Significance
The rate of cesarean section (C-section) delivery worldwide far exceeds the World Health Organization’s recommended rate. C-section delivery has been linked to behavioral effects in the offspring. This suggests effects on the brain, but human studies are confounded by the medical complications, altered birth timing, and maternal factors associated with C-section delivery. We addressed these limitations in a carefully controlled study in mice. Vaginally-born offspring exhibited an acute decrease in cell death across the brain that was absent in C-section–born mice. C-section delivery also was associated with softer vocalizations, as well as with a reduction in at least one neuronal cell type and increased body weight at weaning. Thus, birth mode has acute effects on neurodevelopment that may lead to lasting changes in the brain and behavior.
Keywords: apoptosis, parturition, C-section, vasopressin, prenatal
Abstract
Labor and a vaginal delivery trigger changes in peripheral organs that prepare the mammalian fetus to survive ex utero. Surprisingly little attention has been given to whether birth also influences the brain, and to how alterations in birth mode affect neonatal brain development. These are important questions, given the high rates of cesarean section (C-section) delivery worldwide, many of which are elective. We examined the effect of birth mode on neuronal cell death, a widespread developmental process that occurs primarily during the first postnatal week in mice. Timed-pregnant dams were randomly assigned to C-section deliveries that were yoked to vaginal births to carefully match gestation length and circadian time of parturition. Compared with rates of cell death just before birth, vaginally-born offspring had an abrupt, transient decrease in cell death in many brain regions, suggesting that a vaginal delivery is neuroprotective. In contrast, cell death was either unchanged or increased in C-section–born mice. Effects of delivery mode on cell death were greatest for the paraventricular nucleus of the hypothalamus (PVN), which is central to the stress response and brain–immune interactions. The greater cell death in the PVN of C-section–delivered newborns was associated with a reduction in the number of PVN neurons expressing vasopressin at weaning. C-section–delivered mice also showed altered vocalizations in a maternal separation test and greater body mass at weaning. Our results suggest that vaginal birth acutely impacts brain development, and that alterations in birth mode may have lasting consequences.
A vaginal birth is accompanied by extraordinary events for the newborn, including marked hormonal changes, mechanical stimuli associated with labor and delivery, exposure to a plethora of microorganisms, and immune activation. All of these events are altered or absent following a cesarean section (C-section) birth. C-section delivery now accounts for approximately 30% of all births in the United States and up to 50% in several other countries (1). Although C-sections can be life-saving, they are performed at a rate far exceeding the recommendations of the World Health Organization (2), and human epidemiologic studies demonstrate some health-related consequences. For example, children born by C-section are at greater risk of developing metabolic and immune-related conditions, including type 2 diabetes, asthma, celiac disease, and obesity (reviewed in ref. 3).
The perinatal period is characterized by crucial neurodevelopmental processes, but little is known about how birth, or birth mode, affects the brain. Recently, differences in behavioral and cognitive development have been reported in children delivered vaginally vs. those delivered by C-section (4–6), suggesting an effect of birth mode on early brain development. These findings are controversial, however, as it is difficult to control for all confounding factors in a study of delivery mode in humans (7). Emergency C-sections are associated with other birth complications, and elective C-sections, which compose an increasing percentage of all C-sections worldwide, are carried out before term and scheduled at a time of day convenient for the doctor and patient. In addition, C-section delivery is almost always associated with maternal effects, including antibiotic treatment of the mother, and alterations in breast milk and feeding schedules (7).
Carefully controlled animal studies can in principle address these limitations, but surprisingly few studies have been reported to date. Previous studies have focused on neurochemistry in adult animals born by C-section (8–10) or have examined a single brain region (the hippocampus) in neonates (11, 12). A recent study suggested that the timing of birth (i.e., gestation length) may be more important than mode of birth per se (12).
Here we took a systematic approach by examining neuronal cell death immediately before and after birth across many brain regions in mice born vaginally or by C-section. Cell death is a major developmental process occurring primarily during the first postnatal week in mice (13, 14) that eliminates approximately 50% of the neurons initially generated (15). C-section births were yoked to vaginal births to precisely match the total gestation length and circadian time (i.e., time of day) of delivery (SI Appendix, Fig. S1), and pups of both sexes were cross-fostered and raised in mixed-group litters to minimize maternal effects. We also investigated whether differential patterns of perinatal cell death in vaginally-delivered and C-section–delivered offspring lead to long-term effects in a population of hypothalamic neurons that regulates the brain’s stress and immune responses. In addition, we examined the effects of birth mode on ultrasonic vocalizations (USVs) following maternal separation (one of the few behavioral tests that can be performed in neonates) and on gross development before weaning.
Results
Because males and females were included for all measures, we initially considered sex as a factor in all analyses. However, an effect of sex was found only for a single measure, and thus results are reported for both sexes combined unless noted otherwise.
Greater Neuronal Cell Death After C-Section Delivery.
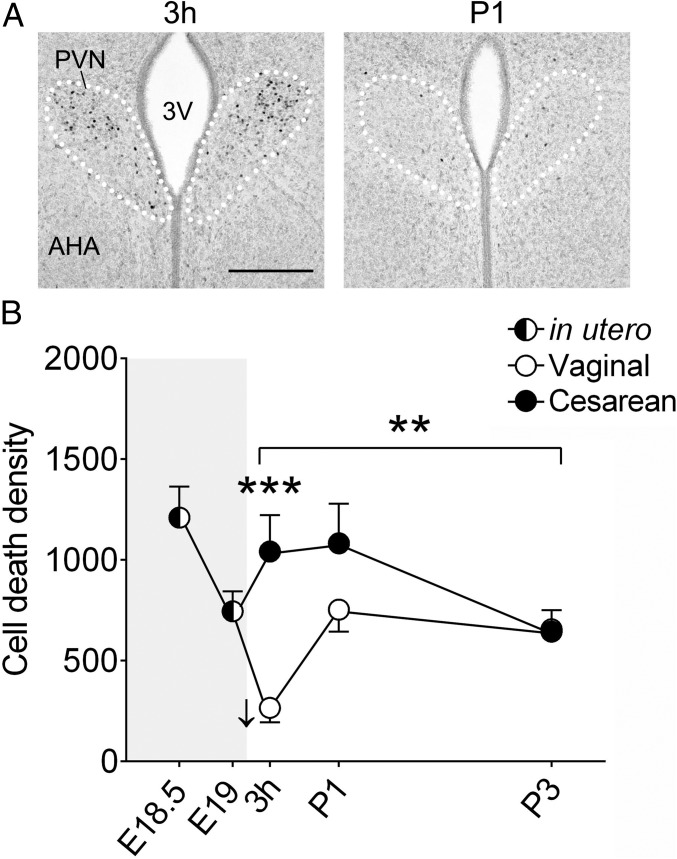
Birth is both stressful and inflammatory for the mammalian fetus and neonate (16, 17). Thus, we first focused on the paraventricular nucleus of the hypothalamus (PVN), which orchestrates the brain’s response to stress and immune challenges. The PVN is known to be strongly activated at birth in sheep (18), and we confirmed this activation in mice (Fig. 1A). Using immunohistochemistry to detect activated caspase-3 (AC3), a marker of cell death, we quantified the density of dying cells in the PVN at two timepoints just before birth, embryonic day (E) 18.5 and E19.0 (mean gestation length is 19.3 d for the C57BL/6 mice used here; ref. 19), and at 3 h postnatally, postnatal day (P) 1, and P3 after a C-section or vaginal delivery (SI Appendix, Fig. S1). We first assessed the acute effects of birth on apoptosis. Vaginally-delivered mice showed an abrupt decline in cell death between E19 and 3 h postnatally that was absent in C-section–delivered pups (Fig. 1B). As a result, cell death density in the PVN was threefold higher in C-section–delivered mice compared with the vaginally-delivered mice at 3 h postnatally (P < 0.0001).
Fig. 1.
(A) Immunohistochemistry for cFos (dark-stained cell nuclei), a marker of neural activation, showing strong activation of the PVN (white dotted lines) at 3 h (Left), but not 1 d (P1; Right), after a vaginal birth. 3V, third ventricle. (Scale bar: 200 μm.) (B) Cell death in the PVN (AC3+ cells/mm3) decreased abruptly following vaginal birth (E19 vs. 3 h; P < 0.05; down-facing arrow), but not after C-section delivery. Across the three postnatal ages, there was an effect of birth mode (asterisks over bracket, **P = 0.001), with greater cell death following C-section delivery. In planned comparisons for individual time points, cell death was greater in the C-section group at 3 h (***P < 0.0001) but not at P1 or P3. Gray shading indicates in utero timepoints. Data are mean ± SEM. n = 12 per group.
In a second analysis, we used two-way ANOVA to determine whether cell death varied significantly between the C-section and vaginal groups over the three postnatal ages. There was both a significant effect of birth mode (P = 0.001) and a birth mode-by-age interaction (P = 0.014) on postnatal cell death in the PVN (Fig. 1B), reflecting the fact that cell death was initially higher in C-section–delivered mice but returned to levels characteristic of vaginally-born mice by P3.
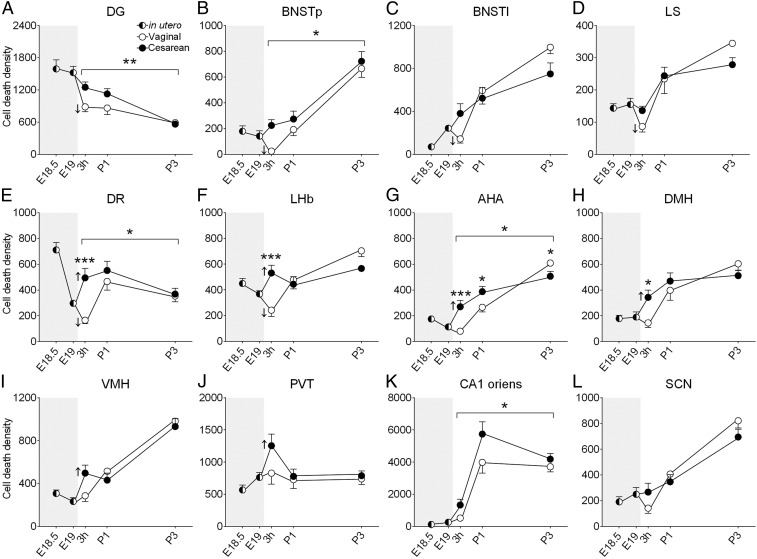
To evaluate whether the effects of birth mode were more widespread, we examined 12 additional brain regions (Fig. 2), focusing on areas linked to physiological functions likely to be influenced by birth (e.g., feeding, thermoregulation, sleep, stress response) and on regions where we had previously mapped the timing of neuronal cell death in neonatal mice (14). As seen in the PVN, vaginally-born pups had an abrupt decline in cell death between E19 and 3 h postnatally in several regions, including the lateral and principal bed nuclei of the stria terminalis (BNSTl and BNSTp, respectively), dentate gyrus (DG), lateral septum (LS), lateral habenula (LHb), and dorsal raphe (DR) (Fig. 2). In contrast, cell death was either unchanged (in the BNSTl, BNSTp, DG, and LS) or increased [in the LHb, DR, anterior hypothalamic area (AHA), dorsomedial hypothalamus (DMH), paraventricular nucleus of the thalamus (PVT), and ventromedial nucleus of the hypothalamus (VMH)] in C-section–delivered offspring (Fig. 2).
Fig. 2.
Cell death (AC3+ cells/mm3) decreased abruptly following vaginal birth in the DG, BNSTp, BNSTl, LS, DR, and LHb (A–F, down-facing arrows indicate significant decline between E19 and 3 h postnatally; P < 0.05 for all), but increased following a C-section birth in the DR, LHb, AHA, DMH, VMH, and PVT (E–J, up-facing arrows; P < 0.05 for all). Asterisks over brackets indicate a significant effect of birth mode on cell death across all postnatal ages (A, B, E, G, K; *P < 0.04; **P < 0.007). No effects were seen in the SCN (L). Asterisks above symbols indicate significant post hoc comparisons (following a significant interaction) for single timepoints (*P < 0.04; ***P = 0.0006). Gray shading indicates in utero timepoints. Data are mean ± SEM. n = 9–12 per group.
Across the three postnatal ages, C-section–born mice had significantly greater cell death than vaginally-born animals in the DG (P = 0.007), CA1 oriens (P = 0.01), BNSTp (P = 0.02), AHA (P = 0.02), and DR (P = 0.004) (Fig. 2). A birth mode-by-postnatal age interaction was found in the AHA (P = 0.0004), DR (P = 0.03), DMH (P = 0.04), and LHb (P = 0.0008), in all cases reflecting higher cell death in C-section–born mice at 3 h postnatally and/or P1 but not at P3 (Fig. 2). In the BNSTl, there was a similar interaction (P = 0.03), but planned comparisons were not significant at any single age (Fig. 2). The effect of birth mode on postnatal cell death was not significant in the LS, suprachiasmatic nucleus (SCN), VMH, and PVT (Fig. 2). Thus, 9 out of 13 brain regions showed greater cell death in neonatal C-section–born mice, and no brain region showed the opposite pattern.
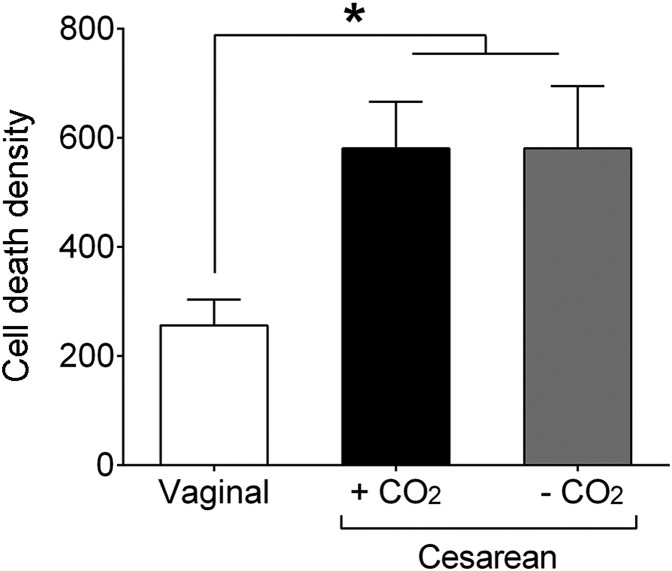
Altered Cell Death Following C-Section Delivery Is Not an Artifact of CO2 Exposure.
It was possible that the differences in cell death between vaginally-born and C-section–born mice was due to the use of CO2 for the C-section procedure. Newborn mice are refractory to CO2 (20) and are immediately responsive on C-section delivery following maternal CO2 exposure, with no apparent ill effects. Nonetheless, to test the effects of CO2 directly, we compared cell death in mice born vaginally, those born by C-section with CO2 exposure as above, and those born by C-section with no CO2 exposure. We focused on the PVN at 3 h postnatally because this is where we saw the greatest effect of C-section birth on cell death (Fig. 1). Cell death in the two C-section–delivered groups was virtually identical (Fig. 3), and in both cases greater than that in vaginally-born mice (P = 0.02 for each comparison). Thus, the effect of birth mode on cell death was replicated, and CO2 exposure of the dam had no effect on cell death in the offspring.
Fig. 3.
Increased cell death following C-section delivery is not an artifact of CO2 exposure. Compared with vaginally-born neonates, C-section–born mice had increased cell death in the PVN at 3 h after birth, regardless of CO2 use for the C-section procedure. *P = 0.02. Data are mean ± SEM. n = 11–12 per group.
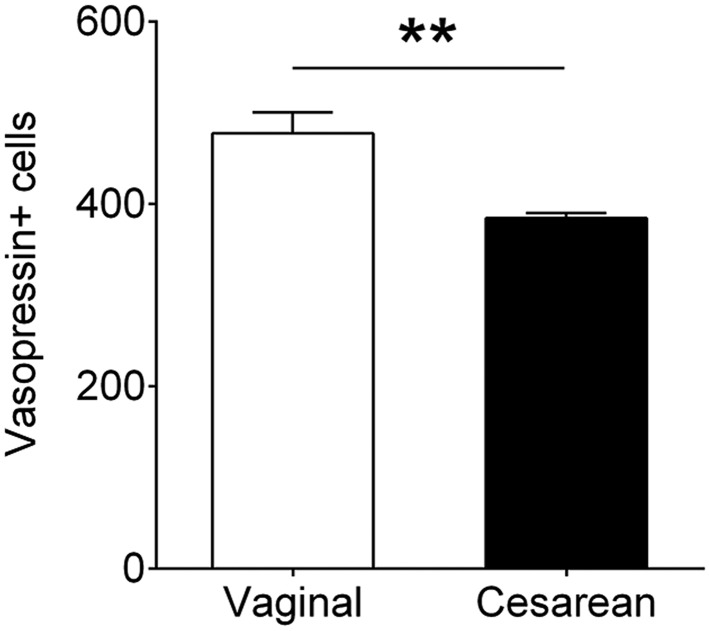
C-Section Birth Causes a Long-Term Change in the PVN.
To determine whether the transient difference in cell death between vaginally-born and C-section–born mice caused long-lasting effects in the brain, we examined total cell number and vasopressin cell number in the PVN at weaning. In humans, vaginal birth is accompanied by a massive release of vasopressin that is not seen following C-section birth (21–23). Based on stereologic cell counts, we found no difference between the groups in total PVN cell numbers at weaning (SI Appendix, Fig. S2); however, there was a significant 20% reduction in the number of cells immunoreactive for vasopressin in the C-section–born mice (P = 0.001; Fig. 4).
Fig. 4.
C-section birth reduced the number of vasopressin neurons in the PVN of weanlings. **P = 0.001. Data are mean ± SEM. n = 8–12 per group.
C-Section–Born Pups Have Decreased USV Amplitudes.
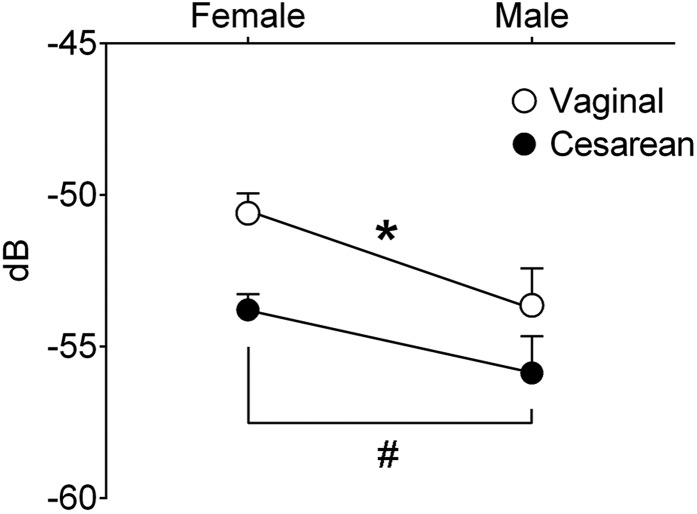
Newborn mice emit ultrasonic distress calls when isolated from their dams and littermates. This behavior reaches a peak at the beginning of the second postnatal week (24) and may provide a measure of affective development (25, 26). We conducted an isolation test on P9 and found reduced call amplitude in C-section–born mice (P = 0.02; Fig. 5). In agreement with a previous report on newborn rats (27), there was also a sex difference in call amplitude, with males emitting softer calls (P = 0.02; Fig. 5). There was no effect of birth mode on the number, duration, or frequency of USVs.
Fig. 5.
C-section–born mice emitted USVs of lower amplitude than vaginally-born mice (*P = 0.02). In addition, males showed lower USV amplitude than females (#P = 0.02). dB, decibels. Data are mean ± SEM. n = 4–6 per group.
Birth Mode Influences Body Weight Gain but Not Other Measures of Gross Development.
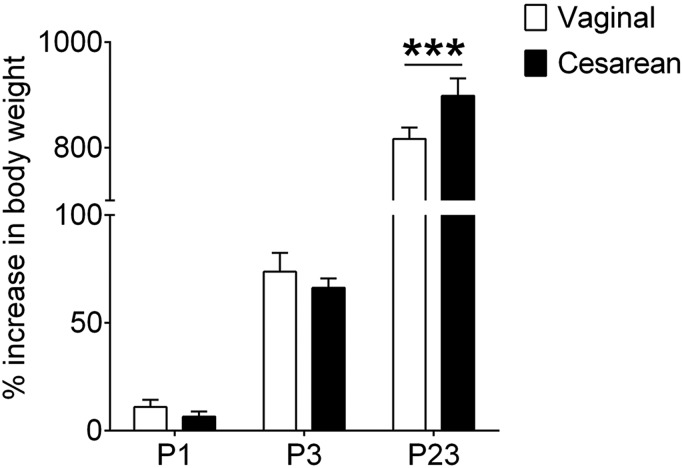
There was no difference in body weight at birth between pups born vaginally and those delivered by C-section (SI Appendix, Fig. S3A). However, C-section–born mice had gained more weight than vaginally-born mice by the time of weaning, leading to a significant effect of birth mode (P = 0.05) and a birth mode-by-age interaction (P = 0.004) on body weight gain (Fig. 6). The timing of eye opening, a measure of general physical maturation, was not affected by birth condition, and overall forebrain size at weaning, a global measure of brain development, also was equivalent in the two groups (SI Appendix, Fig. S3 B and C).
Fig. 6.
C-section–born mice had a significantly greater percent increase in body weight at weaning (P23). ***P = 0.0009. Data are mean ± SEM. n = 10–15 per group.
Discussion
Previous studies examining the acute effects of birth mode on brain development have reported a change in gene expression in hippocampal neurons (11) and a transient effect on the morphology of hippocampal pyramidal cells (12) in animals born by C-section. Here we examined 13 widespread brain regions and found effects of birth mode on a fundamental neurodevelopmental process; in many brain regions, vaginally-born offspring had an acute decrease in cell death shortly after birth that was absent in pups delivered by C-section, whereas in other regions, cell death did not change relative to prenatal levels in vaginally delivered pups, but increased in those delivered by C-section. Although the method we used to assess cell death did not allow us to determine the phenotype of the labeled cells, previous research suggests that the majority of cells positive for AC3 in the neonatal mouse brain are dying neurons, as described in detail previously (14). C-section birth was also associated with fewer vasopressin neurons in the PVN and increased body weight at weaning, as well as with changes in distress calls during the neonatal period.
Effects of Birth on Cell Death.
Cell death in many brain regions decreased abruptly following vaginal birth. To our knowledge, this effect has not been reported previously, and it suggests that labor and vaginal delivery trigger as-yet unidentified mechanisms that inhibit cell death. Remarkably, C-section–born mice never showed the decline in cell death immediately after birth, with brain regions showing either no change or increased cell death at 3 h after birth. Our results do not reflect developmental age or circadian differences between groups, because C-section–born and vaginally-born mice were carefully matched for gestational age and time of birth.
A vaginal birth is a complex event, and it will be important to tease out the mechanism(s) underlying the effects seen here. Labor and delivery are accompanied by hormonal surges, hypoxia, intense squeezing of the head associated with expulsion of the fetus, and colonization of the newborn by maternal microbes. Vaginal birth triggers a massive release of vasopressin in newborn humans and rats (22, 23, 28, 29). Vasopressin levels in the cerebrospinal fluid are closely correlated with plasma levels in human newborns (30, 31), suggesting concomitant central (i.e., brain) and peripheral release. Because vasopressin is neuroprotective in vitro (32) and suppresses neuronal network activity in newborn rat and guinea pig brains (33), the spike in vasopressin may protect the brain from excitotoxicity at birth. Importantly, a much smaller increase in vasopressin is seen following a C-section birth (21–23, 28), at least in humans. This may explain why the decreased cell death seen in many brain regions after vaginal delivery was absent following C-section delivery.
Maternal oxytocin levels also surge during labor, and in slice cultures of the perinatal rat hippocampus, oxytocin orchestrates a transient excitatory-to-inhibitory switch in GABA signaling that may protect neurons (34, 35). The extent to which maternal oxytocin reaches the fetal brain is not clear, however (36, 37), and oxytocin may actually worsen metabolic indicators of hypoxia when administered to full-term rat dams or directly to newborn pups (38). Hypoxia accompanies parturition regardless of birth mode but is greater following a vaginal delivery than after C-section delivery (39). Because hypoxia is typically associated with the death of neurons (40), and we saw less cell death in vaginally-delivered pups compared with C-section–delivered pups, hypoxia itself seems unlikely to account for our findings.
Differences in microbial colonization at birth could contribute to differences in cell death between vaginally-born and C-section–born mice. The microbiota that colonize human newborns differs by birth mode (41, 42; but see ref. 7), which we posit could have rapid effects on brain development. We recently found that neuronal cell death differs within hours of birth in mice born into a germ-free vs. a conventional environment (43). Similarly, perinatal rats show increased expression of cell death genes in the brain at 2–8 h after exposure to lipopolysaccharide, a component of bacterial cell walls (44, 45). Microbial invasion at birth is associated with the release of inflammatory cytokines (17), and vaginally-born and C-section–born subjects have differing blood cytokine levels (46, 47). Because cytokines can cross the blood-brain barrier and in turn induce cell death and/or neuroprotection (48–50), this could also be a mechanism for influencing cell death in a birth mode-dependent manner.
Long-Term Effects of Birth Mode on the Brain and Behavior.
Given the high rate of C-section deliveries worldwide (1), it is important to understand how birth mode affects the developing brain, and whether any of the changes seen have enduring consequences. As adults, rats delivered by C-section have altered dopaminergic function and receptor levels for excitatory amino acids in the brain, as well as behavioral changes in response to stress (8, 10, 51). These findings are important because they establish the long-term neural effects of C-section delivery, but they leave open the question of what developmental processes are affected. We observed effects of birth mode on cell death that had normalized by P3. Although the death of a cell is an irreversible process, and in that sense is permanent, it is possible that compensatory changes occur at later ages.
To address long-term effects, we analyzed the PVN at weaning and found that C-section–born mice had fewer vasopressin-immunoreactive neurons compared with vaginally-born mice. Like most neuronal cell types, vasopressin neurons are pruned by developmental cell death (52), and birth mode may modulate this process. The lack of effect on total PVN cell number may reflect the fact that vasopressin cells are only one of many cell types in the PVN and suggests that the effects of birth mode on cell death are cell-type specific. An important question is whether effects are specific to the magnocellular or parvocelluar populations of vasopressin neurons in the PVN, which project to the posterior pituitary or within the central nervous system, respectively. However, subdivisions of the mouse PVN are less distinct than those of the rat PVN (53), and we could not distinguish between cell types in the present study. We also cannot rule out the possibility that vasopressin expression, not cell death, is affected by C-section delivery. In fact, neonatal stress alters vasopressin expression in the adult PVN (54). Since by several markers, vaginal birth is more stressful for the fetus than C-section birth (16), birth mode may program vasopressin expression during the perinatal period.
Vasopressin is involved in sociality, the immune response, and the stress axis, among other functions (55). Interestingly, a greater risk of autism (4), impaired immune responses (56), and dampening of the stress response (57) have been linked to C-section birth in humans. In addition, a change in vasopressin cell number could also be related to body weight. In this study, C-section–born pups gained significantly more weight than those born vaginally, which is consistent with a recent finding in mice (58), as well as with reports of higher body mass index and higher rates of obesity in humans born by C-section (59, 60). The mechanism underlying this effect is unknown. However, food intake is suppressed when vasopressin neurons in the PVN are activated (61), and thus a reduction in vasopressin neuron number could contribute to increased weight gain. In fact, in a post hoc analysis, we found that the number of vasopressin cells in the PVN was negatively correlated with the percent increase in body weight at weaning across the animals in this study (R = −0.5; P = 0.02).
C-section–born pups also emitted calls of lower amplitude (i.e., softer calls) in a maternal separation test. Chiesa et al. (12) reported no effect of birth mode on USVs of mice but did not measure call amplitude. Interestingly, call amplitude may be the most salient call feature inducing maternal responses in mice (62). Differences in distress cries also have been reported between vaginally-born and C-section–born human newborns, with reductions in cries and time to calm down in the latter (57, 63).
Conclusions
We have found that birth mode alters neonatal brain development and behavior, as well as body weight gain at weaning in mice. Because cell death sculpts neural networks throughout the brain, the widespread effects of C-section delivery on developmental cell death suggest that birth mode may influence diverse neural processes. C-section delivery has been associated with emotional, attentional, and sleep disturbances in human infants and young children (64, 65). Our findings identify a mechanism that could underlie the effects of birth mode on the brain and behavior.
Methods
Animals.
Adult C57BL/6 mice were obtained from our breeding program or purchased from The Jackson Laboratory. Mice were maintained in a 12-h:12-h light:dark cycle with ad libitum access to food and water. All procedures were approved by Georgia State University’s Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Timed Pregnancies and Delivery Mode.
Timed pregnancies (n = 32) were generated by pairing animals within 1 h of lights off and removing males at 1–2 h after lights on. Brain tissue from offspring was collected at two prenatal (E18.5 and E19) and four postnatal (3 h, P1, P3, and P23) ages.
For the E18.5 and E19 timepoints, dams were anesthetized with 2% CO2 and rapidly decapitated. An abdominal incision was made to expose the uterine horns, and fetuses were extruded and weighed, and their brains were collected. Next, beginning on the evening of E18, we performed hourly checks of the remaining dams under red light. When the first dam had vaginally delivered one pup, a dam showing no signs of labor was selected at random and C-sectioned as described above. In this way, vaginal and C-section deliveries were matched for gestation length and circadian time of delivery.
C-section–delivered newborns were stimulated to breathe by cleaning membranes from their muzzles and gently prodding their bodies with a sterile cotton-tipped applicator. Pups born vaginally were removed from dams within 1 h after the appearance of the first pup. All pups were placed with littermates on a heating pad kept at ±32 °C until 3 h postnatally, when brains from a subset of pups were collected. The remaining pups were marked with tattoo ink to indicate delivery mode, and 6–8 pups were randomly assigned to each of 11 foster dams. Pups were killed on P1, P3, or P23, with all collections performed at 4–6 h after lights on.
Effects of CO2.
We established additional timed pregnancies and monitored for vaginal deliveries as described above. When one dam gave birth, a dam showing no signs of labor was selected at random for C-section either after CO2 exposure (as above) or after rapid decapitation with no exposure to CO2. Pups from 11 litters were collected after a 3-h survival period, as above.
Choice of Cell Death Marker.
We used immunohistochemistry for AC3 to label dying cells. Caspase-3 is initially present in an inactive form and is cleaved (activated) in cells undergoing apoptosis. Although activation of caspase-3 has been seen in some situations in the absence of cell death, the vast majority of AC3+ cells in the neonatal rodent central nervous system colabel with other markers of apoptosis and are neurons (66, 67). In addition, virtually all AC3+ cells are eliminated in the brains of perinatal mice lacking the pro-death gene Bax (13).
Brain Collection and Immunohistochemistry.
Brains were fixed in 5% acrolein for 24 h and then transferred to cryoprotectant solution at −20 °C until sectioning. Brains were coronally frozen-sectioned into four 40-μm series for perinatal animals (E18.5, E19, 3 h postnatally, P1, and P3) and three 30-μm series for weanlings (P23). Sections were processed for detection of AC3 (E18.5, E19, 3 h postnatally, P1, and P3) or vasopressin (P23 only) as reported previously (13, 14, 43). AC3-labeled tissue was counterstained with thionin.
Quantification of AC3 Labeling.
Slides were scanned using a Hamamatsu Nanozoomer slide scanner (Hamamatsu Photonics). Aperio Image Scope software (Leica Biosystems) was used to draw contours around areas of interest, and the number of AC3+ cells within each contour was recorded. AC3 labeling was expressed as a density (AC3+ cells per mm3), and all analyses were performed by investigators blinded to experimental condition.
We analyzed 13 brain regions that are reliably identifiable in the newborn brain based on thionin staining. These included telencephalic, hypothalamic, thalamic, epithalamic, and midbrain regions. The CA1 oriens and the DG were defined as described previously (14). For the LS, we included sections from the point at which the corpus callosum connects at the midline to the point at which the medial septum disappears. For the DR, we included sections from its most rostral appearance to the point at which the interfascicular part of the DR disappears. All sections were included for all other regions of interest.
Total and Vasopressin Cell Numbers in the PVN.
Brain sections of mice at P23 were stained with thionin. Stereologic cell counts were made using Stereo Investigator software (MBF Biosciences), using the optical fractionator function (counting frame, 25 μm × 25 μm; sampling grid, 80 μm × 80 μm). We also used Stereo Investigator to count the total number of vasopressin cells in the PVN.
Forebrain Size and Eye Opening.
Forebrain size was determined as described previously (43). For eye opening, pups were monitored twice daily for separation of the eyelid margins. The day of eye opening was considered the day at which a pup had opened both eyes.
Maternal Separation Test.
We recorded USVs during separation from the dam and littermates on P9. Dams were removed at 15–30 min before testing (approximately 6 h after lights on), and cages containing all littermates were placed on a heating pad maintained at ±32 °C. Individual pups were then placed in a glass dish containing bedding material in a recording chamber heated to 32 °C. A microphone was positioned 21.5 cm from the pup, and USVs were recorded for 3 min after which pups were returned to their littermates. USV analysis was performed using Avisoft SAS Lab Pro (Avisoft Bioacoustics) as described previously (68). We identified all USVs of at least 5-ms duration with frequencies of 50–80 kHz, in accordance with previous studies in postnatal mice (25).
Data Analyses.
One-way ANOVA was used to evaluate acute effects of birth as well as potential effects of anesthetic exposure on cell death. Two-way ANOVA was used to evaluate postnatal group differences in cell death, USVs, and body weight. When applicable, ANOVA was followed by Fisher’s least significant difference post hoc tests. The t test was used to evaluate group differences in regional volume, total and vasopressin cell number in the PVN, forebrain size, and day of eye-opening. Data transformations and nonparametric tests (Kruskal–Wallis test followed by Dunn’s tests) were performed as necessary (this applied only to cell death data).
Supplementary Material
Acknowledgments
We thank Rex Burch, Alex Strahan, Dean Blake, and Michael Black for their technical assistance and Geert de Vries, Laura Cortes, Carla Cisternas, Ilona Golynker, Oliver Davidson, Janique de Vries, and James Taylor for their helpful comments on this manuscript. This work was supported by the National Science Foundation (Grant IOS-1743673, to N.G.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11664.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811962115/-/DCSupplemental.
References
- 1.Betrán AP, et al. The increasing trend in caesarean section rates: Global, regional and national estimates, 1990-2014. PLoS One. 2016;11:e0148343. doi: 10.1371/journal.pone.0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betran AP, Torloni MR, Zhang JJ, Gülmezoglu AM. WHO Working Group on Caesarean Section WHO statement on caesarean section rates. BJOG. 2016;123:667–670. doi: 10.1111/1471-0528.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tribe RM, et al. Parturition and the perinatal period: Can mode of delivery impact on the future health of the neonate? J Physiol. March 13, 2018 doi: 10.1113/JP275429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curran EA, et al. Research review. Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. J Child Psychol Psychiatry. 2015;56:500–508. doi: 10.1111/jcpp.12351. [DOI] [PubMed] [Google Scholar]
- 5.Yip BHK, et al. Caesarean section and risk of autism across gestational age: A multi-national cohort study of 5 million births. Int J Epidemiol. 2017;46:429–439. doi: 10.1093/ije/dyw336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polidano C, Zhu A, Bornstein JC. The relation between cesarean birth and child cognitive development. Sci Rep. 2017;7:11483. doi: 10.1038/s41598-017-10831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stinson LF, Payne MS, Keelan JA. A critical review of the bacterial baptism hypothesis and the impact of cesarean delivery on the infant microbiome. Front Med (Lausanne) 2018;5:135. doi: 10.3389/fmed.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boksa P, El-Khodor BF. Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: Possible implications for schizophrenia and other disorders. Neurosci Biobehav Rev. 2003;27:91–101. doi: 10.1016/s0149-7634(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 9.El-Khodor BF, Boksa P. Differential vulnerability of male versus female rats to long-term effects of birth insult on brain catecholamine levels. Exp Neurol. 2003;182:208–219. doi: 10.1016/s0014-4886(03)00079-7. [DOI] [PubMed] [Google Scholar]
- 10.El-Khodor BF, Flores G, Srivastava LK, Boksa P. Effects of birth insult and stress at adulthood on excitatory amino acid receptors in adult rat brain. Synapse. 2004;54:138–146. doi: 10.1002/syn.20073. [DOI] [PubMed] [Google Scholar]
- 11.Simon-Areces J, et al. UCP2 induced by natural birth regulates neuronal differentiation of the hippocampus and related adult behavior. PLoS One. 2012;7:e42911. doi: 10.1371/journal.pone.0042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiesa M, et al. Term or preterm cesarean section delivery does not lead to long-term detrimental consequences in mice. Cereb Cortex. May 16, 2018 doi: 10.1093/cercor/bhy112. [DOI] [PubMed] [Google Scholar]
- 13.Ahern TH, et al. Cell death atlas of the postnatal mouse ventral forebrain and hypothalamus: Effects of age and sex. J Comp Neurol. 2013;521:2551–2569. doi: 10.1002/cne.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosley M, et al. Patterns of cell death in the perinatal mouse forebrain. J Comp Neurol. 2017;525:47–64. doi: 10.1002/cne.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 16.Lagercrantz H. The good stress of being born. Acta Paediatr. 2016;105:1413–1416. doi: 10.1111/apa.13615. [DOI] [PubMed] [Google Scholar]
- 17.Marchini G, Berggren V, Djilali-Merzoug R, Hansson LO. The birth process initiates an acute phase reaction in the fetus-newborn infant. Acta Paediatr. 2000;89:1082–1086. doi: 10.1080/713794557. [DOI] [PubMed] [Google Scholar]
- 18.McDonald TJ, Nathanielsz PW. Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. Am J Obstet Gynecol. 1991;165:764–770. doi: 10.1016/0002-9378(91)90325-l. [DOI] [PubMed] [Google Scholar]
- 19.Murray SA, et al. Mouse gestation length is genetically determined. PLoS One. 2010;5:e12418. doi: 10.1371/journal.pone.0012418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchett K, Corrow D, Stockwell J, Smith A. Euthanasia of neonatal mice with carbon dioxide. Comp Med. 2005;55:275–281. [PubMed] [Google Scholar]
- 21.Wellmann S, et al. High copeptin concentrations in umbilical cord blood after vaginal delivery and birth acidosis. J Clin Endocrinol Metab. 2010;95:5091–5096. doi: 10.1210/jc.2010-1331. [DOI] [PubMed] [Google Scholar]
- 22.Chard T, Hudson CN, Edwards CR, Boyd NR. Release of oxytocin and vasopressin by the human foetus during labour. Nature. 1971;234:352–354. doi: 10.1038/234352a0. [DOI] [PubMed] [Google Scholar]
- 23.Polin RA, Husain MK, James LS, Frantz AG. High vasopressin concentrations in human umbilical cord blood: Lack of correlation with stress. J Perinat Med. 1977;5:114–119. doi: 10.1515/jpme.1977.5.3.114. [DOI] [PubMed] [Google Scholar]
- 24.Elwood RW, Keeling F. Temporal organization of ultrasonic vocalizations in infant mice. Dev Psychobiol. 1982;15:221–227. doi: 10.1002/dev.420150306. [DOI] [PubMed] [Google Scholar]
- 25.Hofer MA, Shair HN, Brunelli SA. Ultrasonic vocalizations in rat and mouse pups. Curr Protoc Neurosci. 2002;Chapter 8:Unit 8.14. doi: 10.1002/0471142301.ns0814s17. [DOI] [PubMed] [Google Scholar]
- 26.Winslow JT. Ultrasonic vocalizations by infant mice: An ethological expression of separation anxiety. Neuromethods. 2009;42:67–84. [Google Scholar]
- 27.Bowers JM, Perez-Pouchoulen M, Edwards NS, McCarthy MM. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J Neurosci. 2013;33:3276–3283. doi: 10.1523/JNEUROSCI.0425-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellmann S, Bührer C. Who plays the strings in newborn analgesia at birth, vasopressin or oxytocin? Front Neurosci. 2012;6:78. doi: 10.3389/fnins.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summanen M, Bäck S, Voipio J, Kaila K. Surge of peripheral arginine vasopressin in a rat model of birth asphyxia. Front Cell Neurosci. 2018;12:2. doi: 10.3389/fncel.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartrons J, Figueras J, Jiménez R, Gaya J, Cruz M. Vasopressin in cerebrospinal fluid of newborns with hypoxic-ischemic encephalopathy: Preliminary report. J Perinat Med. 1993;21:399–403. doi: 10.1515/jpme.1993.21.5.399. [DOI] [PubMed] [Google Scholar]
- 31.Carson DS, et al. Plasma vasopressin concentrations positively predict cerebrospinal fluid vasopressin concentrations in human neonates. Peptides. 2014;61:12–16. doi: 10.1016/j.peptides.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Aguilera G. Vasopressin protects hippocampal neurones in culture against nutrient deprivation or glutamate-induced apoptosis. J Neuroendocrinol. 2010;22:1072–1081. doi: 10.1111/j.1365-2826.2010.02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spoljaric A, et al. Vasopressin excites interneurons to suppress hippocampal network activity across a broad span of brain maturity at birth. Proc Natl Acad Sci USA. 2017;114:E10819–E10828. doi: 10.1073/pnas.1717337114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyzio R, et al. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 35.Tyzio R, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 36.Brown CH, Grattan DR. Does maternal oxytocin protect the fetal brain? Trends Endocrinol Metab. 2007;18:225–226. doi: 10.1016/j.tem.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Carbillon L. Comment on “Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery”. Science. 2007;317:197, author reply 197. doi: 10.1126/science.1141090. [DOI] [PubMed] [Google Scholar]
- 38.Boksa P, Zhang Y, Nouel D. Maternal oxytocin administration before birth influences the effects of birth anoxia on the neonatal rat brain. Neurochem Res. 2015;40:1631–1643. doi: 10.1007/s11064-015-1645-7. [DOI] [PubMed] [Google Scholar]
- 39.Berger N, Vaillancourt C, Boksa P. Interactive effects of anoxia and general anesthesia during birth on the degree of CNS and systemic hypoxia produced in neonatal rats. Exp Brain Res. 2000;131:524–531. doi: 10.1007/s002219900305. [DOI] [PubMed] [Google Scholar]
- 40.Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29:551–563. doi: 10.1016/j.ijdevneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wampach L, et al. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front Microbiol. 2017;8:738. doi: 10.3389/fmicb.2017.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dominguez-Bello MG, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castillo-Ruiz A, et al. The microbiota influences cell death and microglial colonization in the perinatal mouse brain. Brain Behav Immun. 2018;67:218–229. doi: 10.1016/j.bbi.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eklind S, et al. Effect of lipopolysaccharide on global gene expression in the immature rat brain. Pediatr Res. 2006;60:161–168. doi: 10.1203/01.pdr.0000228323.32445.7d. [DOI] [PubMed] [Google Scholar]
- 45.Sharangpani A, Takanohashi A, Bell MJ. Caspase activation in fetal rat brain following experimental intrauterine inflammation. Brain Res. 2008;1200:138–145. doi: 10.1016/j.brainres.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ly NP, et al. Mode of delivery and cord blood cytokines: A birth cohort study. Clin Mol Allergy. 2006;4:13. doi: 10.1186/1476-7961-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malamitsi-Puchner A, et al. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum Dev. 2005;81:387–392. doi: 10.1016/j.earlhumdev.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Correale J, Villa A. The neuroprotective role of inflammation in nervous system injuries. J Neurol. 2004;251:1304–1316. doi: 10.1007/s00415-004-0649-z. [DOI] [PubMed] [Google Scholar]
- 49.Lambertsen KL, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sedel F, Béchade C, Vyas S, Triller A. Macrophage-derived tumor necrosis factor α, an early developmental signal for motoneuron death. J Neurosci. 2004;24:2236–2246. doi: 10.1523/JNEUROSCI.4464-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boksa P, Wilson D, Rochford J. Responses to stress and novelty in adult rats born vaginally, by cesarean section or by cesarean section with acute anoxia. Biol Neonate. 1998;74:48–59. doi: 10.1159/000014010. [DOI] [PubMed] [Google Scholar]
- 52.de Vries GJ, et al. Sexual differentiation of vasopressin innervation of the brain: Cell death versus phenotypic differentiation. Endocrinology. 2008;149:4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biag J, et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: A study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murgatroyd C, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 55.Mavani GP, DeVita MV, Michelis MF. A review of the nonpressor and nonantidiuretic actions of the hormone vasopressin. Front Med (Lausanne) 2015;2:19. doi: 10.3389/fmed.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208:249–254. doi: 10.1016/j.ajog.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Taylor A, Fisk NM, Glover V. Mode of delivery and subsequent stress response. Lancet. 2000;355:120. doi: 10.1016/S0140-6736(99)02549-0. [DOI] [PubMed] [Google Scholar]
- 58.Martinez KA, 2nd, et al. Increased weight gain by C-section: Functional significance of the primordial microbiome. Sci Adv. 2017;3:eaao1874. doi: 10.1126/sciadv.aao1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darmasseelane K, Hyde MJ, Santhakumaran S, Gale C, Modi N. Mode of delivery and offspring body mass index, overweight and obesity in adult life: A systematic review and meta-analysis. PLoS One. 2014;9:e87896. doi: 10.1371/journal.pone.0087896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan C, et al. Association between Cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 2016;170:e162385. doi: 10.1001/jamapediatrics.2016.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol Metab. 2014;3:209–215. doi: 10.1016/j.molmet.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wohr M, Oddi D, D’Amato FR. Effect of altricial pup ultrasonic vocalization on maternal behavior. Hbk Behav Neurosci. 2010;19:159–166. [Google Scholar]
- 63.Olza Fernández I, et al. Mode of delivery may influence neonatal responsiveness to maternal separation. Early Hum Dev. 2013;89:339–342. doi: 10.1016/j.earlhumdev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Adler SA, Wong-Kee-You AM. Differential attentional responding in caesarean versus vaginally delivered infants. Atten Percept Psychophys. 2015;77:2529–2539. doi: 10.3758/s13414-015-0969-3. [DOI] [PubMed] [Google Scholar]
- 65.Kelmanson IA. Emotional and behavioural features of preschool children born by caesarean deliveries at maternal request. Eur J Dev Psychol. 2013;10:676–690. [Google Scholar]
- 66.Srinivasan A, et al. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- 67.Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner. Neuroscience. 2011;199:535–547. doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paul MJ, et al. Atypical social development in vasopressin-deficient Brattleboro rats. eNeuro. 2016;3:e0150–0115.2016. doi: 10.1523/ENEURO.0150-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.