Abstract
Background:
Wild boars (Sus scrofa) are distributed worldwide and found in many parts of Iran. Although S. scrofa is reservoirs for many parasites, there is little data on helminthic prevalence in them. We aimed to survey the status of helminthic infections in S. scrofa in the Mazandaran Province of northern Iran.
Methods:
Twenty-one wild boars were captured and examined for helminth infection during Dec 2012–Mar 2014. Adult worms such as Macracanthorhynchus hirudinaceus were identified by helminth size and shape, and the arrangement of the proboscis hooks. The sedimentation and flotation techniques were used to detect parasite eggs and larvae in faecal samples. Muscle samples were also surveyed for Trichinella larvae by artificial digestion method.
Results:
Of the 21 samples, 13 (61.9%) were infected with one or more helminth species. Seven helminth types were identified in the alimentary track, comprising 5 nematodes, 1 trematode, and 1 acanthocephalan, with prevalence rates of Macracanthorhynchus hirudinaceus (57.14%), Globocephalus spp. (33.33%), Trichuris suis (19.04), Gongylonema pulchrum (14.28%), Fasciola hepatica (14.28%), Dioctophyma renale (4.76%), and Ascaris suum (4.76%).
Conclusion:
Wild boars might be involved in transmitting zoonotic parasites to humans. The abundance of these animals near human habitation creates favorable conditions for infection. So the risk of parasitic helminth diseases increases in other animals and humans.
Keywords: Wild boar, Parasitic helminths, Prevalence, Iran
Introduction
Wild boars (Sus scrofa) are distributed worldwide (1) and are omnivorous, with diets comprising insect larvae, amphibians, reptiles, mushrooms, birds, bird eggs, small rodents, fruits, etc. (2). Owing to their natural feeding habits, S. scrofa is host and reservoir of many parasites; they play an important role in the transmission of infections to domestic animals and humans (2).
Sus scrofa may pass feces including infectious agents in the farming fields and cause to contaminate water resources and the subsequent these agents influence to crops and plants. Therefore, people who eat these plants are infected (3).
Wild boars are found in many parts of Iran, especially in the mountainous areas. They usually cause destruction of crops and are often killed by farmers or hunted for meat by Christian Armenians (3). Although S. scrofa is widely distributed in northern Iran, little information is available about the parasitic infections in wild boars in northern Iran.
We determined the status of helminthic infections in wild boars in the Mazandaran Province of northern Iran.
Materials and Methods
Ethical approval
Ethical approval of present study was received from the Ethics Committee in Mazandaran University of Medical Sciences, Mazandaran, Iran.
Study area
This study was conducted in the suburbs of 3 different cities (Sari, Savadkuh, and Behshahr) in the Mazandaran Province, Iran. The Mazandaran province (36° 33′ 56″ N 53° 03′ 32″ E) is located in northern Iran on the southern coast of the Caspian Sea (Fig. 1). It covers an area of approximately 23842 km2 and has a population of 2,922,432 individuals. This province has a moderate and subtropical climate and is geographically divided into the three regions: plains, forests, and mountains.
Fig. 1:
Map of Mazandaran Province, northern Iran and 3 different cities (Sari, Savadkuh, and Behshahr), Iran
Sample collection
Twenty-one wild boars were captured from Dec 2012 to Mar 2014. During sampling, the shooting site, sampling time, sex, and age of each boar were recorded. Based on tooth shape and development and physical appearance, the samples were categorized into three age groups (4). The abdominal viscera of the animals were removed immediately after capture, placed in plastic bags, labeled, and sent to the parasitology laboratory, Mazandaran University, Iran. Necropsy was performed in the laboratory, and in the first step, internal organs such as oesophagus, stomach, small and large intestines, liver, and muscles, including the diaphragm were examined macroscopically for the presence of helminthic parasites. All the parasite worms were identified (5). For example in present study, M. hirudinaceus was identified according to helminth size and shape, and the arrangement of the probosci’s hooks.
To recover non-visible helminths, the stomach and intestine were separately washed in buckets with tap water and squeezed through 500-μm and 150-μm sieves. The sedimentation and flotation techniques were used to detect parasite eggs and larvae in faecal samples collected from the intestinal tract (6). The ova were identified based on morphological features (shape, structure, and presence of larva) (7). In addition, muscle samples were surveyed for Trichinella larvae by two direct and artificial digestion methods.
Statistical analysis
SPSS version 16.0 (Chicago, IL, USA) was used for all statistical analyses. Chi-squared (χ2) test was performed to investigate the significance of the differences in prevalence of helminth infections between age and sex in the different groups. The level of significance was P<0.05 for all calculations.
Results
Of the 21 wild boars (11 females and 10 males) examined for helminthic infections, 13 (61.9%) were infected with one or more helminth species. Seven helminth types were identified, comprising 5 nematodes, 1 trematode, and 1 acanthocephalan. The prevalence of helminth species in the suburbs of 3 different cities in the Mazandaran Province are shown in Table 1. The highest prevalence was observed for M. hirudinaceus (57.14%) (Fig. 2), while the lowest was recorded for Dioctophyma renale (4.76%) and Ascaris suum (4.76%). Helminth infections were found in 10 of 11 wild boars (90.9%) in Savadkuh, 3 of 5 (60.0%) in Behshahr, and 0 of 5 (0%) in Sari. Of the 13 (61.9%) wild boars infected with helminth species, Poly-parasitism was more common (84.62%) than mono-parasitism (15.38%). Poly-parasitism with M. hirudinaceus was the most frequent (Table 2). The age of the wild boars could not influence the prevalence of infection. In addition, there was no significant difference in the prevalence rates of infection in males and females (Table 3).
Table 1:
Prevalence of helminth infections in Sus scrofa in Mazandaran, northern Iran
| Parasites | Infected wild boars number | Prevalence (%) | ||
|---|---|---|---|---|
| Behshahr City | Sari City | Savadkuh City | ||
| M. hirudinaceus | 9 | 0 | 3 | 57.14 |
| Globocephalus spp. | 7 | 0 | 0 | 33.33 |
| T. suis | 3 | 0 | 1 | 19.04 |
| G. pulchrum | 3 | 0 | 0 | 14.28 |
| F. hepatica | 2 | 0 | 1 | 14.28 |
| Dioctophyma renale | 1 | 0 | 0 | 4.76 |
| A. suum | 1 | 0 | 0 | 4.76 |
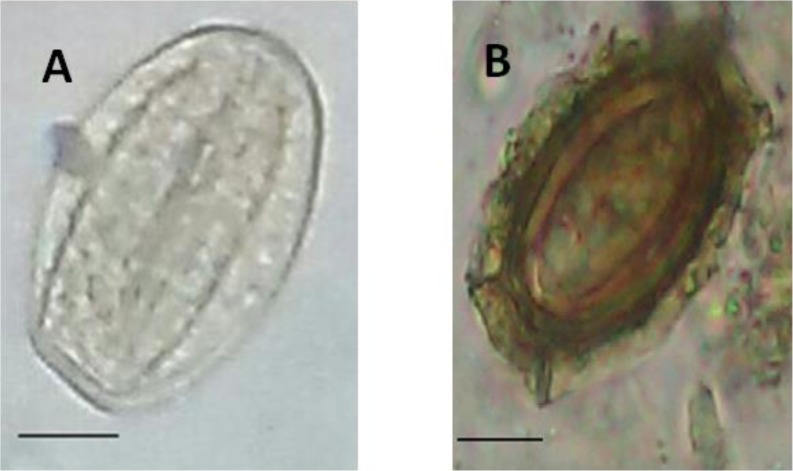
Fig. 2:
Endoparasites of isolated from Sus scrofa
A: Egg of Macracanthorhynchus hirudinaceus; B: Egg of Trichuris suis. Bars=15 μm. Eggs are shown by ×40 magnification (Original)
Table 2:
Prevalence of multiple infections (helminths) in 21 wild boars in Mazandaran, northern Iran
| Species of parasite per wild boar | Number of infected wild boars |
|---|---|
| M. hirudinaceus | 2 |
| M. hirudinaceus + Globocephalus spp. | 2 |
| M. hirudinaceus + T. suis | 1 |
| M. hirundinaceus + F. hepatica | 1 |
| Globocephalus spp. + A. suum | 1 |
| M. hirudinaceus + Globocephalus spp. + G. pulchrum | 2 |
| M. hirudinaceus + Globocephalus spp. + T. suis | 1 |
| M. hirudinaceus + T. suis + F. hepatica | 1 |
| M. hirudinaceus + F. hepatica + G. pulchrum | 1 |
| M. hirudinaceus + Globocephalus spp. + T. suis + Dioctophyma renale | 1 |
| Total number of infected wild boars | 13 |
Table 3:
Prevalence of helminth infections of wild boars in relation with age and sex
| Variable | Number examined | Number infected | Percentage (%) | P-value |
|---|---|---|---|---|
| Age (yr) | > 0.05 | |||
| ≤ 1 | 9 | 3 | 33.3 | |
| 1< and <3 | 6 | 4 | 66.6 | |
| 3–10 | 6 | 6 | 100 | |
| Sex | > 0.05 | |||
| Male | 10 | 8 | 80 | |
| Female | 11 | 5 | 45.4 | |
| Total | 21 | 13 | 61.9 |
Discussion
There is little data available on helminth prevalence in S. scrofa in Iran, and there have been no comprehensive studies on worm prevalence in the Mazandaran Province. The results of this study showed a helminth prevalence of 61.9% in 21 S. scrofa from the Mazandaran Province of Iran. This prevalence was higher than that reported from Talesh City, in the Guilan Province of Iran (34%) (8), but was almost similar to results (1), who reported a prevalence of 74% in the north, northeast, and southwest of Iran, and another (9), who reported a prevalence of 58.3% in Luristan in western Iran. Similarly, in Tamil Nadu, south India opined which majority of wild boars surveyed (62%) had at least one helminth species in the internal organs (10). The reasons for such similarity in Tamil Nadu and Mazandaran might be assigned to the feeding regime of wild boars and climatic conditions of these two areas.
In the present study, we found seven types of helminths in wild boars, while 16 species of worms were reported in 57 wild boars (1). Of the 7 important nematode species included in the veterinary report (11), two were found in our study: A. suum and T. suis.
Our results also showed that M. hirudinaceus was the most prevalent helminth, followed by Globocephalus spp. While this is consistent with the other results (9), it is in contrast to the results (1), who reported that Globocephalus spp. was the most prevalent helminth in wild boars. M. hirudinaceus is distributed worldwide and has been reported in different hosts such as dogs, pigs, and even humans (12). While this parasite has been frequently reported in kinds of dogs and wild boars in Iran (13), human infection has not yet been reported. Definitive hosts have been known to be infected after ingestion of different beetle species (14). In this study, 12 of the 21 (57.14%) S. scrofa were infected with this parasite, indicating that these animals could play an important role in the infection of humans with M. hirudinaceus in Iran, especially in the suburban and rural areas.
In this study, the prevalence rate of 33.33% was recorded for Globocephalus spp. The moderately high prevalence of Globocephalus spp. might be due to the suitable conditions for resistance against the infectious larvae in this region. Wild boars infected with Globocephalus spp. suffer from anaemia and pathologic changes in the mucosa (15).
T. suis (Fig. 2), found in this study, is an intestinal nematode found in wild boars (16), and is similar to Trichuris trichura in humans, with respect to life cycle, morphology, and symptoms (17). Additionally, the successful transmission of this nematode from pigs to humans was reported (18).
G. pulchrum is another nematode identified in the present study with a prevalence of 14.28%. A higher prevalence (35%) of this worm was reported (1). Humans could be accidentally infected with G. pulchrum (19).
F. hepatica, a liver fluke identified in the current study with a prevalence of 14.28%, was reported to have a prevalence of 4% (1). In the northwest region of Spain, a prevalence of 11.2% for F. hepatica was found in 358 wild boars from Galicia (20). This was the first study to suggest that wild boars could be primary hosts for F. hepatica. As people consume forest herbs and plants in the northern regions of Iran, wild boars might be considered reservoir host of fascioliasis for human infection.
Among the helminths identified in this study, egg Dioctophyma renale was also observed in the faeces; while eggs of mentioned species are shed in the urine, there is the possibility that the eggs passed through the alimentary system of wild boar after the consumption of infected host.
Furthermore, the prevalence of 4.76% recorded for A. suum in this survey was almost the same as that reported in three geographical areas (north, northeast, and southwest) of Iran (5%) (1), but was lower than that reported in Eastern Ghana (12.7%) (21) and Botswana (54.6%) (22). Although the prevalence of A. suum recorded in the present study was not very high, it is nevertheless important because this parasite has been known to cause severe visceral larval migration, liver and lung lesions, and eosinophilic pneumonia in humans (23).
However trichinelliasis frequently occurs in extensive varieties of canids in Iran (24), in this study, no wild boars were infected.
In the three regions of the Mazandaran Province studied, only Sari city had no boars with helminthic infection. This may be due to the different climatic, geographical, and environmental conditions of this region, compared to the other regions. Sari city has comparatively lesser vegetation than the two other cities and is located in a flat area.
Conclusion
Wild boars might play an important role in transmitting zoonotic parasites to humans. The abundance of these animals near human habitation creates favorable conditions for infection. Therefore, the risk of protozoan diseases increases in other animals and humans.
Acknowledgements
The authors would like to appreciate very much for kind collaboration of Dr. Badali. They also would like to thank the financial support by Vice Chancellors for Research of Mazandaran University of Medical Sciences, Iran (project no. 901).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Eslami A, Farsad-Hamdi S. Helminth parasites of wild boar, Sus scrofa, in Iran. J Wildl Dis. 1992; 28(2):316–8. [DOI] [PubMed] [Google Scholar]
- 2.Mowlavi G, Massoud J, Mobedi I, et al. Very highly prevalent Macracanthorhynchus hirudinaceus infection of wild boar Sus scrofa in Khuzestan province, south-western Iran. Helminthologia. 2006;43(2):86–91. [Google Scholar]
- 3.Yaghoobi K, Sarkari B, Mansouri M, Motazedian MH. Zoonotic intestinal protozoan of the wild boars, Sus scrofa, in Persian Gulf’s coastal area (Bushehr province), Southwestern Iran. Vet World. 2016; 9(10):1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anezaki T. Estimating age at death in Jomon Japanese wild boar (Sus scrofa leucomystax) based on the timing of molar eruption in recent comparative samples. Mammal Study. (2009);34(2):53–63. [Google Scholar]
- 5.Mehlhorn H, Düwel D, Raether W. Atlas de parasitolog´ıa veterinaria. Grass, Barcelona: 1992; p. 436. [Google Scholar]
- 6.Ziomko I, Cencek T. Outline of laboratory diagnosis of farm animal parasitoses. Publisher National Veterinary Research Institute, Pulawy; 1995. [Google Scholar]
- 7.Thienpont D, Rochette F, Vanparijs O. Diagnosing helminthiasis by coprological examination. Published in Beerse by Janssen Research Foundation; 1986. [Google Scholar]
- 8.Garedaghi Y, Mojallal S, Ouzbandi A. Helminth Parasites of a hunted-wild boar (Sus scrofa) in the Talesh City, North of Iran. Bull Env Pharmacol. Life Sci. 2014;3(3):247–250. [Google Scholar]
- 9.Solaymani-Mohammadi S, Mobedi I, Rezaian M, et al. Helminth parasites of the wild boar, Sus scrofa, in Luristan province, western Iran and their public health significance. J Helminthol. 2003; 77(3):263–7. [DOI] [PubMed] [Google Scholar]
- 10.Boon A, Jayathangaraj MG, Palanivelrajan M, et al. Prevalence of helminthic fauna in wild pigs in comparison with domestic pigs: A study in the adjoining areas of Mudumalai, Anamalai and Sathyamangalam tiger reserves, Tamil Nadu south India. J. Parasitol. Vector Biol. 2015; 7(3): 46–52. [Google Scholar]
- 11.Nansen P, Roepstorff A. Parasitic helminths of the pig: factors influencing transmission and infection levels. Int J Parasitol. 1999; 29(6):877–91. [DOI] [PubMed] [Google Scholar]
- 12.Leng YJ, Huang WD, Liang PN. Human infection with Macracanthorhynchus hirudinaceus Travassos, 1916 in Guangdong Province, with notes on its prevalence in China. Ann Trop Med Parasitol. 1983; 77(1):107–9. [DOI] [PubMed] [Google Scholar]
- 13.Arbabi M, Doroodgar A, Hooshyar H, et al. Macracanthorhynchus hirudinaceus in carnivores of Kashan region, Iran. In Third National Congress on Medical Parasitology, Sari, Iran 2001. [Google Scholar]
- 14.Mobedi I, Arfaa F, Vedadi L. The possible role of dung-pusher beetles in the epidemiology of intestinal helminths. Bull Soc Pathol Exot Filiales. 1971; 64(6):924–5. [PubMed] [Google Scholar]
- 15.Soulsby eJL. Helminths, arthropods and protozoa of domesticated animals. Can Vet J. 1968; 10(8):223. [Google Scholar]
- 16.Pattison HD, Thomas RJ, Smith WC. A survey of gastrointestinal parasitism in pigs. Vet Rec. 1980; 107(18):415–8. [DOI] [PubMed] [Google Scholar]
- 17.Holland C. Neglected infections-trichuriasis and strongyloidiasis. Impact of Helminth Infections on Human Nutrition. London: Taylor and Francis; 1987; 161–201. [Google Scholar]
- 18.Beer RJ. Experimental infection of man with pig whipworm. Br Med J. 1971; 2(5752):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleier T, Hetzel U, Bauer C, Behlert O, Burkhardt E. Gongylonema pulchrum infection and esophageal squamous cell carcinoma in a vari (Lemur macaco variegata; Kehr 1792). J Zoo Wildl Med. 2005; 36(2):342–5. [DOI] [PubMed] [Google Scholar]
- 20.Mezo M, González-Warleta M, Castro-Hermida JA, et al. The wild boar (Sus scrofa Linnaeus, 1758) as secondary reservoir of Fasciola hepatica in Galicia (NW Spain). Vet Parasitol. 2013; 198(3–4):274–83. [DOI] [PubMed] [Google Scholar]
- 21.Tiwari k. P, Chikweto A, Belot G, et al. Prevalence of intestinal parasites in pigs in Grenada, West Indies. W Indian Vet J. 2009; 9(1):22–27. [Google Scholar]
- 22.Nsoso SJ, Mosala KP, Ndebele RT, Ramabu SS. The prevalence of internal and external parasites in pigs of different ages and sexes in Southeast District, Botswana. Onderstepoort J Vet Res. 2000; 67(3):217–20. [PubMed] [Google Scholar]
- 23.Kakihara D, Yoshimitsu K, Ishigami K, et al. Liver lesions of visceral larva migrans due to Ascaris suum infection. Abdom Imaging. 2004; 29(5):598–602. [DOI] [PubMed] [Google Scholar]
- 24.Mobedi I, Arfaa F, Madadi H, Movafagh K. Sylvatic focus of trichinosis in the Caspian region, northern Iran. Am J Trop Med Hyg. 1973; 22(6):720–2. [DOI] [PubMed] [Google Scholar]