Abstract
Objective
Autonomic nervous system is involved at the onset of Parkinson disease (PD), and alpha‐synuclein (α‐Syn) and its phosphorylated form (p‐αSyn) have been detected in dermal autonomic nerve fibers of PD. We assessed disease specific conformation variant of α‐Syn immunoreactivity in cutaneous nerves and characterized skin denervation patterns in PD and atypical parkinsonism (AP).
Methods
We enrolled 49 subjects, 19 with PD, 17 age‐matched healthy controls, and 13 with AP. The manifestations of disease were rated on clinical scales. Skin biopsies from ankle, thigh, and neck were analyzed by immunofluorescence for p‐αSyn, 5G4 as a conformation specific antibody to pathogenic α‐Syn and PGP9.5 as axonal marker. Intraepidermal nerve fiber density was measured in all anatomical sites as marker of neurodegeneration. Thirteen of the 19 PD underwent a 1 year follow‐up visit plus skin biopsies.
Results
PD subjects displayed more severe cervical skin denervation (P < 0.03), which correlated to disease duration and worsened between initial and follow‐up examination (P < 0.001). p‐αSyn and 5G4 were equally sensitive and specific for the diagnosis of PD (area under the ROC was 0.839 for p‐αSyn and 0.886 for 5G4). PD and AP with possible alpha‐synucleinopathies share the features of marked cervical denervation and the presence of 5G4. In contrast AP with possible tauopathies were normal.
Interpretation
Conformational specific forms of α‐Syn are detectable in skin biopsy by immunofluorescence in PD, with a promising diagnostic efficiency similar to p‐αSyn. Cervical cutaneous denervation correlates with disease duration and increases over time standing out as a potential biomarker of PD progression.
Introduction
The diagnosis of PD still relies exclusively on clinical signs of motor involvement that appear after significant neurodegeneration has already occurred. Hence, there is a strong need for reliable biomarkers, so that treatment can be started early for maximum impact and clinical trials of disease‐modifying drugs can be conducted. Many studies have revealed Lewy bodies, the hallmark of PD pathology, in peripheral tissues, and Braak intriguingly hypothesized that alpha‐synuclein (α‐Syn) pathology may begin in the peripheral nervous system well before it appears in the brain.1 In fact, non‐motor autonomic symptoms, including gastrointestinal and cutaneous manifestations, are common in early PD.2 Nearly two‐thirds of patients with PD complain of thermoregulatory, cutaneous vasomotor, and sweating disturbances.3
α‐Syn deposits have been demonstrated by immunohistochemistry in cutaneous autonomic fibers in PD patients, and the amount of α‐Syn correlates with measures of autonomic dysfunction.4 Phosphorylated α‐Syn (p‐αSyn) deposition in PD patients has been found in the autonomic nerves surrounding sweat glands and pilomotor muscles.5, 6, 7 Positive skin biopsies for p‐αSyn have been found in 73–75% of patients with PD or multiple system atrophy (MSA)8 and in patients with Lewy body dementia.9 Two promising studies have shown that dermal p‐αSyn can be detected in a subgroup of patients with REM‐sleep behavioral disturbances (RBD), potentially representing prodromal PD.10, 11 Another important point emerging from these studies is that most PD patients displayed cutaneous somatic and autonomic denervation.4, 6, 12, 13, 14, 15, 16 However, the potential relationship between pathological α‐Syn deposits and the loss of small nerve fibers is still unclear.
Thus even if skin biopsy is capable to distinguish PD from healthy subjects, it lacks adequate sensitivity and specificity and further studies are therefore needed to achieve standardization of anatomical site and of immunohistochemical process including the choice of the antigen.17
Soluble oligomeric forms of misfolded proteins are the early pathogenic species not only in PD but also in Alzheimer disease and other proteinopathies. In particular misfolded α‐Syn protein forms small oligomers that are neurotoxic both in vivo an in vitro18, 19 . α‐Syn oligomers have been isolated from postmortem brains20, 21 and, more importantly in the plasma22 and cerebrospinal fluid of living PD patients.23, 24
Since p‐αSyn is present in cutaneous nerves of PD, we hypothesize that other disease specific forms of α‐Syn may be present there as well. In particular we exploit 5G4 antibody for the first time in skin biopsies: this is a conformation‐specific monoclonal antibody with high reactivity for disease specific forms of α‐Syn including oligomers in brain and low reactivity for monomeric α‐Syn.25, 26 We investigate both the aggregates and phosphorylated α‐Syn and innervation rate in skin biopsies in three anatomical sites in PD, atypical parkinsonism (AP), and healthy controls, plus a follow‐up analysis at 12 months in PD. Our main aims are: (1) to test whether α‐Syn aggregates detection by 5G4 in skin biopsies can serve as a biomarker of PD and/or AP and compare it to p‐αSyn detection, (2) to determine the diagnostic accuracy of skin biopsies at different anatomical sites, (3) to measure skin innervation at baseline and its variation with time at different anatomical sites as a biomarker of disease progression.
Methods
Subjects
The Cantonal Ethics Committee approved the study protocol and all enrolled subjects gave written informed consent to the study. Twenty‐two consecutive patients with idiopathic PD and 13 with AP followed by the movement disorders outpatient clinic at NSI Lugano were enrolled from July 2015 to December 2017. Inclusion criteria for PD were: a definite clinical diagnosis according to the UK Brain Bank diagnostic criteria, disease duration at least 3 years, no family history, and no major cognitive impairment or major dysautonomic symptoms in the history. In seven cases, the diagnosis was supported by 123I‐FP‐CIT (DaTSCAN; GE Healthcare, Buckinghamshire, UK) showing typical dopaminergic nigrostriatal denervation. Seventeen age‐matched healthy controls (HC) for the PD patients were recruited among hospital staff and patients’ partners. The AP group comprised patients in two subgroups: seven with AP with possible alphasynucleinopathy pathology (AP‐SYN), including five with probable multiple system atrophy and two with probable Lewy body dementia according to published diagnostic criteria,27, 28 and six with AP with possible tauopathy pathology (AP‐TAU), including four with probable progressive supranuclear palsy and two with possible corticobasal degeneration.29, 30 During the screening visit, all subjects underwent a blood test for known causes of neuropathy (glycated hemoglobin, creatinine, vitamin B12, TSH, serum immunofixation, HIV, HCV, syphilis, and borreliosis), and three subjects (all PD group) who tested positive were excluded. Scores on the following clinical diagnostic instruments were determined: the Hoehn and Yahr scale (H&Y) and MDS‐UPDRS for the gravity of disease (assessed by movement disorders experts), the Mini‐Mental State Examination (MMSE), the Montreal Cognitive Assessment (MOCA), the Beck Depression Inventory‐II (BDI), and the REM Sleep Behavior Disorder Screening Questionnaire. The levodopa equivalent daily dose (LEDD) was calculated for the PD patients.31
Thirteen of the 19 subjects with PD were reevaluated in a follow‐up visit at 12 months (T12) and underwent a second round of skin biopsies and clinical assessment scales as above. Of the remaining six patients, four were lost to follow‐up (because of disease progression in one case and personal reasons in three cases), and two were enrolled less than 12 months before the analysis.
Skin biopsy
Each subject underwent six (3 mm‐diameter) skin biopsies at three anatomical sites (two biopsies from the skin of the neck at C8 dermatomal level), two from the thigh 10 cm below the trochanter, and two from the leg 10 cm above lateral malleolus) on the side which was clinically more affected, according to our standard technique.32
For each anatomical site, one biopsy was fixed in PLP2% fixative for immunofluorescence studies and one biopsy was frozen and subsequently a RIPA extract was prepared for biochemistry analysis.
Skin innervation
Skin innervation was assessed with a standard indirect immunofluorescence technique on 50 μm thin tissue sections stained by free floating with an antibody specific for the neuronal marker PGP9.5 (rabbit polyclonal, Abcam, Cambridge UK, 1:1000). Cell nuclei were counterstained for DAPI. PGP9.5 positive structures were counted to determine the linear intraepidermal nerve fiber density (IENFD) according to published standard protocols and expressed as number of fibers/mm33, 34 (Fig. 4A).
Figure 4.
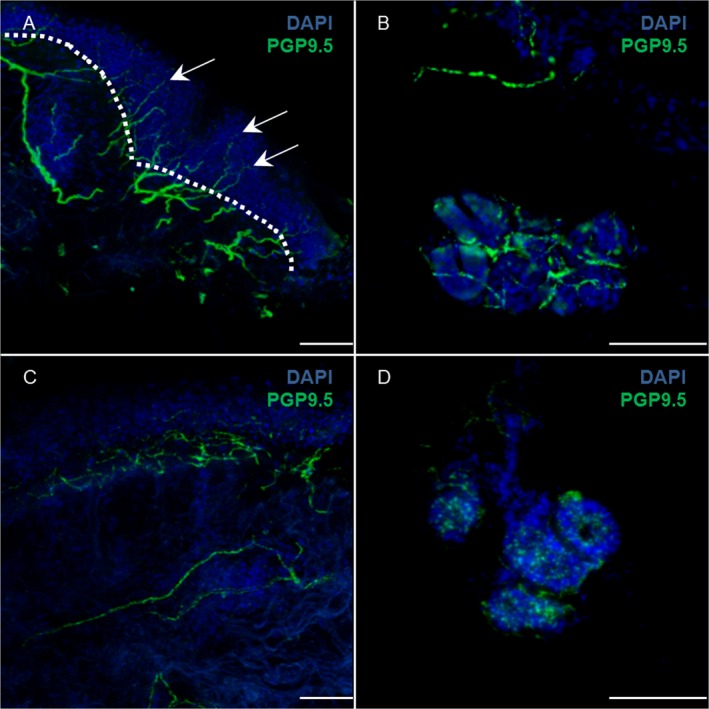
Skin innervation: intraepidermal nerve fiber density (IENFD). Immunofluorescence staining with anti‐PGP9.5 (green) and DAPI (blue) of cervical skin in an healthy subject (A) and PD (C); in A the dotted white line shows the border between epidermis and dermis: IENFD is calculated as the number of nerve fibers (arrows) crossing the border per mm. Dermal nerve fibers around sweat glands are shown in an healthy subject (B) and PD (D). The pictures shows skin denervation in PD more evident at level of autonomic nerve fibers of sweat gland. Scale bar 50 μm.
The same rater blind to the disease group, assessed all skin biopsies at baseline and follow‐ups.
Phosphorylated alpha synuclein and 5G4 immunofluorescence
Eight nonconsecutive 50 μm skin sections per site were incubated overnight with a panel of primary antibodies: PGP9.5, anti‐p‐αSyn, and 5G4 (Data S1).
Western blot analysis
The total amount of protein in the RIPA extracts obtained from each skin biopsy was determined with the QuantiPro BCA assay kit; 30 μg of protein was then subjected to SDS polyacrylamide electrophoresis, transferred to PVDF membranes, and probed with antibody against PGP9.5 and β‐actin (mouse monoclonal, Sigma Aldrige, Saint Louis, MO, 1:10.000). PGP9.5 quantification in skin extracts was determined by fluorescence intensity with an Odyssey CLx system (Li‐cor Biosciences, Lincoln, Nebraska) and normalized to β‐actin signal for each subject at each site.
Statistical analysis
Comparisons across groups of patients were performed with Welch t‐tests. Analyses of denervation at different sites were run with mixed models including the subject as a random factor (Data S1).
Results
Patients
The demographic data and clinical assessments in each group are summarized in Table 1. AP‐TAU subjects result statistically older than HC group (P = 0.031), more depressed (P = 0.008), and with a greater cognitive impairment (P = 0.002) compared to PD and AP‐SYN. AP groups share a more severe disease gravity measured by H&Y compared to PD subjects (AP‐SYN P < 0.001, AP‐TAU P < 0.001).
Table 1.
Demographic data and clinical scores of patients with PD, AP and healthy controls
| PD T0 | PD T12 | AP‐SYN | AP‐TAU | HC | |
|---|---|---|---|---|---|
| Number and gender |
19 13 Male, 6 Female |
13 10 Male, 3 Female |
7 3 Male, 4 Female |
6 2 Male, 4 Female |
17 9 Male, 8 Female |
|
Median age (years) (quartiles) |
66 (56.5/73) |
67 (61/80) |
72 (66/73.5) |
76.5 * (71.5/77.8) |
57 (54/72) |
| Median disease duration (years) (quartiles) |
4.5 (3/7.8) |
6 (4/9) |
5.5 (2.8/7.5) |
4 (3.3/4.8) |
– |
|
Median H&Y Scale (quartiles) |
2 (1/3) |
2 (2/2) |
5 *** (3.5/5) |
4.5 *** (4/5) |
– |
| % Patients treated with L‐DOPA | 73.7% | 69% | – | – | – |
|
LEDD (mg) (quartiles) |
587.5 (337.5/762.5) |
495 (300/700) |
– | – | – |
| Median Beck Depression Scale (quartiles) |
6 (2.5/8.5) |
9 (4/12) |
8 (3.8/13) |
14 ** (11.8/15.5) |
– |
| Median MDS‐UPDRS (quartiles) | 22.50 (15.3/30.5) |
19 (13.5/26) |
– | – | – |
|
Median RBD (quartiles) |
3 (1.5/4) |
3 (2/5) |
3 (1.3/4.8) |
2.5 (0.3/4.8) |
– |
|
Median MMSE (quartiles) |
29 (28.5/30) |
30 (28/30) |
28 (26.3/29) |
24 ** (22/28) |
– |
|
Median MOCA (quartiles) |
27 (23.5/28.5) |
27 (20/28) |
24.5 (21.5/26) |
22 (16/23.5) |
– |
PD, control, and AP‐SYN groups did not differ in age, (Tukey post hoc test : |t 45| ≤ 2.02, P ≥ 0.19), while the APTAU subjects were significantly older than the healthy controls (Tukey post hoc test: t 45 = 2.85, P = 0.03). AP‐TAU and AP‐SYN had a more severe disease as measured by H&Y compared to PD subjects (P < 0.001). The sex ratio did not differ across patient groups (binomial GLM: χ2 3 = 3.01, P = 0.39). AP‐TAU subjects had significantly lower MMSE scores (Tukey post hoc test: t 27 = −3.74, P = 0.002) and significantly higher scores on the Beck Depression Inventory (Tukey post hoc test: t 28 = 3.23, P = 0.01) compared to PD group, while AP‐SYN subjects had intermediate values of both scores, not differing significantly from either the AP‐TAU group or the PD group (Tukey post hoc test: |t 27| ≤ 2.05, P ≥ 0.12). There was no significant difference in disease duration in the PD, AP‐SYN, and AP‐TAU groups (Poisson GLM: χ2 2 = 1.22, P = 0.54). *P < 0.05; **P < 0.01; ***P < 0.01.
PD, Parkinson Disease; AP, atypical parkinsonism; AP‐SYN, atypical parkinsonism with synucleinophaties; AP‐TAU, Atypical Parkinsonism with tauopathies; HC, healthy control; H&Y scale: Hoen and Yahr scale; LEDD, levodopa equivalent daily dose; MDS‐UPDRS, Movement Disorder Society ‐ Unified Parkinson's Disease Rating Scale; RBD, REM sleep behavior disorder screening questionnaire; MMSE, minimental state examination; MOCA, montreal cognitive assessment.
Phosphorylated alpha synuclein and 5G4 positive deposits are significantly more expressed in PD
In PD subjects, pathological α‐Syn was significantly more highly expressed, being detected mainly in dermal nerve fascicles innervating autonomic structures, namely sweat glands, muscle arrector pilorum (MAP), and arterioles, while no staining of epidermal nerve fibers was found in any group (Figs. 1, 2). P‐αSyn was found to a significantly greater extent in the PD group than in all others (z ≥ 2.64, P (FDR) ≤ 0.03); the extent of its presence did not vary significantly among sites (likelihood‐ratio test: χ2 2 = 1.58, P = 0.45) (Fig. 1I–J) or between the two sexes (χ2 1 = 0.34, P = 0.56). In PD group, 5G4‐positive structures were expressed to a significantly greater extent than in the healthy subjects or in the AP‐TAU patients (z ≥ 2.87, P (FDR) = ≤0.01), and in AP‐SYN patients more than in the AP‐TAU ones (z ≥ 2.29, P (FDR) = 0.04). Moreover, AP‐SYN patients expressed 5G4 positive structures at similar levels to PD patients (z ≥ 1.98, P (FDR) = 0.07) but to a lesser extent in cervical area (Fig. 2I–J). Finally, 5G4 positive structures were more highly expressed in male than in female patients (χ2 1 = 0.4.75, P = 0.03), but at similar levels at all three sites (likelihood‐ratio test: χ2 2 = 0.38, P = 0.83).
Figure 1.
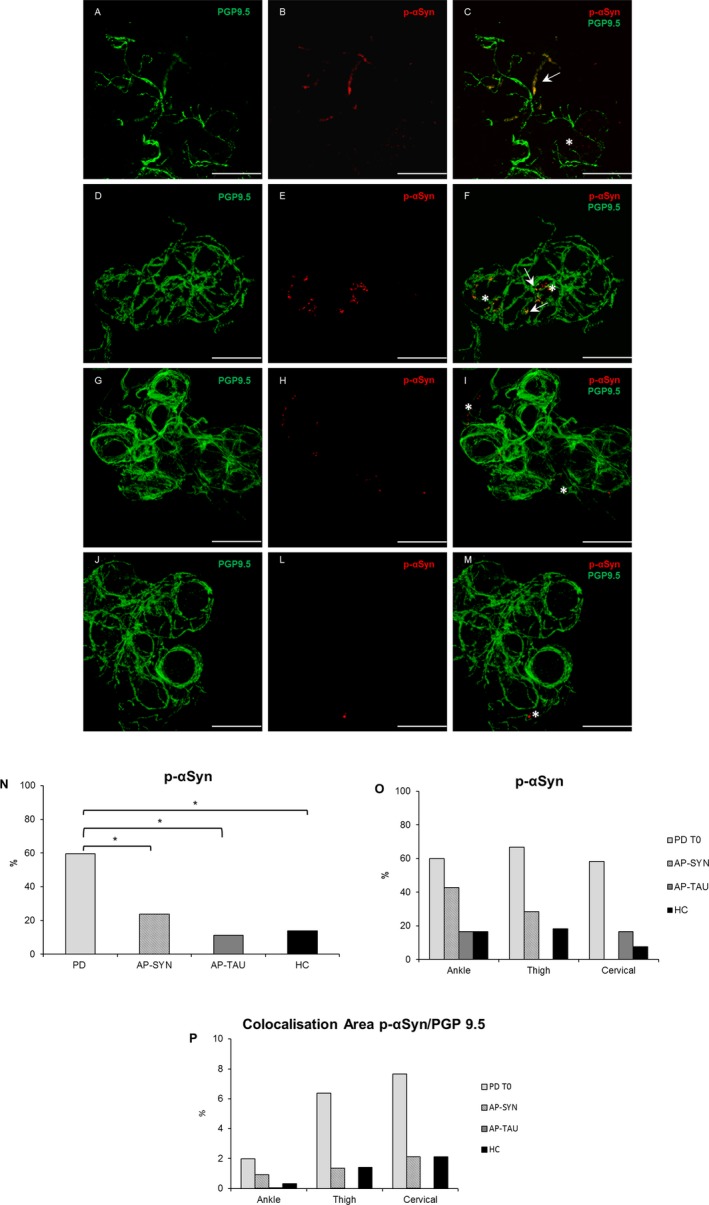
Phosphorylated alpha synuclein deposits are more expressed in PD. Confocal images of immunofluorescence with PGP9.5 (green) and p‐αSyn (red) of dermal nerves around sweat glands in PD T0 (A–B), in AP‐SYN (D–E), AP‐TAU (G–H), and healthy subject (J–L). In yellow colocalization of p‐αSyn and PGP 9.5 (C,F,I,M) along axons. Scale bar 50 μm. White arrows indicate positive structures, asterisks indicate unspecific staining in non‐neuronal structures. The percentage of p‐αSyn is higher in PD T0 patients compared to other groups (P = 0.028) (N), but is not different among localizations (O). A major colocalization area p‐αSyn/PGP9.5 was measured in PD with a proximal to distal gradient (P). PD, Parkinson disease.
Figure 2.
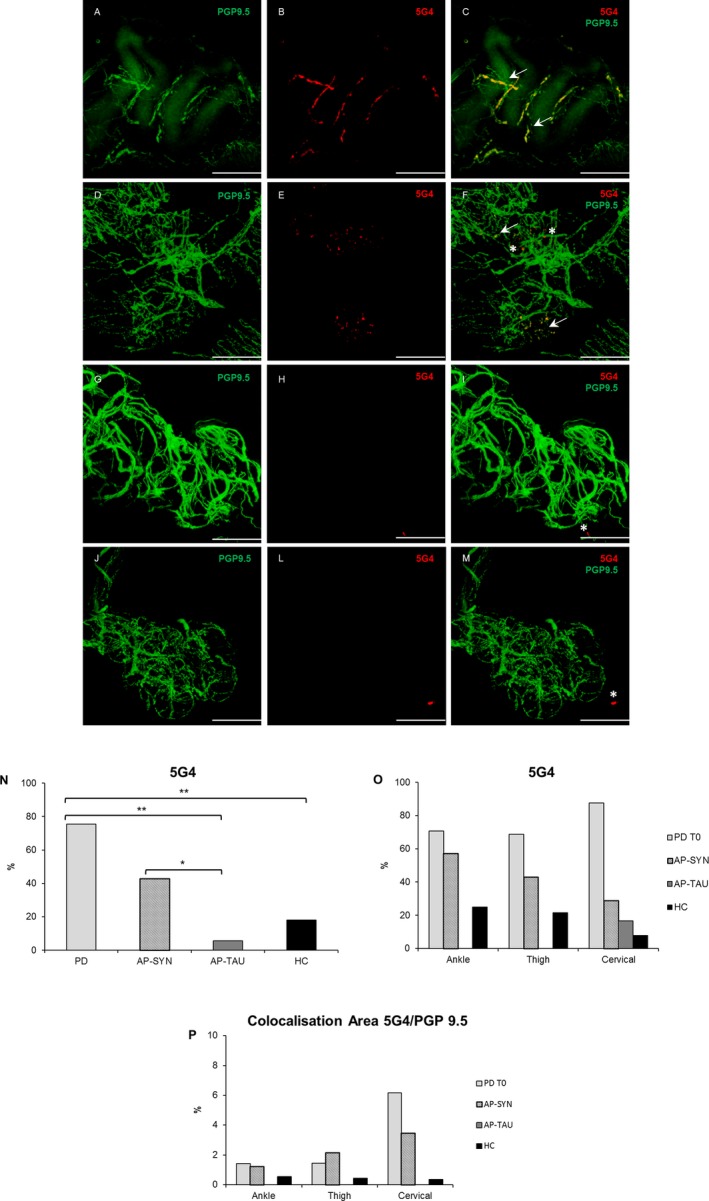
5G4 deposits are more expressed in PD and AP‐SYN patients. Confocal images of immunofluorescence with PGP9.5 (green) and 5G4 (red) of dermal nerves around sweat glands in PD T0 (A–B), AP‐SYN (D–E), AP‐TAU (G–H), and healthy subject (J–L). In yellow colocalization of 5G4 and PGP 9.5 (C,F,I,M) along axons. Scale bar 50 μm. White arrows indicate positive structures, asterisks indicate unspecific staining in non‐neuronal structures. The percentage of 5G4 is higher in PD T0 compared to HC and AP‐TAU (P FDR < 0.012), but not compared to AP‐SYN, and in AP‐SYN more than in AP‐TAU (P FDR = 0.04) (N); no significant differences among localizations but a tendency of higher 5G4 at cervical site in PD (O). A major colocalization area 5G4/PGP9.5 was measured in PD with a proximal to distal gradient (P).
A major colocalization area was measured in PD for both 5G4 and p‐αSyn with a proximal to distal gradient (Figs. 1P and 2P). However this analysis is limited by the fact that sweat glands were not available for all subjects and that a sweat gland denervation would increase the ratio.
No significant difference in p‐αSyn deposits was found at T12 (|z| ≤ 0.95, P ≥ 0.35), while temporal changes in the expression of 5G4 deposits between T0 and T12 differed among sites (likelihood‐ratio test for the effect of the site x time interaction: χ2 2 = 10.18, P = 0.006). However, post hoc tests did not demonstrate any significant pairwise difference between sites (|z| ≤ 0.04, P ≥ 0.97), perhaps because of the small number of skin biopsies available for this analysis.
5G4 versus p‐αSyn detection: sensitivity and specificity
When considering at least two positive sites for p‐αSyn, we found that nine out of 16 PD (56%) were positive, 0/12 in controls, 0/7 in AP‐SYN and 0/6 in AP‐TAU; for 5G4 in PD group 13/16 were positive (81.3%), 2/14 (14%) in controls, 4/7 in AP‐SYN (57.1 %), and 0/7 in AP‐TAU.
Thus for PD group p‐αSyn had 56% of sensitivity and 100% of specificity respect controls and AP‐SYN and AP‐TAU; 5G4 had 81% of sensitivity and 86% specificity respect healthy controls, 43% specificity respect AP‐SYN, 100% specificity respect AP‐TAU. In AP‐SYN 5G4 had a sensitivity of 57% and specificity of 19% respect PD and 86% respect controls, 100% respect AP‐TAU
We calculated the sensitivity and specificity of p‐αSyn and 5G4 in diagnosing PD at each anatomical site, as summarized in Figure 3A. The worst performance was with p‐αSyn in the thigh and the best performance was with 5G4 in the neck (P = 0.054). The diagnostic yield of a combination of tests run with p‐αSyn, 5G4, and both antibodies at all anatomical sites was assessed by ROC curves (Fig. 3B). The area under the ROC was 0.839 for p‐αSyn (SE = 0.066; 95% CI = 0.699–0.951) and 0.886 for 5G4 (SE = 0.054; 95% CI = 0.759–0.970). The difference between areas was 0.047 (SE = 0.058; 95% CI = −0.067 to 0.164; P = 0.42). The AUC of a test employing both antibodies was 0.863 (SE = 0.069; 95% CI = 0.713–0.983), and did not differ significantly from the AUC of the tests with p‐αSyn or 5G4 alone (P ≥ 0.34).
Figure 3.
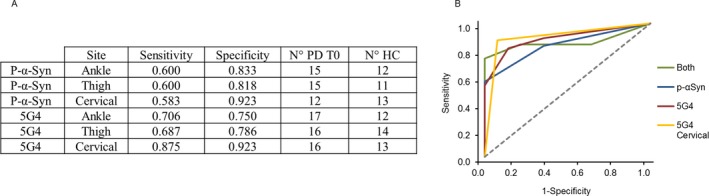
ROC analysis for p‐αSyn and 5G4 in PD. Sensitivity and specificity tests with each antibody in each anatomical site. N° PD T0 and N° HC are the number of subjects for which each test is available (A). Comparison of the diagnostic yield of test performed with p‐αSyn (blue), 5G4 (red), both markers (green) and 5G4 in cervical area only (yellow) (B). Statistical power of the ROC curves was: p‐αSyn (0.946), 5G4 (0.987), both markers (0.987) and 5G4 in cervical area only (0.998).
Since the best results were achieved with 5G4 in the neck, we also assessed the diagnostic efficiency of testing both antibodies in the neck only. The AUC was 0.899 for 5G4 (SE = 0.056, 95% CI = 0.774–1.000), 0.753 for p‐αSyn (SE = 0.081, 95% CI = 0.589–0.900) and 0.871 for p‐αSyn + 5G4 (SE = 0.055, 95% CI = 0.750–0.965).
Cervical skin denervation is a biomarker of PD progression
PD group shows reduced epidermal nerve fibers density at all anatomical sites, and gender affects denervation
In Figure 4 immunofluorescence images of innervation of epidermis and dermal autonomic structures are shown for PD and healthy controls. The results of the mixed‐model analysis of IENFD are summarized in Table 2. In all groups, the IENFD depends significantly on the site of nerve biopsy, with a greater epidermal innervation density in the neck (Tukey post hoc tests: t 72.8 ≥ 6.11, P < 0.001) and a proximal‐to‐distal gradient effect in accordance with previous report (Fig. 5C). The IENFD was lower in the PD group than in the HC and AP‐TAU groups (t 40.8 < −2.33, P < 0.03), but not the AP‐SYN group (t 38.4 = 0.44, P = 0.66), independently of anatomical site, as indicated by the nonsignificant site x group interaction. The IENFD of AP‐TAU patients also tended to be higher than that of AP‐SYN ones, although this difference was not statistically significant (t 41.8 = 1.71, P = 0.09) (Fig. 5A). Difference in IENFD among groups also varied according to sex. Indeed, the IENFD was significantly lower in women in the PD group than in healthy women (t 34.9 = 3.4, P = 0.002) and in AP‐TAU women (t 36.2 = 4.04, P < 0.001), but not in women in the AP‐SYN group (t 36.6 = 1.60, P = 0.12). Women with PD also had lower IENFD values than men with PD (t 35.6 = 2.64, P = 0.01). In addition, the IENFD values of women in the AP‐SYN group were lower than those of women in the AP‐TAU group (t 37.9 = −2.24, P = 0.03). In contrast, there was no significant difference across groups for male subjects (t 40.1 = 0.93, P ≥ 0.36) (Fig. 5B). Age did not significantly affect IENFD values.
Table 2.
IENFD analysis in PD, AP and HC subjects
| Variable | Sum Sq | Mean Sq | NumDF | DenDF | F | P |
|---|---|---|---|---|---|---|
| Site | 2281.66 | 1140.83 | 2 | 74.568 | 37.040 | <0.001 |
| Group | 300.81 | 100.27 | 3 | 38.540 | 3.256 | 0.032 |
| Gender | 26.35 | 26.35 | 1 | 40.352 | 0.855 | 0.361 |
| Age | 0.12 | 0.12 | 1 | 38.251 | 0.004 | 0.951 |
| Site*Group | 284.70 | 47.45 | 6 | 73.050 | 1.541 | 0.177 |
| Site*Gender | 38.61 | 19.31 | 2 | 74.550 | 0.627 | 0.537 |
| Group*Gender | 355.36 | 118.45 | 3 | 38.928 | 3.846 | 0.017 |
| Site*Group*Gender | 249.64 | 41.61 | 6 | 73.035 | 1.351 | 0.246 |
Results of mixed‐model analysis of IENFD in relation to anatomical site, patient group, gender, and age and to the two and three way interactions among anatomical site and patient group and gender. Patient identity was included as a random grouping factor. Age was included as a covariate.
Figure 5.
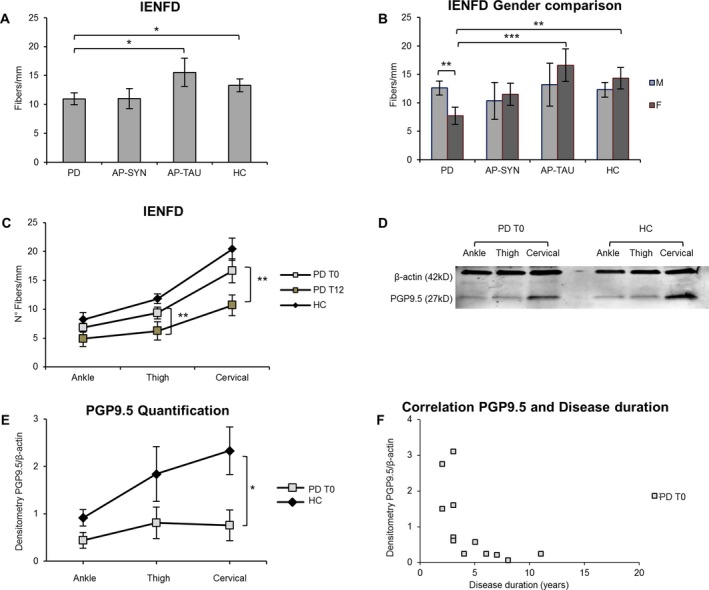
Cervical skin denervation correlates to disease duration and significantly increases at 12 months follow‐up. IENFD is lower in PD T0 patients compare to AP‐TAU and HC subjects (P < 0.03), but not compared with AP‐SYN (A). Total IENFD is significantly reduced in PD females versus PD males (P = 0.012), in PD females versus HC females (P = 0.002) and versus AP‐TAU (P < 0.001) (B). In PD after 12 months IENFD is significantly lower at the thigh (P = 0.002) and at the cervical site (P = 0.001) (C). Western blot of skin lysates (an example) (D). Densitometry of PGP9.5 and β‐actin bands measured by LICOR software (n = 12 PD, n = 8 HC) PGP9.5 values normalized to β‐actin, show a major denervation at cervical site (P < 0.05) (E). PGP9.5 quantification negatively correlates to disease duration at cervical site in PD at T0 (F).
Cervical cutaneous denervation is correlated with disease duration and increases over time
Differences between PD patients and healthy controls in the PGP9.5 measurements obtained by Western blot were significantly larger at cervical site, as indicated by the significance of the site x group interaction (df = 7, F = 7.09, P = 0.02, Fig. 5D–E). Cervical skin denervation was correlated with disease duration (Spearman's correlation: r = −0.59, n = 13, P = 0.03, Fig. 5F).
On follow‐up at 12 months (T12), skin biopsies showed that, in the PD group, the IENFD was significantly lower than at baseline on the neck (difference = −12.0 ± 2.6 SE, t 40.6 = 4.63, P < 0.001 at Tukey post hoc tests) and the thigh (−7.7 ± 2.4 SE, t 39.6 = 3.24, P = 0.002), but not the leg (−0.1 ± 2.2 SE, t 39.6 = 0.03, P = 0.98) (Fig. 5C).
Cervical cutaneous denervation is more pronounced in patients with more severe PD
We split the PD group at T0 into two subgroups according to disease severity (H&Y score). Six patients had more severe disease (H&Y ≥ 3), and 13 had milder disease (H&Y < 3). As shown in Figure 6, the subjects in the first group were significantly older and had a shorter disease duration (age: t 12.759 = 4.08, P = 0.001; disease duration: t 13.595 = −2.74, P = 0.02). No difference in LEDD was found (t 13.049 = −0.001, P = 0.999). Mixed models accounting for biopsy site and sex showed that, on assessment at T0, the group with more severe PD displayed similar levels of positivity to p‐αSyn or 5G4 and similar IENFD values to patients with less severe disease (|t 16.01| ≤ 1.25, P ≥ 0.23). However, a comparison of the denervation values of the same patients at T0 and T12 in a mixed model accounting for patient sex and disease severity showed a significant worsening of denervation at the cervical site only (t 41.1 = −5.06, P < 0.001; |t 41.1| ≤ 1.56, P ≥ 0.13 for the other areas) (Fig. 6D). The group with more severe disease is significantly older and age can affect innervation, but at T12 a significant denervation was measured selectively at cervical site only and not at distal sites where we would attend a major denervation due to ageing since distal axons are more exposed to metabolic and oxidative stress. Finally IENFD did not correlate at any anatomical site with LEDD at T0 and T12.
Figure 6.
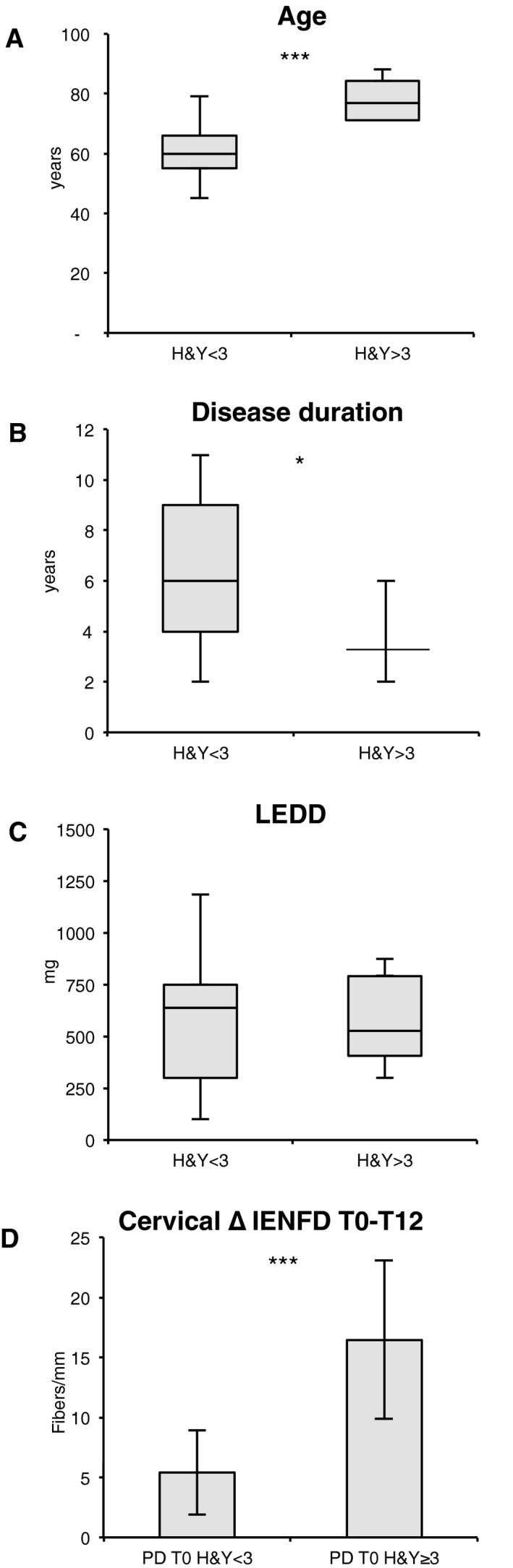
Cervical skin denervation in PD differs according to disease gravity. PD patients were divided in two groups based on H&Y severity. The group with H&Y ≥ 3 is significant older (P = 0.005) (A), with a lower disease duration (P = 0.036) (B), and no difference in LEDD (C). After 12 months IENFD denervation is greater in more disabled patients at cervical site (P = 0.019) (D).
Discussion
The main new aspects of this study are the investigation of disease associated α‐Syn forms other than p‐αSyn in peripheral skin nerves of patients with PD and AP, the finding that cervical skin denervation is a biomarker of PD progression, and the further finding that gender affects skin denervation. Aggregated α‐Syn was detected with 5G4, an antibody whose specificity and sensitivity for oligomeric α‐Syn were shown in previous studies.25, 26, 35 This is a novel, highly sensitive biomarker for PD to be used in skin biopsies. We found that 5G4 positive deposits are present in skin dermal nerves, especially in the nerves surrounding sweat glands and other autonomic structures of dermis (a spatial distribution resembling that of p‐αSyn), and that they are as sensitive and specific as p‐αSyn for the clinical diagnosis of PD. Both p‐αSyn and 5G4 had highly favorable ROC curves (Fig. 3) with only a small difference between their AUCs that cannot be interpreted as showing the superiority of either antibody. In addition, 5G4 immunofluorescence for cervical site only is just as effective in diagnosing PD as immunofluorescence of samples from all three anatomical sites. This is an important finding for simplifying the procedure of sampling skin biopsies toward an effective and less invasive biomarker search, and matches the results of major denervation in the cervical area as measured by Western blot of whole skin (epidermis and dermis) lysates. While several independent groups have found p‐αSyn in dermal nerves (albeit with different specificity and sensitivity) and others in the gastrointestinal nervous plexus, submandibular glands,17, 36 to our knowledge this is the first evidence of aggregated α‐Syn evidence in cutaneous nerves. Counterintuitively we found 5G4 positive staining also in heathy subjects, even if to a significantly lesser extent. These results may have several methodological explanations, alternatively, the presence of 5G4 positive samples in the elderly healthy population may be correct. In fact, incidental Lewy body disease (ILBD) defined as cerebral Lewy body pathology, is identified at autopsy in subjects without evidence of antemortem neurological disorders. ILBD is found in 5 to 24% of people >60 years of age.37 Furthermore, aggregated αSyn has been detected also in gastrointestinal mucosa of healthy subjects36, 38Conformation specific antibody to pathogenic α‐Syn can bring important advantages in the field of peripheral biopsies in neurodegenerative disease. In fact care must be taken when evaluating immunoreactivities with p‐αSyn (at Ser129) antibody, since it may cross‐react with phosphorylated low‐molecular weight neurofilament subunits, and cellular stresses or insults can greatly increase the phosphorylation state of many proteins, including p‐αSyn at Ser129.39, 40 Plus αSyn oligomers are thought to be an early neurotoxic agent that plays a crucial role at the beginning of the neurodegenerative cascade leading to PD while the pathogenic role of p‐αSyn is more controversial. Marked accumulation of it (>90%) has been reported in brain Lewy bodies, which implies an important role in the regulation of α‐Syn aggregation, yet it is still debated whether phosphorylation at the residue S129 enhances or suppresses α‐Syn aggregation and toxicity.41 Indeed, p‐αSyn could be a late event in disease progression, promoting the “disaggregation” of inclusion bodies and enhancing their degradation.42 Hence, the high sensitivity and non‐inferiority of α‐Syn aggregates detected by 5G4 antibody vs p‐αSyn in cervical cutaneous nerves, if are replicated in other studies, especially in early and premotor PD, may represent an important step toward the detection of an early biomarker of PD disease. In fact, in this analysis we focused on patients with well established clinical diagnoses and a long duration of disease in order to avoid confounding data in cases of uncertain diagnosis, particularly in the early phases.
Another important finding of this study is that patients with PD display marked epidermal denervation as measured by IENFD. The more severe denervation in cervical skin biopsies as revealed by Western blot in comparison to IENFD may be explained by the fact that the entire tissue samples with both epidermal and dermal nerve fibers are measured by Western blot, while the IENFD measures epidermal innervation only. Perhaps neurodegeneration is more severe in the dermis, which is also the area where the deposits of pathological misfolded α‐Syn are mainly found.7, 43 Moreover, cervical skin denervation positively correlates with disease duration and is a marker of disease severity, as shown by the fact that more severely affected patients had greater denervation at this site on follow‐up at 12 months. Thus, cervical skin denervation alone is an interesting potential marker of neurodegeneration and disease progression in PD independently of pathological α‐Syn deposits. As we excluded confounding factors such as vitamin deficiency or levodopa therapy, it is unlikely that this finding is due to other factors than the disease itself. The finding of a trend toward greater accumulation of pathologic α‐Syn in the skin of the neck was statistically insignificant; however, it remains to be clarified whether denervation is a direct local effect of α‐Syn aggregation or an indirect neurotoxic effect mediated by long‐distance cell‐body signaling leading to the accumulation of pathogenic α‐Syn. It has been shown that α‐Syn preferentially aggregates in neurons with long, hyperbranching, thin, and unmyelinated axons, such as the axons of the nigrostriatal projection, the cardiac sympathetic system, the vagal intestinal autonomic system,1, 39, 44 and skin. Orimo et al44 showed that α‐Syn aggregates in cardiac sympathetic distal axons precedes the accumulation in neuronal cell bodies and heralds centripetal axonal degeneration, since we measured intraepidermal somatic sensory axons density while aggregated α‐Syn were found mainly in autonomic nerve structures, a quantification of autonomic nerves is mandatory to verify this theory in skin.
This study shows a robust denervation in PD patients at all skin sites that is most pronounced on the neck, with a proximal‐to‐distal gradient, the opposite of what is classically observed in dying‐back, length‐dependent peripheral neuropathy. This supports a possible centrifugal spread of pathology from the central nervous system toward the periphery, mainly along the autonomic and sensory nerves. This hypothesis, however, contrasts with other studies on the diffusion of misfolded α‐Syn from the gastrointestinal peripheral system through the vagus nerve by retrograde axonal transport to the medulla oblongata. A multihit hypothesis has been proposed suggesting that α‐Syn deposition is not monofocally initiated but may be induced by multiple independent factors (e.g. infective agents or viruses) outside the central nervous system through the nasal cavity or the digestive tract.39
Several genetic PD are characterized by considerable variation in neuropathology and no α‐Syn aggregates in CNS. Our study included only sporadic PD, but it is known that LRRK2 mutations are found in 1–2% of sporadic PD.45 However from cellular and animal models it appears that LRRK2 kinase activity leads to phosphorylation of α‐Syn46 thus it is still possible that LRRK2 mutation carriers do harbor α‐Syn pathology.
These data show that gender influences epidermal innervation and pathological α‐Syn accumulation: epidermal denervation was more severe in women with PD and AP‐SYN, while the presence of 5G4 at baseline and the extent of denervation on follow‐up at 12 months were greater in men. These results are hard to explain in the light of current knowledge, but they highlight the need to take account of sex in the design of clinical trials and biomarkers search studies. They are consistent with evidence of sex differences in the prevalence, clinical course, and motor and cognitive manifestations of PD.47 In addition, it is known that gender influence epidermal nerve fibers density in healthy subjects,34 and females are more prone to develop idiopathic small fiber neuropathy.48 P‐αSyn presence did not vary significantly between the two sexes (P = 0.56) while 5G4 positive structures were more highly expressed in male than in female patients (P = 0.03). We can speculate that being 5G4 deposits present presumably at the beginning of disease they are less detectable after neurodegeneration has occurred and since women showed a major denervation, 5G4 deposits were fewer, however our analysis is underpowered to answer the question if the differential denervation among sexes is due to different presence of P‐αSyn/5G4
Finally, skin denervation and pathological α‐Syn may also be useful as biomarkers for atypical parkinsonism, as suggested by previous studies.8, 10 In particular, they can be used to distinguish between AP‐TAU and AP‐SYN. In fact, PD and AP‐SYN share the common features of significant skin denervation and 5G4 positivity with respect to healthy controls and AP‐TAU, in accordance with their common pathologic background linked to the presence of alpha‐synuclein accumulation. In particular, p‐αSyn is more specific for PD, while 5G4 presence did not statistically differ in AP‐SYN but displays a lower expression and a different distribution among skin biopsy sites, being found mainly on the neck in PD and on the thigh and leg in AP‐SYN. Our group of AP‐SYN, albeit small, included a majority of probable‐MSA (5 out of 7). The presence of p‐αSyn in MSA has been debated and conflicting results have been reported,6, 8 due to different anatomical site of biopsy and different protocols of IF analysis.49 Our study protocol is in line with the paper by Doppler8 and results are concordant, however larger studies and unified protocols are strongly needed for definitive evidence. We also found that 5G4 in MSA in contrast to PD is found mainly at distal sites: αSyn accumulates in neurons and neurites in PD while in MSA it accumulates in oligodendrocytes as glial cytoplasmic inclusions. Interestingly it has been recently demonstrated that distinct αSyn strains are generated by different intracellular milieus.50 We can speculate that the pathological spreading across the nervous system is also differently influenced by different αSyn strains.
In conclusion, these results highlight the importance of investigating different pathological antigens including protein aggregates with different types of antibody in order to improve the diagnostic yield of skin biopsy in PD and AP.
Author Contributions
GM: conception and study design, acquisition and analysis of data, writing the manuscript. EV: acquisition and analysis of data, drafting figures. VB: acquisition and analysis of data. SG, CS: enrollment and clinical assessment of patients. RA: statistical analysis of data. AK: study design, enrollment and clinical assessment of patients, analysis of data, drafting the manuscript.
Conflict of Interest
The authors do not have any conflict of interest to declare.
Supporting information
Data S1. (Methods) Phosphorylated alpha synuclein and aggregated alpha synuclein immunofluorescence and statistical analysis.
Acknowledgments
We are very grateful to all the patients and their relatives who participated to this study. We are grateful to Mrs. Nicole Vago, research nurse, for her valuable work on the clinical database and to Dr. Ethan Taub for editing the article. We thank Parkinson Schweiz and ABREOC (the Scientific Research Advisory Board of the Ente Ospedaliero Cantonale) for their financial support of this study.
Funding Information
We thank Parkinson Schweiz and ABREOC (the Scientific Research Advisory Board of the Ente Ospedaliero Cantonale) for their financial support of this study.
Funding Statement
This work was funded by Parkinson Schweiz grant ; ABREOC (the Scientific Research Advisory Board of the Ente Ospedaliero Cantonale) grant .
References
- 1. Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 2003;110:517–536. [DOI] [PubMed] [Google Scholar]
- 2. Kihara M, Kihara Y, Tukamoto T, et al. Assessment of sudomotor dysfunction in early Parkinson's disease. Eur Neurol 1993;33:363–365. [DOI] [PubMed] [Google Scholar]
- 3. Swinn L, Schrag A, Viswanathan R, et al. Sweating dysfunction in Parkinson's disease. Mov Disord 2003;18:1459–1463. [DOI] [PubMed] [Google Scholar]
- 4. Wang N, Gibbons CH, Lafo J, Freeman R. alpha‐Synuclein in cutaneous autonomic nerves. Neurology 2013;29:1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doppler K, Ebert S, Uceyler N, et al. Cutaneous neuropathy in Parkinson's disease: a window into brain pathology. Acta Neuropathol 2014;128:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zange L, Noack C, Hahn K, et al. Phosphorylated alpha‐synuclein in skin nerve fibres differentiates Parkinson's disease from multiple system atrophy. Brain 2015;138(Pt 8):2310–2321. [DOI] [PubMed] [Google Scholar]
- 7. Donadio V, Incensi A, Piccinini C, et al. Skin nerve misfolded alpha‐synuclein in pure autonomic failure and Parkinson disease. Ann Neurol 2016;79:306–316. [DOI] [PubMed] [Google Scholar]
- 8. Doppler K, Weis J, Karl K, et al. Distinctive distribution of phospho‐alpha‐synuclein in dermal nerves in multiple system atrophy. Mov Disord 2015;30:1688–1692. [DOI] [PubMed] [Google Scholar]
- 9. Donadio V, Incensi A, Rizzo G, et al. A new potential biomarker for dementia with Lewy bodies: Skin nerve alpha‐synuclein deposits. Neurology 2017;89:318–326. [DOI] [PubMed] [Google Scholar]
- 10. Doppler K, Jentschke HM, Schulmeyer L, et al. Dermal phospho‐alpha‐synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson's disease. Acta Neuropathol 2017;133:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antelmi E, Donadio V, Incensi A, et al. Skin nerve phosphorylated alpha‐synuclein deposits in idiopathic REM sleep behavior disorder. Neurology 2017;88:2128–2131. [DOI] [PubMed] [Google Scholar]
- 12. Dabby R, Djaldetti R, Shahmurov M, et al. Skin biopsy for assessment of autonomic denervation in Parkinson's disease. J Neural Transm (Vienna) 2006;113:1169–1176. [DOI] [PubMed] [Google Scholar]
- 13. Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain 2008;131(Pt 7):1903–1911. [DOI] [PubMed] [Google Scholar]
- 14. Giannoccaro MP, Donadio V, Incensi A, et al. Skin biopsy and I‐123 MIBG scintigraphy findings in idiopathic Parkinson's disease and parkinsonism: a comparative study. Mov Disord 2015;30:986–989. [DOI] [PubMed] [Google Scholar]
- 15. Nolano M, Provitera V, Manganelli F, et al. Loss of cutaneous large and small fibers in naive and l‐dopa‐treated PD patients. Neurology 2017;89:776–784. [DOI] [PubMed] [Google Scholar]
- 16. Nolano M, Provitera V, Stancanelli A, et al. Small fiber pathology parallels disease progression in Parkinson disease: a longitudinal study. Acta Neuropathol 2018; 10.1007/s00401-018-1876-1. [DOI] [PubMed] [Google Scholar]
- 17. Lee JM, Derkinderen P, Kordower JH, et al. The search for a peripheral biopsy indicator of alpha‐synuclein pathology for Parkinson Disease. J Neuropathol Exp Neurol 2017;76:2–15. [DOI] [PubMed] [Google Scholar]
- 18. Kalia LV, Kalia SK, McLean PJ, et al. Alpha‐Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol 2013;73:155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Danzer KM, Haasen D, Karow AR, et al. Different species of alpha‐synuclein oligomers induce calcium influx and seeding. J Neurosci 2007;27:9220–9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharon R, Bar‐Joseph I, Frosch MP, et al. The formation of highly soluble oligomers of alpha‐synuclein is regulated by fatty acids and enhanced in Parkinson's disease. Neuron 2003;37:583–595. [DOI] [PubMed] [Google Scholar]
- 21. Roberts RF, Wade‐Martins R, Alegre‐Abarrategui J. Direct visualization of alpha‐synuclein oligomers reveals previously undetected pathology in Parkinson's disease brain. Brain 2015;138(Pt 6):1642–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El‐Agnaf OM, Salem SA, Paleologou KE, et al. Detection of oligomeric forms of alpha‐synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J 2006;20:419–425. [DOI] [PubMed] [Google Scholar]
- 23. Tokuda T, Qureshi MM, Ardah MT, et al. Detection of elevated levels of alpha‐synuclein oligomers in CSF from patients with Parkinson disease. Neurology 2010;75:1766–1772. [DOI] [PubMed] [Google Scholar]
- 24. Majbour NK, Vaikath NN, van Dijk KD, et al. Oligomeric and phosphorylated alpha‐synuclein as potential CSF biomarkers for Parkinson's disease. Mol Neurodegener 2016;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kovacs GG, Wagner U, Dumont B, et al. An antibody with high reactivity for disease‐associated alpha‐synuclein reveals extensive brain pathology. Acta Neuropathol 2012;124:37–50. [DOI] [PubMed] [Google Scholar]
- 26. Kovacs GG, Breydo L, Green R, et al. Intracellular processing of disease‐associated alpha‐synuclein in the human brain suggests prion‐like cell‐to‐cell spread. Neurobiol Dis 2014;69:76–92. [DOI] [PubMed] [Google Scholar]
- 27. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017;32:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kipfer S, Stephan MA, Schupbach WM, et al. Resting tremor in Parkinson disease: a negative predictor of levodopa‐induced dyskinesia. Arch Neurol 2011;68:1037–1039. [DOI] [PubMed] [Google Scholar]
- 32. Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008;131(Pt 7):1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lauria G, Cornblath DR, Johansson O, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 2005;12:747–758. [DOI] [PubMed] [Google Scholar]
- 34. Provitera V, Gibbons CH, Wendelschafer‐Crabb G, et al. A multi‐center, multinational age‐ and gender‐adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol 2016;23:333–338. [DOI] [PubMed] [Google Scholar]
- 35. Kiely AP, Ling H, Asi YT, et al. Distinct clinical and neuropathological features of G51D SNCA mutation cases compared with SNCA duplication and H50Q mutation. Neuropath Appl Neuro 2015;41:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruffmann C, Bengoa‐Vergniory N, Poggiolini I, et al. Detection of alpha‐synuclein conformational variants from gastro‐intestinal biopsy tissue as a potential biomarker for Parkinson's disease. Neuropathol Appl Neurobiol 2018; 10.1111/nan.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dickson DW, Fujishiro H, DelleDonne A, et al. Evidence that incidental Lewy body disease is pre‐symptomatic Parkinson's disease. Acta Neuropathol 2008;115:437–444. [DOI] [PubMed] [Google Scholar]
- 38. Stokholm MG, Danielsen EH, Hamilton‐Dutoit SJ, Borghammer P. Pathological alpha‐synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol 2016;79:940–949. [DOI] [PubMed] [Google Scholar]
- 39. Uchihara T, Giasson BI. Propagation of alpha‐synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 2016;131:49–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kovacs GG. Molecular pathological classification of neurodegenerative diseases: turning towards precision medicine. Int J Mol Sci 2016;17:E189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oueslati A. Implication of alpha‐synuclein phosphorylation at S129 in synucleinopathies: what have we learned in the last decade? J Parkinsons Dis 2016;6:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Inglis KJ, Chereau D, Brigham EF, et al. Polo‐like kinase 2 (PLK2) phosphorylates alpha‐synuclein at serine 129 in central nervous system. J Biol Chem 2009;284:2598–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Donadio V, Incensi A, Rizzo G, et al. Spine topographical distribution of skin alpha‐synuclein deposits in idiopathic parkinson disease. J Neuropathol Exp Neurol 2017;76:384–389. [DOI] [PubMed] [Google Scholar]
- 44. Orimo S, Uchihara T, Nakamura A, et al. Axonal alpha‐synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson's disease. Brain 2008;131(Pt 3):642–650. [DOI] [PubMed] [Google Scholar]
- 45. Marras C, Schule B, Munhoz RP, et al. Phenotype in parkinsonian and nonparkinsonian LRRK2 G2019S mutation carriers. Neurology 2011;77:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal‐dominant parkinsonism with pleomorphic pathology. Neuron 2004;44:601–607. [DOI] [PubMed] [Google Scholar]
- 47. Miller IN, Cronin‐Golomb A. Gender differences in Parkinson's disease: clinical characteristics and cognition. Mov Disord 2010;25:2695–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Samuelsson K, Kostulas K, Vrethem M, et al. Idiopathic small fiber neuropathy: phenotype, etiologies, and the search for fabry disease. J Clin Neurol 2014;10:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doppler K, Volkmann J, Sommer C. Skin biopsies in the differential diagnosis of parkinsonism: are we ready for simplified protocols? Brain 2016;139(Pt 1):e5. [DOI] [PubMed] [Google Scholar]
- 50. Peng C, Gathagan RJ, Covell DJ, et al. Cellular milieu imparts distinct pathological alpha‐synuclein strains in alpha‐synucleinopathies. Nature 2018;557:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. (Methods) Phosphorylated alpha synuclein and aggregated alpha synuclein immunofluorescence and statistical analysis.


