Abstract
Objective
The efficacy of deep brain stimulation in disorders of consciousness remains inconclusive. We investigated bilateral 30‐Hz low‐frequency stimulation designed to overdrive neuronal activity by dual pallido‐thalamic targeting, using the Coma Recovery Scale Revised (CRS‐R) to assess conscious behavior.
Methods
We conducted a prospective, single center, observational 11‐month pilot study comprising four phases: baseline (2 months); surgery and titration (1 month); blind, random, crossover, 1.5‐month ON and OFF periods; and unblinded, 5‐month stimulation ON. Five adult patients were included: one unresponsive‐wakefulness‐syndrome male (traumatic brain injury); and four patients in a minimally conscious state, one male (traumatic brain injury) and three females (two hemorrhagic strokes and one traumatic brain injury). Primary outcome measures focused on CRS‐R scores. Secondary outcome measures focused notably on baseline brain metabolism and variation in activity (stimulation ON – baseline) using normalized fluorodeoxyglucose positron emission tomography maps. Statistical analysis used random‐effect models.
Results
The two male patients (one minimally conscious and one unresponsive wakefulness syndrome) showed improved mean CRS‐R scores (stimulation ON vs. baseline), in auditory, visual and oromotor/verbal subscores, and visual subscores respectively. The metabolism of the medial cortices (low at baseline in all five patients) increased specifically in the two responders.
Interpretation
Our findings show there were robust but limited individual clinical benefits, mainly in visual and auditory processes. Overall modifications seem linked to the modulation of thalamo‐cortico‐basal and tegmental loops activating default mode network cortices. Specifically, in the two responders there was an increase in medial cortex activity related to internal awareness.
Introduction
Only 22% of minimally conscious state (MCS; minimal and inconsistent awareness) and 17% of unresponsive wakefulness syndrome (UWS; vegetative state; open‐eyes, no evidence of awareness) patients return to an approximation of their former levels of functioning 4 years after injury1; bearing in mind that disorder of consciousness (DOC) is associated with an increased risk of death,2, 3, 4 and that exceptionally, late, satisfactory recovery occurs (e.g.5, 6, 7, 8). Among foremost concerns are expectations of significant recovery offset by very low rates of discharge to the home environment without disability, and the predicament of permanent DOC, which should be considered at 6 months post stroke, 12 months post trauma in UWS, and between three and 5 years or less in MCS.9 Expectations come from drug‐based,10 physical and non‐pharmacological interventions.11 Seven studies (58 UWS or MCS patients; only two series in excess of three patients) used deep brain stimulation (DBS) to trigger recovery.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Stimulation frequencies were mostly low 25 Hz‐50 Hz13, 14, 15, 16, 17, 18, 19 (two times at 100 Hz21, 22 and one at 250 Hz12). Unilateral (left‐side two case‐reports; right‐side one series) or bilateral (two case‐reports; one series) implantations were performed (data unknown in one series). The most common targets were the reticular polar, anterior and laminar (centromedian and parafascicular) thalamic nuclei, and the so‐called central thalamus.23 The tegmentum (in two patients)17 and the right pallidum (in one patient; combined with the left anterior thalamus)13 were very seldom targeted. The overall results in terms of clinical benefit are as yet inconclusive due to patient heterogeneity, means of assessment (scales, follow‐ups), time since injury (1 month to 6 years), devices, and contact locations. In this context we aimed to conduct a prospective 11‐month observational pilot study designed to assess the relevance of DBS at the bedside, using the Coma Recovery Scale Revised (CRS‐R).24 Anoxic patients were excluded because of high long term disability and low expectancy of recovery.25, 26 CRS‐R was administered repeatedly during each phase of the study taking into consideration the variability of signs of awareness.27 With respect to inclusion we considered neither structural nor functional brain imaging regarding the lack of consensus on this aspect, however identification of covert activity is promising.11, 28, 29 The thalamus and the pallidum were targeted because experimental stimulation has evoked cortical activity resembling consciousness.30, 31 Hence dual‐site electrode implantation was used, hypothesizing that it would be more effective in boosting overall brain hypo activity, and could further enable independent dual‐site modulation of the cognitive and motor thalamo‐cortico‐basal loops involved in DOC paradigms.32, 33, 34 We considered that either activation of preserved loops or reactivation of silent loops, would induce modifications of CRS‐R score. 30‐Hz low frequency stimulation was selected according to clinical13, 14, 15, 16, 17, 18, 19 and experimental data.31, 35 Our goal was to override remote cortical structures in the frequency band where beta and gamma waves, both of which are linked with consciousness processes,36 overlap, while also overriding pallidal activity. In this study, surgery was conducted at a single center, and an ecological approach was adopted at each center where patients were recruited and monitored. Primary measure was based on CRS‐R scores, and secondary outcome measures on CRS‐R subscores, open‐eye condition at night, and fluorodeoxyglucose (FDG) positron emission tomography, obtained prior to and during DBS. The impact on sleep, of continuous, twenty‐four hours a day, stimulation, was checked at night, looking at open‐eye condition.
Patients and Methods
Study design and participants
Five patients were consecutively included from April 2012 to February 2015 (Table 1; Fig. 1), fulfilling inclusion criteria (age ≥ 18 years; stable, chronic DOC status of UWS or MCS, ≥6 months after cerebrovascular accident and ≥1 year after traumatic brain injury) with no evidence of exclusion criteria (cerebral death, anoxic diagnosis, locked‐in syndrome, blindness or deafness); study authorizations (Appendix S1). The protocol was divided into four phases: (1) Baseline condition (baseline), lasting at least 2 months, using FDG and magnetic resonance imaging (MRI); (2) DBS surgery and titration, lasting 1 month; (3) blind, 3‐month crossover (CO) phase, with two randomized periods of 1.5 months, 1.5‐month ON (CO‐ON) and OFF (CO‐OFF); (4) unblinded stimulation phase of at least 5‐months (DBS‐ON) using FDG. The two randomized crossover periods were termed first period (just after titration) and second period (before DBS‐ON), whatever the CO‐ON or CO‐OFF status.
Table 1.
Characteristics of the five patients
| Clinical consciousness status, gender, age (year), handedness; time elapsed since initial injury | Neurologic status | Surgery | Comorbidities | Diagnosis | Date | |
|---|---|---|---|---|---|---|
| P1 | UWS, male, 32, right handed; 12 years and 2 months | hypertonic quadriplegia predominant on right‐side; flexion or extension of upper limbs, and extension of lower limbs; chewing | May 2012, replacement of right VAS by left VPS (SC valve); July 2012, DBS surgery | right VAS, tracheostomy and gastrostomy post injury | traumatic head injury; left intracerebral hematoma; intraventricular and subarachnoid hemorrhage; multiple cerebral contusions; hydrocephalus | Dec 1999 |
| P2 | MCS‐, female, 62, right handed; 1 year and 2 months | hypertonic triplegia, left hemibody and right lower limb; flexion of lower limbs, and extension of the left upper limb; chewing | November 2012, replacement of right VAS by left VPS (SC valve); Dec 2012, DBS surgery | right VAS, gastrostomy, tracheostomy, right cranioplasty, and left neurogenic paraosteopathy (lower limbs) post hemorrhage; essential hypertension; migraine; hepatitis C; cataract right eye | rupture of right sylvian aneurysm; endovascular occlusion; intracerebral hematoma, intraventricular and subarachnoid hemorrhage; right sylvian artery vasospasm; right decompressive craniectomy; hydrocephalus | July 2011 |
| P3 | MCS‐, male, 24, left handed; 3 years and 1 month | hypertonic triplegia, left hemibody and right lower limb; flexion of the lower limbs and the left upper limb | November 2012, repair of right cranioplasty and repositioning ofVPS valve in the SC region; March 2013, DBS surgery | bilateral decompressive craniotomy followed by cranioplasty, right VPS, bilateral pulmonary embolism, tracheostomy and gastrostomy post injury; multidrug‐resistant bacteria; depression and schizophrenia without medication; neurogenic pain of the right inferior limb; pollen allergy | traumatic head injury; brain edema; right and left subdural hemorrhage; cervical spine injury and C2 fracture; hydrocephalus; pneumothorax | Nov 2009 |
| P4 | MCS‐, female, 22, right handed; 4 years | quadriplegia; flaccid paralysis of axial muscles and lower limbs; slight hypertonia of the upper limbs; chewing | Jan 2015, DBS surgery | tracheostomy, gastrostomy, lobar pneumonias (multidrug‐resistant bacteria) and suspected partial epileptic seizure post injury; unstable diabetes mellitus type 1; asthmatic bronchitis | traumatic head injury; polytrauma; bradycardia and asystole with 15 min of cardiopulmonary resuscitation; subarachnoid hemorrhage; multiple cerebralcontusions; bilateral pneumothorax; right pulmonary embolism | Nov 2010 |
| P5 | MCS‐, female, 47, right handed; 2 years and 3 months | hypertonic quadriplegia predominant on left; trismus; chewing; grunt | May 2015, DBS surgery | right VPS, tracheostomy and gastrostomy post injury; chronic consumption of alcohol and tobacco; chronic depression; eczema and keratitis in 2014‐2015; pollen allergy | rupture of right internal carotid artery termination aneurysm; endovascular occlusion; right frontal hematoma, intraventricular and subarachnoid hemorrhage; hydrocephalus | Dec 2012 |
VAS, ventriculoatrial shunt; VPS, ventriculoperitoneal shunt; SC subclavicular; UWS, Unresponsive Wakefulness Syndrome; MCS‐, Minimally Conscious State minus (patient demonstrating pursuit eye movement, ability to localize (and orient) noxious stimuli, and appropriate movements or affective behaviors).49
Figure 1.
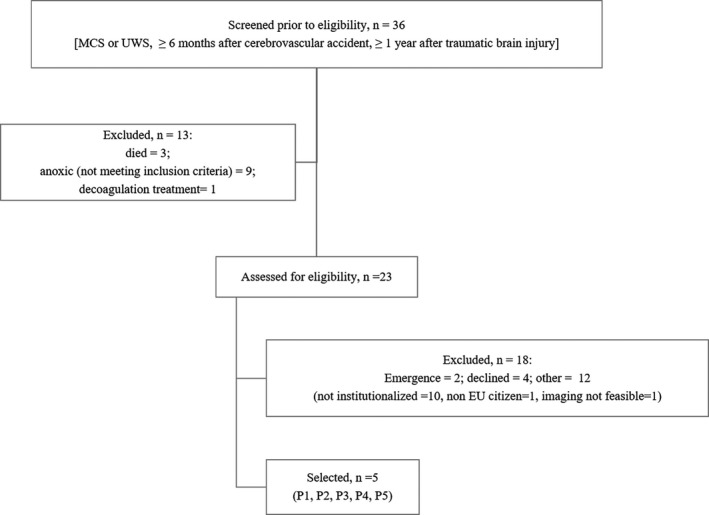
Enrolment of patients. MCS, Minimally Conscious State; UWS, Vegetative State; EU, European Union.
Procedures and outcomes
Three patients were operated prior to imaging and electrode implantation (Table 1): the cerebrospinal fluid shunt valve was moved from the skull to the subclavicular region, enabling artefact‐free brain MRI (P1, P2, P3); the right cranioplasty of P3 was corrected for further electrode implantation. Electrodes (DBS 3389, Medtronic, Minneapolis, MN, USA) were positioned under general anesthesia. The intended targets were: the medial pallidum, around the midpoint of its anteromedial‐posterolateral axis, with the electrode tip in a ventral position; the anterior intralaminar region midway up the thalamus (central‐anterior thalamus). The targeting was personalized (Appendix S1). Immediately after surgery a postoperative CT‐Scan (voxel size = 0.488 mm × 0.488 mm × 0.625 mm) was coregistered with preoperative stereotactic imaging (Fig. 2A) for electrode contact location. The active contacts (1.27 mm diameter, 1.5 mm length; inter‐contact distance 0.5 mm) were positioned within the targets. We performed double‐contact monopolar stimulation 14 times (on five occasions the small size of the anatomical target precluded double‐contact monopolar stimulation) out of the 19 leads implanted (no pallidum in P1) (Fig. 2B). Two neuropacemakers (ACTIVA SC® or ACTIVA PC®, Medtronic, Minneapolis, MN, USA, respectively, depending on unilateral or bilateral electrodes) were implanted subcutaneously under general anesthesia, one in the chest wall (subclavicular fossa) connected to the pallidal electrodes, and one in the abdomen wall connected to the thalamic electrodes. Titration of stimulation parameters was carried out 1 month after surgery for 5 days, by pairs (right and left) of thalamic or pallidal electrodes, to evaluate observable clinical tolerance using targeted values as follows: 30‐Hz, pulse duration = 60 microseconds and voltage = 6 V. These parameters were identical to those used in DBS, except in P5, whose voltage was set at 4V due to facial tension and limb hypertonia at 6V.
Figure 2.
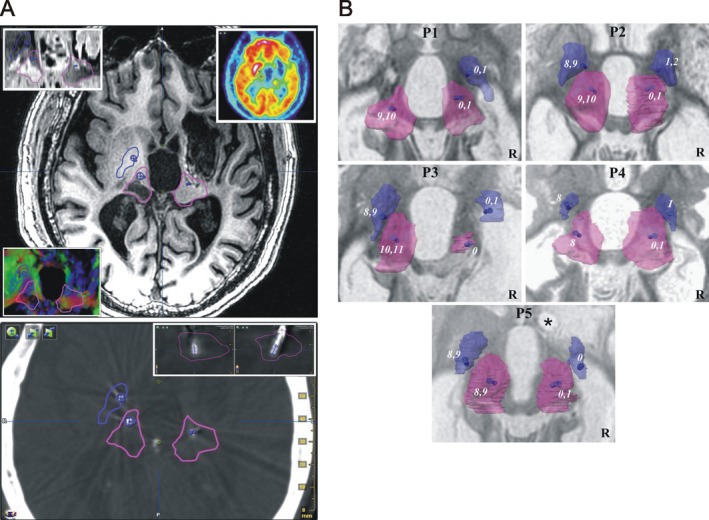
Contacts. (A) Deep brain targets and electrode positioning: Example (P1) of right and left targeted structures, pallidum (blue) and thalamus (pink) and location of effective (used in chronic stimulation; blue segments) contacts: top, T1 axial slice (4 mm above the anterior‐posterior commissure plane; inlays, clockwise from top left, Inversion‐Recovery sequence, FDG, and color‐coded diffusion tensor map); bottom, coregistered (with preoperative imaging) postoperative CT‐Scan, axial slice going through the center of the two effective contacts of the left thalamus, inlays, top‐right, sagittal and coronal slices going through the effective contacts within the thalamus. (B) Contact locations: Contacts (number, from distal to proximal, right from 0 to 3, left from 4 to 11) within the pallidum (blue) and thalamus (pink) of the 5 patients (P): superior view of 3D (semi‐transparent) anatomical structures, the effective contacts (numbers) are displayed as blue segments; background axial T1‐weighted MRI slice (inverted gray scale) going through anterior‐posterior commissure (ACPC) plane; *coil artefact; R, right.
The primary outcome measure was CRS‐R scores across the different phases. This enabled (a priori definition) the identification of responder, that is. patient whose mean CRS‐R score increases significantly from baseline to unblinded DBS‐ON; the crossover phase was used to observe early‐acute effects. We used the French version37 of the CRS‐R. Scores vary from 0 to 23, with six subscores quantifying auditory (au; 0 to 4), visual (vi; 0 to 5), motor (mo; 0 to 6), oromotor‐verbal (ov; 0 to 3), communication (co; 0 to 2) and arousal (ar; 0 to 3) processes; MCS status is evoked when au = 3, vi = 2, mo = 3, ov = 3 or co = 1 (MCS‐thresholds), and emergence from MCS when mo = 6 or co = 2 (emergence‐thresholds). At night, spontaneous eye‐opening was observed (yes or no response). CRS‐R subscores, MCS‐thresholds and emergence‐thresholds and open‐eye state were analyzed as secondary outcomes. Bedside clinical evaluation was performed by the qualified medical and healthcare care staff of each clinical facility; we aimed to perform CRS‐R and eye‐opening assessments twice a week. Data collection time was established at the time of inclusion, according to each individual care protocol (in line with patient and medical unit habits). The procedure was the same for each patient; the nurses were trained by the same person. Only the time during the day, or the day during the week, was slightly different for each patient according to the organization of teams (work schedule; care schedule according to patient, visits of relatives…). However, the timetable of data collection were regular for each patient (e.g. Monday and Thursday at 10 a.m.). The measure of FDG metabolism during baseline and DBS‐ON phases (secondary outcome) was the only functional imaging technique authorized for use in implanted stimulation devices, and enabling comparison of phases. The voxels of normalized FDG PET were colored according to the relative activity, red for high and blue for low (Fig. 3A; Appendix S1). We computed (voxel‐based) maps of metabolic variation DBS‐ON – Baseline, depicting increase (DBS‐ON > Baseline) in green, and decrease (Baseline > DBS‐ON) in beige (Fig. 3A; Appendix S1). Activity (low, high or intermediate) and variation (upward, increased activity; downward, decreased activity) were specified regarding 23 cortical regions of the right and left hemispheres in all five patients (n = 230) by 3D‐rendering, then the 23 regions were grouped into three cortical areas for further analysis (Fig. 3B; Appendix S1): the internally‐oriented area including the limbic and medial cortices of the default mode network38 (DMN), related to internal awareness39; the externally‐oriented area, including lateral association fronto‐parietal cortices of the DMN,38 related to external awareness39; and other areas.
Figure 3.
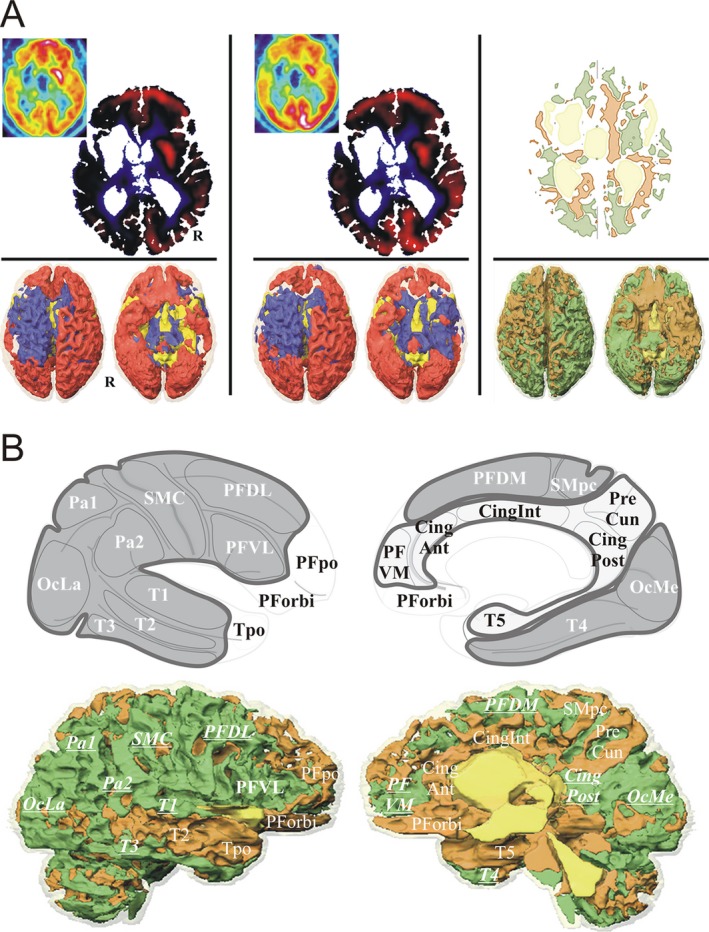
FDG methods. (A) FDG normalization: Baseline (left column) and DBS‐ON (intermediate column) normalized FDG, and DBS‐ON minus Baseline normalized FDG variation (right column), example for P1: top row (4 mm above the anterior‐posterior commissure plane), left and intermediate columns (inlay, raw FDG slices), standardized uptake values of cerebral metabolic rate of glucose normalized by cerebral global mean (CGM) activity (red, above CGM; blue, below CGM), and right column, variation map (green, increase; beige, decrease); bottom row, 3D rendering (superior and inferior views) of normalized data (R, right hemisphere). (B) FDG metabolism by cortical region and area: Top row, cortical areas and regions: internal area (light gray) with prefrontal ventromedial (PFVM), precuneal (PreCun), cingulate anterior (CingAnt), intermediate (CingInt) and posterior (posterior, retrosplenial and isthmus; CingPost) and temporo mesial para hippocampal (T5) cortices; external area (dark gray) with prefrontal dorsolateral (PFDL), ventrolateral (PFVL) and dorsomedial (PFDM), sensorimotor central (SMC) and paracentral (SMpc), parietal superior (Pa1) and inferior (Pa2), occipital lateral (OcLa) and medial (OcMe), temporal T1 (T1), T2 (T2), T3 (T3) and fusiform gyrus (T4) cortices; other area (white) with prefrontal orbital (PForbi) and polar (PFpo), temporopolar (Tpo) and cerebellar (Cerb) cortices. Bottom row, specification of FDG activities by region: example P1, right hemisphere; bold underlined increased activity (upward variation).
Figure 4.
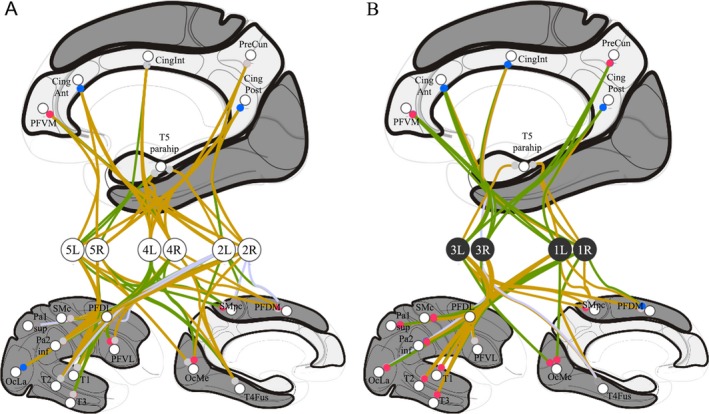
FDG results. Individual analysis of right (R) and left (L) hemispheres of the five patients (see Figure 3.B. for abbreviations): A, non‐responders, P2, P4 and P5, (white circles); B, responders, P1 and P3, (black circles); top, medial view showing the internally‐oriented cortices (light gray); bottom, lateral (left) and medial (right) views showing the externally‐oriented cortices (dark gray); FDG metabolism variations are represented by connection lines toward cortices, green when the metabolism increased (DBS‐ON > Baseline) and beige when the metabolism decreased (Baseline > DBS‐ON) (connections with lesioned cortices are in light purple); baseline FDG metabolism is represented by colored circle, red for high relative activity and blue for low relative activity (gray for near cerebral global mean activity); the two responders exhibited significant increased FDG activity in the internally‐oriented area.
Statistical analysis
Statistical analysis (Stata software; Version 13, StataCorp, College Station, TX) was conducted based on a two‐sided Type I error of 5%. Continuous data were described as mean ± standard‐deviation or median [interquartile range] according to statistical distribution, and categorical parameters as the number of patients and associated percentages. Individual and group analysis of primary outcome CRS‐R scores and subscores was performed, using random effects (mixed) models accounting for between‐ and within‐patient variability (repeated measures or longitudinal data collected from the same individual). The secondary outcome was FDG imaging (globally and by hemisphere) and the same analysis (random‐effects models) was conducted for the three cortical areas (internal, external and other): (1) at baseline, based on the number of high, intermediate, low, and lesioned cortical regions by area and by CRS‐R‐based response, that is. responder status; (2) following DBS (DBS‐ON – Baseline), based on the number of increased and decreased cortical regions by area and by CRS‐R‐based response, and for the external and internal cortical areas, by CRS‐R‐based response and FDG PET variation, with or without adjustment by baseline FDG.
Results
Group level analyses of overt behaviors and ‐ CRS‐R subscores, MCS‐threshold and emergence‐threshold – tables, are in the Appendix S1.
Baseline
DOC status before inclusion was confirmed in all five patients; mean baseline CRS‐R scores (Table 2) varied from 4.2 (P5) to 11.7 (P3) and overall MCS status relied largely on auditory, visual and motor subscores. High FDG activity predominated in the left hemisphere (58.26%; P = 0.004) in the 5‐patient group. Internally‐oriented and externally‐oriented areas were the source of primarily low (40%) and high (47%) activity (P < 0.001) respectively; the five patients shared these FDG features (P < 0.001).
Table 2.
Mean CRS‐R scores during baseline, DBS‐ON, CO‐ON and CO‐OFF phases (the first period of the crossover phase is highlighted in gray); values are expressed as mean ± SD (number of assessments)
| Patient | Baseline | DBS‐ON | CO‐ON | CO‐OFF |
|---|---|---|---|---|
| P1 | 6.1 ± 1.3 (15) | 8.4 ± 1.8 (41)a | 7.3 ± 1.9 (11) | 8.6 ± 2.0 (11)a |
| P2 | 9.6 ± 2.7 (17) | 9.5 ± 1.5 (34) | 10.3 ± 1.3 (11)b | 8.5 ± 1.9 (8) |
| P3 | 11.7 ± 1.6 (18) | 13.8 ± 2.2 (40)a | 15.1 ± 1.9 (7)a | 14.0 ± 3.4 (10)a |
| P4 | 4.8 ± 1.8 (57) | 4.3 ± 1.8 (28) | 3.9 ± 1.6 (11)b | 6.6 ± 1.5 (14)a |
| P5 | 4.2 ± 3.3 (34) | 3.0 ± 1.7 (27) | 4.7 ± 2.0 (15) | 5.1 ± 2.4 (10) |
| All | 6.3 ± 3.5 (141) | 8.4 ± 4.2 (170) | 7.5 ± 4.1 (55) | 8.4 ± 3.7 (53)a |
| All ‐ P5 | 6.9 ± 3.3 (107) | 9.4 ± 3.8 (143)a | 8.6 ± 4.2 (40) | 9.2 ± 3.5 (43)a |
Significant difference (P < 0.05) with baseline
Significant difference (P < 0.05) of CO‐ON with CO‐OFF.
Severe clinical adverse events
There was no surgery‐related mortality. P4 (medical history of pneumonia and type‐1 diabetes) developed postoperative bronchopulmonary infection (12‐day stay in intensive care unit). At the end of the study (June 2016) four patients were alive. P4 died in April 2016, 15 months after surgery and 5 years and 3 months after initial trauma. She died suddenly with no underlying infection or acute neurological signs (no autopsy in line with wishes of relatives). P5 was diagnosed with chronic filamentary keratitis seven months after surgery (DBS‐ON phase) resulting in permanent palpebral closure.
CRS‐R scores
Mean scores, DBS‐ON in P1 (P < 0.001; responder) and P3 (P = 0.001; responder), CO‐ON (first period, 15.1 ± 1.9) (P < 0.01) in P3, CO‐OFF in P1 (second period, P < 0.001), P3 (second period, P = 0.006) and P4 (first period, P < 0.001), were superior to baseline scores (Table 2). Within crossover, the mean value of CO‐ON (second period) was superior to CO‐OFF in P2 (P = 0.04) and, conversely, inferior in P4 (P = 0.001).
CRS‐R subscores
Mean subscores, visual in P1 (P < 0.001) and P3 (P = 0.03), auditory (P < 0.001) and oromotor‐verbal (P = 0.006) in P3, increased from baseline to DBS‐ON. Conversely mean subscores ‐ auditory (P = 0.01), oromotor‐verbal (P = 0.001), and arousal (P = 0.02) in P4, and motor in P5 (P = 0.04) ‐ decreased. Within crossover, the mean value of CO‐ON subscores ‐ motor in P1 (first period, P = 0.002) and P4 (second period, P < 0.001) and visual in P5 (second period, P = 0.009) ‐ was lower than the mean value of CO‐OFF subscores and, conversely, motor subscores in P2 (second period, P = 0.03) and P3 (first period, P = 0.03) were higher.
MCS‐threshold and emergence
There was an individual increase during DBS‐ON in visual in P1 (P < 0.001) and auditory in P3 (P < 0.001) MCS‐thresholds. Occurrence of MCS‐thresholds was more frequent ‐ motor in P4 (P = 0.003) and visual in P5 (P = 0.001) ‐ during CO‐OFF (second period).
Open‐eye state at night
Open‐eye state at night (Table 3) was reported in P1, P2, P3 and P4 (n = 272 values; P5 showed no evidence of open‐eye ‐ technical issues and keratitis). We observed that the eyes were open 47 times, 17.3% (data missing in one case). P2 (P = 0.03) and P3 (P = 0.001) showed a decrease in frequency of open‐eye state at night during DBS‐ON versus baseline; this was also true in P3 during crossover, in both CO‐ON (first period, P = 0.05) and CO‐OFF (second period, P = 0.008).
Table 3.
Open‐eye state at night during baseline, DBS‐ON, CO‐ON and CO‐OFF phases (the first period of the crossover phase is highlighted in gray); values are expressed in number of observations (out of number of assessments; and percentage)
| Patient | Baseline | DBS‐ON | CO‐ON | CO‐OFF |
|---|---|---|---|---|
| P1 | 1 (14; 7.1%) | 6 (25; 24%) | 4 (14; 28.6%) | 2 (10; 20%) |
| P2 | 6 (24; 25%) | 1 (38; 2.6%)a | 2 (8; 25%) | 0 (9; 0%) |
| P3 | 11 (16; 68.8%) | 1 (40; 2.5%)a | 1 (6; 16.7%)a | 2 (13; 15.4%)a |
| P4 | 2 (14; 14.3%) | 3 (22; 13.6%) | 3 (13; 23.1%) | 3 (6; 50%) |
| P5 | n.a. | |||
| All ‐ P5 | 20 (68; 29.4%) | 11 (125; 8.8%)a | 10 (41; 24.4%) | 7 (38; 18.4%) |
Significant difference (P < 0.05) with baseline.
Variation in Brain Metabolism following DBS
The number of upward and downward variations in cortical areas (increased activity of internally‐oriented area, 38%, externally‐oriented, 42% and other areas, 53%; P = 0.35) and in CRS‐R‐based responses (increased activity of responder, 46%, and non‐responder, 40%; P = 0.39) was equal in all five patients. However, the two responders (P1, P3) exhibited increased FDG activity during DBS‐ON in the internally‐oriented area (responder, 64%, and non‐responder, 22%; P = 0.002; P = 0.007 when adjusted to baseline FDG activity), whereas variations in the externally‐oriented area were unchanged (responder, 39%; non‐responder, 44%; P = 0.64; P = 0.85 when adjusted to baseline FDG activity). Interaction between the externally‐oriented area and CRS‐R‐based responses was significant (P = 0.006).
Discussion
The main finding in this trial is that low frequency DBS provoked tangible and reproducible clinical modifications (CRS‐R items), long after the injury, with an increase in FDG metabolism of the internally‐oriented cortex for the two responders. All five surgical interventions were safe (nil mortality), bearing in mind that only one case of intracerebral hematoma had been reported in earlier series.22 However, our case of transient pulmonary infection shows that the most fragile patients are more prone to complications. Broadly our results corroborate with the largest pioneering series,15, 16, 17, 18, 19, 20 providing clues (institutional scales; prospective case series) that about 40–50% of patients may respond to low frequency DBS, and more precisely both UWS and MCS patients from our series. However, the overall clinical benefit of all trials, including ours, is limited. None of our five patients attained a level of verbal communication, but they sustained severe orofacial and language dysfunctions (Table 1; Appendix S1) which hindered progress. Improvement in verbal communication seems more attainable in less severe condition21 than our patients. Indeed reasonable expectations following DBS in severe patients focused primarily on communication,18 in any event. Our patients globally made progress in terms of auditory, visual, oromotor‐verbal, motor and communication processes, and visual, auditory and communication MCS‐thresholds (Appendix S1), most modifications having been perceived favorably by relatives and local healthcare staff. Specifically, the responders made progress regarding visual function and motor MCS‐threshold (P1), and auditory, visual and oromotor‐verbal functions and auditory MCS‐threshold (P3). Indeed, visual and auditory attention would appear to be the most common improvement, more particularly in the two responders, facilitating both communication and spatial exploration. Visual modifications are of particular importance since visual CRS‐R subscores have the highest diagnostic sensitivity among MCS and UWS patients.37 We have also found that DBS modified open‐eye state at night, possibly reflecting changes in sleep patterns, even if it was only significant on an individual basis in one responder (P3) and one non‐responder (P2). Globally if we found across groups that CRS‐R scores increased from baseline to crossover and DBS‐ON phases (Appendix S1), it is when investigating individuals that detailed interpretation occurs. Indeed, changes in individual CRS‐R scores observed during the DBS‐ON phase were not as a matter of course either totally favorable or absent. Decreases in auditory, oromotor‐verbal, arousal and motor, subscores in P4 and P5 demonstrated the diversity of DBS effects according to each specific type of brain damage. However, these unexpected effects were observed in non‐responders alone, whose baseline CRS‐R scores were the most severe and in whom the damaged brains were the smallest; P4 was a particularly severe case, cumulating trauma and anoxia and extensive higher function impairments (Appendix S1) with hypotonic quadriplegia. Our results also point to the spontaneous variability of global CRS‐R scores due to the inconsistency of clinically observable awareness,27 advocating recordings of repeated clinical status assessment in clinical studies. On the basis of our FDG metabolism data we found that MCS and UWS patients had preserved FDG activity (assuming spontaneous near at resting condition) in default mode network (DMN) cortices,38 notably the lateral associative fronto‐parietal cortices featuring MCS.29 The plainly left‐sided predominance of preserved metabolism has been interpreted, thus far, as a mere coincidence of lesion topography across patients. The spontaneous imbalance of internally‐oriented and externally‐oriented areas in the five patients, at the source of mainly low and high FDG activity respectively, echoes the link between cortices related to internal awareness and the severity of DOC,40, 41 as well as the increase in FDG activity of the internally‐oriented cortical area of the two responders.
There are limitations to our pilot study. The results we derived from a limited number of patients provide insight into the potential clinical benefit of DBS, but also raise issues. More specifically, analysis of the crossover phase failed to show conclusive results when comparing first and second periods. This could be due to carryover DBS effect, possibly spanning several weeks, in association with lesion effects (surgery and titration lasting about one month, directly followed by the crossover phase). Indeed, only P2 and P4, non‐responders, manifested significant changes in mean CO‐ON versus CO‐OFF CRS‐R scores (CO‐ON was the second period in both patients). The design of future crossover phases should likely be modified, example. assessment of DBS 3 months after surgery and 2 months after titration. Analysis of overt conscious behavior was only based on the CRS‐R scoring, which is robust and standardized, yet less reliable regarding visual, oromotor verbal and arousal processes.11, 24 Moreover, analysis of behavior through distinct processes involving overlapping brain functions, impaired specifically because of extensive lesions (e.g. dyspraxia, dysgnosia and language disorders), likely influences sub‐scale scores in a complex way. Our inclusion criteria were exclusively clinical and further studies should benefit from functional imaging according to clinical consensus,29 along the same lines as our findings regarding FDG.
That being so, our findings contribute to an understanding of the mechanisms underlying low frequency DBS modulation. Cortico‐basal ganglia‐thalamo‐cortical loops seem to activate affected networks, notably through thalamo‐ and pallido‐ cortical direct and indirect pathways in DOC models.11, 23, 42, 43, 44 This effect would appear to be diffuse (FDG modifications within the 23 regions studied lacked statistical significance). In any case, DBS‐mediated activation had overdriven internally‐oriented areas, which may account for the recovery of conscious behavior. Modifications in open‐eye state at night also suggest that pallido‐tegmental circuits45, 46 may have been activated (along with thalamo‐cortico‐basal loops) particularly those involving the tegmental pedunculopontine nucleus. Neurons of the latter discharge specifically at low frequencies,47 and the pedunculopontine nucleus is involved to the same extent in arousal‐attention, paradoxical sleep, locomotor behaviors and tonus (see e.g.35, 48). 100‐Hz thalamus DBS can modify, partially alleviating22 or increasing,21 muscle tone in severe DOC patients. In our series, there was an increase in P5 muscle tone subsequent to implantation of pallidal or thalamic electrodes (titration phase). It is conceivable that different frequency settings, in the pallidum and/or the thalamus, may modulate tone in keeping with clinical context as hypertonia and pathologic posturing predominate in this population, aside from DOC.
In summary, recovery of certain brain processes that have been dysfunctional for many years in DOC patients is possible using low frequency DBS. Clinical response criteria comprise: UWS or MCS patients with high mean baseline CRS‐R scores (of at least 2 months); multiple focal lesions following traumatic injury or hemorrhagic stroke with documented activity of DMN cortices. We stress that our results thus far have demonstrated limited individual benefits and quality of life remains unknown. Further lines of research should ideally decipher complex pathophysiology, refine techniques and indications, and include requisite societal debate that goes hand in hand with therapeutic advances. Meanwhile DBS techniques could be discussed in line with the rules governing deontology and ethics, on case‐by‐case basis, taking into account both the burden and the vulnerability of the population of permanent DOC patients, over long periods of rehabilitation that can last for decades.
Author Contribution
JJL performed surgical procedures, prepared the figures, analyzed and interpreted the data; AS, BeP and SR, collected clinical data, organized imaging examinations, hospitalization and follow‐up care; JG, AS, AK, TG and BJ performed structural (MRI, CT‐Scan) and functional (FDG PET) imaging; BrP undertook statistical analysis; BeP and JJL performed titration; JC performed electrophysiological recordings, and JC and SR analyzed and interpreted the electrophysiological data; GC and AC performed preliminary surgery; FF and BR conceptualized and conducted PET FDG processing; JJL, BeP, AS, CS, JC, TG, FF and BrP conceptualized the study; JJL, AS, BeP, BrP, JC, CS, GC and FF drafted and revised the manuscript.
Conflict of Interest
None declared.
Supporting information
Appendix S1. Supplementary Material
Acknowledgments
This study was funded by the Fondation de l'Avenir (Paris, France; ET1‐615 2011; “Deroche Legacy” Grant BO4‐001; wages of AS; patient transportation and MRI costs) and the University Hospital of Clermont‐Ferrand (waived hospital inpatient expenses, endorsed study insurance, trial promoter). Medtronic France supplied DBS devices (without charge), and the Centre Jean Perrin provided FDG Pet‐Scan exams (without charge). JJL declares grant from the Fondation de l'Avenir and Medtronic France. BeP declares grants from the Fondation de l'Avenir. JJL declares non‐financial support from Saint‐Jude Medical (grant awarded to the academic entity; travel expenses) and Medtronic (travel expenses). The remaining authors declare no conflicts of interest. We extend our thanks to: A Momon and JO Dolomie (anesthesiologists); healthcare and medical staff and radiology technicians; J Luaute (neurorehabilitation; MD, PhD), T Sarraf (neurorehabilitation; MD), S Laureys (neurology; MD, PhD), and D Fontaine (neurosurgery; MD, PhD) for their input. We would also like to thank family members of patients for their trust.
Funding Information
This study was funded by the Fondation de l'Avenir (Paris, France; ET1‐615 2011; “Deroche Legacy” Grant BO4‐001; wages of AS; patient transportation and MRI costs) and the University Hospital of Clermont‐Ferrand (waived hospital inpatient expenses, endorsed study insurance, trial promoter). Medtronic France supplied DBS devices (without charge), and the Centre Jean Perrin provided FDG Pet‐Scan exams (without charge). JJL declares grant from the Fondation de l'Avenir and Medtronic France. BeP declares grants from the Fondation de l'Avenir. JJL declares non‐financial support from Saint‐Jude Medical (grant awarded to the academic entity; travel expenses) and Medtronic (travel expenses).
Funding Statement
This work was funded by Fondation de l'Avenir grants ET1‐615 2011 and Grant BO4‐001; Centre Jean Perrin grant ; Medtronic grant ; University Hospital of Clermont‐Ferrand grant .
References
- 1. Katz DI, Polyak M, Coughlan D, et al. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1‐4 year follow‐up. Prog Brain Res 2009;177:73–88. [DOI] [PubMed] [Google Scholar]
- 2. Ventura T, Harrison‐Felix C, Carlson N, et al. Mortality after discharge from acute care hospitalization with traumatic brain injury: a population‐based study. Arch Phys Med Rehabil 2010;91:20–29. [DOI] [PubMed] [Google Scholar]
- 3. Luauté J, Maucort‐Boulch D, Tell L, et al. Long‐term outcomes of chronic minimally conscious and vegetative states. Neurology 2010;75:246–252. [DOI] [PubMed] [Google Scholar]
- 4. Harrison‐Felix C, Pretz C, Hammond FM, et al. Life expectancy after inpatient rehabilitation for traumatic brain injury in the United States. J Neurotrauma 2015;32:1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avesani R, Gambini MG, Albertini G. The vegetative state: a report of two cases with a long‐term follow‐up. Brain Inj 2006;20:333–338. [DOI] [PubMed] [Google Scholar]
- 6. Sarà M, Sacco S, Cipolla F, et al. An unexpected recovery from permanent vegetative state. Brain Inj 2007;21:101–103. [DOI] [PubMed] [Google Scholar]
- 7. McMillan TM, Herbert CM. Further recovery in a potential treatment withdrawal case 10 years after brain injury. Brain Inj 2004;18:935–940. [DOI] [PubMed] [Google Scholar]
- 8. Fins JJ, Schiff ND, Foley KM. Late recovery from the minimally conscious state: ethical and policy implications. Neurology 2007;68:304–307. [DOI] [PubMed] [Google Scholar]
- 9. Prolonged disorders of consciousness: national clinical guidelines [Internet]. RCP London 2015;[cited 2016 May 18] Available from: https://www.rcplondon.ac.uk/guidelines-policy/prolonged-disorders-consciousness-national-clinical-guidelines
- 10. Giacino JT, Whyte J, Bagiella E, et al. Placebo‐controlled trial of amantadine for severe traumatic brain injury. N Engl J Med 2012;366:819–826. [DOI] [PubMed] [Google Scholar]
- 11. Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol 2014;10:99–114. [DOI] [PubMed] [Google Scholar]
- 12. McLardy T, Ervin F, Mark V, et al. Attempted inset‐electrodes‐arousal from traumatic coma: neuropathological findings. Trans Am Neurol Assoc 1968;93:25–30. [PubMed] [Google Scholar]
- 13. Hassler R, Ore GD, Dieckmann G, et al. Behavioural and EEG arousal induced by stimulation of unspecific projection systems in a patient with post‐traumatic apallic syndrome. Electroencephalogr Clin Neurophysiol 1969;27:306–310. [DOI] [PubMed] [Google Scholar]
- 14. Sturm V, Kühner A, Schmitt HP, et al. Chronic electrical stimulation of the thalamic unspecific activating system in a patient with coma due to midbrain and upper brain stem infarction. Acta Neurochir (Wien) 1979;47:235–244. [DOI] [PubMed] [Google Scholar]
- 15. Cohadon F, Richer E, Rougier A, et al. Deep Brain Stimulations in Cases of Prolonged Post‐traumatic Unconsciousness In: Gybels J., Hitchcock E. R., Ostertag C., Rossi G. F., Siegfried J., Szikla G., eds. Advances in stereotactic and Functionnal Neurosurgery 6. pp. 535–537. Vienna: Springer‐Verlag, 1984. [Google Scholar]
- 16. Cohadon F, Richer E, Rougier A, et al. Deep brain stimulation in cases of prolonged post‐traumatic unconsciousness In: Lazorthes Y., Upton A. R. M., eds. Neurostimulation: an Overview. pp. 247–250. Mt. Kisco, New York: Futura Publishing Company, 1985. [Google Scholar]
- 17. Tsubokawa T, Yamamoto T, Katayama Y, et al. Deep‐brain stimulation in a persistent vegetative state: follow‐up results and criteria for selection of candidates. Brain Inj 1990;4:315–327. [DOI] [PubMed] [Google Scholar]
- 18. Cohadon F, Richer E. Deep cerebral stimulation in patients with post‐traumatic vegetative state. 25 cases. Neurochirurgie 1993;39:281–292. [PubMed] [Google Scholar]
- 19. Yamamoto T, Katayama Y, Kobayashi K, et al. DBS therapy for a persistent vegetative state: ten years follow‐up results. Acta Neurochir Suppl 2003;87:15–18. [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto T, Katayama Y, Kobayashi K, et al. Deep brain stimulation for the treatment of vegetative state. Eur J Neurosci 2010;32:1145–1151. [DOI] [PubMed] [Google Scholar]
- 21. Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 2007;448:600–603. [DOI] [PubMed] [Google Scholar]
- 22. Magrassi L, Maggioni G, Pistarini C, et al. Results of a prospective study (CATS) on the effects of thalamic stimulation in minimally conscious and vegetative state patients. J Neurosurg 2016;000:1–10. [DOI] [PubMed] [Google Scholar]
- 23. Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci 2008;1129:105–118. [DOI] [PubMed] [Google Scholar]
- 24. Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale‐revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85:2020–2029. [DOI] [PubMed] [Google Scholar]
- 25. Estraneo A, Moretta P, Loreto V, et al. Late recovery after traumatic, anoxic, or hemorrhagic long‐lasting vegetative state. Neurology 2010;75:239–245. [DOI] [PubMed] [Google Scholar]
- 26. Giacino JT, Kalmar K. The vegetative and minimally conscious states: a comparison of clinical features and functional outcome. J Head Trauma Rehabil 1997;12:36. [Google Scholar]
- 27. Cortese MD, Riganello F, Arcuri F, et al. Coma recovery scale‐r: variability in the disorder of consciousness. BMC Neurol 2015;15:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Perri C, Bahri MA, Amico E, et al. Neural correlates of consciousness in patients who have emerged from a minimally conscious state: a cross‐sectional multimodal imaging study. Lancet Neurol 2016;15:830–842. [DOI] [PubMed] [Google Scholar]
- 29. Stender J, Gosseries O, Bruno M‐A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 2014;384:514–522. [DOI] [PubMed] [Google Scholar]
- 30. Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1949;1:455–473. [PubMed] [Google Scholar]
- 31. Dieckmann G. Cortical synchronized and desynchronized responses evoked by stimulation of the putamen and pallidum in cats. J Neurol Sci 1968;7:385–391. [DOI] [PubMed] [Google Scholar]
- 32. Lutkenhoff ES, Chiang J, Tshibanda L, et al. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann Neurol 2015;78:68–76. [DOI] [PubMed] [Google Scholar]
- 33. Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol 2014;10:99–114. [DOI] [PubMed] [Google Scholar]
- 34. Koch C, Massimini M, Boly M, Tononi G. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci 2016;17:307–321. [DOI] [PubMed] [Google Scholar]
- 35. Steriade M. Central core modulation of spontaneous oscillations and sensory transmission in thalamocortical systems. Curr Opin Neurobiol 1993;3:619–625. [DOI] [PubMed] [Google Scholar]
- 36. Dehaene S, Changeux J‐P. Experimental and theoretical approaches to conscious processing. Neuron 2011;70:200–227. [DOI] [PubMed] [Google Scholar]
- 37. Schnakers C, Majerus S, Giacino J, et al. A french validation study of the coma recovery scale‐revised (CRS‐R). Brain Inj 2008;22:786–792. [DOI] [PubMed] [Google Scholar]
- 38. Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci USA 2001;98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vanhaudenhuyse A, Demertzi A, Schabus M, et al. Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci 2011;23:570–578. [DOI] [PubMed] [Google Scholar]
- 40. Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ‐F, et al. Default network connectivity reflects the level of consciousness in non‐communicative brain‐damaged patients. Brain 2010;133(Pt 1):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silva S, de Pasquale F, Vuillaume C, et al. Disruption of posteromedial large‐scale neural communication predicts recovery from coma. Neurology 2015;85:2036–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saunders A, Oldenburg IA, Berezovskii VK, et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 2015;521:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crone JS, Lutkenhoff ES, Bio BJ, et al. Testing proposed neuronal models of effective connectivity within the cortico‐basal ganglia‐thalamo‐cortical loop during loss of consciousness. Cereb Cortex 2017;27:2727–2738. [DOI] [PubMed] [Google Scholar]
- 44. Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci 2016;16:533–541. [DOI] [PubMed] [Google Scholar]
- 45. Shink E, Sidibé M, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: II. Topography and synaptic organization of pallidal efferents to the pedunculopontine nucleus. J Comp Neurol 1997;382:348–363. [PubMed] [Google Scholar]
- 46. Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract‐tracing methods. J Comp Neurol 1994;344:210–231. [DOI] [PubMed] [Google Scholar]
- 47. Garcia‐Rill E, Simon C, Smith K, et al. The pedunculopontine tegmental nucleus: from basic neuroscience to neurosurgical applications. J Neural Transm 2011;118:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones BE. The organization of central cholinergic systems and their functional importance in sleep‐waking states. Prog Brain Res 1993;98:61–71. [DOI] [PubMed] [Google Scholar]
- 49. Bruno M‐A, Vanhaudenhuyse A, Thibaut A, et al. From unresponsive wakefulness to minimally conscious PLUS and functional locked‐in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 2011;258:1373–1384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Material


