total mucosal thickness, the severity of villus infection and the distribution scores (12). Villus height was determined by measuring the length of 10 intact villi, from the crypt mouth to the apical villus region. Tip of villus to the base of the crypt was the criteria for total mucosal thickness. The percentage of the parasitized villi in four microscopic fields was used for determination of the severity of villus infection. The presence of parasite stage with ×10 magnification in four microscopic fields was used for establishing the distribution score (12).
Statistical analysis
ANOVA, two-way t-test and Tukey’s multiple range tests were used to determine the significance of differences among groups (SPSS 15.0, Chicago, IL, USA) at P≤0.05.
Results
Growth performance
All of the unchallenged groups including treated and untreated had the highest average weight gain (352–371g) while the lowest level (148 g) was recorded for the untreated challenge group (P<0.05). The average weight gain of challenged groups either treated with salinomycin or amprolium+ ethopabate was significantly lower than unchallenged groups. However, salinomycin was more efficient weight gain in the experimental challenge. The same results were obtained for feed conversion ratio (Table 2).
Table 2:
Evaluation of intestinal morphometry in chicken with experimental coccidiosis receiving dietary anticoccidials
| Experimental group | Average weight gain (g) | Oocyst index | Feed conversion ratio (g/g) | Gross Lesion score | Villus height (μm) | Total mucosal thickness (μm) | Severity of villus infection | Distribution score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Eimeria mix sp. isolate | Salinomycin | 230.5b | 2b | 1.74c | 1.5b | 556a | 884a | 1.89a | 1.7a |
| 2 | Eimeria mix sp. isolate | Amprolium + ethopabate | 172.7c | 2.1b | 2.1b | 2.1b | 531a | 856b | 2.16a | 1.97a |
| 3 | Eimeria mix sp. isolate | Untreated | 148d | 2.6a | 2.75a | 2.3a | 456b | 822c | 1.9a | 1.7a |
| 4 | unchallenged | Salinomycin | 368a | 0 | 1.55c | - | 498b | 833c | 0 | 0 |
| 5 | unchallenged | Amprolium+ethopabate | 352a | 0 | 1.58c | - | 552a | 874a | 0 | 0 |
| 6 | unchallenged | Untreated | 371a | - | 1.65c | - | 477b | 808d | 0 | 0 |
Means sharing the same superscripts within each section do not differ (P≤0.05).
Oocyst scores and gross lesions
As it was expected no oocyst was observed in unchallenged groups, while the oocyst index in challenged untreated group was significantly higher than challenged treated groups (P<0.05). The challenged untreated group had the highest gross lesion score (2.3) and the lowest GLS was determined for birds challenged-treated with amprolium+ethopabate (2.1) and challenged-treated with salinomycin (1.5), respectively. The intestinal gross examination of birds showed varying degrees of lesions in different parts of the intestines. These differences were significant between treated and untreated challenged groups. Gross lesions were varying from few scattered petechiae, ladder-like necrosis lesions to ballooned intestine, severe hemorrhage, and cecal cord.
Intestinal histomorphometry
In this study, histopathological findings including villus height, total mucosal thickness, the severity of villus infection, and distribution score were measured. The distribution score and the severity of villus infection in different groups were between 1.7–1.97 and 1.9–2.16 respectively, which the highest value for both indexes belonged to amprolium+ethopabate treated groups.
No differences were observed in distribution score and the severity of villus infection among different treatments while the total mucosal thickness showed significant differences between different groups. Comparing the total mucosal thickness in different groups reveals better results in the two treated groups in comparison with the control group. The highest mucosal thickness was recorded for the challenged birds receiving salinomycin and unchallenged birds treated with ethoamprox. The lowest mucosal thickness was seen in the control group (unchallenged-untreated birds).
The villus height in birds challenged and treated with both of drugs and unchallenged birds treated with ethoamprox were significantly higher than the control group, the unchallenged birds treated with kimiamycin and the challenged-untreated birds, while no difference was observed between the two treated groups. There was a significant difference in villus height of unchallenged birds treated with ethoamprox (552μm) comparing with unchallenged either untreated or treated birds with kimiamycin (498 μm) (Table 2).
Histopathologic alterations
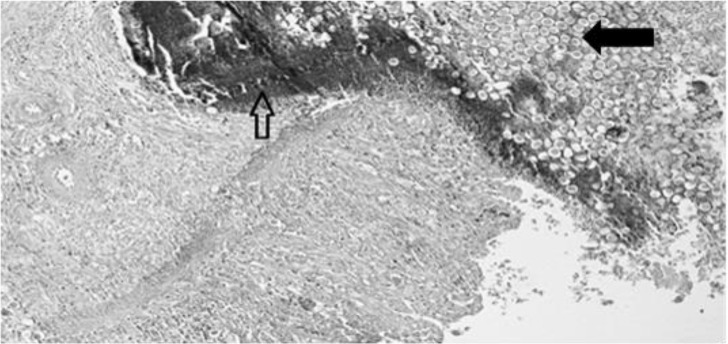
In challenged untreated groups small intestine histopathological observation included diffusely infiltrating and severely expanding lamina propria with marked mixed inflammatory cell infiltrate mostly composed of lymphoblasts, with fewer histiocytes, plasma cells, and eosinophils. This inflammatory reaction extended multifocally and transmurally with mild accumulations on the serosal surface. Within the luminal epithelial cells, coccidian parasites at various stages of development were obvious. Abundant macrogamonts characterized by a peripheral ring of large eosinophilic granules, less abundantly microgamonts and schizonts were seen. Tip of villi was mostly broken off leading to truncation and fusion of villi and thickening of the mucosa. In some cases, severe damage of mucosa leading to hemorrhagic enteritis were noted (Fig. 1).
Fig. 1:
Histopathological section (H&E staining) (200×). Hemorrhagic enteritis, diffuse infiltration, severally expanding lamina propria (hollow arrow) and numerous coccidian parasites at various stage of development (filled arrow) are visible
Discussion
Infection with Eimeria causes morphologic alteration in intestinal mucosa resulting in the reduction of absorptive surface. Various methods including gross and microscopic lesion scoring can be applied to evaluate the severity of coccidiosis, different drug’s efficacy rate and routine coccidiosis screening in broiler flocks (13, 14). We tried to assay the intestinal morphometric alteration in experimental coccidiosis in broilers treated with salinomycin and amprolium+ ethopabate.
Administration of both drugs improved growth performance, gross lesion score and different microscopic lesions measured in this study, in comparison to challenged untreated group. No significant differences in the average weight gain and FCR were noted between unchallenged birds treated with either drug with the control group. Salinomycin had a better effect on average weight gain and feed conversion ratio. These results are corresponding to the findings (15), that reported amprolium+ethopabate has a good effect in mean body weight, FCR, villus height and mucosal thickness and (16), salinomycin could induced growth promotor effects and improve feed conversion and body weight gain via changing the composition and activities of gut microflora. A study on the effect of four anti-coccidial drugs on the normal chicken intestinal morphology concluded that anticoccidial drugs diclazuril, semduramicin, salinomycin, and maduramicin in the absence of coccidiosis had adverse effects on chicken performance and intestinal morphology (17). Likewise, salinomycin may suppress weight gain and FCR in the absence of coccidiosis (18), in this experiment unchallenged birds receiving salinomycin had average weight gain and FCR comparable to the negative control group.
Macroscopically amprolium+ethopabate and salinomycin significantly reduced coccidiosis gross lesions in challenged birds comparing to untreated birds. Microscopically anticoccidial administration in the presence of infection has significantly increased the villus length of the intestinal mucosa. Long villi and shallow crypts are essential for normal nutrient absorption in birds. Additionally, the presence of amprolium+ethopabate in unchallenged birds has greatly increased the mucosal thickness and villi height in comparison to the control group. Increase of villus height in the unchallenged group treated with amprolium+ethopabate was due to direct drug effect (19), about diclazuril. Regarding salinomycin in unchallenged birds, the total mucosal thickness was increased but the villus height was not significantly influenced. The severity and distribution scores, indicative of parasite reproduction, were not affected by anticoccidials used in this study. Gross lesion alone can underestimate Eimeria infection within broiler flocks (20); hence checking microscopic lesions may be used complementarily for monitoring coccidiosis and efficacy of different control measures implied (6). Microscopy has added benefits to gross lesion scoring as it readily detects not only oocysts but also developmental stages of the parasite (5).
In a study designed to assess the potential impact of litter fermentation and diclazuril administration on intestinal macroscopic and microscopic lesions caused by E. acervulina, both measurements reduced villus atrophy and induced greater absorptive area (19). Villus cell proliferation or apoptosis is an adaptive reaction to alterations imposed by different stimuli or pathogens (21). Broilers with dietary salinomycin had vaguely enhanced FCR but reduced villus height (22). With Eimeria proliferating in the mucosa, the effect of anticoccidials can be attributable to their effect on the parasite life cycle (18, 19) while with the absence of parasite the drugs may induce different host interaction in the intestinal mucosa. Feed components, pathogens and etc. interact with the normal chicken growth rate by modifying proliferation and maturation of cells in the small intestinal mucosa (21, 23). As anticoccidials are used as feed additives and can influence the intestinal cell proliferation studying the effects of these compounds in the presence and absence of Eimeria infection may lead to a better therapeutic outcome.
Conclusion
Microscopic lesions, as gross lesions, in the intestinal mucosa of broilers infected with Eimeria sp. is directly influenced by anticoccidial feed additives. The anticoccidial can induce some histological changes in the mucosa when there is no parasite to be affected. Some of these outcomes may be advantageous for the integrity of the intestinal epithelium and hence the birds’ production performance.
Acknowledgements
The authors would like to thank the faculty of Veterinary Medicine, University of Tehran for funding the project number 12/6/7506009.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 2002; 15 (1): 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav A, Gupta SK. Study of resistance against some ionophores in Eimeria tenella field isolates. Vet Parasitol. 2001; 102: 69–75. [DOI] [PubMed] [Google Scholar]
- 3.Pirali-Kheirabadi K, Zamani-Moghadam A, Abdi F, Bahonar AR. The effect of administration of anticoccidial drugs on oocysts shedding and performance in experimental coccidiosis in broiler chicken. Int J Vet Res. 2008; 2: 67–73. [Google Scholar]
- 4.Amerah AM, Ravindran V. Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poult Sci. 2015; 94(4):673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin MA, Brown J, Bounous DI. Use of microscopic lesion scores, gross lesion scores and oocyst count scores to detect Eimeria maxima in chickens. Avian Pathol. 1998; 27 (4): 405–408. [DOI] [PubMed] [Google Scholar]
- 6.Orengo J, Buendía AJ, Ruiz-Ibáñez MR, et al. Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Vet Parasitol. 2012; 185(2–4): 158–163. [DOI] [PubMed] [Google Scholar]
- 7.Holdsworth PA, Conway DP, McKenzie ME, et al. World association for advancement of veterinary parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet Parasitol. 2004; 121: 189–212. [DOI] [PubMed] [Google Scholar]
- 8.Conway DP, McKenzie ME. Poultry coccidiosis diagnosis and testing procedures. Iowa: Blackwell; 2007. [Google Scholar]
- 9.Arabkhazaeli F, Nabian S, Modirsaneii M, et al. Biopathologic characterization of three mixed poultry Eimeria spp. isolates. Iran J Parasitol. 2011; 6 (4): 23–32. [PMC free article] [PubMed] [Google Scholar]
- 10.Daugschies A, Gässlein U, Rommel M. Comparative efficacy of anticoccidials under the conditions of commercial broiler production and in battery trials. Vet Parasitol. 1998; 76: 163–171. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JK, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970; 28: 30–36. [DOI] [PubMed] [Google Scholar]
- 12.Idris AB, Bounous DI, Goodwin MA, et al. Lack of correlation between microscopic lesion scores and gross lesion scores in commercially grown broilers examined for small intestinal Eimeria spp. coccidiosis. Avian Dis. 1997; 388–391. [PubMed] [Google Scholar]
- 13.Chapman HD. Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl. Int J Parasitol. 1998; 28: 1141–1144. [DOI] [PubMed] [Google Scholar]
- 14.Conway DP, Dayton AD, McKenzie ME. Comparative testing of anticoccidials in broiler chickens: The role of coccidial lesion scores. Poult Sci. 1999; 78: 529–535. [DOI] [PubMed] [Google Scholar]
- 15.Bahadoran S, Hassanpour H, Pirali-Kheirabadi K, et al. Effect of Clopidol and Amprolium/Ethopabate on Performance and Intestinal Morphology of Chickens with Experimental Coccidiosis. Kafkas Üniversitesi Veteriner Fakültesi Dergisi. 2014; 20(4): 571–576. [Google Scholar]
- 16.Diarra MS, Silversides FG, Diarrassouba F, et al. Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, Clostridium perfringens and Enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escherichia coli isolates. Appl Environ Microbiol. 2007; 73(20): 6566–6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassanpour H, Bahadoran S, Koosha S, et al. Effect of diclazuril, semduramicin, salinomycin and maduramycin as preventive anticoccidial drugs on chicken intestinal morphology. Global Vet. 2010; 5: 1–5. [Google Scholar]
- 18.Tipu MA, Pasha TN, Zulfiqar A. Comparative efficacy of salinomycin sodium and neem fruit (Azadirachta indica) as feed additive anticoccidials in broilers. Int J Poultry Sci. 2002; 4: 91–93. [Google Scholar]
- 19.Assis RC, Luns FD, Beletti ME, et al. Histomorphometry and macroscopic intestinal lesions in broilers infected with Eimeria acervulina. Vet Parasitol. 2010; 168(3-4): 185–189. [DOI] [PubMed] [Google Scholar]
- 20.Conway DP, McKenzie ME, Dayton AD. Relationship of coccidial lesions scores and weight gain in infections of Eimeria acervulina, E. maxima and E. tenella in broilers. Avian Pathol. 1990; 19: 489–496. [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa T, Masuda T, Tsukahara T, et al. Morphometric and Histopathological Evaluation of a Probiotic and its Synergism with Vaccination against Coccidiosis in Broilers. Anim Sci Let. 2014; 1(1): 33–49. [Google Scholar]
- 22.Czerwiński J, Højberg O, Smulikowska S, et al. Effects of sodium butyrate and salinomycin upon intestinal microbiota, mucosal morphology and performance of broiler chickens. Arch Anim Nutr. 2012; 66:102–116. [DOI] [PubMed] [Google Scholar]
- 23.Uni Z, Zaiger G, Gal-Garber O, et al. Vitamin A deficiency interferes with proliferation and maturation of cells in the chicken small intestine. Br Poult Sci. 2000; 41: 410–415. [DOI] [PubMed] [Google Scholar]