Abstract
Introduction:
Autologous platelet-based concentrates represent increasingly popular adjuncts to a variety of medical, surgical and aesthetic interventions. Their beneficial potential rests on the ability to deliver a high concentration of growth factors to the target tissues. There are currently no reports in the literature appraising the evidence behind the use of platelet-rich plasma (PRP) in scar management.
Methods:
A detailed English literature review was conducted using PubMed Medline, Embase and Web of Science; the manuscripts were appraised and classified according to the Joanna Briggs Institute Levels of evidence. The results are presented in descending order of evidence separately for atrophic, keloid, surgical and traumatic scars.
Discussion:
On the basis of level 1 evidence currently available, it appears that PRP can improve the quality of atrophic acne scars treated with ablative fractional CO2 laser and decrease the duration of laser-related side effects including oedema and erythema. Regarding surgical scars, the current data suggest that PRP may improve wound healing and early scar quality; furthermore, incorporation of PRP in fat-grafting procedures undertaken in conjunction with non-ablative, fractional laser can contribute to better wound healing as well as a significant improvement in texture, colour and contour in traumatic scar resurfacing. There are no high level studies at present to support the incorporation of autologous platelet-based concentrates in the management of keloid scars.
Conclusion:
PRP is a promising adjunct in scar management practice. Further research with long-term follow-up is warranted to delineate the value of this modality in different subtypes of scars.
Keywords: Atrophic, concentrate, keloid, management, platelet, platelet-rich plasma, PRP, scar, traumatic
Lay summary
Platelet-rich plasma (PRP) is an increasingly popular product used in a variety of medical, surgical and aesthetic interventions; it is derived by spinning down a patient’s own blood and applying it back to an area of the body undergoing an intervention. We undertook this study to find out whether the use of PRP can have a beneficial effect on scars. We conclude that at present there is some evidence that it may improve the quality of depressed acne scars and ameliorate the duration of side effects associated with fractional laser treatment. Furthermore, PRP can improve healing parameters and early scar quality following a Caeserean section as well as enhance outcomes if used in combination with fat grafting and fractional laser for the revision of traumatic scars. The evidence behind the role of PRP for the management of keloid scars is low at present. Most studies do not assess long-term results, so further research is needed before PRP is widely adopted in scar management protocols.
Introduction
Platelet-rich plasma (PRP) is an autologous blood-derived product enriched in platelets, growth factors and chemo/cytokines delivered in a concentrated volume of plasma. Since the 1970s, PRP has received significant attention as applied to tissue repair and regeneration.1,2 Initial studies focused predominantly on applications within the musculoskeletal and maxillofacial fields; however, in recent years, it has been used for a range of dermatological indications including wound healing, fat grafting, alopecia, scar management as well as soft-tissue volume augmentation.3
PRP has the potential to deliver a high concentration of growth factors to target tissues by virtue of the contents within the alpha and dense granules.
a) Alpha granules contain seven fundamental growth factors: platelet-derived growth factors (PDGFaa, PDGFbb and PDGFab); transforming growth factor beta (isoforms TGFβ1 and 2); epithelial growth factor (EGF); and vascular endothelial growth factor (VEGF). These modulate cell proliferation, differentiation, angiogenesis and chemotaxis;4,5
b) The dense granules contain bioactive agents including serotonin, histamine, dopamine, calcium and adenosine; these can increase membrane permeability and modulate inflammatory processes.6,7
Degranulation of these organelles results in the release of pre-packaged growth factors, many of which have short half-lives; therefore, greater effectiveness may result if they are activated at or just before application. PRP has 3–5 times the concentration of platelets normally found in wounds and the resulting growth factor release following activation can further stimulate cell proliferation and differentiation towards tissue regeneration.8
PRP is prepared either manually or using automated devices or kits. In the manual method, blood is withdrawn from the patient, an anticoagulant is added and the mixture is centrifuged. In the double spin-method, blood is separated into three layers: platelet-poor plasma (PPP) at the top of the tube; PRP in the middle; and red blood cells (RBCs) at the bottom (image 1). The RBCs are discarded and following the second centrifugation, the PPP is discarded. The end product consists predominantly of PRP and thrombin or calcium chloride is subsequently added as a platelet activator.7,8 The value of the second centrifugation relates to the concept that after a single spin, the RBCs interfere with the fine separation of the platelets regardless of the rate or the time of centrifugation, hence producing a preparation with less percentage of PRP.9,10
Image 1.
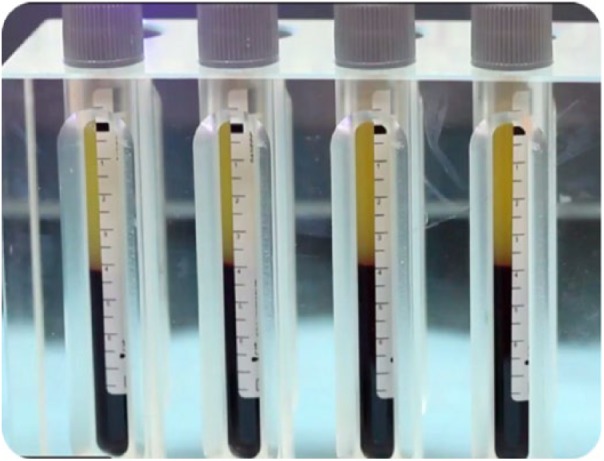
Appearance of whole blood centrifugates illustrating the separation of platelet (top) and red blood cell (bottom) portions before the platelet rich plasma (PRP) is extracted for clinical use.
Various automated devices and kits have been manufactured in the last few years in order to facilitate the delivery of ready-to-apply PRP without the need for manual separation. The limitation associated with the wide range of available automated devices/kits is that their products are likely to have different growth factor concentrations; this poses challenges in comparing clinical efficacy. Some of them have been approved by the U.S. Food and Drug Association (FDA), e.g. Smart PReP® (Harvest Technologies Inc, Plymouth, MA, USA), PCCS® (3i Implant Innovations Inc, West Palm Beach, FL, USA), BioMet GPSII®, etc.11
The current consensus classification system for the four main categories of autologous platelet concentrates is based on their fibrin architecture and cell content:
1. Pure PRP (P-PRP) or leukocyte-poor PRP: this is by far the most common category used in clinical practice in either liquid or gel form;
2. Leukocyte- and PRP (L-PRP): this can be delivered in either liquid or gel form;
3. Pure platelet-rich fibrin (P-PRF) or leukocyte-poor PRF: this is a solid preparation and therefore has limited clinical applications;
4. Leukocyte- and PRF (L-PRF) available in gel form only.
PRP can be applied as an isolated modality or as an adjunct to minimally invasive and surgical interventions given its potential to improve repair and regeneration.13–15 Scar management is an expanding field encroaching a number of disciplines including plastic surgery, dermatology and physical therapy to mention a few.
A number of animal models have been used over the last few years to assess the effectiveness of PRP in the treatment of different types of scars. There is preliminary evidence in a canine model16 that application of autologous PRP using the subcutaneous infiltration route at the wound margins can produce a significant enhancement of wound re-epithelisation and a reduction of scar formation at weeks 1 and 3 in comparison to controls. Malondialdehyde (MDA) concentration, which is a marker of oxidative damage, was also significantly decreased in PRP-treated wounds in this work; furthermore, the activity of matrix metalloproteinase 9 (MMP-9) reached its peak at the second week and was significantly higher in the PRP-treated group.
In another experimental rat uterine horn adhesion model, the inflammatory cytokine tumour growth factor 1 beta (TGF-1beta) expression was significantly reduced in the PRP-treated group compared to the control and hyaluronic acid groups; these findings prompted the authors to conclude that PRP is a promising and effective adjunct in the prevention of postoperative adhesion development.17 The aim of this article is to review the current available evidence on the efficacy of platelet preparations in the management of scars and present future directions for research in the field.
Methodology
A detailed English literature review was conducted of the following databases: PubMed Medline; Embase; and Web of Science. The following keywords were used: ‘platelet-rich plasma AND scar’; ‘PRP AND scar’; ‘PRP AND keloid’; ‘hypertroph* AND platelet-rich AND plasma’; ‘platelet-rich plasma AND hypertroph* AND scar’. Our search retrieved 52 citations; all abstracts were screened by both authors to ensure relevance. A total of 36 articles were selected for inclusion into our work; following full detailed manuscript review, 20 were deemed relevant to be presented in our analysis. The selected papers were appraised and classified according to the Joanna Briggs Institute Levels of evidence with the help of an independent research consultant in evidence synthesis.18
We present our findings based on a categorisation system according to the type of scars platelet-rich preparations were applied to, namely atrophic, keloid, surgical and traumatic scars.
Results
Atrophic acne scars
Atrophic scars are the most common type of acne scars; they can be further subdivided into ice pick, boxcar and rolling subtypes.16 A variety of management modalities have been employed including laser resurfacing, chemical peeling, dermal fillers, dermabrasion, needling, subcision and punch excision.17 PRP is a recently introduced adjunct and has been used predominantly in combination with other modalities.
Level 1
Lee et al.19 carried out a split face study in 14 Korean patients who underwent ablative fractional laser resurfacing and were randomly assigned to receive either injectable PRP or normal saline following the treatment episode. The PRP was extracted with a double spin-method and produced 6 mL for each patient; injection points were spaced 1.5–2 cm apart.
The laser settings were: pulse energy 25 mJ per fixed 150-μm diameter microbeam and a density of 400 MTZ/cm2 with concurrent forced air cooling for epidermal protection; assessment was performed with the help of a standardised photographic method by blinded dermatologists. Erythema on the PRP side improved faster compared to controls and was significantly less at day 4 as confirmed by chromometer readings (P = 0.01 and 0.047, respectively). Duration of oedema as well as crusting were significantly shorter in the PRP group (6.1 ± 1.1 vs 7.1 ± 1.5 days and 5.9 ± 1.1 vs. 6.8 ± 1.0 days, P = 0.04) and the overall clinical improvement four months after treatment was superior on the PRP arm (2.7 ± 0.7 vs. 2.3 ± 0.5, P = 0.03).
In another study, Gawdat et al.20 compared the efficacy of topical versus intradermal PRP (double-spin method) after ablative fractional CO2 laser (FCL) using a single-blind randomised split-face study design in 30 patients with facial atrophic acne scars (Fitzpatrick skin types III to V). Group 1 underwent ablative fractional CO2 laser followed by intradermal PRP on one side and intradermal saline on the other; Group 2 had FCL followed by intradermal on one side and topical PRP on the other side. Each patient received three treatment sessions at monthly intervals. The settings for the laser were as follows: 15 W, dwell time 600 ms, spacing 700 μm, smart stack level 2. The injectable PRP and saline were administered in 0.2-mL volumes and injected at 1.5 cm apart; topical PRP was applied in 2-mL volumes. Photographic assessment comparison by a blinded physician using a four-point scale (excellent to poor) at three months after the last session showed that the combination of ablative fractional CO2 laser and PRP (topical and intradermal administration) showed significantly better results than ablative fractional CO2 laser alone (P = 0.03). Interestingly, no significant difference was observed between topical and intradermal PRP adjuvant administration (P = 0.10). Results based on a patient clinical satisfaction scale showed a similar trend to the above physician-reported outcomes. Side effects including erythema, oedema, post-inflammatory hyperpigmentation and acneiform reaction were all of a significantly shorter duration in the PRP-treated areas (P = 0.02) leading to significantly shorter downtime. Results regarding scar depth using optical coherence tomography showed that the PRP-treated areas were improved in a statistically significant manner (P = 0.01). In conclusion, this study showed that the combination of PRP and ablative fractional CO2 laser produced a better resurfacing response, fewer side effects and quicker recovery than laser alone; additionally, given that there was no statistically significant difference between the results obtained with topical versus intradermal PRP, the authors advocate the topical administration in order to minimise discomfort associated with the injectable route.
Level 2
Nofal et al.8 conducted a quasi-experimental prospective controlled study on 45 patients with atrophic acne scars of varying severity. The cohort was divided into three groups of 15 patients each undergoing one of the following treatments: (1) intradermal injection of PRP; (2) application of 100% trichloroacetic acid (TCA)-CROSS; and (3) combination therapy of skin microneedling and topical PRP. Each patient underwent three sessions at two-weekly intervals. Results were assessed using the qualitative global acne scarring grading system (QGSGS) by two blinded dermatologists using photographs before and two weeks after the last treatment. Patient satisfaction ratings were also obtained. QGSGS findings suggested that a highly significant improvement in scar severity was seen after all modalities (P < 0.001); namely, an excellent to very good rating was found in 46.7% of the PRP group, 26.7% of the TCA CROSS group and 60% of the PRP with microneedling group. Nevertheless, none of the three treatments were significantly superior in comparison (P = 0.87) with regards to the quartile grading scale as well as patient satisfaction ratings. Limitations of this study include apart from the small cohort size, the large number of variables (including the number of treatment sessions used, the depth of needling as well as the mode of PRP administration), which make any valid conclusions challenging.
Similarly, Ibrahim et al.21 compared microneedling alone to combined microneedling and PRP for post-acne atrophic scars as part of a split-face comparative study involving 35 patients. All patients (Fitzpatrick skin types I–IV) were treated with four sequential microneedling sessions using a 1.5 mm dermaroller to pinpoint bleeding alone on the right side of the face, and a combination of microneedling and topical PRP on the left side with an interval of three weeks between sessions. The follow-up period was three months following which, two blinded dermatologists performed photographic evaluation using the Goodman and Baron grading system. Patients also graded their response as poor, good, very good or excellent. Both treatment modalities produced a significant improvement in the global acne scoring system, namely from 3.2 ± 0.7 to 1.8 ± 0.6 for the right side and 2.1 ± 1.1 for the left side (P < 0.001); nevertheless, the difference between both modalities were not statistically significant (P = 0.73). A similar trend was observed with regards to the patient satisfaction scores for both modalities. Interestingly, there was a statistically significant difference in the post-procedural erythema and oedema in favour of the PRP-treated side (P < 0.001); hence the authors concluded that PRP can minimise side effects of microneedling in acne management.
Another split-face randomised controlled study was performed by Faghihi et al.22 in which 16 patients (Fitzpatrick skin type II–IV) with atrophic acne scars received ablative fractional CO2 laser combined with intradermal PRP treatment on one half of their face and laser with intradermal normal saline (NS) on the other half. The PRP was prepared using a two-stage centrifugation process and was injected intradermally within 2-cm intervals to an overall volume of 0.2-mL following ablative CO2 laser session (settings: power 25 W; duration of 3; energy 30 mJ; pixel pitch of 1; and ablation depth of 600 μm). Participants received two treatment cycles one month apart. Serial digital photography at baseline, one month after the first session and four months after the second session were obtained and a quartile improvement grading scale was used by two blinded dermatologists to evaluate the overall clinical improvement. Participants were asked to grade overall satisfaction based on a range between 0–3 (slightly to very satisfied) and a 0–10 visual analogue scale was used to record adverse effects (erythema and oedema). The overall clinical improvement of acne scars was higher on the PRP-laser treated side, but the difference was not statistically significant either at one month following the first session or four months following the final session (P = 0.15 and 0.23). Moreover, the adverse effects including erythema and oedema were more severe and of longer duration in the PRP-laser treated side in a statistically significant manner. In this work, PRP addition to the laser modality appears to produce more severe side effects and longer downtime.
Abdel et al.23 conducted a similar study in which he treated 30 patients suffering from post-acne scars with ablative fractional CO2 laser (settings: 15 W, 600 ms dwell time, spading 700 μm, smart stack level 3); Only the right side of the face received intradermally injected autologous PRP (0.1 mL per point separated by 1–1.5cm). The laser was applied in two separate sessions (every 3–4 weeks) and patient’s follow-up was completed six months after the final laser session. Assessment was done by two blinded dermatologists based on digital photographs; additionally patients filled out a questionnaire to grade their improvement. The overall improvement of the right side based on the Qualitative Global Grading System was better than on the left side (P < 0.001). The resolution of erythema following the laser was faster on the PRP-treated side (P = 0.0052) and post-inflammatory pigmentation did not occur on the treated side; the occurrence of acneiform eruption was also significantly lower on the treated side. In addition, patient satisfaction was also higher on the PRP-treated side (P < 0.001).
Level 3
Chawla24 performed a study to investigate the efficacy of PRP versus vitamin C as adjuncts to microneedling for the treatment of atrophic post-acne scars as part of a split-face prospective study. Four sessions separated by a four-week interval were offered to 30 patients (Goodman and Baron grades II–IV), 23 of which completed the study. A double-spin method was used for the PRP and 1.5-mm needling rollers were used. At the end of the four treatments, photographic assessment was undertaken by the patient and treating physician and improvement was graded on a scale from poor to excellent. Results suggest that PRP compared favourably as contributing to an excellent outcome by the physician (18.5% vs. 7%) and also to those who had a poor response (37% vs. 22.2%); additionally, patient scores indicated that patients were more satisfied with the PRP adjunct (P = 0.01).
Level 4
Zhu et al.25 examined the combination of topical PRP with erbium fractional laser for the treatment of 22 patients (Fitzpatrick type III or IV) with facial atrophic acne scars. The settings for the laser treatment were: pulse duration 300–600 ms; pulse energy 600–1200 mJ; microbeam diameter 2–7 mm; and penetration depth 18–24 μm. At 1–3 month follow up, digital photography assessed by two blinded dermatologists showed difference of 2.77 ± 0.39 corresponding to moderate improvement; additionally, self-evaluation using a quartile grading scale was shown to have improved by 3.3 ± 0.36 and 91% of the patients were ‘very satisfied’.
Nita et al.26 treated 64 patients suffering from 43 atrophic and 21 ‘contractile’ scars involving different body parts with combination of ablative fractional CO2 laser, PRP and autologous fat grafting. Standard fat harvesting and the Coleman technique were employed for the lipofilling technique and fractional CO2 laser settings (power 9–12 W, time 4 ms, medium density) were matched to skin type. The PRP was obtained after two centrifugations (GLOFIN, Salo, Finland kit) and injected in the mid to deep dermis. At six-month follow-up, the overall patient satisfaction rate was > 50% (55.81% for atrophic and 52.38% for contractile scars). The authors proposed that the combination of the three modalities seems to be an effective approach for scars. Table 1 summarises the salient literature reports relating to the use of PRP and atrophic acne scars.
Table 1.
A summary of the different studies investigating the role of PRP in the management of atrophic acne scars.
| Author, reference | Level of evidence | Patient clinical criteria | Study design | Follow-up | Outcomes |
|---|---|---|---|---|---|
| Lee et al.19 | Split-face RCT (1c) | N = 14 Patients with post-acne atrophic scars |
Patients underwent ablative fractional laser (pulse energy 25 mJ per fixed 150-μm diameter microbeam and a density of 400 MTZ/cm2) resurfacing and were randomly assigned to receive either injectable PRP or normal saline following the treatment episode | Assessment was performed on days 0, 2, 4, 6, 8, 15 and 30 through standardised photographic assessment by blinded dermatologists | The overall clinical improvement four months after treatment was better on the PRP arm (2.7 ± 0.7 vs. 2.3 ± 0.5, P = 0.03). Erythema on the PRP side improved faster compared to controls and was significantly less at day 4 and confirmed by chromometer (P = 0.01 and 0.047, respectively). Duration of oedema as well as crusting were significantly shorter in the PRP groups (6.1 ± 1.1 vs. 7.1 ± 1.5 days and 5.9 ± 1.1 vs. 6.8 ± 1.0 days, P = 0.04) |
| Gawdat HI et al.20 | RCT (1c) | N = 30 Patients with post-acne atrophic scars |
30 patients were randomly divided into two groups. Group 1 was administered fractional carbon dioxide laser (15 W, dwell time 600 ms, spacing 700 μm, smart stack level 2) followed by intradermal PRP on one side and fractional carbon dioxide laser followed by intradermal saline on the other. In group 2, one cheek was treated with fractional CO2 laser followed by intradermal PRP, and the other received fractional CO2 laser followed by topical PRP | Each patient received three treatment sessions at monthly intervals. Photographic assessment comparison by a blinded physician using a four-point scale (excellent to poor) at 3 months after the last session |
The combination of ablative fractional CO2 laser and PRP (topical and intradermal) showed significantly better results than ablative fractional CO2 laser alone (P = 0.03); nevertheless, no significant difference was observed between topical and intradermal PRP adjuvant administration (P = 0.10) |
| Nofal E et al.8 | Quasi-experimental prospectively controlled study (2c) | N = 45 Patients with atrophic post-acne scars |
Patients were randomly assigned to three equal groups: group A (intra-dermal PRP); group B (100% TCA peel); group C (combined skin needling and topical PRP). Results were assessed using the qualitative global acne scarring grading system (QGSGS) and patient satisfaction ratings. | Digital colour facial photographs were taken at baseline, and at the end of follow-up (2 months after the last session) | QGSGS findings suggested that a highly significant improvement in scar severity was seen after all modalities (P < 0.001), the highest was 60% with the PRP with microneedling group; nevertheless, none of the three treatments were significantly superior in comparison (P = 0.87) with regards to both outcomes from the quartile grading scale and patient satisfaction |
| Ibrahim MK et al.21 | Quasi-experimental prospectively controlled study (2c) | N = 35 Patients with post-acne atrophic scars |
All patients were treated with four sequential microneedling sessions using a 1.5mm dermaroller alone on the right side of the face, and combination of microneedling and topical PRP (double-spin method) on the left side with an interval of 3 weeks | The follow-up period was 3 months following which, two blinded dermatologists performed photographic evaluation using the Goodman & Baron grading system | Both treatment modalities produced a significant improvement in the global acne scoring system, namely from 3.2 ± 0.7 to 1.8 ± 0.6 for the right side and 2.1 ± 1.1 for the left side (P < 0.001); nevertheless, the difference between both modalities were not statistically significant (P = 0.73). Interestingly, there was statistically significant erythema and oedema in the PRP-treated side (P < 0.001) |
| Faghihi G et al.22 | Quasi-experimental prospectively controlled study (2c) | N = 16 Patients with post-acne atrophic scars |
Patients received ablative fractional CO2 laser (power 25 W, duration of 3, energy 30 mJ, pixel pitch of 1 and ablation depth 600 μm) combined with intradermal PRP treatment on one half of their face and laser with intradermal normal saline (NS) on the other half | Serial digital photography at baseline, 1 month after the first session and 4 months after the second session were obtained and a quartile improvement grading scale was used by two blinded dermatologists to evaluate the overall clinical improvement | The overall clinical improvement of acne scars was higher on the PRP-laser treated side, but the difference was not statistically significant either one month after the first session or four months after the final session (P = 0.15 and 0.23). Moreover, the adverse effects including erythema and oedema were more severe and of longer duration than the NS-laser-treated side in a statistically significant manner |
| Abdel Aal AM et al.23 | Quasi-experimental prospectively controlled study (2c) | N = 30 Patients with post-acne scars |
Patients were treated with ablative fractional CO2 laser (15 W, 600 ms dwell time, spading 700 μm, smart stack level 3) on both sides of the face and then only the right side received intradermally injected autologous PRP (0.1ml per point separated by 1–1.5cm. The laser was applied in two separate sessions (every 3–4 weeks) | Follow-up was completed 6 months after the final laser session. Assessment was done by two blinded dermatologists based on digital photographs; additionally patients filled out a questionnaire to grade their improvement | The overall improvement of the right side based on the QGSGS was better than on the left side (P < 0.001). The resolution of erythema following the laser was faster on the PRP-treated side (P = 0.0052) and post-inflammatory pigmentation did not occur on the treated side ; the occurrence of acneiform eruption was also significantly lower on the treated side. Patient satisfaction was also higher on the PRP treated side (P < 0.001) |
| Chawla24 | Observational split-face study (3) | N = 30 (23 completed the study) Patients with post-acne atrophic scars | Patients were offered four sessions of microneedling with PRP on one side and microneedling with vitamin C on other side of the face with an interval of 4 weeks between sessions. | At the end of the four treatment sessions, photograph assessment was undertaken by the patient and treating physician and improvement was graded on a scale from poor to excellent | PRP compared favourably as contributing to an excellent outcome by the physician (18.5% vs. 7%) and also to those who had a poor response (37% vs. 22.2%); additionally, patient scores indicated that patients were more satisfied with the PRP adjunct (P = 0.01) |
| Zhu JT et al.25 | Case series (4c) | N = 22 Patients with post-acne scars |
PRP combined with erbium fractional laser therapy (300–600 ms, pulse energy 600–1200 mJ, microbeam diameter 2–7 mm, penetration depth 18–24 μm) | Follow-up after 1–3-month interval, based on digital photographs assessed by two blinded dermatologists | The magnitude of difference was found to be 2.77 ± 0.39 corresponding to moderate improvement. Self-evaluation using a quartile grading scale improved by 3.3 ± 0.36 and 91% of the patients were ‘very satisfied’ |
| Nita AC et al.26 | Case series (4c) | N = 64 Patients with atrophic and ‘contractile’ scars |
Patients were treated with combination of ablative fractional CO2 laser (power 9–12 W, time 4 ms, medium density) with PRP and autologous fat graft | The follow-up took place after 1 week, 1, 3 and 6 months, using digital photographs | At six-month follow-up, the overall patient satisfaction rate was > 50% (55.81% for atrophic and 52.38% for ‘contractile’ scars). |
Keloid scars
Level 4
Keloids represent a particularly challenging subset of scars for which a variety of therapeutic approaches have been described including surgical excision and radiotherapy, intralesional steroid injection and cryotherapy.27 Jones et al.28 recruited 40 patients (with 44 keloid scars) and treated them using a combination of:
Extralesional surgical excision;
PRP (2–3 mL) applied to the excision wound bed and incision site; and
Postoperative superficial photon X-ray radiation therapy within 72 h of excision (cumulative dose of 13–18 Gy delivered as 2–3 fractions).
All patients were advised to use a corticosteroid-based cream (0.5% hydrocortisone) twice daily for the first three months; additionally, triamcinolone injections were administered to four patients who were scored ‘poor’ on the Kyoto Scar Assessment Scale (KSAS); the latter was employed to assess scar outcomes in the study. This novel protocol approach achieved a recurrence rate of 4.5% (defined as induration, hypertrophy or extraordinary erythema beyond the site of excision) at a minimum three-month follow-up (range = 3–11 months). Assessment using the KSAS revealed that 61% achieved an excellent rating, 24% good, 3% fair and 12% poor. Radiation-induced hyperpigmentation was noted in all patients in the study. Limitations of the study included the short follow-up period, the non-standardised radiation protocol used as well as the variable use of triamcinolone; these factors make it very challenging to identify the exact contribution of PRP in the final recurrence rate. The same author29 reported results of a retrospective study on 49 patients with 50 ear keloids treated with the same combination therapy as described above and achieved a 6% recurrence rate with a two-year follow-up period.
Currently, there is an ongoing randomised controlled trial (RCT) to assess the efficacy of autologous PRP administered immediately after complete surgical excision and then subsequently within the first month postoperatively on three occasions.30 Given the limitations of the reported studies so far, results are eagerly awaited to assess the role of PRP in keloid scar management. Table 2 summarises the salient literature reports relating to the use of PRP and keloid scars.
Table 2.
A summary of the different studies investigating the role of PRP in the management of keloid scars.
| Author, reference | Level of evidence | Patient clinical criteria | Study design | Follow-up | Outcomes |
|---|---|---|---|---|---|
| Jones ME et al.16 | Case series (4c) | N = 40 Patients with 44 keloid scars |
Patients with keloid scars were treated using surgical excision, PRP and postoperative superficial photon X-ray radiation therapy within 72 h of excision (cumulative dose of 13–18 Gy delivered as 2–3 fractions) | Assessment was performed on day 10 and at 1, 3, 6 and 9 months; recurrence was determined by examination and photo documentation | This approach achieved a 4.5% recurrence rate at a minimum of 3 months follow-up. Assessment using the Kyoto Scar Assessment Scale (KSAS) revealed that 61% achieved an excellent rating, 24% good, 3% fair and 12% poor |
| Jones ME et al.17 | Case series (4c) | N = 49 Patients with 50 ear keloids |
Patients were treated with extralesional surgical excision of keloids localised to the ear followed by the application of autologous PRP to wound site and postoperative superficial photon X-ray radiation therapy within 72 h of excision (cumulative dose of 13–18 Gy delivered as 2–3 fractions) | On completion of initial protocol, patients are instructed to follow-up on day 10 and 1, 3, 6, 9 and 12 months postoperatively | This approach achieved a 6% recurrence rate on follow-up over a 2-year period |
Surgical scars
Surgical or postoperative scars have received special attention in the literature in the last few years and a limited number of studies have investigated the role of PRP in optimising final scar quality.
Level 1
Tehranian, et al.31 conducted a RCT involving 140 patients undergoing elective Caesarean delivery. They were randomly allocated into two groups; the intervention group received PRP applied to the subcutaneous tissues of the wound before closure, whereas the control group received the usual care (i.e irrigation of the wound with saline before closure). Patients were examined by blinded physicians on days 1 and 5 as well as eight weeks after the procedure using a visual analogue scale for postoperative pain (VAS) and the Redness, Oedema, Ecchymosis, Discharge, Approximation (REEDA) scale to assess wound-healing progress; the Vancouver Scar Scale (VSS) was additionally used to grade the quality of scar formation. The authors identified that patients who were treated with topical PRP had a significant reduction in the REEDA score (85.5% for PRP vs. 72% for control group, P < 0.0001) implying better healing progress. Regarding the VSS, treatment with PRP had a significant effect on reducing the score beginning on the fifth day and continuing with a stable trend at the end of the eight weeks (54% vs. 18% reduction, P < 0.001). Furthermore, based on the VAS score for pain, PRP contributed to statistically significant reduction of pain experienced at the end of the follow-up period (93% vs. 79%, P < 0.001). Limitations of this study include the very short follow-up, which did not extent into the remodelling phase of scar maturation.
Level 4
Azzena et al.32 reported a case of a painful adherent postoperative scar following a shoulder replacement surgery in which they injected a gel mixture of autologous adipose tissue combined with PRP into a subcutaneous pocket using a novel in vivo adipocyte delivery system. The patient reported complete remission of pain and ultrasonography performed six months and one year after treatment showed enhanced fat survival and resolution of the adhesion with the underlying fascia. Table 3 summarises the salient literature reports relating to the use of PRP and surgical scars.
Table 3.
A summary of the different studies investigating the role of PRP in the management of surgical scars.
| Author (ref.) | Level of evidence | Patient clinical criteria | Study design | Follow-up | Outcomes |
|---|---|---|---|---|---|
| Tehranian A et al.31 | RCT (1c) | N = 140 Patients who underwent elective Caesarean delivery |
Patients were randomly allocated into two groups; the intervention group received PRP applied to the subcutaneous tissues of the wound before closure, whereas the control group received the usual care (irrigation of the wound with saline before closure) | Patients were examined by blinded physicians on days 1 and 5 as well as 8 weeks following the procedure using a visual analogue scale (VAS) for postoperative pain, the Redness, Oedema, Ecchymosis, Discharge, Approximation (REEDA) scale to assess wound healing progress and the Vancouver Scar Scale (VSS) for the quality of scar formation | Topical PRP had a significant effect on reducing the REEDA score (85.5% for PRP vs. 72% for control group, P < 0.0001), implying better healing progress. Additionally, PRP significantly reduced the VSS score beginning on day 5 and continued with a stable trend at the end of week 8 (54% vs. 18% reduction, P < 0.001). Furthermore, pain was significantly reduced at the end of the follow-up period in the PRP group. (93% vs. 79%, P < 0.001) |
| Azzena B et al.32 | Case study (4d) | N = 1 Patient with postoperative scar following shoulder replacement surgery |
A single painful adherent postoperative scar treated with a gel mixture of autologous adipose tissue combined with PRP using an in vivo adipocyte delivery system | 6 months and 12 months after treatment using pain assessment and ultrasound imaging | Complete remission of pain; ultrasonography performed 6 months and 12 months after treatment showed fat survival and resolution of the adhesions |
Traumatic scars
Scarring following trauma can lead to both aesthetic and functional sequelae for patients;33 a small number of studies have investigated the role of PRP in this subset of scars.
Level 1
Cervelli et al.33 recruited 60 patients (Fitzpatrick skin types II–IV) affected by traumatic scars of varying aetiology in different bodily parts. They were randomly allocated to one of three groups (20 patients each): group A was treated with fat grafts mixed with PRP at one and three months; group B underwent four sessions of 1540-nm non-ablative laser alone (settings: 20–40 J/cm2 using a 10-mm fractional handpiece); and group C was treated with a combination of both procedures (laser at one and three months delivered seven days after the graft/PRP). The PRP was extracted with a single-spin preparation and was added to the fat (harvested using the Coleman technique) before injection at volumes in the range of 5–50 mL depending on the defect. The combined modality group showed greater overall clinical improvement in comparison to the other groups as assessed using the Manchester Scar Scale (MSS). The most effective scar treatment was the combination of the fat graft-PRP and non-ablative laser resurfacing in group C, which had increases in wound healing of 22% and 11% compared with groups A and B, with significant improvement in texture, colour and scar contours on MSS. The authors concluded that the addition of PRP to a combination of fat grafting and non-ablative 1540-nm laser increases the efficacy of the combined scar management strategy.
Level 2
Gentile et al.34 conducted a comparative study on 20 patients with burn and traumatic scars using either stromal vascular fraction (SVF)-enhanced autologous fat grafts or 1 mL of Coleman-based fat grafting mixed with 0.5 mL of PRP; in this study a control group of 10 patients were treated with centrifuged fat without PRP addition. The fat re-implantation was performed following scar subcision with 1.5-mm diameter cannulas. In both groups (study and control), one operation was required in six cases and two in four cases. Evaluation was performed using photographic team evaluation and radiological assessment as well as patient self-evaluation. In patients treated with PRP-enriched fat, 69% maintenance of contour and three-dimensional volume after one year was observed in comparison to the control group. Magnetic resonance imaging (MRI) additionally showed that patients treated with PRP as well as SVF-enriched fat showed lower fat absorption. The authors concluded that use of PRP during fat grafting improves adipose tissue maintenance and survival.
Level 4
Majani et al.35 evaluated the results of lipografting in 28 patients with different types of scars (including burn cicatricial, traumatic and postoperative scars). Eleven patients (group 1) received lipografting only and 11 (group 2) were treated with PRP 7–10 days before the surgery; six patients (group 3), who had symmetrical scars, were treated on the left side only with lipografting and on the right side with a combination therapy of PRP and lipografting. The PRP was obtained using a single-spin technique and infiltrated into the scars in volumes in the range of 1–8 mL and fat was injected in volumes in the range of 8–37 mL. Patients were photographed at 30, 90 and 180 days postoperatively. Thirty days following lipografting, all patients showed better scar elasticity and the treated area showed evidence of aesthetic improvement. Ninety days after surgery, in three patients from group 1 and one patient from group 2, there was absorption of the injected fat. In patients from group 3, the increase was most evident on the right side. The authors concluded that a suitable preparation of the treated areas with the combination therapy of PRP and lipografting resulted in more durable corrections, particularly in situations where vascularization is more impaired. Table 4 summarises the salient literature reports relating to the use of PRP and traumatic scars.
Table 4.
A summary of the different studies investigating the role of PRP in the management of traumatic scars.
| Author, reference | Level of evidence | Patient clinical criteria | Study design | Follow-up | Outcomes |
|---|---|---|---|---|---|
| Cervelli V et al.33 | RCT (1c) | N = 60 Patients with traumatic scars |
Patients were randomly allocated to one of three groups (20 patients each): group A was treated with fat grafts mixed with PRP at months 1 and 3; group B was treated with four sessions treatment with 1540-nm non-ablative laser (20–40 J/cm2 using a 10-mm fractional handpiece) alone; and group C was treated with both procedures (laser at 1 and 3 months delivered 7 days after the graft/PRP) | The overall degree of patient satisfaction was assessed at 6-month follow-up, using a structured questionnaire grading the aesthetic and functional quality of the scar as excellent, good, fair or poor. Postoperative follow-up examinations were performed at weeks 1, 2, 4 and 8, and months 3 and 6 | The most effective scar treatment was the combination of the fat graft, PRP and non-ablative laser resurfacing in group C, which produced increases in wound healing of 22% and 11% compared with groups A and B, with significant improvement in texture, colour and scar contours on Manchester Scar Scale (MSS). At 6 months, 84% patients evaluated the scar appearance as good to excellent and 16% as poor to fair |
| Gentile P et al.34 | Quasi-experimental prospectively controlled study (2c) | N = 20 Patients with burn and traumatic scars |
Patients were treated using either stromal vascular fraction (SVF)-enhanced autologous fat grafts or 1 mL of Coleman-based fat grafting mixed with 0.5 mL of PRP. There was a control group of 10 patients who were treated with centrifuged fat without PRP addition. The fat re-implantation was performed following scar subcision with 1.5-mm diameter cannulas | Evaluation was performed via photographic team evaluation, radiological assessment (MRI and ultrasound) as well as patient self-evaluation | In patients treated with PRP-enriched fat, a 69% maintenance of contour and 3D volume after 1 year was seen compared to the control group. Additionally, MRI showed that patients treated with PRP as well as SVF-enriched fat showed lower fat absorption |
| Majani et al.35 | Case series (4c) | N = 28 Patients with different types of scars (6 cicatricial burn scars, 12 scars from previous plastic surgery, 2 post-Caesarean section, 4 post-general surgery and 4 patients traumatic scars) |
All patients were treated using lipografting: 11 patients (group 1) without PRP; 11 patients (group 2) were treated with PRP (7–10 days before); 6 patients (group 3), with symmetrical scars, were treated on the left side with lipografting and on the right side with a combination of PRP and lipografting | Patients were followed-up and photographed at 30, 90 and 180 days postoperatively | 30 days following lipografting, better scar elasticity and evidence of aesthetic improvement was observed. 90 days after surgery, in three patients from group 1 and one patient from group 2, there was absorption of the injected fat. In patients from group 3, the increase was most evident on the right side (i.e PRP and lipografting). |
Discussion
Based on the studies available in the field of atrophic acne scarring, there is level 1 evidence that PRP in conjunction with ablative fractional CO2 laser treatment may improve the overall clinical response/quality of scars attained and decrease the duration of laser related side effects including erythema and duration of oedema.19,20 Additionally, there appears to be no statistically significant difference between the intradermal and topical application of PRP after fractional CO2 laser treatment in terms of efficacy.20 Level 2 studies suggest that PRP can decrease erythema and oedema following 1.5-mm microneedling in a statistically significant manner.21 Another study in this category of evidence failed to show statistical superiority of PRP and skin microneedling compared with TCA CROSS and isolated intradermal PRP administration on the basis of QGSGS ratings.8 Furthermore, two other level 2 studies provided contradictory results around the role of PRP in ameliorating post-fractional CO2 side effects including erythema resolution.22,23 Some of the main limitations of the studies available in this arena include the small cohort sizes as well as their short follow-up period (range = 2–6 months); this is very limited given that the timescale of scar remodelling is considered to span over at least 12 months. Furthermore, the heterogeneity of treatment parameters (volume/concentration of PRP, laser settings, Fitzpatrick skin types) may render generalised conclusions challenging.
There is weak evidence (level 4) for the inclusion of PRP in the management of keloid scars employing surgery and radiotherapy. The non-comparative design of the two available studies, the selective use of steroids to a significant percentage of patients as well as the short follow-up period in one report (deviating significantly from the widely accepted minimum two-year period to assess long-term efficacy reliably) make valid conclusions challenging to draw.27,28 The results of the currently conducted RCT are eagerly awaited to appraise the value of PRP in keloid scar management.
Regarding surgical scars, there is level 1 evidence that autologous platelet preparations may improve wound healing and scar quality at eight weeks as well as mediate a reduction in postoperative pain following a Caesarean section; nevertheless, the short follow-up in the study does not provide an indication of the possible contribution of PRP towards better long term scar quality.31
Reviewing studies in resurfacing of traumatic scars, the addition of PRP in fat-grafting procedures combined with non-ablative, fractional 1540-nm laser appears to contribute to better wound healing compared to isolated modalities (PRP-enriched fat grafting and laser alone). Furthermore, MSS scores indicated a significant improvement in texture, colour and scar contours at six-month follow-up.33 Another level 2 study showed that PRP enrichment of autologous fat grafts can improve adipose tissue survival and maintenance during fat grafting at 12-month follow-up.34
Concluding remarks
This work appraised the literature concerning the use of PRP in scar management. The use of autologous plasma derived adjuncts has a number of potential advantages by virtue of the ability to deliver a high concentration of growth factors to target tissues and potentially improve wound healing and scarring parameters. The majority of studies currently available focus on the adjunctive use of PRP in the management of atrophic acne scars; nevertheless, there are significant shortcomings in this arena including the small cohort sizes appraised, the short follow-up periods as well as the heterogeneity of treatment parameters employed, which render generalised conclusions challenging. Further high-quality studies are eagerly awaited in order to further delineate to role of autologous platelet-derived adjuncts in scar management protocols.
Acknowledgments
We would like to acknowledge Ewelina Rogozinska (research consultant in evidence synthesis) for her assistance with the categorisation of the included studies into levels of evidence and Alistair Turner, BTI Biotechnology Institute, UK for providing the image included in the manuscript.
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Osaid H. Alser  https://orcid.org/0000-0001-6743-803X
https://orcid.org/0000-0001-6743-803X
References
- 1. Pierce GF, Mustoe TA, Altrock BW, et al. Role of platelet-derived growth factor in wound healing. J Cell Biochem 1991; 45: 319–326. [DOI] [PubMed] [Google Scholar]
- 2. Pierce GF, Mustoe TA, Lingelbach J, et al. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol 1989; 109: 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lynch MD, Bashir S. Applications of platelet-rich plasma in dermatology: A critical appraisal of the literature. J Dermatolog Treat 2016; 27(3): 285–289. [DOI] [PubMed] [Google Scholar]
- 4. Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents 2012; 26 (2 Suppl 1): 3s–22s. [PubMed] [Google Scholar]
- 5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 2004; 62: 489–496. [DOI] [PubMed] [Google Scholar]
- 6. Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009; 37: 2259–2272. [DOI] [PubMed] [Google Scholar]
- 7. Leo MS, Kumar AS, Kirit R, et al. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol 2015; 14(4): 315–323. [DOI] [PubMed] [Google Scholar]
- 8. Nofal E, Helmy A, Nofal A, et al. Platelet-rich plasma versus CROSS technique with 100% trichloroacetic acid versus combined skin needling and platelet rich plasma in the treatment of atrophic acne scars: a comparative study. Dermatol Surg 2014; 40(8): 864–873. [DOI] [PubMed] [Google Scholar]
- 9. Marx R. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent 2001; 10: 225–228. [DOI] [PubMed] [Google Scholar]
- 10. Nagata M, Messora M, Flávia A, et al. Effectiveness of two methods for preparation of autologous platelet-rich plasma: an experimental study in rabbits. Eur J Dent 2009; 4: 395–402. [PMC free article] [PubMed] [Google Scholar]
- 11. Kumaran MS. Platelet-rich plasma in dermatology: boon or a bane? Indian J Dermatol Venereol Leprol 2014; 80(1): 5–14. [DOI] [PubMed] [Google Scholar]
- 12. Dohan Ehrenfest DM, Andia I, Zumstein MA, et al. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J 2014; 4(1): 3–9. [PMC free article] [PubMed] [Google Scholar]
- 13. Alsousou J, Thompson M, Hulley P, et al. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br 2009; 91: 987–996. [DOI] [PubMed] [Google Scholar]
- 14. Alsousou J, Ali A, Willett K, et al. The role of platelet-rich plasma in tissue regeneration. Platelets 2013; 24: 173–182. [DOI] [PubMed] [Google Scholar]
- 15. Mosca MJ, Rodeo SA. Platelet-rich plasma for muscle injuries: game over or time out? Curr Rev Musculoskelet Med 2015; 8: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farghali HA, AbdElKader NA, Khattab MS, et al. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci Rep 2017; 37(2): BSR20160503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oz M, Cetinkaya N, Bas S, et al. A randomized controlled experimental study of the efficacy of platelet-rich plasma and hyaluronic acid for the prevention of adhesion formation in a rat uterine horn model. Arch Gynecol Obstet 2016; 294(3): 533–540. [DOI] [PubMed] [Google Scholar]
- 18. The Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. Supporting Document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation. Adelaide: The Joanna Briggs Institute, 2014. Available at: joannabriggs.org. [Google Scholar]
- 19. Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg 2011; 37(7): 931–938. [DOI] [PubMed] [Google Scholar]
- 20. Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg 2014; 40(2): 152–161. [DOI] [PubMed] [Google Scholar]
- 21. Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat 2018; 29: 281–286. [DOI] [PubMed] [Google Scholar]
- 22. Faghihi G, Keyvan S, Asilian A, et al. (2016). Efficacy of autologous platelet-rich plasma combined with fractional ablative carbon dioxide resurfacing laser in treatment of facial atrophic acne scars: A split-face randomized clinical trial. Indian J Dermatol Venereol Leprol 2016; 82(2): 162–168. [DOI] [PubMed] [Google Scholar]
- 23. Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet-rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther 2018; 20(2): 106–113. [DOI] [PubMed] [Google Scholar]
- 24. Chawla S Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg 2014; 7(4), 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu JT, Xuan M, Zhang YN, et al. The efficacy of autologous platelet-rich plasma combined with erbium fractional laser therapy for facial acne scars or acne. Mol Med Rep 2013; 8(1): 233–237. [DOI] [PubMed] [Google Scholar]
- 26. Nita AC, Orzan OA, Filipescu M, et al. Fat graft, laser CO2 and platelet-rich-plasma synergy in scars treatment. J Med Life 2013; 6(4): 430–433. [PMC free article] [PubMed] [Google Scholar]
- 27. Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg 2010; 125(2): 557–568. [DOI] [PubMed] [Google Scholar]
- 28. Jones ME, Hardy C, Ridgway J. Keloid management: a retrospective case review on a new approach using surgical excision, platelet-rich plasma, and in-office superficial photon X-ray radiation therapy. Adv Skin Wound Care 2016; 29(7): 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones ME, McLane J, Adenegan R, et al. Advancing keloid treatment: a novel multimodal approach to ear keloids. Dermatol Surg 2017; 43(9): 1164–1169. [DOI] [PubMed] [Google Scholar]
- 30. Regen Lab SA. Available at: https://clinicaltrials.gov/ct2/show/NCT02922972 (retrieved 7 February 2018).
- 31. Tehranian A, Esfehani-Mehr B, Pirjani R, et al. Application of autologous platelet-rich plasma (PRP) on wound healing after Caesarean section in high-risk patients. Iran Red Crescent Med J 2016; 18(7): e34449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azzena B, Mazzoleni F, Abatangelo G, et al. Autologous platelet-rich plasma as an adipocyte in vivo delivery system: case report. Aesthet Plast Surg 2008; 32(1): 155–158. [DOI] [PubMed] [Google Scholar]
- 33. Cervelli V, Nicoli F, Spallone D, et al. Treatment of traumatic scars using fat grafts mixed with platelet-rich plasma, and resurfacing of skin with the 1540 nm nonablative laser. Clin Exp Dermatol 2012; 37(1): 55–61. [DOI] [PubMed] [Google Scholar]
- 34. Gentile P, De Angelis B, Pasin M, et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical evaluation for cell-based therapies in patients with scars on the face. J Craniofac Surg 2014; 25(1): 267–272. [DOI] [PubMed] [Google Scholar]
- 35. Majani U, Majani A. Correction of scars by autologous fat graft and platelet rich plasma (PRP). Acta Med Mediterr 2012; 28: 99–100. [Google Scholar]
How to cite this article
- Alser OH and Goutos I. The evidence behind the use of platelet-rich plasma (PRP) in scar management: a literature review. Scars, Burns & Healing, Volume 4, 2018. DOI: 10.1177/2059513118808773; [DOI] [PMC free article] [PubMed] [Google Scholar]


