In the PNAS paper “Polo-like kinase 4 inhibition produces polyploidy and apoptotic death of lung cancers,” Kawakami et al. (1) treat lung cancer cell lines and mouse tumor models with the small molecule CFI-400945 and interpret its effects as being solely due to inhibition of Polo-like kinase 4 (PLK4), the kinase essential for centrosome duplication (2). We believe that this interpretation, unambiguously stated in the title and reiterated throughout the abstract and text, is incorrect because there is compelling evidence that CFI-400945 has profound cellular effects that cannot be attributed to PLK4 inhibition.
To analyze the consequences of centrosome removal, we developed the potent and selective PLK4 inhibitor, centrinone (3). The biochemical and cellular specificity of centrinone has been validated using enzymatic assays and centrinone-resistant cells generated by introducing an active site mutation into the endogenous PLK4 locus (3). We and others have used centrinone to deplete centrosomes from >40 different cell lines (3–7). For example, after centrinone treatment, there is virtually complete centrosome loss from human HeLa cervical carcinoma, U2OS osteosarcoma, and MDA-MB-231 breast adenocarcinoma cells (3, 7). By contrast, CFI-400945 causes these cell lines to accumulate excess centrosomes and become grossly multinuclear (3, 8). This latter phenotype is not observed with centrinone and likely results from cytokinesis failure due to off-target inhibition of the mitotic kinase Aurora B by CFI-400945 (8). Since PLK4 phosphorylates itself to promote its own degradation (2), incomplete inhibition elevates PLK4 protein levels, leading to centrosome amplification rather than loss. Therefore, the excess centrosomes caused by CFI-400945 are likely due to partial PLK4 inhibition convolved with persistent cytokinesis failure resulting from Aurora B inhibition (8, 9).
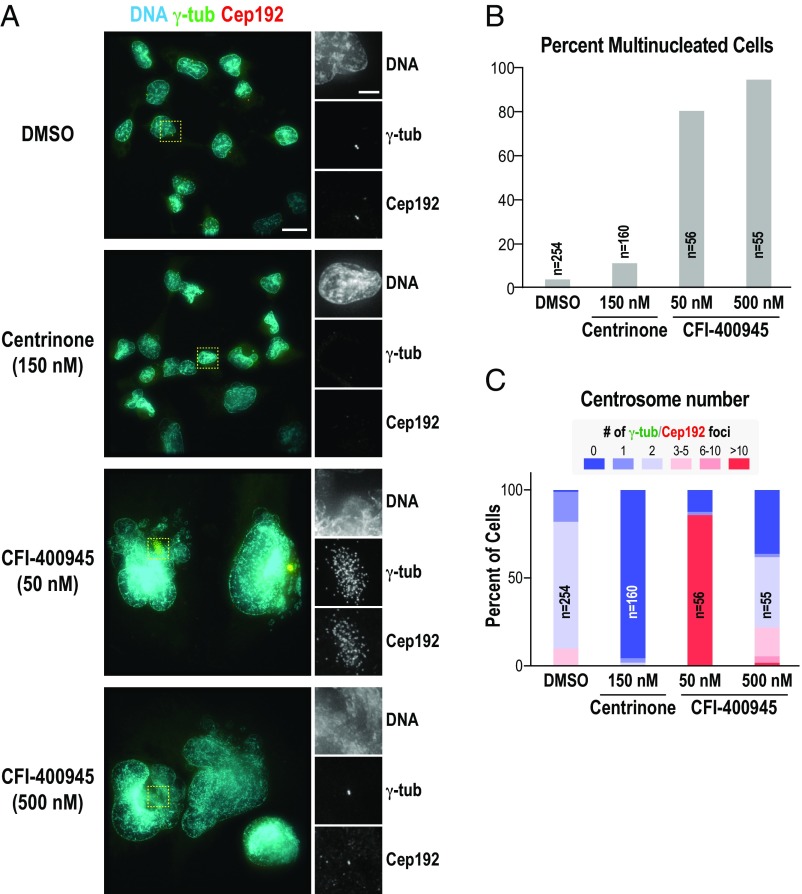
We compared the effects of centrinone and CFI-400945 on NCI-H1299, one of the lung cancer lines analyzed by Kawakami et al. (1). At a concentration that leads to >95% complete centrosome removal, centrinone treatment led to a minor increase (11% versus 4% in DMSO control) in mild multinucleation (Fig. 1 A and B). In stark contrast, 50 and 500 nM CFI-400945 caused extensive multinucleation, with nearly all cells exhibiting extremely large multilobed nuclei (Fig. 1 A and B). This phenotype is consistent with penetrant Aurora B inhibition and with figures 2 and 3A of ref. 1. As in figure 3 B and C of ref. 1, 50 nM CFI-400945 caused centrosome amplification (Fig. 1 A and C), likely due to partial PLK4 inhibition (8, 9). At 500 nM CFI-400945, our conditions favored greater PLK4 inhibition than in Kawakami et al. (figure 3 B and C of ref. 1) and resulted in a mixture of cells with zero, two, and more than two centrosomes (Fig. 1 A and C).
Fig. 1.
Centrinone and CFI-400945 have different effects on NCI-H1299 lung cancer cells. (A) NCI-H1299 cells (American Type Culture Collection; grown on plastic in RPMI medium with 10% FBS) were stained for DNA (cyan), γ-tubulin (green), and Cep192 (red) after treatment with DMSO [0.02% (vol/vol)], centrinone (150 nM), or CFI-400945 (50 or 500 nM) for 7 d. Cells were replated on poly-d-lysine–coated glass coverslips, processed for immunofluorescence (3), and imaged on a DeltaVision Elite (GE Healthcare Life Sciences) equipped with a scientific complementary metal-oxide–semiconductor camera (pco.edge 5.5; PCO) using a 60× 1.42 N.A. PlanApo N or a 40× 1.35 N.A. UApo objective (Olympus). Lower magnification merged panels (Left) and magnified single-channel images of the regions in the yellow dashed boxes (Right) are shown. (Scale bars: Left, 20 μm; Right, 5 µm.) (B and C) Quantification of percent multinucleation (B) and centrosome number (γ-tubulin and Cep192-colabeled foci) (C). This manual analysis does not consider the extent of multinucleation. CFI-400945–treated cells are massively multinucleated; centrinone-treated cells are not.
While the robust effects of CFI-400945 on tumor growth and its potential utility in cancer therapy are exciting and deserve attention, we believe it is misleading and inaccurate to present the effects of CFI-400945 as being solely due to PLK4 inhibition. This is an important point not only because it influences interpretation of the effects of CFI-400945 in cell biological and in vivo studies (10) but also because of its implications for PLK4 inhibitor therapeutic programs and the validity of PLK4 as a drug discovery target (9).
Footnotes
Conflict of interest statement: A.K.S. is a coinventor on a patent application filed by the Ludwig Institute for Cancer Research (US62/149,292) related to the structures, syntheses, and uses of centrinone and chemically related Plk4 inhibitors.
References
- 1.Kawakami M, et al. Polo-like kinase 4 inhibition produces polyploidy and apoptotic death of lung cancers. Proc Natl Acad Sci USA. 2018;115:1913–1918. doi: 10.1073/pnas.1719760115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitouni S, Nabais C, Jana SC, Guerrero A, Bettencourt-Dias M. Polo-like kinases: Structural variations lead to multiple functions. Nat Rev Mol Cell Biol. 2014;15:433–452. doi: 10.1038/nrm3819. [DOI] [PubMed] [Google Scholar]
- 3.Wong YL, et al. Cell biology. Reversible centriole depletion with an inhibitor of Polo-like kinase 4. Science. 2015;348:1155–1160. doi: 10.1126/science.aaa5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Copeland SJ, Thurston SF, Copeland JW. Actin- and microtubule-dependent regulation of Golgi morphology by FHDC1. Mol Biol Cell. 2016;27:260–276. doi: 10.1091/mbc.E15-02-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guizzunti G, Seemann J. Mitotic Golgi disassembly is required for bipolar spindle formation and mitotic progression. Proc Natl Acad Sci USA. 2016;113:E6590–E6599. doi: 10.1073/pnas.1610844113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meitinger F, et al. 53BP1 and USP28 mediate p53 activation and G1 arrest after centrosome loss or extended mitotic duration. J Cell Biol. 2016;214:155–166. doi: 10.1083/jcb.201604081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing M, et al. GOLPH3 drives cell migration by promoting Golgi reorientation and directional trafficking to the leading edge. Mol Biol Cell. 2016;27:3828–3840. doi: 10.1091/mbc.E16-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason JM, et al. Functional characterization of CFI-400945, a Polo-like kinase 4 inhibitor, as a potential anticancer agent. Cancer Cell. 2014;26:163–176. doi: 10.1016/j.ccr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Holland AJ, Cleveland DW. Polo-like kinase 4 inhibition: A strategy for cancer therapy? Cancer Cell. 2014;26:151–153. doi: 10.1016/j.ccr.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sredni ST, et al. Inhibition of polo-like kinase 4 (PLK4): A new therapeutic option for rhabdoid tumors and pediatric medulloblastoma. Oncotarget. 2017;8:111190–111212. doi: 10.18632/oncotarget.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]