Abstract
IN BRIEF Type 2 diabetes can be prevented or delayed in people with prediabetes through participation in an intensive lifestyle change program (LCP), particularly one based on the Diabetes Prevention Program research study. Digital health offers opportunities to extend the reach of such LCPs and possibly improve on these programs, which traditionally have been delivered in person. In this review, we describe the current state of evidence regarding digital health–supported LCPs and discuss gaps in research and opportunities for future efforts.
The United States is confronting a type 2 diabetes epidemic; over 30 million Americans have diabetes, about 95% of whom have type 2 diabetes (1). The prevalence of type 2 diabetes will likely continue to grow because 84 million American adults have prediabetes (1). Prediabetes can be diagnosed through laboratory testing in “individuals whose glucose levels do not meet the criteria for diabetes, but are too high to be considered normal,” according to the American Diabetes Association (2). In addition to being associated with elevated risks for negative cardiovascular outcomes (3), prediabetes is associated with a high risk of progression to type 2 diabetes. Progression rates vary across populations; one estimate reports an incidence rate of type 2 diabetes of 35.6/1,000 person-years among people with prediabetes, whereas another observed that 29% of people with prediabetes progressed to type 2 diabetes within 3 years (4–6). An estimated 74% of people with pre-diabetes develop type 2 diabetes in their lifetime (7).
Thankfully, many people with prediabetes can prevent or delay type 2 diabetes by participating in an intensive lifestyle change program (LCP) modeled after the Diabetes Prevention Program (DPP) research study (8). Briefly, the DPP randomized, controlled trial (RCT) included 3,234 people with impaired fasting glucose or impaired glucose tolerance. Participants were randomized to receive one of three interventions: an in-person LCP that fostered the development of skills to achieve a healthy lifestyle, metformin therapy, or a placebo/standard of care control. The LCP and metformin reduced the incidence of type 2 diabetes after 3 years by 58 and 31%, respectively relative to placebo. Longer-term outcomes were reported in the Diabetes Prevention Program Outcomes Study (DPPOS) (9), which demonstrated that those who participated in the LCP experienced a 27% reduced incidence of type 2 diabetes relative to placebo 15 years later.
The original DPP LCP has been translated for more practical use and tested in a variety of settings using both health care professionals and lay individuals to deliver the program to individual participants and groups (10–13).
The Centers for Disease Control and Prevention (CDC) oversees the National Diabetes Prevention Program (National DPP), which harnesses the success of the DPP and DPPOS studies, as well as related translational evidence. The goals of the National DPP are to expand access to LCPs, increase program uptake and retention, and provide quality assurance for these programs via the Diabetes Prevention Recognition Program (DPRP) (14).
The CDC DPRP grants recognition to LCPs that deliver an approved curriculum and achieve other program standards, including an average 5% weight loss among participants. As of March 2018, the CDC recognized 1,779 organizations delivering in-person LCPs and at least 120 organizations delivering LCPs via other modes, including online delivery or distance learning (15). More than 160,000 people have participated in the National DPP as of 9 March 2018 (K.K., P. Schumacher, K. Henriksen, personal communication), which is a remarkable achievement. Yet, millions more Americans with prediabetes could still benefit from the program.
As the CDC and its partner organizations, including the American Medical Association (AMA), work to scale the National DPP, digital health modes of LCP delivery offer opportunities to extend the reach of the program and possibly improve LCPs. The AMA classifies digital health solutions into seven categories: remote monitoring for efficiency, remote monitoring and management for improved care, clinical decision support, patient engagement, tele-visits, point-of-care, and tools providing consumer access to clinical data (16). Digital health solutions that incorporate remote monitoring, patient engagement, and tele-visits are particularly relevant to LCPs. Here, we describe the evidence regarding digital health–supported delivery of LCPs intended to prevent type 2 diabetes and discuss gaps in research and future opportunities.
Evidence Review
The key components of LCPs include coaching; self-monitoring of diet, physical activity, and weight; skills development; and group support (Table 1). Digital health can supplement or replace standard delivery modes of these LCP components. CDC-recognized LCPs can offer course sessions through a desktop computer, laptop, tablet, or mobile phone with coaching interactions conducted through phone, email, texting, or online messaging; the CDC refers to these programs as “online” programs. Alternatively, LCPs can also provide access to a lifestyle coach conducting in-person sessions in another location via remote classrooms or telehealth; the CDC refers to these as “distance-learning” programs.
TABLE 1.
LCP Components Delivered in Person or via Digital Health
| Component | Standard Delivery Mode* | Potential Digital Delivery Modes |
|---|---|---|
| Didactic curriculum | In-person session | • Video conferencing • Telephone conferencing • Text messaging • Mobile app • Web platform |
| Individual skill development and problem-solving | In-person session | • Video conferencing • Telephone calls • Text messaging • Mobile app • Web platform |
| Self-monitoring of diet and physical activity | Manual documentation/data entry | • Mobile app • Wearable tracking • Photo/video logs |
| Motivation and peer support | In-person session | • Video conferencing • Text messaging • Group chat |
| Weight tracking | In-person session | • Wireless device • Mobile app |
Standard delivery modes are nondigital modes of delivery. LCPs that are considered “in person” typically deliver most forms of the program via the standard mode but may deliver some components of the program digitally.
For this evidence review, we conducted a literature search in PubMed inclusive of articles published from 2000 to February 2018 that evaluated the effectiveness of digital health–supported LCPs based on the DPP research study. These programs may or may not have met the CDC Recognition Program standards and operating procedures (17). The following search terms were used: “Diabetes Prevention Programs and digital,” “DPP and virtual,” “DPP and telehealth,” and “DPP and distance learning.” Reference lists were reviewed to identify additional articles for inclusion.
In considering the effectiveness of digital health–supported LCPs, we primarily focus on the outcome of weight loss because it is an excellent proxy for the risk of developing type 2 diabetes in the future (18). As previously noted, a 5% loss of body weight is one of the CDC’s program standards and is widely considered a clinically meaningful amount of weight loss (8,14).
Comprehensive Digital Programs
Some CDC-recognized providers offer comprehensive digital LCPs: Omada Health, Inc., and Noom are two examples. Participants complete the curriculum on their own time (asynchronously) and use digital self-monitoring tools such as smart scales to monitor weight or wearables to track physical activity. Personalized health coaching and group support occur via messaging.
Three-year data from a single-arm trial of the Omada program demonstrated an average weight loss of 4.7% at 1 year (P <0.0001) and 3.0% at 3 years (P = 0.0009) (19). Another nonrandomized trial of the Omada program among people with prediabetes found that 31% lost 5% of their body weight compared to 20% of people in a matched control group (P = 0.001) (20). Finally, a single-arm retrospective analysis of 500 Medicare-age adults with prediabetes or metabolic syndrome demonstrated a mean 7.5% weight loss among the 86% of participants who completed 1 year of the program (21). Noom had similar results regarding program effectiveness in a pilot study of 43 employees of a large insurance company (mean age 51 years); 83% completed the program, losing a mean 7.5% of body weight at 6 months (22). Other small trials or pilot studies have tested similar interventions and report significant weight loss that varies in magnitude. However, many studies lack a comparison group (23–25).
Programs Using Less Comprehensive Digital Solutions
One feasibility RCT tested the combination of in-person course sessions of an LCP supplemented with mobile phone tracking, reminders, and messaging to enhance and reinforce the content from the in-person component. The control group received a pedometer and an educational brochure. Results showed that the intervention group lost an average 6.8% of body weight at 5 months compared to a 0.3% weight gain in the control group (P = 0.001) (26).
Another pilot took the opposite approach, completely automating the coaching component of the LCP (27). Participants received 1 year of regular contact through individually tailored goal-setting, automated emails, and automated motivational coaching phone calls. Despite the lack of a human coach, 70.6% of individuals remained engaged in the sixth and final month of the LCP, and mean body weight loss was 3.60% at 6 months compared to 1.32% in the control group (P <0.001).
Digital Programs Using Video or Telephone Delivery
LCPs delivered via tele-visit approaches demonstrate mixed results. A randomized comparative effectiveness trial tested a text message–based LCP in which participants received six text messages each week for 1 year. Text message content was based on the DPP LCP curriculum and focused on nutrition, physical activity, and motivation, and participants were asked once weekly for their current weight. Participants also had the option to receive weekly telephone motivational interviewing sessions, and both the intervention and control groups were offered several weight loss resources delivered by a local health care system (including an in-person DPP-based LCP); 29.2 and 35.6% of the respective groups reported using another weight loss program during the study period. The results showed no difference between groups in the proportion achieving a 5% weight loss, with <20% of all participants achieving this outcome. However, there was a significant between-group difference in the proportion achieving a 3% weight loss (28).
In another pilot study conducted in a remote frontier community, a DPP-based LCP was delivered simultaneously to an in-person group and an off-site group via video conferencing. The two groups achieved similar weight loss after the 16-week core curriculum program; >40% lost 7% of their body weight (29).
Another randomized comparative effectiveness trial (30) compared an LCP delivered via telephone to individual participants versus to groups. The DPP curriculum was adapted to be delivered by a primary care provider educator through group conference calls or individual phone calls during the first year of the study; the second year used a modified 12-session curriculum based on DPP materials. Completion rates and mean percentage of weight loss were similar for the individual and group interventions at 12 months (4.2 and 4.5%, respectively), but the group participants experienced greater weight loss at 2 years (1.8 vs. 5.6%, P = 0.016).
Research in Specific Populations
Limited available research suggests that digital LCPs intended to prevent type 2 diabetes can be tailored to specific populations. A randomized pilot of a video-conferencing LCP targeted to obese men led to an average weight loss of 3.5% of body weight at the end of the intervention period (31). Omada conducted a feasibility study of a Spanish-language LCP in a low-income Spanish-speaking population and found high engagement after 4 weeks of engaging with the program, but some participants had difficulty with the technology (32).
Participant Satisfaction and Engagement
Participants in digital LCPs report high satisfaction and positive perceptions of these programs. Surveys and focus groups suggest that people are willing to engage with LCPs using a variety of digital health solutions and see potential for this to provide real-time professional and peer support, as well as an opportunity to model and practice weight loss skills and healthy behaviors (33,34). One study of a 6-month LCP reported >75% participant satisfaction rates with the program’s entire “virtual package,” as well as high ratings of individual components such as virtual small group dynamics and the technology used (35). Another study of women veterans enrolled in the Omada LCP found that participants perceived that the program was a good fit for their health needs and integrated easily into their daily life (36).
Digital LCPs also offer an opportunity to collect granular data regarding participant engagement, providing insights into which components of an LCP drive effectiveness. A Noom study (37) found that both food tracking and group participation positively predicted weight loss. An Omada study (19) also found that group participation predicted weight loss, but tracking and completion of lessons was not associated with weight loss.
Cost Outcomes Associated With Digital LCPs
Omada has published two studies using economic simulations to predict the potential cost savings associated with its digital LCP. The first study examined a general participant population with prediabetes (38), and the second focused on a Medicare-age population with prediabetes (39). For the general prediabetes population, the model predicted that the digital LCP would result in cumulative per-person medical savings of $1,533, $3,317, and $10,043 at 3, 5, and 10 years, respectively, among participants who completed at least four lessons. Predicted savings was greater among participants who completed at least nine lessons. In the Medicare population, the model predicted that the digital LCP would result in a cumulative per-person medical savings of $1,720, $3,840, and $11,550 at the same time points. This was a relatively conservative estimate in which it was assumed that participants would regain at least some weight over time. In the general population, the medical savings exceeded the intervention costs around the 3-year time point. The break-even point occurred earlier in the Medicare population, at around 1–2 years.
Discussion
Digital health–supported LCPs can be delivered through a variety of modes, including telephone or video conferencing, text messaging, mobile apps, and online platforms. Although the quality of the published research on digital LCPs varies substantially, there is fair- to good-quality evidence that these programs are effective in achieving clinically significant weight loss and often have high engagement rates.
It should be noted that many studies recruited participants directly and thus may have incurred a selection bias for those patients who would prefer a digital delivery method. Although selection bias may be a threat to the validity of these studies, it may not actually represent a substantial limitation because it reflects the real-world implementation of LCPs, in which individuals self-select whether they will participate.
Very few studies of digital LCPs included participants who reflect the demographics of the general U.S. population. Most studies included populations that are largely Caucasian, female, college-educated, and located close to an urban area. A broad review of technology-based interventions to reduce cardiovascular risk among priority populations such as racial and ethnic minorities and individuals of lower socioeconomic status found that technology offers the opportunity of tailoring interventions to specific populations. However, available research is sparse and mostly limited to pilot studies involving Latino and non-Hispanic black participants (40).
Outside of the United States, a trial conducted in a South Asian Indian population with prediabetes found good evidence supporting a monthly telephone-delivered LCP, which resulted in a reduction in relative risk of type 2 diabetes of 28.5% at 3 years (41). However, this LCP was a significant departure from that of the original DPP research study, and it is not clear whether it would translate well to South Asian populations in the United States.
Noom and Omada offer their programs in a variety of languages in addition to English, although no studies have evaluated the cultural competence of digital health–supported LCPs. Although it could be hypothesized that digital translations would not differ significantly from in-person programs in this context, research would be helpful to confirm this hypothesis.
Many experts are optimistic that digital health–supported LCPs offer opportunities to reach and tailor content to underserved populations, including racial and ethnic minorities, men, and rural residents. However, without a sound evidence base, clinicians cannot confidently recommend digital health–supported LCPs to some of these priority populations, and further research is urgently needed.
We identified no direct comparisons of a digital health–supported LCP to an in-person LCP for the purposes of preventing type 2 diabetes, nor did we find a comparison of a comprehensive digital LCP to a program that supplemented in-person sessions with digital tools. These comparisons could be beneficial in designing future programs to expand the reach of LCPs.
However, the question of whether an in-person LCP or a digital LCP is more effective across a broad population is likely not a particularly important question. Rather, as the number of LCP options continues to grow, clinicians need evidence to guide individual patients in selecting the LCP in which they personally will be most likely to succeed. In other words, what are the key LCP and patient criteria that determine the best LCP-patient match, if any? Also, do patient outcomes improve if patients are offered a choice of LCP delivery methods and can select a program that best meets their needs? The option for patients to customize their delivery modes and digital tools may pave the way to personalized LCPs and possibly even increased participation in LCPs.
Limited research demonstrates positive patient experiences and perceptions of digital LCPs, and several studies show high participant engagement with these programs. Participant-level data from the CDC’s DPRP demonstrate a dose-response relationship between the number of LCP sessions that participants attend and the weight loss they achieve (42). When considering in-person LCPs, the most straightforward measurement of participant engagement is the number of sessions attended. However, comprehensive digital LCPs collect large quantities of granular data regarding participant engagement with several components of their programs. This creates an opportunity to develop a more nuanced understanding of different forms of participant engagement and of how quantity and type of engagement interact to affect weight loss outcomes.
Omada and Noom have begun to collect and publish data on the effects of specific components of their programs that can influence weight loss but are just beginning to scratch the surface of this new terrain. Research that more deeply evaluates participant engagement could shape the next generation of not only digital LCPs, but also all LCPs in the National DPP.
The original DPP trial provided one-on-one coaching to participants and was highly effective. However, delivering an LCP on an individual basis was quite labor-intensive and expensive, so translation of the DPP curriculum to a group setting was a key step to begin scaling the National DPP to a larger audience. Digital health–supported LCPs can mimic one-on-one coaching interactions through their individualized platforms and are able to automate certain components of the program, such as feedback on dietary and physical activity tracking data or reminders to participants, thereby reducing the human resources required to deliver an LCP.
If we seek to reach the 84 million Americans with prediabetes, there is no question that digital health–supported LCPs are an essential and growing piece of the National DPP initiative. Additionally, digital LCPs can deliver personalized coaching and simultaneously provide opportunities for the group interactions and support that are a valuable component of LCPs. In other words, digital LCPs do not require a choice between individual or group-based interactions because both are possible without significantly changing the structure or cost of the program.
Digital LCPs that involve mobile components also quite literally extend the program into the community. Participants interact with the program to implement healthy lifestyle changes at the actual places and times they need help with behavior modifications. This model aligns directly to a key tenet of the National DPP, which is to be grounded in the community and to meet participants when and where they need assistance. In this way, digital health–supported LCPs offer an exciting and scalable means to form stronger linkages for our patients between the clinical world and the day-to-day community setting.
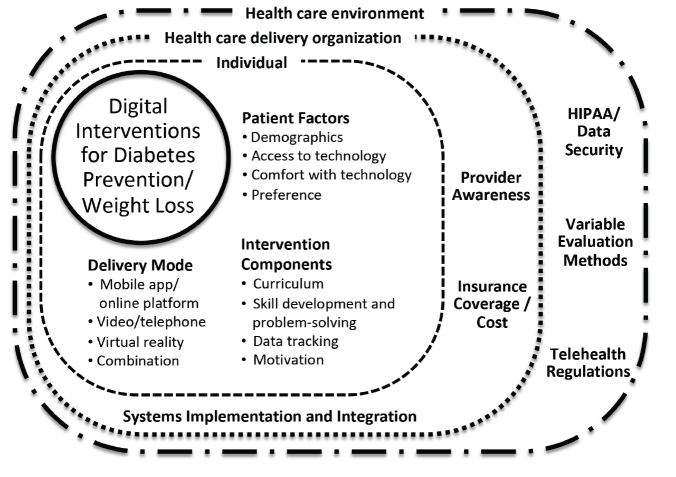
Despite the gaps in literature described above, digital health solutions to prevent type 2 diabetes have a more robust evidence base than the vast majority of digital health solutions targeted toward consumers (43). However, in addition to the need to broaden the evidence base, a number of other practical challenges impede the adoption of digital LCPs. An AMA survey (16) determined that clinicians have four key questions about digital health: “Does it work?” “Does it work in my practice?” “Will I get paid?” and “Will I get sued?” The first question has been discussed here extensively, and the other questions speak to issues that need to be addressed before digital health– supported LCPs can be adopted widely. There are several factors that influence uptake, including lack of clinician awareness, implementation challenges (such as referral processes), and varying levels of insurance coverage and costs. Superimposed on these factors, the general digital health environment in the country is evolving, and issues around the Health Insurance Portability and Accountability Act (HIPAA), data security, and digital health regulations are in constant flux. Figure 1 depicts the relationship of these factors to the adoption of digital LCPs in the clinical setting. As health care delivery organizations successfully address these barriers to implementing digital health solutions, it is imperative that they publish their experiences so that best practices for the adoption of digital LCPs are documented and spread.
FIGURE 1.
Factors influencing the adoption of digital health–supported LCPs.
It is worth noting that digital LCPs have the potential to generate cost savings for the health care system (37,38,42). Whether this will translate to more consistent insurance coverage for digital LCPs remains to be seen. Digital health–supported LCPs are currently excluded from the Medicare coverage for type 2 diabetes prevention LCPs that began in 2018 because they were not included in the model test that led to payment for in-person diabetes prevention LCPs (44).
Conclusion
Digital LCPs can expand access for patients who struggle to attend an in-person LCP or prefer a digital health solution. Gaps in evidence and implementation include a dearth of research regarding program effectiveness and implementation best practices in priority populations and guidance for clinicians seeking to determine the best patient-LCP match. Deeper exploration is also needed to characterize how participant engagement with different components of digital LCPs affects outcomes. Future work should address these gaps using pragmatic study designs that examine the clinical and organizational context for the adoption of digital health LCPs for the prevention of type 2 diabetes. As the United States strives to reduce the burden of type 2 diabetes, digital health–supported LCPs can innovatively bridge the clinical and community settings and are an important addition to our national type 2 diabetes prevention strategy.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the American Medical Association.
Acknowledgments
We are indebted to Annalynn Skipper for her assistance with the literature review and thoughtful editing and to Benjamin O’Brien for his creative figure development and formatting. We are also deeply grateful to Meg Barron, Chelsea Katz, Kim Brunisholz, and Karen Kmetik for invaluable feedback.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
K.K. and N.S. researched data and wrote and edited the manuscript. K.K. is the guarantor of this work and, as such, had full access to all the data contributing to the literature review and takes full responsibility for the accuracy of the content summarized here.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States. Atlanta, Ga., U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2017 [Google Scholar]
- 2.American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S13–S27 [DOI] [PubMed] [Google Scholar]
- 3.Kodama S, Saito K, Tanaka S, et al. . Fasting and post-challenge glucose as quantitative cardiovascular risk factors: a meta-analysis. J Atheroscler Thromb 2012;19:385–396 [DOI] [PubMed] [Google Scholar]
- 4.Morris DH, Khunti K, Achana F, et al. . Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 2013;56:1489–1493 [DOI] [PubMed] [Google Scholar]
- 5.Khan T, Tsipas S, Wozniak G. Medical care expenditures for individuals with prediabetes: the potential cost savings in reducing the risk of developing diabetes. Popul Health Manag 2017;20:389–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen SS, Johansen NB, Witte DR, et al. . Incidence of register-based diabetes 10 years after a stepwise diabetes screening programme: the ADDITION-Denmark study. Diabetologia 2016;59:989–997 [DOI] [PubMed] [Google Scholar]
- 7.Ligthart S, van Herpt TTW, Leening MJG, et al. . Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol 2016;4:44–51 [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Conner E, Fowler SE, et al. ; DPP Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DPP Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community: the DEPLOY pilot study. Am J Prev Med 2008;35:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amundson HA, Butcher MK, Gohdes D, et al. ; Montana Cardiovascular Disease and Diabetes Prevention Program Workgroup. Translating the Diabetes Prevention Program into practice in the general community: findings from the Montana Cardiovascular Disease and Diabetes Prevention Program. Diabetes Educ 2009;35:209–223 [DOI] [PubMed] [Google Scholar]
- 12.O’Brien MJ, Perez A, Scanlan AB, et al. . PREVENT-DM comparative effectiveness trial of lifestyle intervention and metformin. Am J Prev Med 2017;52:788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moin T, Damschroder LJ, AuYoung M, et al. . Diabetes Prevention Program translation in the Veterans Health Administration. Am J Prev Med 2017;53:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention National Diabetes Prevention Program: what is the National DPP? Available from www.cdc.gov/diabetes/prevention/about/index.html. Accessed 10 March 2018.
- 15.Centers for Disease Control and Prevention National Diabetes Prevention Program: Registry of all recognized organizations. Available from nccd.cdc.gov/DDT_DPRP/Registry.aspx. Accessed 10 March 2018
- 16.American Medical Association Digital Health Study: Physicians’ Motivations and Requirements for Adopting Digital Clinical Tools. Available from www.ama-assn.org/sites/default/files/media-browser/specialty group/washington/ama-digital-health-report923.pdf. Accessed 15 December 2017
- 17.Centers for Disease Control and Prevention Diabetes Prevention Recognition: program standards and operating procedures. Available from www.cdc.gov/diabetes/prevention/pd f/dprp-standards.pdf. Accessed 28 June 2018
- 18.Maruthur NM, Ma Y, Delahanty LM, et al. . Early response to preventive strategies in the diabetes prevention program. J Gen Intern Med 2013;28:1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sepah SC, Jiang L, Ellis RJ, McDermott K, Peters AL. Engagement and outcomes in a digital Diabetes Prevention Program: 3-year update. BMJ Open Diabetes Res Care 2017;5:e000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson MG, Castro Sweet CM, Edge MD, et al. . Evaluation of a digital behavioral counseling program for reducing risk factors for chronic disease in a workforce. J Occup Environ Med 2017;59:e150–e155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro Sweet CM, Chiguluri V, Gumpina R, et al. . Outcomes of a digital health program with human coaching for diabetes risk reduction in a Medicare population. J Aging Health 2018;30:692–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaelides A, Raby C, Wood M, Farr K, Toro-Ramos T. Weight loss efficacy of a novel mobile Diabetes Prevention Program delivery platform with human coaching. BMJ Open Diabetes Res Care 2016;4:e000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McTigue KM, Conroy MB, Hess R, et al. . Using the Internet to translate an evidence-based lifestyle intervention into practice. Telemed J E Health 2009;15:851–858 [DOI] [PubMed] [Google Scholar]
- 24.Cha E, Kim KH, Umpierrez G, et al. . A feasibility study to develop a diabetes prevention program for young adults with prediabetes by using digital platforms and a handheld device. Diabetes Educ 2014;40:626–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijsman CA, Westendorp RG, Verhagen EA, et al. . Effects of a web-based intervention on physical activity and metabolism in older adults: randomized controlled trial. J Med Internet Res 2013;15:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuoka Y, Gay CL, Joiner KL, Vittinghoff E. A novel diabetes prevention intervention using a mobile app: a randomized controlled trial with overweight adults at risk. Am J Prev Med 2015;49:223–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block G, Azar KM, Romanelli RJ, et al. . Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res 2015;17:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer HH, Fischer IP, Pereira RI, et al. . Text message support for weight loss in patients with prediabetes: a randomized clinical trial. Diabetes Care 2016;39:1364–1370 [DOI] [PubMed] [Google Scholar]
- 29.Vadheim LM, McPherson C, Kassner DR, et al. . Adapted Diabetes Prevention Program lifestyle intervention can be effectively delivered through telehealth. Diabetes Educ 2010;36:651–656 [DOI] [PubMed] [Google Scholar]
- 30.Weinstock RS, Trief PM, Cibula D, Morin PC, Delahanty LM. Weight loss success in metabolic syndrome by telephone interventions: results from the SHINE study. J Gen Intern Med 2013;28:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azar KM, Aurora M, Wang EJ, Muzaffar A, Pressman A, Palaniappan LP. Virtual small groups for weight management: an innovative delivery mechanism for evidence-based lifestyle interventions among obese men. Transl Behav Med 2015;5:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontil V, McDermott K, Tieu L, et al. . Adaptation and feasibility study of a digital health program to prevent diabetes among low-income patients: results from a partnership between a digital health company and an academic research team. J Diabetes Res 2016;2016:8472391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Napolitano MA, Hayes S, Russo G, Muresu D, Giordano A, Foster GD. Using avatars to model weight loss behaviors: participant attitudes and technology development. J Diabetes Sci Technol 2013;7:1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuoka Y, Kamitani E, Bonnet K, Lindgren T. Real-time social support through a mobile virtual community to improve healthy behavior in overweight and sedentary adults: a focus group analysis. J Med Internet Res 2011;13:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azar KM, Koliwad S, Poon T, et al. . The Electronic CardioMetabolic Program (eCMP) for patients with cardiometabolic risk: a randomized controlled trial. J Med Internet Res 2016;18:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moin T, Ertl K, Schneider J, et al. . Women Veterans’ experience with a web-based diabetes prevention program: a qualitative study to inform future practice. J Med Internet Res 2015;17:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Faw M, Michaelides A. Mobile but connected: harnessing the power of self-efficacy and group support for weight loss success through mHealth intervention. J Health Commun 2017;22:395–402 [DOI] [PubMed] [Google Scholar]
- 38.Su W, Chen F, Dall TM, Iacobucci W, Perreault L. Return on investment for digital behavioral counseling in patients with prediabetes and cardiovascular disease. Prev Chronic Dis 2016;13:E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen F, Su W, Becker SH, et al. . Clinical and economic impact of a digital, remotely-delivered intensive behavioral counseling program on Medicare beneficiaries at risk for diabetes and cardiovascular disease. PLoS One 2016;11:e0163627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linke SE, Larsen BA, Marquez B, Mendoza-Vasconez A, Marcus BH. Adapting technological interventions to meet the needs of priority populations. Prog Cardiovasc Dis 2016;58:630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 42.Ely EK, Gruss SM, Luman ET, et al. . A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017;40:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.IQVIA The Growing Value of Digital Health: Evidence and Impact on Human Health and the Healthcare System. Available from www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-growing-value-of-digital-health.pdf?_=1520967035346. Accessed 13 March 2018
- 44.Centers for Medicare & Medicaid Services Fact sheet: final policies for the Medicare Diabetes Prevention Program expanded model in the calendar year 2018 physician fee schedule final rule. Available from innovation.cms.gov/Files/fact-sheet/mdpp-cy2018fr-fs.pdf. Accessed 13 March 2018