Significance
Previously we demonstrated that IL-15 by continuous infusion at 2 μg/kg/d for 10 days induced a 38-fold increase in circulating natural killer (NK) cells and a 358-fold increase in CD56bright NK cells. In the present study we demonstrated that IL-15 enhanced antibody-dependent cellular cytotoxicity (ADCC) of tumor-directed monoclonal antibodies in two systems. Both NK cells and macrophages were required for optimal therapeutic responses. These studies support clinical trials of IL-15 combined with tumor-directed monoclonal antibodies. In translation of this study, a phase I trial of IL-15 combined with alemtuzumab has been opened for patients with adult T cell leukemia (ATL) NCT02689453.
Keywords: cancer immunotherapy, antibody-dependent cellular cytotoxicity, interleukin-15, NK cells, macrophages
Abstract
The goal of cancer immunotherapy is to stimulate the host immune system to attack malignant cells. Antibody-dependent cellular cytotoxicity (ADCC) is a pivotal mechanism of antitumor action of clinically employed antitumor antibodies. IL-15 administered to patients with metastatic malignancy by continuous i.v. infusion at 2 μg/kg/d for 10 days was associated with a 38-fold increase in the number and activation status of circulating natural killer (NK) cells and activation of macrophages which together are ADCC effectors. We investigated combination therapy of IL-15 with rituximab in a syngeneic mouse model of lymphoma transfected with human CD20 and with alemtuzumab (Campath-1H) in a xenograft model of human adult T cell leukemia (ATL). IL-15 greatly enhanced the therapeutic efficacy of both rituximab and alemtuzumab in tumor models. The additivity/synergy was shown to be associated with augmented ADCC. Both NK cells and macrophages were critical elements in the chain of interacting effectors involved in optimal therapeutic responses mediated by rituximab with IL-15. We provide evidence supporting the hypothesis that NK cells interact with macrophages to augment the NK-cell activation and expression of FcγRIV and the capacity of these cells to become effectors of ADCC. The present study supports clinical trials of IL-15 combined with tumor-directed monoclonal antibodies.
The goal of cancer immunotherapy is to stimulate the host immune system to attack cancer cells. Recombinant interleukin-2 (IL-2) is a prototypic immunotherapeutic treatment for patients with metastatic malignancies (1). Despite its accepted role, IL-2 has limitations. IL-2 has a dual role first as an immunomodulator stimulating the proliferation of effector cells that kill cancer cells but also a second role to prevent autoimmunity by acting as a checkpoint or suppressor of the immune system by the maintenance of inhibitory CD25+ Foxp3+ T-regulatory cells (Tregs) and by the activation-induced cell death (AICD) (2, 3). Furthermore, IL-2 has been associated with capillary leak syndrome. These issues prompted a search for other immunotherapeutics with the benefits of IL-2 but with fewer negative adverse events.
IL-15, a 14- to 15-kDa member of the 4α-helix bundle family of cytokines that was described nearly simultaneously by the Waldmann Laboratory (4) and Grabstein et al. (5) signals through a heterotrimeric receptor. IL-2 and IL-15 in their heterotrimeric receptors use cytokine-specific receptor α-chains, IL-2 receptor α (IL-2Rα/CD25) for IL-2 and IL-15Rα (CD215) for IL-15. They share the IL-2/IL-15Rβ chain (CD122) and with IL-4, IL-7, IL-9, and IL-21 the common γ-chain (CD132) (3). Both cytokines stimulate proliferation of T cells, induce generation of cytotoxic lymphocytes (CTLs), and stimulate the expansion of natural killer (NK) cells (2, 3). However, in many adaptive immune responses IL-2 and IL-15 have distinct roles. In contrast to IL-2, IL-15 inhibits IL-2–mediated AICD and does not activate functional Tregs nor does it cause a major capillary leak syndrome in mice or nonhuman primates (6–8). IL-15 predominately acts as a cell-surface molecule as part of an immunological synapse with IL-15Rα on antigen-presenting cells providing IL-15 in trans to mononuclear cells such as NK and CD8 memory cells (9, 10). In preclinical toxicology studies, IL-15 induced prolonged expansion and activation of NK cells and CD8 memory T cells (11, 12). On the basis of these distinctions, we suggested that IL-15 might be better than IL-2 as a cancer immunotherapeutic.
In a number of murine models, IL-15 proved to be of value in the therapy of neoplasia (13–18). A study of IL-15 safety was performed in rhesus macaques and the only toxicity was neutropenia due to a transient redistribution of neutrophils from the circulation to the tissues (11). A 12-d bolus i.v. infusion of 20 µg/kg/d of IL-15 to rhesus macaques was associated with a 4- to 8-fold increase in the number of circulating NK cells (11, 19). When administered by continuous i.v. infusion at 20 µg/kg/d for 10 d, IL-15 led to a 10-fold increase in the number of circulating NK cells, a 15-fold increase in the number of circulating monocytes, and a massive 80- to 100-fold increase in the number of circulating effector memory CD8 T cells (12). Our s.c. infusions to the nonhuman primates at 20 µg/kg/d for 10 d led to a 10-fold expansion in the number of circulating effector memory T cells.
On the basis of murine models of cancer, great interest has been generated in bringing IL-15 to the clinic for patients with metastatic malignancies. We have completed patient accrual in three clinical trials of IL-15 with 18–22 patients each, with different dosing strategies: one by bolus infusion, one by s.c. administration, and the third by continuous i.v. infusion of Escherichia coli-produced rhIL-15 to patients with metastatic malignancies. With bolus infusions at a dose of 3 µg/kg/d, there was a greater than 10-fold expansion of NK cells (20). When IL-15 was administered s.c. 5 days a week for 2 wk at 3 µg/kg/d, there was a 10.8-fold increase in the number of circulating NK cells. Finally, when it was administered at 2 µg/kg/d by continuous i.v. infusion, in the 3 d immediately following termination of the infusions there was a 38-fold increase in the number of circulating NK cells, and a 358-fold increase in the number of CD56bright NK cells (21).
The true IL-15 cytokine may not be an IL-15 monomer but rather may be considered an IL-15Rα/IL-15 heterodimeric cytokine. To address this issue, IL-15/IL-15Rα and ALT-803, a complex of an IL-15 mutein (N72D) bound to the sushi domain of IL-15Rα, fused to the Ig G1Fc, were generated and entered into clinical trials (22–25). The heterodimeric agents extend the in vivo half-life of IL-15. However, due to limits in the doses that were utilized to avoid unwanted toxicity, there was only a 10-fold increase in the NK numbers compared with 38-fold increase when rhIL-15 is administered by continuous i.v. infusion. ALT-803 in combination with nivolumab for patients with refractory and relapsed cancer showed evidence of antitumor activity (24). Furthermore, the combination of the IL-15 receptor superagonist complex ALT-803 with the therapeutic (anti-CD20) monoclonal antibody for patients with relapsed or refractory indolent non-Hodgkin’s lymphoma (NHL) significantly augmented NK and CD8+ T cell numbers and enhanced functional responses (degranulation and IFN-γ production) against anti-CD20 mAb-coated NHL targets (25).
The observation following IL-15 administration stimulated efforts to translate the effects of IL-15 on NK cells into an effective anticancer strategy.
Since the first report of the successful use of a monoclonal antibody in the treatment of human B-cell lymphoma in 1982 (26), a large number of monoclonal antibodies have become standard effective treatment for cancer (27). These antibodies act by diverse mechanisms, including induction of apoptosis of tumor cells, blockade of cytokine-mediated signaling pathways, interruption of checkpoint inhibitors, complement fixation, and especially antibody-dependent cellular cytotoxicity (ADCC) (27, 28). One of the most effective approaches to increase the effectiveness of monoclonal antibodies was to augment their interaction with Fc receptors on innate immune effector cells, to increase the effectiveness of ADCC (28). In most cases these modifications involved the increase of the affinity of the monoclonal antibody in its binding to activating receptors FcγRI and FcγRIII. For these antibodies, the protective response to tumor challenge was abolished in activating FcγR-deficient mice (29–32). Alternatively, for anti-TNF superfamily antibodies (e.g., anti-CD40) effectiveness was augmented by increasing their affinity for the inhibitory receptor FcγRIIB (33, 34). In the present study we investigated an alternative strategy to increase ADCC by the use of a cytokine to increase the number and state of activation of the cells involved in the ADCC process, NK cells, and monocytes/macrophages. IL-2, IL-12, and IL-21 have been shown to augment ADCC (35–37). Furthermore, exploring predominantly in vitro analysis, enhanced ADCC has been demonstrated against B-cell lymphoma by interleukin-15 (38, 39). In addition, Vincent et al. (40) reported that a fusion protein of rituximab with IL-15/IL-15Rα showed enhanced therapeutic efficacy against human B-cell lymphoma in SCID mice.
The predominant approaches with IL-15 discussed above are based on the hypothesis that the host is making an immune response albeit inadequate to its tumor and that this response can be augmented by IL-15. However, we propose that IL-15 may have its major impact in combination therapy with agents that have some efficacy and specificity toward the tumor. As noted above, IL-15 administration has the capacity to dramatically increase the number of activated NK cells. However, with autologous NK cells, there is an inhibition of their cytotoxic action by the interaction of tumor-expressed host class I MHC (class 1a and HLA-E) with negative regulatory elements on the surface of the NK cells, including KIRs and NKG2A, respectively (41). We proposed that to circumvent this impediment, a very attractive antitumor strategy would involve combination therapy utilizing IL-15 with its augmentation of NK cells in conjunction with cancer-directed monoclonal antibodies to augment their ADCC and thereby increase their antitumor efficacy. The present study was directed toward testing this hypothesis. The combination of human IL-15 (hIL-15) with the anti-CD20 monoclonal antibody rituximab was evaluated in a syngeneic system, wherein EL4 cells transfected with human CD20 were the tumor targets. Such combination therapy markedly increased the magnitude and duration of the antitumor responses compared with either agent alone. Furthermore, the additivity/synergy was shown to be associated with augmented ADCC. In addition, we demonstrated that combination therapy of hIL-15 with the anti-CD52 alemtuzumab (Campath-1H) led to regression of a human ATL in a xenograft mouse model, supporting this combination strategy.
In the present study, we also addressed the issue of the nature of the ADCC effector cells (NK cell or macrophage). We demonstrated that both NK cells and macrophages were critical links in the chain of effectors involved in optimal therapeutic responses mediated by rituximab in a combination regimen with hIL-15. The present studies support human clinical trials of the combination of IL-15 with antitumor monoclonal antibodies. On the basis of the present study, a clinical trial was opened: s.c. recombinant human IL-15 (s.c. rhIL-15) and alemtuzumab for patients with refractory or relapsed chronic and acute adult T cell leukemia (ATL).
Results
The Inclusion of hIL-15 in Combination Therapy Increased the Therapeutic Efficacy of Rituximab with the EL4-hCD20 Tumor in C57BL/6 WT Mice.
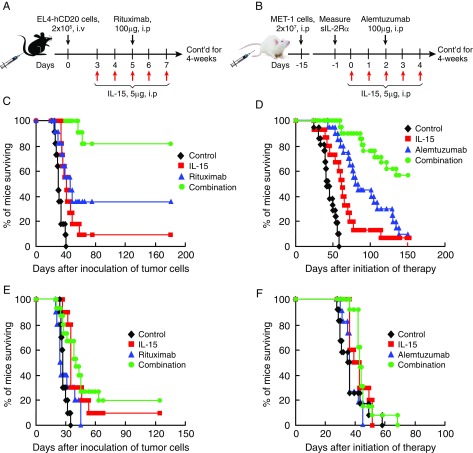
Among the several mechanisms of rituximab’s antitumor action, ADCC is believed to be of particular importance (42–44). In the previous studies, IL-15 increased the proliferation and activation of NK cells, monocytes, and macrophages, the effector cells involved in ADCC (11, 13, 20, 21, 38). We evaluated the therapeutic efficacy of the combination regimen of hIL-15 with rituximab in a syngeneic tumor model that involved the i.v. administration into immunologically intact mice of EL4-hCD20 cells which express human CD20 on their surface. The therapeutic protocol is shown in Fig. 1A. Treatment with either hIL-15 alone at a dose of 5 μg per mouse, i.p., 5 days a week for 4 wk or with rituximab alone at a dose of 100 μg per mouse, i.p., weekly for 4 wk was associated with modest prolongation of the survival of EL4-hCD20 tumor-bearing mice compared with survival of the mice in the PBS control group (Fig. 1C, P < 0.01). Critically, therapeutic activity was markedly augmented as defined by prolongation of survival of the mice when the two agents were administered together compared with monotherapy with either hIL-15 (Fig. 1C, P < 0.001) or rituximab (Fig. 1C, P < 0.05) alone. All mice in the PBS control group died from tumor progression by day 40. The combination treatment resulted in a highly significant prolongation of survival, with 80% of the mice in the combination group remaining alive at day 180 (Fig. 1C), whereas only 10% of the mice in the hIL-15 group and 30% in the rituximab group were alive by that day (Fig. 1C). The therapeutic study was repeated with comparable results as shown in SI Appendix, Fig. S1A. These results suggested the hypothesis that the addition of IL-15 to cancer-directed monoclonal antibodies can increase their anticancer efficacy.
Fig. 1.
The inclusion of hIL-15 in combination therapies increased the therapeutic efficacy of antitumor monoclonal antibodies in syngeneic and xenograft tumor models in WT mice, but not in FcγR−/− mice. (A) Experimental schema of the EL4-hCD20 tumor model. (B) Experimental schema of the xenograft model of human ATL (MET-1). (C) Kaplan–Meier survival plot of the mice in one of the therapeutic studies of the EL4-hCD20 tumor model in C57BL/6 WT mice (n = 11). Treatment with hIL-15 alone (red line) at a dose of 5 μg per mouse, i.p., 5 days a week for 4 wk and treatment with rituximab (blue line) at a dose of 100 μg per mouse, i.p., weekly for 4 wk modestly prolonged the survival of the EL4-hCD20 tumor-bearing mice compared with the mice in the PBS control group (black line) (P < 0.01). Furthermore, the combination therapy (green line) with both hIL-15 and rituximab provided greater therapeutic efficacy than any of the other groups as demonstrated by the fact that 80% of the mice in the combination group remained tumor-free for the 180-d observation period, a significantly higher rate than the 10% and 30% in the hIL-15 (P < 0.0001) and rituximab (P < 0.05) groups, respectively. (D) Kaplan–Meier survival plot of the mice in the therapeutic studies of the MET-1 model in SCID/NOD WT mice (n = 15–20). Treatment with hIL-15 alone (red line) at the same dose and dosing schedule as above prolonged the survival of the MET-1 tumor-bearing mice compared with the mice in the PBS control group (black line) (P < 0.001). Treatment with alemtuzumab (blue line) at a dose of 100 μg per mouse weekly for 4 wk provided greater therapeutic efficacy as seen by prolonged survival of the MET-1 tumor-bearing mice compared with the mice in the PBS control (P < 0.001) or hIL-15 alone group (P < 0.05). Furthermore, the combination therapy (green line) with both hIL-15 and alemtuzumab provided greater therapeutic efficacy as demonstrated by the fact that more than 50% of the mice in the combination group were alive at day 150, whereas fewer than 10% of the mice in the hIL-15 alone (P < 0.0001) or alemtuzumab alone (P < 0.001) group and none of the mice in the PBS control group (P < 0.0001) were alive at that time. The experiment was repeated and the results of the two experiments were pooled together. (E) Kaplan–Meier survival plot of the mice in one of the therapeutic studies of the EL4-hCD20 tumor model in C57BL/6 FcγR−/− mice (n = 10–15). The treatment with hIL-15 alone and the combination regimen had a therapeutic efficacy as seen by the prolongation of survival of the EL4-hCD20 tumor-bearing mice compared with those in the control and rituximab groups (P < 0.05). Treatment with rituximab did not show any therapeutic effect compared with the mice in the PBS control group (P = 0.265). The combination regimen showed a similar therapeutic efficacy as the hIL-15 did (P = 0.61). The therapeutic efficacy of the combination therapy in the FcγR−/− mice was markedly reduced compared with mice with intact FcγR (compare Fig. 1E with Fig. 1C). (F) The therapeutic study was performed in the MET-1 tumor-bearing FcγR−/− SCID/NOD mice (n = 10–13). The survival times of the MET-1 tumor-bearing FcγR−/− mice among the four groups were not statistically different and the median survival durations were 36, 41, 36, and 43 d for the control, IL-15, alemtuzumab, and combination groups, respectively. These survivals in FcγR−/− mice were markedly reduced compared with those in mice with wild-type FcγR (compare Fig. 1F with Fig. 1D). The experiment was repeated and the results of the two experiments were pooled together.
The Inclusion of hIL-15 in Combination Therapy Increased the Efficacy of Alemtuzumab in a Murine Model of Human ATL.
Previously, we demonstrated that the main antitumor cytotoxic mechanism with the anti-CD52 antibody, alemtuzumab, in vivo required FcγR-containing receptor expression on mononuclear cells, including macrophages (30). In the present study, we extended the investigation of the therapeutic efficacy of the combination regimen of hIL-15 with alemtuzumab in a mouse xenograft model of human ATL (MET-1). The therapeutic protocol is shown in Fig. 1B. Therapy started when the serum sIL-2Rα levels of the MET-1 tumor-bearing mice were more than 1,000 pg/mL. The dose and dosing schedule with hIL-15 and the antibody were the same as used above. Treatment with hIL-15 alone was associated with a prolongation of survival of MET-1 tumor-bearing mice compared with mice in the PBS control group (Fig. 1D, P < 0.001). Treatment with alemtuzumab alone significantly prolonged survival of the MET-1 tumor-bearing mice compared with the mice in either the PBS control (Fig. 1D, P < 0.0001) or hIL-15 alone group (Fig. 1D, P < 0.05). Critically, the combination therapy with both hIL-15 and alemtuzumab provided greater therapeutic efficacy than those observed with monotherapy with either hIL-15 or alemtuzumab (Fig. 1D, P < 0.001). All mice in the PBS control group died from tumor progression within 2 mo. More than 80% of the mice in hIL-15 alone and 50% of the mice in alemtuzumab alone groups died from tumor progression by day 80 (Fig. 1D). In comparison, treatment with the combination of hIL-15 and alemtuzumab resulted in a significantly longer survival, with 90% of the mice in the combination group alive at day 80 and more than 50% alive at day 150 (Fig. 1D, P < 0.001 compared with either the hIL-15 or alemtuzumab alone group). The therapeutic study was repeated with comparable results and the results were pooled together.
FcγR Receptors Were Required for the Therapeutic Efficacy Mediated by the Antibodies Alone and the Combination Regimens.
Antitumor monoclonal antibodies can mediate antitumor effects by a variety of mechanisms, including signaling resulting in cell cycle arrest, direct induction of apoptosis, sensitization to cytotoxic drugs, complement-dependent cytotoxicity (CDC), antibody-dependent phagocytosis, and ADCC. In the present study, to define the role of ADCC, we performed therapeutic trials in the EL4-hCD20 tumor model using C57BL/6 FcγR−/− mice and in the MET-1 model using SCID/nonobese diabetic (NOD) FcγR−/− mice. In the EL4-hCD20 model, rituximab lost efficacy and the combination regimen showed much reduced therapeutic efficacy in the FcγR−/− mice (Fig. 1E, P < 0.01) compared with those observed in the WT mice (Fig. 1C), although the same dose and dosing schedule of hIL-15 and rituximab was used as shown in Fig. 1A. The therapeutic study in the EL4-hCD20 tumor-bearing FcγR−/− mice was repeated and comparable results were obtained as shown in SI Appendix, Fig. S1B. In the MET-1 model, survival time among all groups of the MET-1 tumor-bearing FcγR−/− mice was not statistically different and the median survival durations were 36, 41, 36, and 43 d for the control, hIL-15, alemtuzumab, and combination groups, respectively (Fig. 1F). Again, the anticancer monoclonal antibody lost efficacy and the combination therapy showed much less efficacy in the FcγR−/− mice (Fig. 1F, P < 0.001) compared with that observed in the WT mice (Fig. 1D). These results support the view that FcγR receptors are required for antitumor efficacy and that the enhanced therapeutic efficacy of the combination regimen of IL-15 with antibodies mainly depends on ADCC.
Treatment with hIL-15 Enhanced the ex Vivo Rituximab-Mediated ADCC of NK Cells from WT Mice, but Not the NK Cells from FcγR−/− Mice.
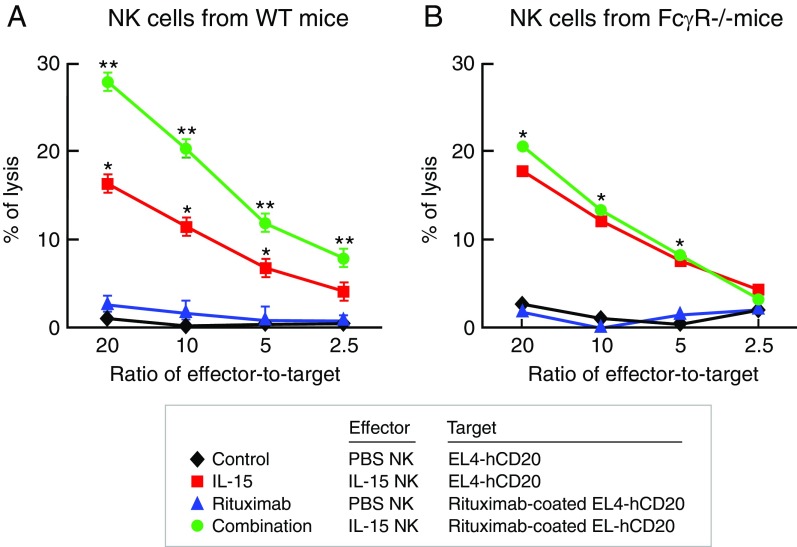
The in vivo therapeutic studies above demonstrated that treatment with either hIL-15 or rituximab alone inhibited EL4-hCD20 tumor growth in WT mice and that the combination regimen with both agents showed greater therapeutic efficacy compared with monotherapy with either hIL-15 or rituximab (Fig. 1C and SI Appendix, Fig. S1A). However, rituximab lost efficacy and the combination regimen showed much reduced therapeutic efficacy in FcγR−/− mice (Fig. 1E and SI Appendix, Fig. S1B) compared with those observed in the WT mice (Fig. 1C and SI Appendix, Fig. S1A). To determine whether the increased antitumor efficacy mediated by the combination regimen in vivo was associated with increased ADCC directly mediated by ex vivo effectors, the lysis activity of NK cells toward the EL4-hCD20 cells was examined. 51Cr-labeled EL4-hCD20 cells were coated or uncoated with rituximab at a concentration of 10 µg/mL at 4 °C for 30 min and then coincubated at various effector-to-target ratios with NK cells for 5 h. The NK cells were freshly isolated from the EL4-hCD20 tumor-bearing WT or FcγR−/− mice that had received either hIL-15 or PBS treatments. The NK cells isolated from both WT and FcγR−/− mice in the PBS groups did not effectively lyse either EL4-hCD20 or rituximab-coated EL4-hCD20 cells, whereas the NK cells from the hIL-15–treated mice efficiently lysed the EL4-hCD20 cells (Fig. 2, red line). Furthermore, the NK cells from the hIL-15–treated WT mice showed enhanced cytolytic activity when the EL4-hCD20 tumor cells were coated with rituximab (Fig. 2A, green line). However, the NK cells from the hIL-15–treated FcγR−/− mice showed similar cytolytic activity toward both rituximab-coated and uncoated EL4-hCD20 cells (Fig. 2B, green and red lines), supporting the view that IL-15 enhanced the ADCC of NK cells.
Fig. 2.
Treatment with hIL-15 was associated with enhanced ex vivo rituximab-mediated ADCC of the NK cells from WT mice, but not the NK cells from FcγR−/− mice. To determine whether the increased antitumor efficacy mediated by the combination regimen in vivo was associated with increased ADCC mediated by NK cells, the lytic activity of NK cells toward the EL4-hCD20 cells was examined ex vivo. 51Cr-labeled EL4-hCD20 cells were coated with rituximab or uncoated at the concentration of 10 µg/mL at 4 °C for 30 min and then coincubated at various effector-to-target ratios with NK cells for 5 h. The NK cells were freshly isolated from the EL4-hCD20 tumor-bearing mice that had received hIL-15 or PBS treatments. (A). The cytolytic activity assay of NK cells isolated from WT mice. The ex vivo NK cells isolated from the mice in the PBS group did not effectively lyse either EL4-hCD20 (black line) or rituximab-coated EL4-hCD20 cells (blue line), whereas the NK cells from the hIL-15–treated mice efficiently lysed the EL4-hCD20 cells (red line). Furthermore, the NK cells from the hIL-15–treated mice showed enhanced cytolytic activity toward rituximab-coated EL4-hCD20 cells (green line). (B). The cytolytic activity assay of NK cells isolated from FcγR−/− mice. In contrast to the NK cells from WT mice, the NK cells from FcγR−/− mice that received IL-15 showed comparable enhanced cytolytic activity toward EL4-hCD20 (red line) or rituximab-coated EL4-hCD20 cells (green line). *P < 0.01 IL-15 was compared with the control or rituximab and **P < 0.01 combination was compared with IL-15.
Both NK Cells and Macrophages Were Required for the Optimal Therapeutic Efficacy Mediated by Rituximab and by the Combination Regimen.
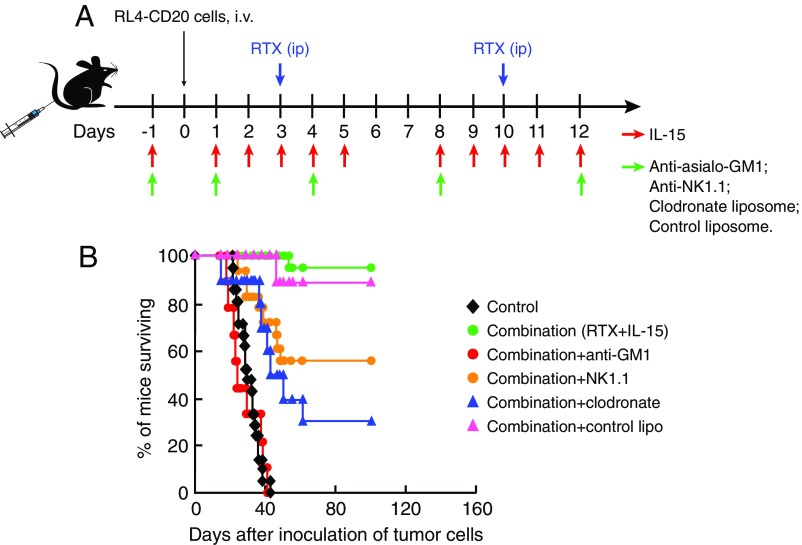
To investigate the requirements for NK cells, macrophages, or both as effectors in the antitumor activity mediated by the combination regimen, we treated the EL4-hCD20 tumor-bearing WT mice with anti-asialo-GM1, anti-NK1.1 monoclonal antibody, or clodronate liposomes to eliminate NK cells or macrophages, respectively, together with the combination therapeutic regimen (IL-15 plus rituximab). The experimental protocol is shown in Fig. 3A. One dose of the anti-asialo-GM1, anti-NK1.1 antibody, or the clodronate liposomes was administered 1 d before the initiation of the combination therapy and subsequent doses were administered twice weekly for 2 wk. The antitumor efficacy of the combination regimen was lost in the anti-asialo-GM1–treated mice (Fig. 3B, P < 0.0001) and reduced in the anti-NK1.1 antibody (Fig. 3B, P < 0.01)- or clodronate liposome-treated mice (Fig. 3B, P < 0.0001), indicating that both NK cells and macrophages were required for optimal generation of ADCC. The administration of the control liposomes did not affect the therapeutic efficacy mediated by the combination regimen (Fig. 3B, P = 0.55). A similar pattern of results was obtained with a second in vivo cell depletion study which was performed in the EL4-hCD20 tumor-bearing WT mice that were treated with rituximab alone (SI Appendix, Fig. S2), again supporting the view that both NK cells and macrophages are required for optimal ADCC.
Fig. 3.
Both NK cells and macrophages are necessary for the optimal therapeutic efficacy mediated by the combination regimen (n = 9–22). The EL4-hCD20 tumor-bearing WT mice were treated with anti-NK1.1 antibody (100 µg) or anti-asialo-GM1 (50 μL) or clodronate liposomes (200 μL) to eliminate NK cells or macrophages, respectively, together with the combination regimen of hIL-15 and rituximab. (A) Experimental schema. (B) Kaplan–Meier survival plot of the mice in the therapeutic study. The combination therapy showed great therapeutic efficacy as seen by the prolonged survival of the EL4-hCD20 tumor-bearing mice compared with the mice in the PBS control group (P < 0.0001). Compared with the group receiving the combination therapy alone, depletion of macrophages with clodronate or depletion of NK cells with anti-NK1.1 antibody was associated with a significant but incomplete reduction of the antitumor efficacy (P < 0.01), whereas administration of anti-asialo-GM1 to eliminate NK cells was associated with an abrogation of the antitumor efficacy (P < 0.0001). The control liposome did not affect the therapeutic efficacy mediated by the combination regimen (P = 0.55). The experiment was repeated and the results of the two experiments were pooled together.
Cell Depletion Reagents Affected the Numbers and Activation Status of NK Cells Mediated by hIL-15 in Vivo.
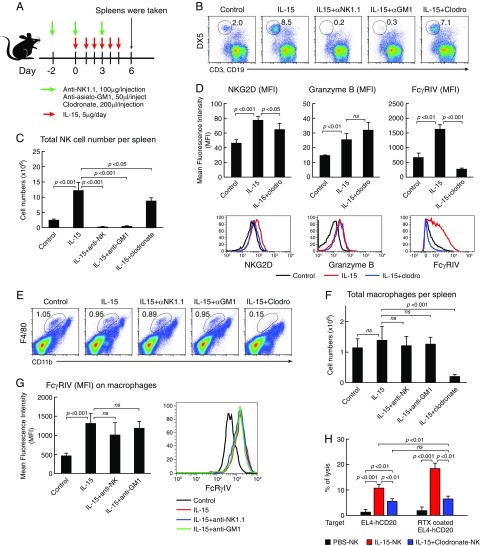
NK cells isolated from the hIL-15–treated mice showed stronger cytotoxic activity to the EL4-hCD20 target cells ex vivo, especially to the rituximab-coated target cells (Fig. 2A). To evaluate the effect of the cell depletion reagents (the anti-asialo-GM1, anti-NK1.1 antibody, and clodronate liposomes) on the numbers and activation status of NK cells as well as macrophages mediated by hIL-15, we treated WT C57BL/6 mice with hIL-15 together with or without the cell depletion reagents for 6 d as shown in Fig. 4A. At day 6 after initiation of the therapy, all mice (three mice per group) were killed. The percentages of different cell populations in spleens were analyzed by flow cytometry and the absolute numbers of NK cells, macrophages, and T cells were calculated. Compared with the mice in the control group, treatment with hIL-15 increased the total cell numbers in spleens (SI Appendix, Fig. S3A, P < 0.01) and especially induced the proliferation of NK cells (Fig. 4 B and C, P < 0.001). Furthermore, treatment with hIL-15 significantly up-regulated the FcγRIV expression on both NK cells and macrophages and increased the expression of NKG2D and granzyme B on NK cells (Fig. 4 D and G, P < 0.01). Treatment with anti-asialo-GM1 and anti-NK1.1 antibody effectively depleted NK cells (Fig. 4 B and C, P < 0.001), but did not deplete macrophages (Fig. 4 E and F). Treatment with anti-asialo-GM1 also reduced the numbers of CD3+CD8+ cells (SI Appendix, Fig. S3B, P < 0.01). Treatment with clodronate liposomes effectively depleted CD11bintF4/80hi macrophages (Fig. 4 E and F, P < 0.001) and reduced NK cell numbers (Fig. 4 B and C, P < 0.05). In addition, clodronate treatment abrogated the expressions of FcγRIV and NKG2D on NK cells induced by hIL-15 (Fig. 4D, P < 0.05), suggesting that NK cells have to interact with macrophages in the context of IL-15 to express FcγRIV. The number of CD3+CD8+ cells in the clodronate group was lower than that in the hIL-15 group (SI Appendix, Fig. S3, P < 0.05). The experiment was repeated with comparable results.
Fig. 4.
Effects of cell depletion reagents on proliferation and activation of NK cells and macrophages induced by hIL-15 (n = 3). WT C57BL/6 mice were treated with hIL-15 together with or without the cell depletion reagents for 6 d. At day 6 after initiation of the therapy, the mice were killed, and spleens were taken. Splenocytes were separated and counted. The percentages of different cell populations in spleens were analyzed by flow cytometry and the absolute numbers of NK cells, macrophages, and T cells were calculated. (A) Experimental schema. (B) Percentage of NK cells. (C) Average absolute NK cell numbers per spleen. (D) Expressions of activation markers on NK cells. (E) Percentage of CD11bintF4/80hi macrophages. (F) Average absolute numbers of macrophages per spleen. (G) FcγRIV expression on macrophages. (H) Cytolytic activity assay of NK cells toward the EL4-hCD20 cells. Treatment with hIL-15 was associated with an increase in the percentages of NK cells in the spleens, thereby resulting in an increased absolute number of NK cells in the spleens compared with that in the control group (P < 0.001). Critically, treatment with hIL-15 significantly up-regulated the FcγRIV expression on both NK cells and macrophages and increased the expression of NKG2D and granzyme B on NK cells (P < 0.01) and increased the killing activity of NK cells toward the EL4-hCD20 and rituximab-coated EL4-hCD20 target cells (P < 0.001). Treatment with anti-asialo-GM1 and anti-NK1.1 antibody effectively depleted NK cells (P < 0.001), but not macrophages. Treatment with clodronate liposomes effectively depleted CD11bintF4/80hi macrophages (P < 0.001). In addition, treatment with clodronate liposomes not only reduced NK-cell numbers (P < 0.05) but also reduced the expressions of FcRγIV and NKG2D on NK cells mediated by hIL-15 (P < 0.05); therefore, the NK cells from IL-15 plus clodronate-treated mice showed similar cytolytic activity to both EL4-hCD20 and rituximab-coated EL4-hCD20 cells.
The Interaction with Macrophages in Vivo Was Necessary for Activation of NK Cells by IL-15.
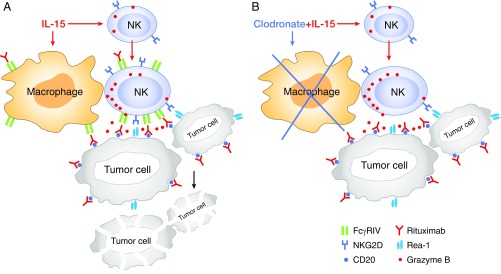
IL-15 induced the proliferation (Fig. 4 B and C) and enhanced the killing activity of NK cells (Fig. 2) and treatment with clodronate in vivo abrogated the expressions of FcγRIV and NKG2D on NK cells induced by hIL-15 (Fig. 4D). We then investigated if the clodronate treatment reduced the killing activity of NK cells mediated by IL-15. The cytolytic activity of NK cells toward the EL4-hCD20 cells was examined ex vivo. WT C57BL/6 mice were treated as above. 51Cr-labeled EL4-hCD20 cells coated or uncoated with rituximab were used as the target. The freshly isolated NK cells from PBS-, or IL-15–, or IL-15 plus clodronate-treated mice were coincubated at an effector-to-target ratio of 10:1 for 5 h. Treatment with hIL-15 increased the cytolytic activity of NK cells toward the EL4-hCD20 cells compared with the NK cells from PBS-treated mice (Fig. 4H, P < 0.001) and the NK cells from IL-15–treated mice showed enhanced cytolytic activity when the EL4-hCD20 tumor cells were coated with rituximab (Fig. 4H, P < 0.01). However, the NK cells isolated from hIL-15 plus clodronate-treated mice showed less killing activity toward EL4-hCD20 cells compared with that of the NK cells in mice treated with IL-15 alone (Fig. 4H, P < 0.01), although these NK cells showed increased killing activity compared with those from PBS-treated control mice (Fig. 4H, P < 0.01). More importantly, the IL-15 plus clodronate-treated NK cells did not show any enhancement of ADCC mediated by rituximab, since these NK cells showed similar killing activity toward both rituximab-coated EL4-hCD20 and uncoated EL4-hCD20 cells, while the NK cells from the mice treated with IL-15 alone showed much stronger killing activity toward rituximab-coated EL4-hCD20 cells than EL4-hCD20 cells (Fig. 4H). We also investigated the ability of NK cells to form conjugates with the target cells ex vivo. NK cells were freshly isolated from WT mice treated with PBS or hIL-15, or with hIL-15 plus clodronate as above, then labeled with CFSE. EL4-hCD20 cells were labeled with eFluor 670 dye, then coated with rituximab or uncoated. The labeled NK cells and EL4-hCD20 cells were cocultured at a ratio of 1:2 at 37 °C for 30 min. Flow cytometry was performed to determine the numbers of double-positive events (NK cell–target cell conjugates). There were more conjugates between IL-15–treated NK and rituximab-coated EL4-hCD20 cells compared with that between PBS-treated NK and rituximab-coated EL4-hCD20 cells (SI Appendix, Fig. S4, P < 0.05). However, clodronate treatment abrogated the IL-15–induced ability of NK cells to form conjugates with rituximab-coated target cells (SI Appendix, Fig. S4, P < 0.001). These results taken together suggested that the interaction with macrophages in vivo was necessary for activation of NK cells by IL-15 as illustrated in Fig. 5.
Fig. 5.
Schema of in vivo working model between NK cells and macrophages during antitumor immune response. (A) With macrophages in the body, IL-15 induced the NK-cell expression of FcγRIV which mediated ADCC, and NKG2D which mediated nonantibody-dependent killing of tumor cells. (B) With macrophage depletion by clodronate, IL-15 did not induce the expression of FcγRIV and NKG2D on NK cells so that they did not show augmentation of ADCC mediated by tumor-directed monoclonal antibody, suggesting that the interaction with macrophages in vivo was necessary for activation of NK cells by IL-15.
Discussion
Recently there have been dramatic advances in cancer immunotherapy which has ascended to a primary focus of new approaches for the treatment of patients with metastatic malignancy. Attractive immunotherapeutic approaches have included cell therapy with chimeric antigen receptors (45–47), as well as agents that reverse the action of checkpoint inhibitors that augment a patient’s response albeit inadequate to their tumor (48). Monoclonal antibodies long have been an element in the armamentarium against cancer. Among the effective monoclonal antibodies are those that directly target the tumor (anti-Fas), those that are directed toward checkpoint inhibitors, those that act by fixing complement, and the majority that function by ADCC (30–32, 49, 50). Previously it was shown that IL-2 (36), IL-15 (38, 39), and IL-21 (37) can enhance the in vitro ADCC activity of trastuzumab, rituximab, and cetuximab, respectively.
One unresolved issue about ADCC concerned which cell type is necessary and sufficient following interaction with an antitumor antibody to mediate this process. Groups using anti-asialo-GM1 to eliminate NK cells reported that NK cells were the primary effectors (51) or those using clodronate liposomes that eliminate macrophages reported that cell as primary effector of ADCC (49, 52) or reported that both populations of cells played roles (53). We considered the possibility that both cells acting as links in a chain might be required for optimal ADCC, that is, that both NK cells and macrophages were necessary but not alone sufficient for an optimal ADCC. In our studies involving anti-asialo-GM1 or anti-NK1.1 antibody in the syngeneic EL4 transfected with human CD20 model with the combination of IL-15 and rituximab regimen, NK cells were shown to be required for ADCC. However, using clodronate liposomes, we demonstrated that macrophages were also required for optimal ADCC. NK cells and macrophages could be acting independently as ADCC effectors. However, in the EL4-hCD20 model with combination therapy, another model of action also existed. NK cells interact with macrophages to augment the capacity of the NK cells as effectors of ADCC as illustrated in Fig. 5 (54, 55).
A number of factors may be involved in the development of effective ADCC and NK-cell cytotoxicity. Fehniger et al. (56) demonstrated that the acquisition of murine NK-cell cytotoxicity requires the translation of a preexisting pool of granzyme B and perforin mRNAs released by NK-cell activation. The red lines in Fig. 2 may reflect such cytotoxicity released by NK-cell activation. The additional event yielding the green line in Fig. 2A involved in the ADCC with rituximab may reflect the expression of FcγRIV. As noted in Fig. 2A, NK cells from untreated wild-type mice did not perform ADCC, even though the target cells were coated with rituximab. As noted in Fig. 4D, such NK cells from control mice express very low level of FcγRIV. However, NK cells from IL-15–treated mice expressed a high level of FcγRIV (red line in Fig. 4D, Lower Right image) and as noted in Figs. 2 and 4H manifest ADCC. However, when IL-15 was administered to mice receiving clodronate to remove macrophages, both the FcγRIV expression (Fig. 4D) and ADCC (Fig. 4H) are lost. FcγRIV-deleted mice have been generated and such FcγRIV-deleted mice lost the ability to perform ADCC (57, 58).
Using hamster antibodies that block FcγRIV, it was demonstrated that FcγRIV was essentially involved in IgG2a- and IgG2b-dependent killing of B cells, melanoma metastases in the lung, and phagocytosis of platelets and red blood cells (57). Furthermore, in FcγRIV-deleted mice a variety of IgG2a- and IgG2b-dependent effector functions were impaired, including antibody-dependent cellular cytotoxicity. Further studies would be required to define the exact role of FcγRIV in activated NK cells. Taken together these results suggest that in the context of IL-15, NK cells interact with macrophages so that the NK cells express FcγRIV required for ADCC.
The primary focus of the present study was to determine if the addition of IL-15 with an antitumor monoclonal antibody would increase the antibody’s ADCC and thereby its antitumor efficacy. We showed that IL-15 administration was associated with an increase in the number and activation status of the cells involved in ADCC. In particular, when IL-15 was administered to rhesus macaques by continuous i.v. infusion, there was a 15-fold increase in the number of circulating monocytes and an 8- to 10-fold increase in the number of NK cells (12). Furthermore, in clinical trials involving patients with metastatic malignancy, when IL-15 was administered by continuous i.v. infusion at 20 µg/kg/d the number of circulating NK cells increased 38-fold and the number of CD56bright NK cells increased 358-fold. In parallel studies, we showed that both CD56lo and CD56hi NK cells were active in natural cytotoxicity, cytotoxicity mediated by Rae-1/NKG2D, as well as by ADCC (21). In the present study, we demonstrated that the IL-15 mediated an increase in the number of NK cells and the augmentation of the state of NK-cell activation that was associated with an increase in ADCC and thereby the therapeutic efficacy of the two antitumor monoclonal antibodies studied. In both the EL4-hCD20 syngeneic model with rituximab and the ATL MET-1 xenograft model with alemtuzumab, the addition of IL-15 significantly augmented the magnitude and duration of the response of animals bearing the appropriate tumor. In FcγR−/− mice in the EL4-hCD20 model, the majority, and in the ATL MET-1 xenograft model, all of the therapeutic efficacy was lost, supporting our view that the antitumor efficacy mediated by the combination of IL-15 with monoclonal antibody was predominantly due to ADCC. In support of this view, ex vivo NK cells from IL-15–treated WT mice, but not FcγR−/− mice, showed an augmentation of the ADCC mediated by rituximab. Translating these results into clinical trials, rhIL-15 by continuous i.v. infusion in cancer patients dramatically increased the number of activated NK cells, especially CD56bright NK cells. IL-15 infusions induced an increase in the expression of NKG2D, NKp30, NKp46, and CD16 (21). The IL-15 infusions increased the cytotoxic abilities of all NK-cell subsets, including those involved in natural cytotoxicity, NKG2D, and ADCC. Thus, our data support the view that IL-15 administration augments the ADCC of the studied antitumor monoclonal antibodies and thereby increases their antitumor efficacy.
To translate our observations with IL-15 plus alemtuzumab in the xenograft model, we have opened patient accrual for a clinical trial evaluating IL-15 with alemtuzumab for patients with chronic and acute HTLV-1–associated ATL. Previously we reported that alemtuzumab treatment was associated with an overall response rate of 52% in 29 patients with ATL. Two of 3 patients with chronic, 12 of 15 patients with acute, and only 1 of 11 patients with lymphomatous ATL manifested a partial or complete response. However, the median duration of the response was only 3.4 mo, with an overall survival of 5.9 mo. It is hoped that the addition of IL-15 to the alemtuzumab regimen will increase the proportion of patients responding and especially the duration of their response.
Furthermore, we have requested permission to initiate additional clinical trials in patients with relapsed and refractory chronic lymphocytic leukemia with IL-15 in association with the optimized anti-CD20 monoclonal antibody obinutuzumab and in patients with renal cancer when rhIL-15 and avelumab (anti–PD-L1) will be coadministered. It is hoped that the addition of IL-15 to the therapeutic regimen will increase the absolute response rate and especially the proportion and duration of complete responses.
In conclusion, our emerging understanding of the IL-15/IL-15R system provides a definition of its actions and its disorders in diseases, including T cell malignancies, and opens distinct possibilities for the development of more rational immune intervention. In particular, the present studies support trials that involve IL-15 administration in association with tumor-directed monoclonal antibodies in the treatment of patients with metastatic malignancy.
Materials and Methods
More details of the materials and methods are presented in SI Appendix, SI Materials and Methods.
Cell Lines.
EL4-hCD20 cells were obtained from Oliver W. Press, Fred Hutchinson Cancer Research Center, Seattle, WA, with permission from Josée Golay, Ospedali Riuniti di Bergamo, Bergamo, Italy (59). The ATL cell line, MET-1, was established from the peripheral blood of a patient with acute ATL as described previously (60).
Mouse Models and Therapeutic Studies.
The EL4-hCD20 tumor model was established by i.v. injection of 2 × 105 EL4-hCD20 cells into C57BL/6 WT or C57BL/6 FcγR−/− mice. The ATL leukemia model was established by i.p. injection of 2 × 107 MET-1 cells into SCID/NOD WT or SCID/NOD FcγR−/− mice as described previously (60). The therapeutic studies were performed in both EL4-hCD20 and MET-1 models using WT and FcγR−/− mice. The therapeutic protocols are shown in Fig. 1 A and C. All mice were purchased from The Jackson Laboratory except for SCID/NOD FcγR−/− mice which were generated in the J.V.R. laboratory. All animal experiments were approved by the National Cancer Institute’s Animal Care and Use Committee (NCI ACUC) and were performed in accordance with NCI ACUC guidelines.
In Vivo Cell Depletion Experiments.
The EL4-hCD20 tumor-bearing WT mice received combination therapy of hIL-15 with rituximab at the same dose and dosing schedules as used in the therapeutic study with the exception that the therapy lasted for 2 wk instead of 4 wk. The in vivo cell depletion experiment protocol is shown in Fig. 3A.
Immune Parameters Following the Treatments with hIL-15 or hIL-15 Combined with Cell Depletion Reagents in C57BL/6 WT Mice.
WT mice were treated with hIL-15, i.p., 5 µg per mouse, every day for 6 d or with hIL-15 combined with cell depletion reagents. Anti-asialo-GM1 (50 μL), anti-NK1.1 monoclonal antibody (100 μg), or clodronate liposomes (200 μL) were given on days −2, 0, and 3. At day 6 after initiation of the treatment, all of the mice (three mice per group) were killed and the spleens were obtained. The proportion of different cell populations and surface markers on NK1.1+ cells and macrophages in the spleens were analyzed by flow cytometry. The absolute numbers of different cell populations were calculated.
Statistical Analysis.
Comparison of the data from ex vivo experiments, cell numbers in spleens, and mean fluorescence intensity of FACS staining for cell-surface and intercellular markers between different treatment groups were analyzed using the Student t test. Statistical significance of differences in survival of mice in different groups was determined by the log-rank test using the GraphPad Prism program (GraphPad Software).
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute (NCI). This project has been funded in part with federal funds from the NCI, NIH, under Contract N01-CO-12400.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811615115/-/DCSupplemental.
References
- 1.Klapper JA, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: Immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 4.Bamford RN, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabstein KH, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 6.Marks-Konczalik J, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: Implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 8.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 9.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 10.Burkett PR, et al. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldmann TA, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117:4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sneller MC, et al. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. 2011;118:6845–6848. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois S, Patel HJ, Zhang M, Waldmann TA, Müller JR. Preassociation of IL-15 with IL-15R α-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi H, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 15.Yu P, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci USA. 2012;109:6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16:6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, et al. Augmented IL-15Rα expression by CD40 activation is critical in synergistic CD8 T cell-mediated antitumor activity of anti-CD40 antibody with IL-15 in TRAMP-C2 tumors in mice. J Immunol. 2012;188:6156–6164. doi: 10.4049/jimmunol.1102604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, et al. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci USA. 2009;106:7513–7518. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lugli E, et al. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood. 2010;116:3238–3248. doi: 10.1182/blood-2010-03-275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlon KC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubois S, et al. IL15 infusion of cancer patients expands the subpopulation of cytotoxic CD56bright NK cells and increases NK-cell cytokine release capabilities. Cancer Immunol Res. 2017;5:929–938. doi: 10.1158/2326-6066.CIR-17-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehniger TA, et al. Abstract CT146: First-in-human phase I combination of the IL-15 receptor super agonist complex ALT-803 with a therapeutic (anti-CD20) monoclonal antibody (mAb) for patients with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL) Cancer Res. 2018;78(Suppl 13):CT146. [Google Scholar]
- 23.Romee R, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131:2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosario M, et al. The IL-15-based ALT-803 complex enhances FcγRIIIa-triggered NK cell responses and in vivo clearance of B cell lymphomas. Clin Cancer Res. 2016;22:596–608. doi: 10.1158/1078-0432.CCR-15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrangle JM, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: A non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19:694–704. doi: 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller RA, Maloney DG, Warnke R, Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982;306:517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- 27.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 28.Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–245. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, et al. Activating Fc receptors are required for antitumor efficacy of the antibodies directed toward CD25 in a murine model of adult t-cell leukemia. Cancer Res. 2004;64:5825–5829. doi: 10.1158/0008-5472.CAN-04-1088. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Zhang M, Goldman CK, Ravetch JV, Waldmann TA. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD52 monoclonal antibody, Campath-1H. Cancer Res. 2003;63:6453–6457. [PubMed] [Google Scholar]
- 31.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 32.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci USA. 1998;95:652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li F, Ravetch JV. Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahan R, et al. Therapeutic activity of agonistic, human anti-CD40 monoclonal antibodies requires selective FcγR engagement. Cancer Cell. 2016;29:820–831. doi: 10.1016/j.ccell.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golay J, et al. Rituximab-mediated antibody-dependent cellular cytotoxicity against neoplastic B cells is stimulated strongly by interleukin-2. Haematologica. 2003;88:1002–1012. [PubMed] [Google Scholar]
- 37.Watanabe M, et al. Interleukin-21 can efficiently restore impaired antibody-dependent cell-mediated cytotoxicity in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2010;102:520–529. doi: 10.1038/sj.bjc.6605502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moga E, et al. Interleukin-15 enhances rituximab-dependent cytotoxicity against chronic lymphocytic leukemia cells and overcomes transforming growth factor beta-mediated immunosuppression. Exp Hematol. 2011;39:1064–1071. doi: 10.1016/j.exphem.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Roberti MP, et al. IL-2- or IL-15-activated NK cells enhance cetuximab-mediated activity against triple-negative breast cancer in xenografts and in breast cancer patients. Breast Cancer Res Treat. 2012;136:659–671. doi: 10.1007/s10549-012-2287-y. [DOI] [PubMed] [Google Scholar]
- 40.Vincent M, et al. Highly potent anti-CD20-RLI immunocytokine targeting established human B lymphoma in SCID mouse. MAbs. 2014;6:1026–1037. doi: 10.4161/mabs.28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 42.Abulayha A, Bredan A, El Enshasy H, Daniels I. Rituximab: Modes of action, remaining dispute and future perspective. Future Oncol. 2014;10:2481–2492. doi: 10.2217/fon.14.146. [DOI] [PubMed] [Google Scholar]
- 43.Weng W-K, Czerwinski D, Timmerman J, Hsu FJ, Levy R. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22:4717–4724. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Weng W-K, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Perera LP, et al. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. Am J Hematol. 2017;92:892–901. doi: 10.1002/ajh.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: Interpreting clinical outcomes to date. Blood. 2016;127:3312–3320. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Killock D. Lung cancer: Anti-PD-1 therapy in the frontline. Nat Rev Clin Oncol. 2016;13:715. doi: 10.1038/nrclinonc.2016.170. [DOI] [PubMed] [Google Scholar]
- 49.DiLillo DJ, Ravetch JV. Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell. 2015;161:1035–1045. doi: 10.1016/j.cell.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiLillo DJ, Ravetch JV. Fc-receptor interactions regulate both cytotoxic and immunomodulatory therapeutic antibody effector functions. Cancer Immunol Res. 2015;3:704–713. doi: 10.1158/2326-6066.CIR-15-0120. [DOI] [PubMed] [Google Scholar]
- 51.Kohrt HE, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchida J, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohrt HE, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117:2423–2432. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Zhou Z, Zhang C, Zhang J, Tian Z. Macrophages help NK cells to attack tumor cells by stimulatory NKG2D ligand but protect themselves from NK killing by inhibitory ligand Qa-1. PLoS One. 2012;7:e36928. doi: 10.1371/journal.pone.0036928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michel T, Hentges F, Zimmer J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front Immunol. 2013;3:403. doi: 10.3389/fimmu.2012.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fehniger TA, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: A novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Nimmerjahn F, et al. FcγRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci USA. 2010;107:19396–19401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Gaetano N, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 60.Phillips KE, et al. IL-2Ralpha-directed monoclonal antibodies provide effective therapy in a murine model of adult T-cell leukemia by a mechanism other than blockade of IL-2/IL-2Ralpha interaction. Cancer Res. 2000;60:6977–6984. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.