Significance
Shortly after its appearance on the drug market, it was found out that thalidomide was highly teratogenic. Although thalidomide passed the safety check in pregnant mice, it was not safe among humans due to different actions of thalidomide among various species. Due to inactivity of immunomodulatory drugs (IMiDs) in mice, preclinical safety checks and clinical investigation of IMiDs is impossible in murine models. Here, we developed a murine model to study IMiDs in vivo and began to unravel the complex IMiD mechanism of action. This model may also permit investigation of the main safety concerns. We further investigated IMiD activity toward different substrates targeted by small molecules. Overall, our study provides an important insight into the study of IMiDs.
Keywords: IMiDs, humanized cereblon, Ikaros 1, casein kinase-1α, DSS colitis
Abstract
Immunomodulatory drugs (IMiDs), including thalidomide derivatives such as lenalidomide and pomalidomide, offer therapeutic benefit in several hematopoietic malignancies and autoimmune/inflammatory diseases. However, it is difficult to study the IMiD mechanism of action in murine disease models because murine cereblon (CRBN), the substrate receptor for IMiD action, is resistant to some of IMiDs therapeutic effects. To overcome this difficulty, we generated humanized cereblon (CRBNI391V) mice thereby providing an animal model to unravel complex mechanisms of action in a murine physiological setup. In our current study, we investigated the degradative effect toward IKZF1 and CK-1α, a target substrate of IMiDs. Unlike WT mice which were resistant to lenalidomide and pomalidomide, T lymphocytes from CRBNI391V mice responded with a higher degree of IKZF1 and CK-1α protein degradation. Furthermore, IMiDs resulted in an increase in IL-2 among CRBNI391V mice but not in the WT group. We have also tested a thalidomide derivative, FPFT-2216, which showed an inhibitory effect toward IKZF1 protein level. As opposed to pomalidomide, FPFT-2216 and lenalidomide degrades CK-1α. Additionally, we assessed the potential therapeutic effects of IMiDs in dextran sodium sulfate (DSS)-induced colitis. In both WT and humanized mice, lenalidomide showed a significant therapeutic effect in the DSS model of colitis, while the effect of pomalidomide was less pronounced. Thus, while IMiDs’ degradative effect on IKZF1 and CK-1α, and up-regulation of IL-2, is dependent on CRBN, the therapeutic benefit of IMiDs in a mouse model of inflammatory bowel disease occurs through a CRBN–IMiD binding region independent pathway.
Thalidomide was developed in 1957 by the German pharmaceutical company Chemie Grünenthal as a sedative and used by pregnant women to ameliorate morning sickness. Although thalidomide was found safe among rodents, it was found to cause severe birth defects when used during the first trimester of pregnancy (1, 2). Cereblon (CRBN) was now known as the primary target of thalidomide’s teratogenicity, although thalidomide-induced oxidative stress and its antiangiogenic effects have previously been advanced to explain thalidomide teratogenicity (3–6). More recently, thalidomide’s degradative effect on CRBN binding protein, CD147, was evidenced as one of the downstream signals for teratogenicity (5). Additionally, thalidomide was shown to inhibit the expression of fibroblast growth factor-8 in the apical ectodermal ridge although it does not have any effect among mice and rats even if mouse/rat CRBN is nearly 95% homologous to human CRBN (5–7). The reason for rodent resistance toward some of the thalidomide’s therapeutic use and its side effects remain unknown.
The immunomodulatory drugs (IMiDs) lenalidomide and pomalidomide promote highly selective recruitment and ubiquitination of proteins to the cullin4A/BCRBN E3 ubiquitin ligase, resulting in the degradation of substrate protein via the 26S proteasome (8, 9). Lenalidomide enables CRBN to target the lymphoid transcription factors IKZF1 and IKZF3, as well as the kinase CK-1α, for proteasomal degradation. IMiDs therapeutic benefit in multiple myeloma (MM) and myelodysplastic syndrome (MDS) del(5q) rely on the inhibition of these proteins (8–10).
CRBN was first described due to its mutation and deletions in patients with hereditary mild mental retardation, but at the time little was known about CRBN’s physiological function (11). Lee et al. (12) showed that CRBN binds and regulates adenosine monophosphate-activated protein kinase. Recently, the acetylated form of glutamine synthetase (13), MEIS2 (14), Rabex-5 (15), and uridine (16) were proved to be substrate proteins that bind to CRBN under physiological condition.
Although thalidomide and its derivatives can bind murine CRBN (6, 14, 17), mouse T cells cannot be stimulated to release IL-2 by IMiDs (17). Additionally, mouse multiple myeloma cells are insensitive to lenalidomide treatment (18).
A single amino acid within the CRBN–IMiD binding region causes mouse CRBN to be resistant toward degradative effects of IMiDs among selected cullin4A/BCRBN E3 ubiquitin ligase substrates (10). As a result, IMiD activity in murine systems cannot be extrapolated to human systems. Krönke et al. (10), shown that lenalidomide treatment of mouse cell line Ba/F3 and primary mouse hematopoietic cells do not change CK-1α levels. To better define the in vivo molecular mechanisms underlying mysterious actions of IMiDs and to delineate species-specific IMiDs actions, we developed an animal model; mice carrying a humanizing mutation [CRBNI391V] at the IMiD binding region.
IMiDs have the therapeutic benefit not only in malignancy but also in diseases characterized by an inflammatory phenotype. Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammation of the gastrointestinal tract. It is generally accepted that colonic inflammation results from dysregulation of the mucosal immune responses to intestinal flora together with genetic and environmental factors (19, 20). Inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-1β, IL-23, and IL-17 have been implicated in the pathogenesis of UC (21, 22). IMiDs antiinflammatory properties gave us the interest to look for their ameliorative effect in IBD and explain their mechanism of action which is unknown. In the present study, we used dextran sodium sulfate (DSS)-induced acute colitis in mice to assess IMiDs’ therapeutic benefit and their mechanism of action.
Results
Generation of Humanized Cereblon Mice, CRBNI391V.
The inability to investigate IMiD properties in murine systems has significantly hampered research into understanding the drug mechanism of action. To overcome this difficulty, we generated mice with cereblon carrying a single amino acid change at position 391 (CRBNI391V), where isoleucine was replaced with the valine amino acid counterpart of human CRBN (SI Appendix, Figs. S1 and S2) using the CRISPR/Cas9 system. A 295-bp region surrounding the I391 coding exon (SI Appendix, Figs. S3 and S4) was amplified and cloned into pCAG–EGxxFP and injected into an oocyte. Mutations screened in offspring by restriction digestion with FspI enzyme and reconfirmed with sequencing (SI Appendix, Figs. S1C and S2). Humanization of cereblon in mice did not result in any observable phenotypic difference.
Humanized Cereblon Mice Respond to IMiD Treatment.
IMiDs coactivate T cells stimulated with anti-CD3ε (23). Although such kind of T cell activation is noticed in a human primary cell or cell line, murine T cells are known to be resistant to IMiD T cell modulation. In this study, T cells derived from humanized CRBN mice (CRBNI391V) were found to be highly sensitive toward IMiD treatment. IMiDs resulted in a higher level of IL-2 production in naïve CD4+ T cells isolated from humanized CRBN mice, whereas WT mice remained unresponsive toward IMiDs activity in an in vitro setting (Fig. 1). Under these conditions, drug treatment did not affect the cell viability (SI Appendix, Fig. S5).
Fig. 1.
Humanized cereblon mice respond to IMiD activity. (A) Naïve CD4+ T cells stimulated with anti-CD3ε (5 µg/mL) and simultaneously treated with DMSO or lenalidomide (10 µM) for the indicated time points. The IL-2 level was measured by ELISA. (B) IL-2 production was measured by RT-PCR at the indicated time points. Data are shown as the mean ± SEM of three independent experiments. (n.s, nonsignificant; P > 0.05, P < 0.05, **P < 0.01, ***P < 0.001; Student’s t test).
Cereblon Modulator, FPFT-2216, and Immune Modulation Activity.
We have tested the immunomodulatory activity of a thalidomide derivative, FPFT-2216 (SI Appendix, Fig. S6). We found that FPFT-2216 highly up-regulates the production of IL-2 although it is less potent than that of pomalidomide (pomalidomide > FPFT-2216 ≥ lenalidomide) (Fig. 2A). Furthermore, FPFT-2216 degrades IKZF1 as well as CK-1α among ubiquitin–proteasomal degradative substrates of IMiDs (Fig. 2B). Immunomodulation activity of FPFT-2216 was comparable in the human cell line, Jurkat T cell line, both in terms of IL-2 production (SI Appendix, Fig. S7A) and the degradation of IKZF1 and CK-1α (SI Appendix, Fig. S7B).
Fig. 2.
Cereblon modulator, FPFT-2216, results in up-regulation of IL-2 production and degradation of IKZF1 and CK-1α. (A) Naïve CD4+ T cells treated with lenalidomide (100 µM), pomalidomide (10 µM), FPFT-2216 (10 µM), and IL-2 production measured by ELISA. P values for WT were nonsignificant for comparison between IMiDs and DMSO (P > 0.05). For I391V, P < 0.05 for all comparisons between IMiDs and DMSO. Student’s t test was used to calculate statistical significance. Data are shown as the mean ± SEM of three independent experiments. (B) Naïve CD4+ T cells were treated as A and Western blot analysis was done for IKZF1 and CK-1α.
In Vivo IMiD-Treated Humanized Cereblon Mice Mirror in Vitro Activity.
To assess in vivo activities of IMiDs in mouse physiological setup, a mouse was administered with lenalidomide (100 mg/kg), pomalidomide (50 mg/kg), and FPFT-2216 (30 mg/kg) for 14 h and naïve CD4+ T cells were isolated from spleen. Western blot analysis revealed significant degradation of CK-1α, and IKZF1 among CRBNI391V but WT mice were resistant to IMiD administration. Pomalidomide treatment did not affect CK-1α level, similar to human cells (10) (Fig. 3). As shown in Fig. 3, the route of administration did not affect the efficacy of IMiDs in mice as the degradative effect toward cullin4A/BCRBN E3 ligase substrates were similar when administered orally (Fig. 3A) or intraperitoneally (Fig. 3B).
Fig. 3.
In vivo administration of immunomodulatory drugs result in degradation of cullin4ACRBN E3 ligase substrates. (A) Mice were administered 200 µL of control, lenalidomide (100 mg/kg), pomalidomide (50 mg/kg), FPFT-2216 (30 mg/kg) solubilized in 0.5% carboxymethylcellulose/sodium and 0.25% Tween 80 for 14 h orally by gavaging. Murine CD4+ T cells were isolated from spleen and Western blot was done. (B) IMiDs were administered intraperitoneally and Western blot analysis was done as in A. The data are representative of three independent experiments. n ≥ 3.
Lenalidomide and Pomalidomide Provide Anti-DSS Colitis Therapeutic Benefit.
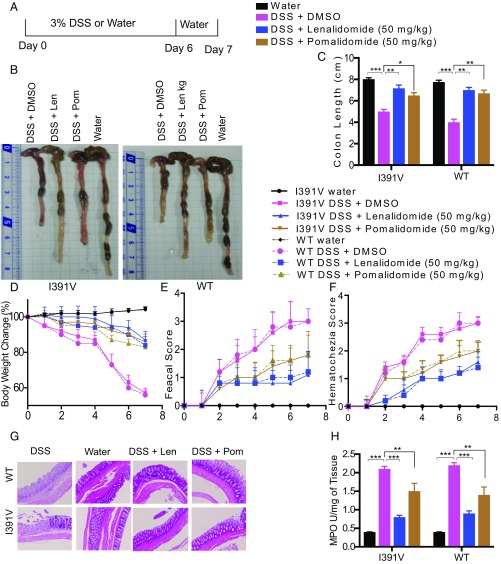
Thalidomide inhibits TNF-α production (24). We investigated IMiD activity toward Toll-like receptor-4 (TLR-4)–activated cytokine production. Peritoneal macrophages were treated with lenalidomide (50 μM), pomalidomide (10 μM), or dimethyl sulfoxide (DMSO) (control), 2 h before stimulation with lipopolysaccharide (LPS). We found that the suppressive effects of IMiDs toward TNF-α production in both CRBNI391V as well as WT mice were comparable (SI Appendix, Fig. S8). To assess the anti-IBD activity of IMiDs, acute colitis was induced in 8-wk-old male CRBNI391V and WT BDF1 mice using 3% DSS (Fig. 4A) to develop typical symptoms of IBD, including body weight loss, diarrhea, and rectal bleeding. DSS administration for 6 d resulted in severe diarrhea, blood in stool, and body weight loss (Fig. 4 D–F).
Fig. 4.
Lenalidomide protects against DSS-induced colitis. (A) DSS-colitis induction. Mice were given 3% DSS for 6 d and water only for the final 1 d, they were simultaneously treated with IMiDs and killed on day 7. (B) Colon isolated from DSS colitis-induced mice. (C) Colon length. (D) Clinical score, change in body weight. P values for WT vs. I391V were nonsignificant for all treatments. P value for DMSO vs. pomalidomide was nonsignificant for WT mice. P value for DMSO vs. lenalidomide was significant for both WT and I391V. P value for DMSO vs. pomalidomide was significant for I391V. (E) Fecal score (stool consistency) and (F) hematochezia (level of blood in stool). P values for WT vs. I391V were nonsignificant for all treatments. P values for DMSO vs. all treatments were significant for both WT and I391V mice (E and F). (G) Histopathological image of colon tissue stained with hematoxylin and eosin. (Scale bar, 10 µm.) (H) Myeloperoxidase activity from colon tissue was measured using a colorimetric method. All experiments were representatives of two independent experiments. Data are expressed as the mean ± SEM (n = 6 per group). Student’s t test was used.
The protective effects of lenalidomide (50 mg/kg) and pomalidomide (50 mg/kg) were evaluated after daily administration to the mice along with DSS. As shown in Fig. 4D, body weight dramatically dropped by DSS induction compared with the vehicle group. The body loss caused by DSS treatment was significantly improved in mice receiving lenalidomide. Pomalidomide helped in regaining the loss of body weight due to DSS administration although its effect was not as potent as that seen in lenalidomide-treated groups (Fig. 4D).
Furthermore, macroscopic examination of the colon revealed that mice treated with DSS had shorter colons than that in the control group. Lenalidomide (50 mg/kg) treatment attenuated colon shortening upon DSS intake (Fig. 4 B and C), indicating less severe inflammation in the colon.
However, pomalidomide (50 mg/kg) treated groups showed little recovery in the colon length (Fig. 4 B and C).
DSS treatment resulted in bloody stool with further colonic inflammation and damage to the intestinal epithelial cell architecture. As shown in the histological image (Fig. 4G), severe crypt destruction and inflammatory cell infiltration were reduced in the histological sections from lenalidomide-treated groups. This ameliorative effect was less observed among pomalidomide-treated mice.
To evaluate the inflammatory cell infiltration in the colon, myeloperoxidase (MPO) activity in mice colonic tissues were examined. Consistent with histological scores, MPO activity in the colon of DSS-treated group was higher than the control group, which was significantly suppressed upon treating mice with lenalidomide (Fig. 4H). Similar to other DSS colitis clinical scores, pomalidomide was less effective in its suppressive effect toward MPO release. Taken together, our results indicate that lenalidomide can markedly reduce inflammatory cell infiltration in the colon of DSS-induced colitis mice.
The infiltration of circulating leukocytes into the colon evokes the release of proinflammatory cytokines such as IL-1β, IL-6, IFN-γ, and TNF-α (21). To determine whether IMiDs abrogate the production of proinflammatory cytokines in the colon, thereby giving a protective advantage to the colonic mucosa, the levels of IL-6, TNF-α, and IL-1β in the supernatant of cultured colons of 3% DSS exposed mice were measured. As shown in SI Appendix, Fig. S9, treatment with lenalidomide reduces the levels of TNF-α, IL-6, and IL-1β although these inhibitions were statistically significant only for TNF-α and IL-1β. These data suggest that lenalidomide may lessen the damaging effects of DSS by suppressing the expression of proinflammatory cytokines. These results agree with Fakhoury et al.’s study (25), where mice were treated with thalidomide, the lead compound of IMiDs family, resulted in significant decrease in the amount of proinflammatory cytokine, TNF-α, IL-6, and IL-1β.
Discussion
In our current study, we revealed that a single amino acid substitution in murine cereblon, CRBNI391V, sensitizes murine CD4+ T cells to IMiDs treatment which are otherwise resistant. While this manuscript was in preparation others have independently confirmed that a single amino acid substitution is sufficient for mice responsiveness to IMiDs activity (26). Although WT mice were resistant to most of the thalidomide’s therapeutic and side effect (5, 10, 17, 18, 27), we found that, upon treating with lenalidomide, pomalidomide, and FPFT-2216, CD4+ T cells from humanized cereblon mice resulted in a higher level of degradation of target IMiD molecule, IKZF1. Concomitantly, CD4+ T cells from humanized cereblon mice produced a higher level of IL-2 upon treatment with lenalidomide, pomalidomide, and FPFT-2216 in an in vitro setting.
We further asked if IMiDs activity can be reproduced in these genetically engineered mice. Humanized cereblon mice treated with IMiDs responded with a higher degree toward CRBN–IMiD target substrates upon drug administration. IMiDs degraded IKZF1 and CK-1α among humanized cereblon mice reconfirming active E3 ubiquitin ligase degradative activity of IMiDs in in vivo experiments. Treating mice per oral (by gavaging) or intraperitoneally did not affect the degree of activity of IMiDs among substrates we have tested.
A number of researchers around the globe have tried to find new CRBN modulatory agents, which may allow a wider range of application in treating more diseases from malignancy to inflammatory diseases. We used a CRBN agent, FPFT-2216, and it showed a potent ability to degrade IKZF1, a target molecule of IMiDs in multiple myeloma. In contrast to pomalidomide, FPFT-2216 triggered the depletion of CK-1α protein level. Since CK-1α protein is encoded by a gene within the common deleted region for del(5q) and haploinsufficient expression increases lenalidomide sensitivity among MDS patients, CK-1α was found to be a mechanistic basis for the therapeutic effect of lenalidomide in del(5q) MDS (10). A strong inhibitory effect on the protein level of CK-1α by FPFT-2216 may make this compound a drug of choice for MDS treatment if tested.
By way of explanation, Chamberlein et al. (17) have reported thalidomide and pomalidomide do not induce the formation of two β-strands in the region of CRBN 346–363. On the other hand, these two β-strands are formed in the presence of lenalidomide (17). These β-strand formations were shown to make structural (geometrical) changes of IMiD binding sites of CRBN and these structural changes might have a contributing factor in the differential substrate specificities among IMiD family drugs. Differential substrate specificities of IMiDs were evidenced by the fact that IKZF1 and CK-1α were degraded by both FPFT-2216 and lenalidomide, whereas pomalidomide was active toward IKZF1 but its action in degrading CK-1α was very weak.
As a lead compound of IMiD family drugs, thalidomide was found to relieve extended complications of Mycobacterium leprae infection, erythema nodosum leprosum (28). This treatment benefit of thalidomide was later found to be due to its inhibitory effect toward TNF-α production (24). Although the mechanistical explanation for TNF-α inhibitory effect of thalidomide remains controversial and unclear (29), other IMiDs, lenalidomide, and pomalidomide, retain this property.
Previously, our study showed that IMiDs inhibit TLR4-induced cytokine production through the suppressive effect on the TRIF/IRF3 pathway (30). These observations were made in murine peritoneal macrophages, and occurred even in CRBN−/− mice. This finding shades a light that IMiDs antiinflammatory effect might not exclusively depend on cereblon-IMiD binding region. In our current study, we found that IMiDs show inhibitory effect toward TLR4-activated cytokine production. TNF-α production was strongly inhibited although its inhibitory effect toward IL-6 was less pronounced.
Humanization of cereblon in mice did not contribute to the inhibitory effect of IMiD toward proinflammatory cytokines we have tested. This current finding and previous work by Millrine et al. (30) strongly suggests that there might be other receptors apart from cereblon involved in the inhibitory effects of IMiDs toward TNF-α production. Notably, Handa and coworkers (6) used a bead coated with the phthalimide side of thalidomide, exposing the glutarimide moiety during their cereblon discovery experiment. Using beads attached to the glutarimide moiety of IMiDs and exposing the phthalimide sidechain might help in finding different possible contributors to the IMiDs mysterious activity. Thus, bias in this screening strategy toward glutarimide binding receptors leaves open the possibility that phthalimide binding receptors might exist.
Thalidomide was known to be effective for CD treatment (25, 31, 32). Although thalidomide was proven to be an effective therapeutic drug for CD, its catastrophic side effects have hampered its use (1, 2). We intended to assess the potential therapeutic activity of lenalidomide and pomalidomide in DSS colitis, an experimental model of IBD (33). Mice treated with 3.0% DSS exhibited the predominant clinical symptoms of colitis: weight loss, diarrhea, bloody feces, crypt distortion, epithelial injury, reduced colon length, increased mucus secretion by goblet cells, and inflammatory cell infiltration. Mice treated with lenalidomide showed an efficient relief from the aforementioned peculiar symptoms of IBD. On the other hand, pomalidomide-treated mice showed lesser recovery from these symptoms compared with lenalidomide-treated groups. Lenalidomide reduced DSS-colitis clinical score, macroscopically visible damage, and reversed inflammation induced shortening of colon length. Lenalidomide-supplemented mice showed colon tissue with healthy histological integrity as well.
In this study, lenalidomide treatment showed a significant rate of reduction in MPO levels, an indicator of neutrophil infiltration, proofing reduction in the infiltration of neutrophils. These results are in line with previous studies where mice (25) or rats (31) were treated with thalidomide, the first generation IMiD from which lenalidomide and pomalidomide derived.
As examined with different parameters, lenalidomide treatment resulted in a higher protective effect toward DSS-induced inflammatory disease. Lenalidomide’s protective effect was similar among CRBNI391V and WT mice groups, suggesting that CRBN–IMiD binding region did not contribute much to IMiDs DSS-colitis amelioration effect. Further studies are required to determine whether CRBN-independent processes are responsible.
The development of this previously unavailable important animal model provides a proof of concept for the development of drugs that direct ubiquitin ligases for specific degradation of different disease-associated proteins. Moreover, it will enable the study of different disease models that can be cured or relieved with IMiDs which otherwise will be impossible to test due to lack of a host animal for drug trial on those specific disease models. As we confirmed in this study, IMiDs’ therapeutic activity in DSS colitis seems independent of amino acid sequences in the CRBN–IMiD binding region. By contrast, a difference in the single amino acid in the CRBN–IMiD binding region affects IMiDs E3 ubiquitin ligase modulation activity for degrading target substrates and up-regulating IL-2 production (SI Appendix, Fig. S10).
Methods and Materials
Humanized Cereblon Mice Generation.
CRBNI391V mice were generated using CRISPR-Cas9. A 295-bp region surrounding the I391 coding exon was amplified and cloned into pCAG-EGxxFP (89684; Addgene) using directional cloning with Nhe1 and BamHI. The optimal Cas9/guide RNA construct was isolated according to a previously described method (34) and injected together with a ssDNA “donor” sequence into BDF1 background oocytes. F0 pups were mated and mutations screened in offspring by sequencing. Details are given in SI Appendix, SI Methods and Materials.
CD4+ T Cell Isolation and Activation.
Mouse CD4+ T cells were isolated by immunomagnetic negative selection (Miltenyi Biotec). Cells were plated at 1 × 106 cells per well in 12-well plates in the presence of anti-CD3ε (5 μg/mL) (clone HIT3a) or (clone 145–2C11). The cells were treated with lenalidomide (100 μM), pomalidomide (10 μM), FPFT-2216 (10, 20, or 40 μM), or 0.1% DMSO for 14 or 24 h.
In Vivo Mice Experiment.
Mice experiments were performed according to ethical guidelines of Osaka University. Mice were administered by gavaging or intraperitoneally with lenalidomide (100 mg/kg), pomalidomide (50 mg/kg), FPFT-2216 (30 mg/kg), or DMSO for 14 h and CD4+ T cells were isolated from the spleen. Cells were lysed and Western blot was done for IKZF1 and CK-1α.
Western Blot, ELISA, and qRT-PCR.
Western blot, ELISA, and qRT-PCR were performed as previously described (15, 30). Details are given in SI Appendix, SI Method and Materials.
DSS-Colitis Induction, Myeloperoxidase Analysis, and Hematoxylin/Eosin Staining.
Colitis was induced by administering 3% dextran sodium sulfate dissolved in fresh water (∼40 kDa; Sigma-Aldrich) to 8-wk-old male CRBNI391V and wild-type BDF1 strain mice for 6 d followed by normal drinking water for 1 d. Lenalidomide (50 mg/kg), pomalidomide (50 mg/kg), or DMSO were administered per oral to the DSS-treated animals. Clinical scores were determined based on previous studies (33, 35, 36). Myeloperoxidase, hematoxylin/eosin staining, and ELISA were done according to previous studies (33, 35, 36). Details are given in SI Appendix, SI Method and Materials.
Cell Culture.
HEK293T cells were grown in DMEM supplemented with 10% (V/V) FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. Mouse primary T cells, peritoneal macrophages, and Jurkat T cells were maintained in RPMI supplemented with 10% (V/V) FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin (Nacalai Tesque).
Statistical Analysis.
P values were calculated using the Student’s t test. P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank the Fujimoto Pharmaceutical Corp. for providing FPFT-2216. This study was supported by the Kishimoto Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814446115/-/DCSupplemental.
References
- 1.Lenz W. A short history of thalidomide embryopathy. Teratology. 1988;38:203–215. doi: 10.1002/tera.1420380303. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV. Thalidomide: Tragic past and promising future. Mayo Clin Proc. 2004;79:899–903. doi: 10.4065/79.7.899. [DOI] [PubMed] [Google Scholar]
- 3.Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med. 1999;5:582–585. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- 4.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichner R, et al. Immunomodulatory drugs disrupt the cereblon-CD147-MCT1 axis to exert antitumor activity and teratogenicity. Nat Med. 2016;22:735–743. doi: 10.1038/nm.4128. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 7.Therapontos C, Erskine L, Gardner ER, Figg WD, Vargesson N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc Natl Acad Sci USA. 2009;106:8573–8578. doi: 10.1073/pnas.0901505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krönke J, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu G, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krönke J, et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature. 2015;523:183–188. doi: 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JJ, Hao J, Kosofsky BE, Rajadhyaksha AM. Dysregulation of large-conductance Ca2+-activated K+ channel expression in nonsyndromal mental retardation due to a cereblon p.R419X mutation. Neurogenetics. 2008;9:219–223. doi: 10.1007/s10048-008-0128-2. [DOI] [PubMed] [Google Scholar]
- 12.Lee KM, Jo S, Kim H, Lee J, Park CS. Functional modulation of AMP-activated protein kinase by cereblon. Biochim Biophys Acta. 2011;1813:448–455. doi: 10.1016/j.bbamcr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen TV, et al. Glutamine triggers acetylation-dependent degradation of glutamine synthetase via the thalidomide receptor cereblon. Mol Cell. 2016;61:809–820. doi: 10.1016/j.molcel.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer ES, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512:49–53. doi: 10.1038/nature13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millrine D, Tei M, Gemechu Y, Kishimoto T. Rabex-5 is a lenalidomide target molecule that negatively regulates TLR-induced type 1 IFN production. Proc Natl Acad Sci USA. 2016;113:10625–10630. doi: 10.1073/pnas.1611751113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann MD, et al. Thalidomide mimics uridine binding to an aromatic cage in cereblon. J Struct Biol. 2014;188:225–232. doi: 10.1016/j.jsb.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain PP, et al. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat Struct Mol Biol. 2014;21:803–809. doi: 10.1038/nsmb.2874. [DOI] [PubMed] [Google Scholar]
- 18.Chesi M, et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood. 2012;120:376–385. doi: 10.1182/blood-2012-02-412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuman MG. Immune dysfunction in inflammatory bowel disease. Transl Res. 2007;149:173–186. doi: 10.1016/j.trsl.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi AK, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4CRBN. Br J Haematol. 2014;164:811–821. doi: 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fakhoury M, Coussa-Charley M, Al-Salami H, Kahouli I, Prakash S. Use of artificial cell microcapsule containing thalidomide for treating TNBS-induced Crohn’s disease in mice. Curr Drug Deliv. 2014;11:146–153. doi: 10.2174/156720181101140212170025. [DOI] [PubMed] [Google Scholar]
- 26.Fink EC, et al. CrbnI391V is sufficient to confer in vivo sensitivity to thalidomide and its derivatives in mice. Blood. 2018;132:1535–1544. doi: 10.1182/blood-2018-05-852798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fratta ID, Sigg EB, Maiorana K. Teratogenic effects of thalidomide in rabbits, rats, hamsters, and mice. Toxicol Appl Pharmacol. 1965;7:268–286. doi: 10.1016/0041-008x(65)90095-5. [DOI] [PubMed] [Google Scholar]
- 28.Sheskin J. Thalidomide in the treatment of lepra reactions. Clin Pharmacol Ther. 1965;6:303–306. doi: 10.1002/cpt196563303. [DOI] [PubMed] [Google Scholar]
- 29.Millrine D, Kishimoto T. A brighter side to thalidomide: Its potential use in immunological disorders. Trends Mol Med. 2017;23:348–361. doi: 10.1016/j.molmed.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Millrine D, et al. Immunomodulatory drugs inhibit TLR4-induced type-1 interferon production independently of Cereblon via suppression of the TRIF/IRF3 pathway. Int Immunol. 2016;28:307–315. doi: 10.1093/intimm/dxw005. [DOI] [PubMed] [Google Scholar]
- 31.Lienenlüke B, et al. Thalidomide impairment of trinitrobenzene sulphonic acid-induced colitis in the rat–Role of endothelial cell-leukocyte interaction. Br J Pharmacol. 2001;133:1414–1423. doi: 10.1038/sj.bjp.0704193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsburg PM, Dassopoulos T, Ehrenpreis ED. Thalidomide treatment for refractory Crohn’s disease: A review of the history, pharmacological mechanisms and clinical literature. Ann Med. 2001;33:516–525. doi: 10.3109/07853890108995961. [DOI] [PubMed] [Google Scholar]
- 33.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15.25.1–15.25.14. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mashiko D, et al. Feasibility for a large scale mouse mutagenesis by injecting CRISPR/Cas plasmid into zygotes. Dev Growth Differ. 2014;56:122–129. doi: 10.1111/dgd.12113. [DOI] [PubMed] [Google Scholar]
- 35.Chinen I, et al. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int Immunol. 2015;27:405–415. doi: 10.1093/intimm/dxv015. [DOI] [PubMed] [Google Scholar]
- 36.Smith P, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.