Abstract
We analyzed the number and functionality of regulatory B (Breg) cells in well‐defined myasthenia gravis patients. We first showed a decreased number of circulating CD19+ CD24++ CD38++ Breg cells and an altered functionality of Breg cells in untreated myasthenia gravis patients. Next, we demonstrated that the proportion of circulating Breg cells was restored in myasthenia gravis patients after thymectomy, probably as Breg cells could be sequestered in the myasthenia gravis thymus. In contrast, corticosteroid treatments did not restore and decreased even more the proportion of Breg cells in myasthenia gravis patients. These results clearly demonstrated that two distinct immunomodulatory therapies affect differentially Breg cells.
Introduction
Acquired myasthenia gravis (MG) is a rare neurological autoimmune disease. MG patients suffer from fluctuating skeletal muscle weaknesses mainly caused by autoantibodies against the acetylcholine receptor (AChR). If the muscle is the target organ, the thymus is the effector organ in the early‐onset form of AChR+ MG. The thymus is characterized by an abnormal recruitment of peripheral B cells leading to ectopic germinal center formation.1 Treatment for MG patients is the anticholinesterase agents but their action is only symptomatic. Thymectomy is also advised to patients, and/or corticosteroids for their anti‐inflammatory properties2, 3
B cells play a negative role in the pathophysiology of MG for their ability to produce pathogenic AChR antibodies. However, B‐cell subsets, called regulatory B (Breg) cells possess immunosuppressive functions: they inhibit the activation of Th1 cells, the differentiation of Th17 cells or of cytotoxic CD8+ T cells, and they can convert CD4+ T cells into suppressive regulatory T cells. The immunoregulatory function of Breg cells is linked to the production of anti‐inflammatory cytokines, mainly interleukin (IL)‐10, and the most studied subtype of Breg cells corresponds to immature CD19+CD38++CD24++ B cells that are found in blood and at sites of inflammation4, 5
Numerical and functional alterations have been observed in multiple sclerosis,6 rheumatoid arthritis7 and systemic lupus erythematosus.8 In recent years, studies on MG demonstrated relatively reduced percentages of Breg cells in MG patients, with an inverse correlation with MG severity9, 10, 11 Moreover, within a Breg cell population, IL‐10 was found to be relatively lesser in MG patients than controls.12 Unfortunately, in all studies on MG and usually other autoimmune diseases, the investigations on Breg cells were done on patients regardless of their immunosuppressive treatments.
Herein, we analyzed in untreated MG patients the percentage of Breg cells and their ability to produce IL‐10. Next, we investigated the impact of two mostly used MG therapies, thymectomy and corticosteroids, on Breg cells and surprisingly demonstrated inversed effects.
Methods
Blood and thymic samples
For blood samples, patients had an early‐onset form of MG (no thymoma) with anti‐AChR antibodies. Patients for global analyses (Figs. 1, 2F) were not thymectomized (n = 25; 11–38 years old) (Table 1). Age‐/sex‐matched healthy controls were obtained from the EFS ((Etablissement Francais du Sang, France)). Additional AChR+ MG patients (no thymoma) with a follow‐up before and after thymectomy (n = 7), and with or without corticosteroids (n = 4) were used and clinical details are given in Tables 2, 3. MG is a rare disease and we were only able to analyze a few patients with a very well‐known follow‐up to investigate the impact of thymectomy or of a corticosteroid treatment on the percentage of Breg cells (Fig. 2A and G).
Figure 1.
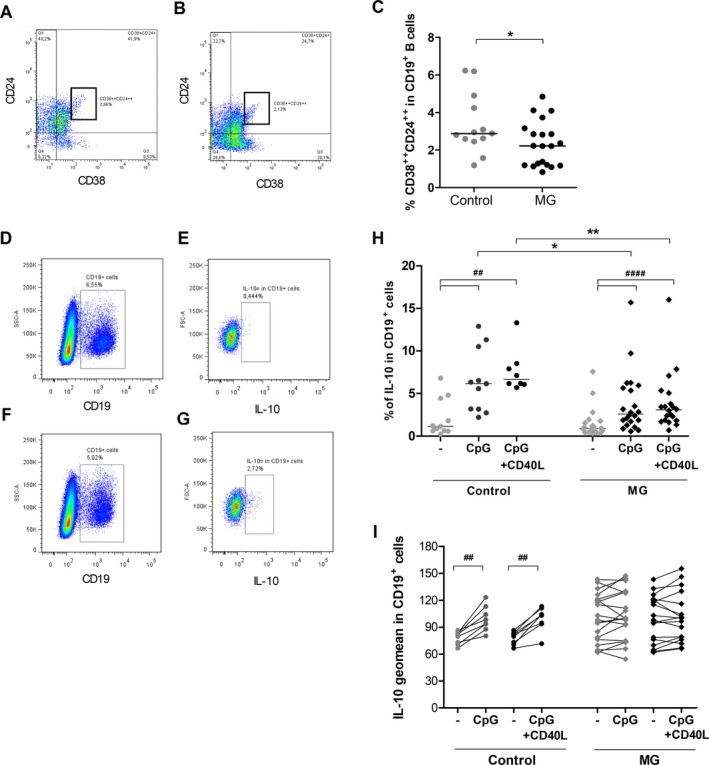
Altered number and function of peripheral Breg cells in MG. (A–C) PBMCs from untreated MG patients (n = 20) and healthy controls (n = 13) were labeled to identify Breg cells by flow cytometry. Representative FlowJo analyses of CD24++ CD38++ cells in CD19+ cells for a healthy donor (A) and an untreated MG patient (B). Percentage of CD24++ CD38++ cells in the CD19+ B cells (C). P‐values were assessed by the Mann–Whitney test (*P < 0.05). (D–I) PBMCs from untreated MG patients (n = 17–22) and healthy controls (n = 8–11) were cultivated 48 hours in resting condition or stimulated with CpG or CpG+CD40L. Representative FlowJo analyses for CD19+ IL‐10+ cells for a control donor in resting condition (D,E) or stimulated with CpG (F,G). PBLs were first gated for singlets on FSC‐H versus FSC‐A and for lymphocytes on SSC‐H vs FSC‐A. Next, CD19+ cells were analyzed in the lymphocyte gate (D,F) and the percentage and geomean for IL‐10‐positive cells in the CD19+ gate extracted (E,G). Global analyses of the percentage of IL‐10+ cells in the CD19 + gate (H) and the geomean of fluorescence for IL‐10 in CD19+ cells (I) in control and MG cells. P‐values were assessed by the Wilcoxon paired test for comparison within each group (## P < 0.01, #### P < 0.001) and by the Mann–Whitney test for comparison between controls and MG patients (*P < 0.05, **P < 0.01).
Figure 2.
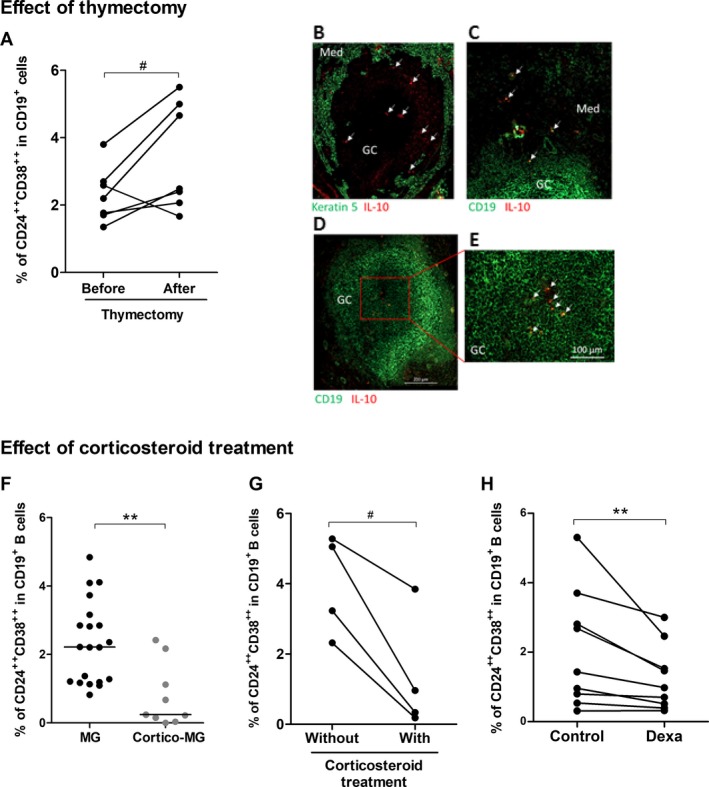
Impact of classical MG therapies on the proportion of Breg cells. Effect of thymectomy: (A) PBMCs from seven MG patients were labeled before and 6–24 months after thymectomy to measure the percentage of CD24++ CD38++ in CD19+ B cells. P‐value was assessed by the paired t‐test (# P = 0.05). Only one patient had an inversed behavior with a decreased percentage of Breg cells after thymectomy. We had no reason to exclude the patient from the analysis but this patient also behave differentially regarding the % of regulatory T cells and Th17 cells compared to other patients. (B–E) Immunofluorescence staining of thymic sections from MG patients with an anti‐CD19 antibody for B cells or an anti‐keratin 5 for thymic medullary epithelial cells (in green), and anti‐IL10 (in red). Images were acquired with a Zeiss Axio Observer Z1 Inverted Microscope. Effect of corticosteroids: (F) PBMCs from MG patients (n = 20) and cortico‐treated MG patients (n = 9) were labeled to analyze the percentage of CD24++ CD38++ cells in the CD19+ B cells. P‐value was assessed by the Mann–Whitney test (**P = 0.002), (G) PBMCs from four MG patients followed up during in the course of their corticosteroid treatment were labeled to measure the percentage of CD24++ CD38++ in CD19+ B cells. P‐value was assessed by the paired t‐test (# P = 0.02). For three patients, the blood samples were initially without treatment and 1–24 months later while they were under corticosteroid treatment. For one patient, the blood sample was initially with corticosteroid treatment and 3 months later without treatment. (H) PBMCs from healthy controls were cultivated 48 hours with dexamethasone (Dexa) or without (control) and the percentages of CD24++ CD38++ cells in the CD19+ B cells were analyzed by flow cytometry. P‐value was assessed by the paired t‐test (**P = 0.008).
Table 1.
List of MG patients included in the study
| Patient | Gender | Age (years) | Anti‐AChR titer (nmol/L) | Anticholinesterase drugs | Corticoids |
|---|---|---|---|---|---|
| 1 | F | 33 | 66.7 | Mestinon | No |
| 2 | F | 38 | 330.0 | None | No |
| 3 | F | 28 | 36.4 | Mestinon | No |
| 4 | F | 34 | > 0.5 | Mestinon | No |
| 5 | M | 33 | 7.0 | Mestinon | No |
| 6 | F | 22 | 10.9 | Mestinon | No |
| 7 | F | 26 | 90.4 | Mytelase | No |
| 8 | M | 19 | 1.5 | None | No |
| 9 | M | 30 | 14.0 | Mestinon | No |
| 10 | F | 26 | 117.0 | Mestinon | No |
| 11 | F | 27 | 2.3 | Mestinon | No |
| 12 | F | 31 | 154.0 | Mestinon | No |
| 13 | M | 29 | 2.4 | Mytelase | No |
| 14 | M | 18 | 21.3 | Mytelase | No |
| 15 | M | 28 | 1.6 | Mytelase | No |
| 16 | F | 27 | 4.9 | None | No |
| 17 | M | 30 | >100 | Mestinon | Yes |
| 18 | F | 33 | 3.7 | Mestinon | Yes |
| 19 | F | 11 | 5.8 | Mestinon | Yes |
| 20 | M | 28 | >100 | Mestinon | Yes |
| 21 | F | 34 | 4.2 | Mestinon | Yes |
| 22 | F | 15 | 15.9 | Mestinon | Yes |
| 23 | M | 21 | 2.9 | None | Yes |
| 24 | F | 17 | 140.0 | Mytelase | Yes |
| 25 | F | 28 | 1.0 | Mestinon | Yes |
Table 2.
List of MG patients before/after thymectomy
| Patient | Gender | Age (years) | Anti‐AChR titer (nmol/L) Before/after thymectomy | Anticholinesterase drugs | Corticoids |
|---|---|---|---|---|---|
| 26 | F | 21 | 1.8/1.1 | Mestinon | No |
| 27 | F | 20 | 12.4/1.2 | Mestinon | No |
| 28 | F | 22 | 8.4/5.8 | Mestinon | No |
| 29 | F | 41 | 0.9/1.7 | Mestinon | No |
| 30 | F | 39 | 3.4/0.8 | Mytelase | No |
| 31 | F | 33 | 117.9/53.5 | Mestinon | No |
| 32 | M | 24 | 126.0/46.1 | Mestinon | No |
Table 3.
List of MG patients with or without corticosteroid treatments
| Patient | Gender | Age (years) | Anti‐AChR titer (nmol/L) With/Without corticosteroids | Anticholinesterase drugs | Corticoids |
|---|---|---|---|---|---|
| 33 | M | 32 | Unknown/13.7 | Mestinon | Yes |
| 34 | M | 44 | 73.0/64.6 | Mytelase | Yes |
| 35 | M | 86 | 34.0/42.2 | Mestinon | Yes |
| 36 | M | 29 | 1.1/1.6 | Mestinon | Yes |
Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll density gradient centrifugation, resuspended in freezing solution and stored in liquid nitrogen.
Thymic fragments were obtained from three early‐onset MG patients only treated with anticholinesterase drugs. Studies on blood and thymic samples were approved by local Ethics Committees [RCB 2006‐A00164‐47 and RCB 2010‐A00250‐39].
Flow cytometry analyses of Breg cells
Frozen PBMCs were thawed, washed in X‐VIVO medium, and stained for 30 min at 4°C with anti‐human monoclonal antibodies: CD19‐ef450, IgD‐ef710, CD138‐PE, CD24‐APC, CD27‐PE‐Cy7, and CD38‐FITC (eBioscience or Immunotech, France). Flow cytometry was performed on a FACS Verse (BD Biosciences) and data were analyzed using the FlowJo software.
Stimulation of IL‐10 expression by B cells
Frozen PBMCs were resuspended in X‐VIVO medium at 106 cells/well in 96‐well plates and stimulated for 48 h with 5 μg/mL of CpG‐ODN‐2137 (Miltenyi‐Biotec, France) at 1 μg/mL of an anti‐human CD40 antibody (CD40L, Biolegend, UK). During the final 5 h, 0.5 μg/mL PMA, 1 μg/mL ionomycin, and 10 μg/mL brefeldin (Sigma, France) were added. Cells were surface stained, fixed, permeabilized and IL‐10 was stained with an anti‐IL10‐PE antibody (eBioscience).
Dexamethasone effects on peripheral Breg cells
Frozen PBMCs from healthy individuals (106 cells/well) were treated with 10 μg of dexamethasone (Sigma) for 48 h. Cultured cells were then stained with antibodies used for Breg cells phenotyping as mentioned above.
Immunohistochemistry on thymic sections
Frozen thymic sections (7 μm) were fixed in ice‐cold acetone for 20 min and stained for 1 h with antibodies: (1) an anti‐human CD19 (eBioscience) followed by an anti‐mouse Alexa Fluor‐488 (Life Technologies, St Aubin, France), and (2) an anti‐IL10 (eBioscience) followed by an anti‐rat Alexa Fluor‐594 (Life Technologies). Two different AChR+ MG patients with an early‐onset form of MG and a thymic hyperplasia characterized by numerous germinal centers were used. Images were acquired with a Zeiss Axio Observer Z1 Inverted Microscope.
Statistical analyses
Statistical analyses were performed using GraphPad Prism. Details on the statistical tests used are given in the figure legends. For very small population, a paired t‐test was used as it is also suitable if the within‐pair correlation is high.13
Results
Reduced number and function of peripheral Breg cells in MG patients
We clearly observed that the well‐characterized immature Breg cells (CD19+CD38++CD24++)5 were significantly lower in MG patients compared to controls (Fig. 1A–C), while the percentages of total, naïve or memory B cells did not change (data not shown). In resting condition, we did not observe any difference in the percentage of CD19+IL‐10+ cells in MG and controls (Fig. 1D–H). However, after stimulations with CpG or CpG/CD40L, we showed a significant increase in the percentage of CD19+IL‐10+ cells in both control and MG donors but the magnitude of the increase was significantly less in MG than control cells (Fig. 1H). In parallel, analyzing the level of IL‐10 expression, we then showed that CpG or CpG/CD40L stimulations always significantly increased IL‐10 expression of about 27% in control cells but not in MG cells (Fig. 1I). This observation explains the lower percentage of CD19+IL‐10+ cells upon stimulation of PBMCs from MG donors (Fig. 1H). It should be noted that using PBMCs from five corticosteroid‐treated MG patients, we observed that they behaved as untreated MG patients with a decreased percentage of CD19+IL‐10+ cells and no stimulation of IL‐10 expression was detected upon stimulation with CpG (data not shown).
Altogether these data demonstrated that MG patients have a decreased number of Breg cells and that IL‐10 was abnormally less expressed upon stimulation reflecting that Breg cells from MG patients were less functional.
Breg cells are normalized in MG after thymectomy
We analyzed the percentage of CD19+CD24++CD38++ Breg cells in PBMCs of seven MG patients before and, 6–24 months, after thymectomy. We observed that, except for one patient, the percentage of Breg cells always increased after thymectomy (Fig. 2A). Analyzing the ratio of Breg cells after/before thymectomy for the six patients who displayed an increased % in Breg cells after thymectomy, we observed a positive correlation with a good evolution of the disease. Patients with the highest ratio showed a clear improvement of the symptoms and two were even in remission (data not shown).
As the percentage of Breg cells increased after thymectomy, Breg cells could be sequestered in the thymus of MG patients. Analyzing by immunofluorescence thymic sections for CD19+IL‐10+ cells, we observed a few CD19+IL‐10+ cells in the medulla and also within germinal centers in MG patients (Fig. 2B–E). In contrast, no CD19+IL‐10+ cells were observed in control thymuses either from infants (n = 2) or adult donors (n = 2) (data not shown).
Corticosteroid treatment reduces peripheral Breg cells in MG patients
We analyzed the percentage of CD19+CD24++CD38++ Breg cells in patients under corticosteroid therapy compared to untreated MG patients and observed that the percentage of Breg cells was significantly decreased in corticosteroid‐treated MG patients (Fig. 2F). We also demonstrated that individually for given patients, a decrease in the percentage of CD19+CD24++CD38++ Breg cells was systematically observed when a patient was under corticosteroids (Fig. 2G). To further investigate the potential impact of corticosteroids on Breg cells, we analyzed the in vitro effects of dexamethasone on PBMCs from healthy individuals. Although the total numbers of CD19+ B cells was comparable among the dexamethasone‐treated and non‐treated cells (data not shown), the dexamethasone treatment induced a significant reduction in the percentage of Breg cells after 48 h in culture (Fig. 2H).
Discussion
We demonstrated that circulating immature (CD19+CD38++CD24++) Breg cells were lower in untreated MG patients than in healthy controls. In addition, we clearly demonstrated for the first time an impaired ability of CD19+ Breg cells from MG patients to produce IL‐10 upon stimulations.
Restoration of the Breg percentage in MG patients after thymectomy
Patients included in our study were early‐onset MG patients. We observed that CD19+IL‐10+ B cells were found in the thymic medulla and within germinal centers of MG patients. Moreover, IL‐10 mRNA expression was increased in MG thymuses with thymic hyperplasia (data not shown). Similarly, in multiple sclerosis, the accumulation of Breg cells has also been observed at the inflammatory sites, in the central nervous system, of the experimental autoimmune encephalomyelitis model induced by immunization with a myelin oligodendrocyte glycoprotein (MOG) peptide.14 We thus hypothesized that thymic inflammation in MG could favor thymic Breg cells recruitment and decrease the proportion of circulating Breg cells. A recent randomized clinical trial has clearly proved the efficacy of thymectomy in reducing MG symptoms.2 Here, we observed that thymectomy was effective in restoring the percentage of circulating Breg cells in MG and this effect may partly account for the efficiency of thymectomy.
Negative effect of corticosteroids on the Breg percentage in MG patients
Surprisingly, we observed that the percentage of Breg cells in corticosteroid‐treated MG patients was significantly lower compared to untreated MG patients. Similarly, a decrease in Breg cells has also been observed in neuromyelitis optica patients and this was exacerbated in patients after methylprednisolone treatment.15 So far, this impact of immunosuppressive treatments on Breg cells has not been taken into account in the majority of studies on patients with autoimmune diseases and patients were usually pooled whatever their treatments.6, 8, 16 This is also true for studies on MG patients.17 Sheng et al. observed a decreased percentage of Breg cells in MG patients and showed that the decrease was stronger in severe patients but four out of six of these severe patients had an immunosuppressive treatment.10 Similarly, Sun et al. also observed a decreased of Breg cells in a cohort of 48 patients with 22 receiving various immunosuppressive or immunomodulatory treatments. They also observed a higher decrease of Breg cells in severely affected MG patients that are usually those receiving immunosuppressive treatments.9 Decreased numbers or function of Breg cells have also been described in various autoimmune diseases, such as multiple sclerosis, systemic lupus erythematosus, rheumatoid arthritis but in these studies patients were often under immunosuppressive treatment.6, 8, 16 Consequently, the decrease associated with the severity need to be further investigated in patients without immunosuppressive treatment to remove the bias due to the direct effect of corticosteroids on Breg cells.
Conclusion
The decrease of peripheral Breg cells in MG patients need to be further defined. Is the decrease involved in the initiation of the disease leading to inflammation, or is it a consequence of the ongoing disease and the associated inflammatory environment? A few animal studies have demonstrated the beneficial effect of adoptive transfer of Breg cells in experimental autoimmune models, including MG.18, 19, 20 This suggests that improving the percentage of Breg cells in autoimmune diseases might exert a beneficial therapeutic effect. However, regarding the opposite effects of thymectomy and corticotherapy on the percentage of Breg cells in MG, it is difficult to consider that improvement in MG patients only relies on Breg cells. Corticosteroids have multiple effects on the immune system and our results demonstrated that the beneficial effect of corticosteroids for MG patients does not seem to be linked to Breg cells. Altogether, our results clearly underline that the implication of Breg cells in MG, and other autoimmune diseases, need to be further investigated in patients taking into account their therapeutic history.
Author Contributions
VY, SM, FD performed and analyzed the experiments, FT collected samples and provided patient information, J‐FR provided thymic biopsies, FB and AB provided blood samples, SB‐A and SM read and revised the manuscript, VY and RLP designed the study, analyzed the experiments and wrote the manuscript.
Conflicts of Interest
The authors have no conflict of interest to declare.
Acknowledgments
We thank Dr Audrey Lupo‐Mansuet for thymic histological analyses. The study was supported by grants from the joint TUBITAK 2219 ‐ Post‐Doctoral Research Fellowship Program (1059B191300672) and from the “Association Française contre les Myopathies” (AFM).
Funding information
The study was supported by grants from the joint TUBITAK 2219 ‐ Post‐Doctoral Research Fellowship Program (1059B191300672) and from the “Association Française contre les Myopathies” (AFM).
Funding Statement
This work was funded by TUBITAK 2219‐Post‐doctoral Research fellowship grant 1059B191300672; Association Française contre les Myopathies grant .
References
- 1. Berrih‐Aknin S, Le Panse R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun 2014;52:90–100. [DOI] [PubMed] [Google Scholar]
- 2. Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mantegazza R, Bonanno S, Camera G, Antozzi C. Current and emerging therapies for the treatment of myasthenia gravis. Neuropsychiatr Dis Treat 2011;7:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol 2008;8:391–397. [DOI] [PubMed] [Google Scholar]
- 5. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015;42:607–612. [DOI] [PubMed] [Google Scholar]
- 6. Knippenberg S, Peelen E, Smolders J, et al. Reduction in IL‐10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naive/memory Breg ratio during a relapse but not in remission. J Neuroimmunol 2011;239(1–2):80–86. [DOI] [PubMed] [Google Scholar]
- 7. Daien CI, Gailhac S, Mura T, et al. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheumatol 2014;66:2037–2046. [DOI] [PubMed] [Google Scholar]
- 8. Blair PA, Norena LY, Flores‐Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010;32:129–140. [DOI] [PubMed] [Google Scholar]
- 9. Sun F, Ladha SS, Yang L, et al. Interleukin‐10 producing‐B cells and their association with responsiveness to rituximab in myasthenia gravis. Muscle Nerve 2014;49:487–494. [DOI] [PubMed] [Google Scholar]
- 10. Sheng JR, Rezania K, Soliven B. Impaired regulatory B cells in myasthenia gravis. J Neuroimmunol 2016;297:38–45. [DOI] [PubMed] [Google Scholar]
- 11. Guptill JT, Yi JS, Sanders DB, et al. Characterization of B cells in muscle‐specific kinase antibody myasthenia gravis. Neurol Neuroimmunol Neuroinflamm 2015;2:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karim MR, Zhang HY, Yuan J, et al. Regulatory B Cells in Seropositive Myasthenia Gravis versus Healthy Controls. Front Neurol 2017;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Winter J. Using the Student's t‐test with extremely small sample sizes. Pract Assessement Res Eval 2013;18:1–10. [Google Scholar]
- 14. Lehmann‐Horn K, Sagan SA, Winger RC, et al. CNS accumulation of regulatory B cells is VLA‐4‐dependent. Neurol Neuroimmunol Neuroinflamm 2016;3:e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quan C, ZhangBao J, Lu J, et al. The immune balance between memory and regulatory B cells in NMO and the changes of the balance after methylprednisolone or rituximab therapy. J Neuroimmunol 2015;15:45–53. [DOI] [PubMed] [Google Scholar]
- 16. Flores‐Borja F, Bosma A, Ng D, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 2013;5:173ra23. [DOI] [PubMed] [Google Scholar]
- 17. Yi JS, Russo MA, Massey JM, et al. B10 cell frequencies and suppressive capacity in myasthenia gravis are associated with disease severity. Front Neurol 2017;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10‐producing B cells. J Exp Med 2003;197:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsushita T, Yanaba K, Bouaziz JD, et al. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 2008;118:3420–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheng JR, Quan S, Soliven B. CD1d(hi)CD5+ B cells expanded by GM‐CSF in vivo suppress experimental autoimmune myasthenia gravis. J Immunol 2014;193:2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]


